Abstract
Heterochromatin protein 1 (HP1), a highly conserved non-histone chromosomal protein in eukaryotes, plays important roles in the regulation of gene transcription. Each of the three human homologs of HP1 includes a chromoshadow domain (CSD). The CSD interacts with various proteins bearing the PXVXL motif but also with a region of histone H3 that bears the similar PXXVXL motif. The latter interaction has not yet been resolved in atomic detail. Here we demonstrate that the CSDs of all three human HP1 homologs have comparable affinities to the PXXVXL motif of histone H3. The HP1 C-terminal extension enhances the affinity, as does the increasing length of the H3 peptide. The crystal structure of the human HP1γ CSD (CSDγ) in complex with an H3 peptide suggests that recognition of H3 by CSDγ to some extent resembles CSD-PXVXL interaction. Nevertheless, the prolyl residue of the PXXVXL motif appears to play a role distinct from that of Pro in the known HP1β CSD-PXVXL complexes. We consequently generalize the historical CSD-PXVXL interaction model and expand the search scope for additional CSD binding partners.
Keywords: chromatin regulation, chromatin structure, heterochromatin, histone, peptide interaction
Introduction
An early study of heterochromatin protein 1 (HP1)2 in Drosophila melanogaster hinted at its possible involvement in the suppression of position effect variegation (1), a mosaic silencing phenomenon by which a euchromatic gene will be silenced when it is located near heterochromatin (2). HP1 is a non-histone chromosomal protein and is evolutionarily conserved from fission yeast to plants and animals (3). It plays important roles in formation and maintenance of higher order chromatin structure and regulation of gene transcription (3–5). There are three human homologs of HP1 proteins, namely HP1α (also known as CBX5), HP1β (CBX1), and HP1γ (CBX3). Null mutation of D. melanogaster HP1 (also known as Su(var)2–5) leads to defects in chromosome segregation and embryonic lethality (6), and knock-out mutation of murine HP1β causes perinatal lethality and defective development of neuromuscular junctions and cerebral cortex (7).
HP1s consist of an N-terminal chromodomain (CD) and a C-terminal chromoshadow domain (CSD), connected by a variable hinge region (HR) (Fig. 1A) (8). All of these three domains critically contribute to HP1 functions: the CD domain recognizes histone H3 methylated at lysine 9 (9, 10); the CSD mediates dimerization of HP1 to form a binding surface for diverse proteins containing the PXVXL motif (11–14); and the HR fragment, which harbors a nuclear localization signal, interacts with DNA and RNA (15, 16). It is believed that chromatin recruits HP1 via interactions between methylated histone H3 lysine 9 and the CD domain (9, 10, 17), but the HR and CSD are also involved (16, 18, 19). Recent studies have shown that the CSD recognizes a region of histone H3 near nucleosomal DNA entry/exit sites and that the CSD may facilitate HP1 recruitment to chromatin via this interaction (20–23). Lavigne et al. (21) found that CSDs of both HP1α and HP1γ bind to a region of histone H3 spanning amino acid residues 35–66, which contains a PXVXL-like PXXVXL motif. V46A mutation or truncation of the histone peptide to amino acid residues 44–66 abrogated binding to HP1α but not to HP1γ (21). Richart et al. (22) narrowed the interaction region of the HP1α CSD to residues 36–58 of histone H3. Dawson et al. (20) found that HP1α, but not HP1β, specifically recognizes the histone H3 fragment around Tyr-41 with its CSD and that this interaction is inhibited following phosphorylation of H3 tyrosine 41, which is catalyzed by Janus kinase 2 (JAK2). Jang et al. (23) discovered that phosphorylation of H3 threonine 45 prevents histone H3 binding to HP1α and HP1β, but not HP1γ.
Figure 1.
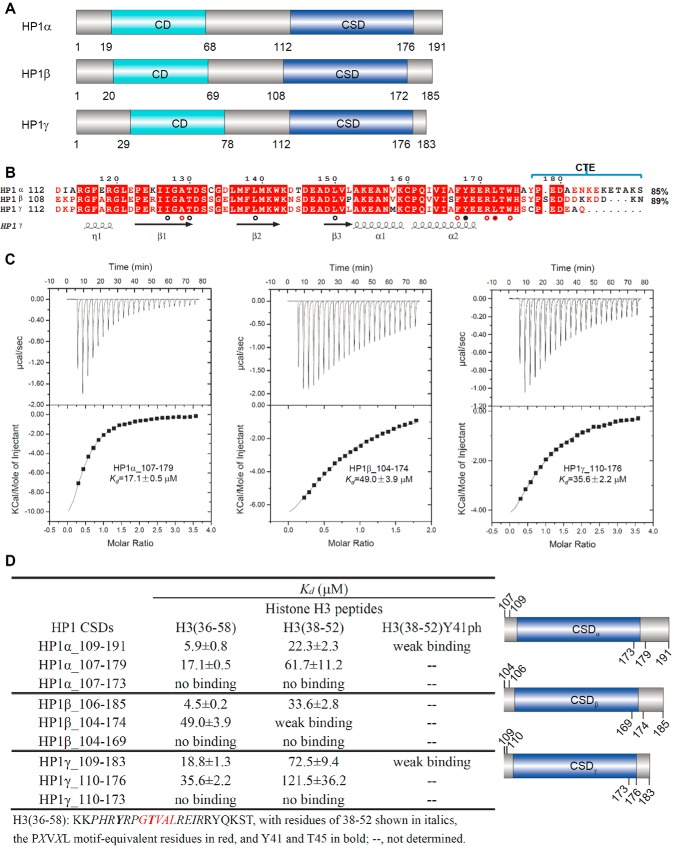
CSDs of HP1α/β/γ similarly bind to H3(36–58). A, schematic representation of the domain structure of human HP1 homologs. B, sequence alignment of human CSDs with structural annotation. Secondary structure elements and residue numbers of the CSDγ are indicated below or above the sequence alignment. Residues interacting with certain positions of the H3 peptide are marked as follows: position 0 (closed black circles), positions +2 and −2 (open red circles), and positions +5 and −5 (open black circles). The identities of CSDs of HP1α and HP1β to HP1γ are indicated at the end of the associated sequence. The alignments were constructed with ClustalW (56) and refined with ESPript (57). C, ITC binding curves of histone H3 peptide H3(36–58) to CSDα/β/γ. D, ITC binding affinities of histone H3 peptides to CSDα/β/γ. All Kd values were calculated from a single measurement, and errors were estimated by curve fitting. A schematic representation of the recombinant HP1 constructs expressed in E. coli for this assay is shown on the right.
Considering that the three mammalian HP1s share ∼65% of amino acid types over the length of the proteins (24), how do their even more highly conserved CSDs bind to histone H3 in different manners? To address this question, we carried out quantitative binding assays and structural analyses of human HP1 homologs' CSDs with the histone H3 PXXVXL motif. Our isothermal titration calorimetry (ITC) binding assays showed that all the three CSDs bind to the PXXVXL-containing fragment of histone H3 with comparable affinities, and the binding affinity is enhanced by both the C-terminal extension (CTE) of the HP1 homologs and the increasing length of the histone peptides. Our crystal structure of the HP1γ CSD (residues 110–176) in complex with an H3 peptide (residues 38–52) provides molecular insights into these observations, and the recognition of the PXXVXL motif by the CSD reveals the binding plasticity of the CSD and suggests an expanded ligand repertoire beyond the canonical PXVXL motif.
Results and discussion
CSDs of HP1α/β/γ bind to the H3 fragment H3(36–58) with a similar affinity
Previous studies have shown that the CSDs of human HP1 homologs recognize a histone H3 region with a PXVXL-like motif around the DNA entry/exit sites of nucleosome but that different CSDs nevertheless behave differently (20, 21, 23). The human genome encodes three HP1 proteins (Fig. 1A), and the amino acid sequence alignment of their CSDs reveals strict conservation in 50 of 65 positions (Fig. 1B). To elucidate specific determinants of their reportedly varying interactions, we performed ITC assays with a histone H3-derived synthetic peptide containing residues 36–58 (H3(36–58)) and recombinant CSDs of HP1α, HP1β, or HP1γ (hereafter referred to as CSDα, CSDβ, and CSDγ, respectively). All of these CSDs bound to the tested H3 peptide with similar affinities (Fig. 1C), which is not surprising given their sequence conservation.
Engagement of CTE strengthens binding
A previous study showed that the CTE plays a role in CSD selectivity and affinity (25). To explore the role of CTE in the interaction with H3 peptides, we performed a series of ITC binding assays using CSDα/β/γ of different C-terminal truncations against the histone H3 peptide H3(36–58). Our binding results support the following three conclusions. 1) The CSD without the CTE is sufficient for binding to the histone H3 fragment. CSDα (aa 107–179), CSDβ (aa 104–174), and CSDγ (aa 110–176), which miss most or all of the CTE, bound to the H3 peptide with comparable affinities (Fig. 1, B and C). H3 binding to these CSDs is reminiscent of PXVXL motif binding by CAF1 in that CAF1 also binds to C-terminally truncated HP1α (22). 2) Compared with the C-terminally truncated CSD constructs, constructs with the complete CTE bound to the histone H3 fragment 2–10-fold more tightly (Fig. 1D), which is consistent with the published pull-down results showing the contribution of the CTE of CSDα to histone binding (22) and also with the previously reported role of the CTE in the interaction between the CSD of DmHP1 and its partners (25). 3) An intact CSD is essential for histone H3 binding. The CSD constructs, which lack the last three residues of the CSD (truncated at Thr-173 of CSDα or corresponding residues of CSDβ or CSDγ), showed no interaction in our assay (Fig. 1, B and D). Taken together, these data indicate that although the CSD without CTE is sufficient for the interaction with histone H3, the CTE strengthens this interaction significantly.
Shorter H3 peptide binds to CSDs less tightly
To measure the effect of the length of histone H3 peptides on the interaction, we performed ITC assays with a shorter H3 peptide (residues 38–52, H3(38–52)) and different CSD constructs. The shorter peptide bound to CSDs less tightly (Fig. 1D), indicating that the residues flanking the PXVXL-like motif also contribute to CSD binding, consistent with the findings of Mendez et al. (25) that the flanking residues of the PXVXL motif affected the binding affinity. Thus, CSDs interact with H3 in a manner preserving some aspects of the CSD interactions with PXVXL motif-containing proteins.
Crystal structure of HP1γ CSD in complex with H3(38–52) peptide
Although the interaction between the CSD and histone H3 has been studied previously (20–23), the molecular mechanism of this interaction is still unknown. We attempted to crystallize several CSD constructs of human HP1 proteins in complex with H3 peptides encompassing either residues 36–58 or 38–52 and succeeded in determining the crystal structure of CSDγ (residues 110–176) in complex with H3(38–52) (Fig. 2 and Table 1).
Figure 2.
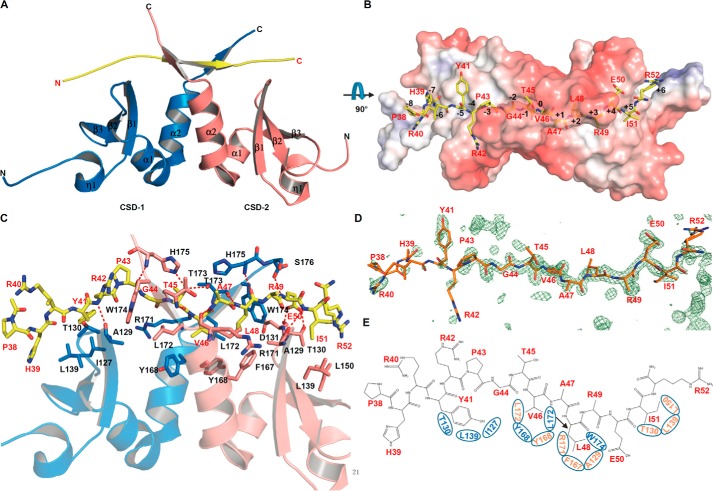
Recognition of the H3(38–52) peptide by CSDγ. A, overall structure of CSDγ-H3 complex. CSDγ dimer and H3 peptide are shown in schematic mode and colored in marine blue (CSD-1), salmon (CSD-2), and yellow (H3). B, surface representation of CSDγ-H3 complex. The electrostatic potential surface was calculated with APBS (58) using coordinates prepared with PDB entry 2PQR (59) at a pH setting of 7 and colored in a red-blue spectrum in the ±7kT/e range. A model of the histone peptide is shown in yellow sticks. C, detailed interactions of the histone H3 peptide with CSDγ. The H3 peptide and H3-interacting CSD residues are shown in stick mode. Hydrogen bonds are shown as red dashes. CSDγ residues are labeled in black, and H3 residues are labeled in red. H3 positions <−3 were poorly resolved by electron density. D, electron density for the H3 peptide. Omit map coefficients were calculated with PHENIX, and a slab of the mFo − DFc map was contoured in PyMOL at level 3. E, schematic view of how hydrophobic pockets of the CSDγ homodimer recognize H3 residues. Interacting residues of CSDγ are contained within ovals and labeled in colors corresponding to CSDs (A). Molecular representations were generated with PyMOL.
TABLE 1.
Data collection and refinement statistics
Values in parentheses are for the highest resolution shell. RMSD, root mean square deviation.
| Parameter | Value |
|---|---|
| Data collection | |
| Radiation wavelength (Å) | 0.9792 |
| Space group | P21 |
| Cell dimensions a, b, c (Å), β (°) | 40.74, 34.97, 45.83, 93.32 |
| Resolution limits (Å) | 45.76–1.60 (1.63–1.60) |
| Unique HKLs | 16,432 (549) |
| Completeness (%) | 95.5 (65.8) |
| Rsym | 0.052 (0.860) |
| I/σ | 14.5 (1.0) |
| Redundancy | 3.4 (2.3) |
| Model refinement | |
| Refinement resolution (Å) | 27.79–1.60 |
| Reflections used/free | 15,332/1,088 |
| Number of atoms/average B-factor (Å2) | 1,246/25.6 |
| Protein | 1,053/25.4 |
| Peptide | 137/27.3 |
| Water | 44/25.4 |
| Others | 12/20.6 |
| Rwork/Rfree | 0.194/0.239 |
| RMSD, bonds (Å)/angles (°) | 0.016/1.8 |
| Molprobity Ramachandran favored/outliers (%) | 100.00/0.00 |
The asymmetric unit of the CSDγ-H3 model comprises two CSDγ molecules (CSD-1 and CSD-2) that form a dimer hinged at their C-terminal α helices (Fig. 2, A–C). Each CSD adopts the canonical conformation of a triple-stranded antiparallel β-sheet, followed by two α helices packed against one side of the β-sheet (Fig. 2A) (26). The H3 peptide, in an extended β-strand-like conformation, is sandwiched by the C termini of both CSD monomers to form another triple-stranded β-sheet, similar to other CSD-peptide complexes (Fig. 2A) (25, 27–29). Analogous to other CSD-peptide complexes, the H3 peptide binds to the CSDγ mainly through the hydrophobic interaction (Fig. 2B) (25, 27–29). The backbones of the H3 peptide-bound and H3 peptide-free (PDB code 3KUP) CSDγ dimers can be superimposed (30) with root mean square deviations around 0.6 and 1.0 Å for the CSD chain A alone and the A/B dimer, respectively, indicating that binding of the peptide does not induce large conformational changes in the protein's backbone or the dimer assembly.
Resemblance to the CSD-PXVXL interaction but apparent non-equivalence of binding of prolyl residues between the histone H3 and the canonical PXVXL motif
We were able to model all residues of the H3(38–52) peptide, although some residues at the N terminus of the peptide have weak density (Fig. 2D). Residues 44–46 (G-T-V) of histone H3 pair with the β strand formed by CSD-2 residues 172–174 (Leu-Thr-Trp; hereafter we refer to H3 residues with single letters and to CSD residues with three-letter abbreviations) antiparallelly, and the H3 residues 46–49 (V-A-L) pack against the corresponding β strand from CSD-1 in parallel (Fig. 2C).
To facilitate the comparison, we adopt notation previously introduced for the CSD-CAF1 complex (27) and designate H3V46 as position 0 (Fig. 2B). Thus, H3V46 is located near the pseudosymmetry axis of the CSD dimer and its side chain is accommodated by a hydrophobic pocket formed by both CSD monomers' Tyr-168 and Leu-172 residues (Fig. 2, C and E). The V46A mutant would interact less effectively with the hydrophobic pocket, consistent with published work (Fig. 2B) (21, 27), whereas the mutation of the corresponding V residue in other PXVXL motifs to E or D probably cannot be accommodated by this small pocket and destroys the interaction between CSD and its partners (11, 12, 31, 32). H3T45 and H3A47 correspond to −1 and +1 of the PXVXL motif, respectively. The side chains of these residues point away from the binding interface, and only the side chain of H3T45 hydrogen bonds with the side chains of His-175 from CSD-2 and of Thr-173 from CSD-1 (Fig. 2C). Hydrogen bonding would be disrupted by the hypothetical phosphorylation of H3T45 due to modified charge distribution, loss of a potential hydrogen bond donor, and/or added steric bulk. In the known CSD-PXVXL complexes, positions −2 and +2 can be occupied by large hydrophobic residues and significantly contribute to the CSD-PXVXL interaction (25, 27–29). In our complex structure, whereas L48 at +2 also inserts into a hydrophobic pocket formed by Leu-172 and Trp-174 of CSD-1 and Ala-129, Phe-167, and Arg-171 of CSD-2 (Fig. 2, C and E), position −2 corresponds to G44. Lacking a side chain, G44 fails to fully exploit the −2 binding pocket of the CSD dimer (Fig. 2C), suggesting that the interaction at −2 may not be as important as previously thought (14, 27, 28). The carbonyl oxygen of H3P43 (position −3) forms a hydrogen bond with the main chain of His-175 from CSD-2, and this hydrogen bond is also conserved in other reported CSD complex structures (Fig. 2C) (27–29).
The side chain of H3E50 (position +4) packs against Trp-174 of CSD-1 and hydrogen-bonds with the side chain of Thr-130 and with the main chain of Asp-131 of CSD-2, whereas the side chain of H3R42 (position −4), poorly resolved, points to the solvent and packs against Trp-174 of CSD-2, with a potential cation-π interaction (Fig. 2C). Residues at positions +5, +6, −5, and −6 (even −7 in the case of a bulged peptide conformation (27)), flanking the PXVXL motif, reportedly play critical roles in binding affinity and selectivity (25, 27). In the present CSDγ-H3 model, H3I51 (position +5) is embraced by the side chains of Thr-130, Leu-139, and Leu-150 from CSD-2 (Fig. 2, C and E). JAK2 phosphorylation target H3Y41 (position −5) (20), only vaguely resolved by electron density, appears surrounded by residues Ile-127, Thr-130, and Leu-139 from CSD-1 (Fig. 2, C and E). Our crystal structure does not conclusively resolve the structural basis for the reported rejection of phosphotyrosine in this position (20), but added bulk or modified electric charge distribution of the added phosphoryl moiety may play a role, because the H3Y41 pocket is largely hydrophobic (Fig. 2B). Accordingly, our ITC studies demonstrate significantly weaker binding of Y41-phosphorylated H3(38–52) peptide to CSDα or CSDγ (Fig. 1D). H3R40 (position −6), H3H39 (position −7), H3P38 (position −8), H3R49 (position +3), and H3R52 (position +6) are poorly resolved, and the nature of their interactions with the CSD, if any, is not elucidated by our crystal structure.
Structural comparison of CSDs of HP1α/β/γ explains why they have similar binding behavior
Previous studies showed that the CSDs of human HP1 homologs recognize the PXVXL-like motif containing histone H3 region differently (20, 21, 23). However, the backbone of the CSDγ dimer superimposes (30) well with CSDα (PDB code 3I3C) and CSDβ (PDB code 3Q6S) dimers, with root mean square deviations around 1.7 and 0.9 Å, respectively, and residues putatively interacting with H3 are strictly conserved (Fig. 1B). These structural comparisons suggest that the CSDs of HP1α/β/γ would recognize histone H3 fragment H3(36–58) similarly, which is confirmed by our ITC assay (Fig. 1D).
Combining our structural analysis and ITC assay, it is clear why removal of Trp-174, His-175, and Ser-176 of CSDγ (or corresponding residues of CSDα or CSDβ) would abolish binding of H3 to HP1s. The absence of Trp-174 would disrupt the binding pocket for H3L48, whereas His-175 and Ser-176 form hydrogen bonds to the H3 peptide (Figs. 1D and 2C). Previous studies also showed that residue Trp-174 plays a critical role for the interaction between CSDs and the PXVXL motif, because the W174A mutant in human HP1α (22), the corresponding W200A mutant in Drosophila HP1a (25), and the W170A mutant in mouse HP1β (26) abrogate the binding of PXVXL peptides to HP1s. Although our crystal structure excludes the CTE, it can still be reasoned that the D/E-rich CTEs (Fig. 1B) would, if present, be in close proximity to histone H3 (Fig. 2C), which is a highly positively charged protein. The negatively charged CTEs can therefore be expected to electrostatically interact with the positively charged histone H3.
Comparison with published CSD-PXVXL complexes
Whereas our model of the CSDγ-H3 complex conserves the general architecture of CSD-PXVXL and related complexes (Fig. 3A), further inspection reveals several differences. As shown in Fig. 3A, the C termini of the PXVXL motif-containing peptides superimpose readily, whereas the N termini diverge. Like the N terminus of CAF1, the poorly resolved N terminus of the H3 peptide appears to bend toward the surface of its CSD receptor, whereas the corresponding segments of Sgo1 and EMSY peptides more closely track the respective C termini of a CSD monomer (Fig. 3A) (27–29). Because the EMSY peptides are presented in the context of a larger protein fragment, the peptides' N termini are somewhat restrained. The N terminus of the Sgo1 peptide, on the other hand, is free and the peptide forms an intermolecular, antiparallel β pair with the C terminus of the CSD and residues of the CTE (29), possibly also explaining enhanced affinity in the presence of the CTE observed in our ITC experiments and by others (22, 25).
Figure 3.
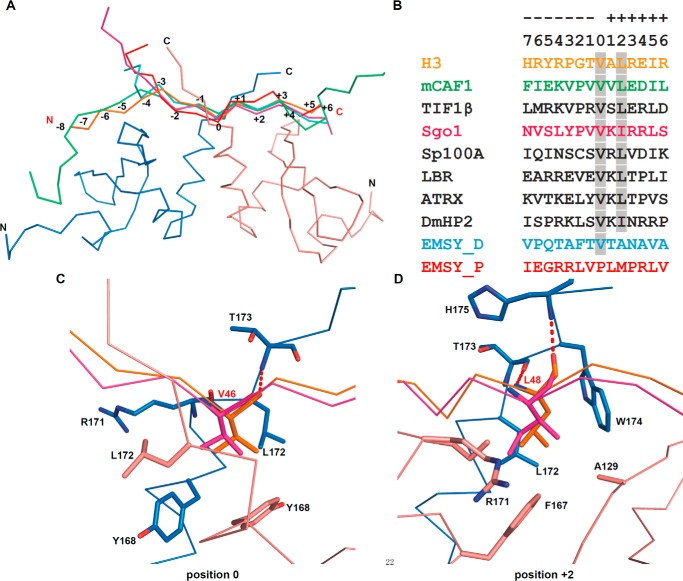
Structural comparison of different CSD-PXVXL complexes. A, structural superimposition of CSDγ-H3 complex (PDB code 5T1I (this work); peptide colored in orange) with the CSDβ-CAF1 complex (PDB code 1S4Z; green), the CSDβ-EMSY complex (PDB code 2FMM, ENT-distal site (cyan), and ENT-proximal site (red)), and the CSDβ-Sgo1 complex (PDB code 3Q6S; pink). For clarity, only the CSDγ is shown in a ribbon and colored as in Fig. 2A because the structures of CSDγ and CSDβ are highly conserved. B, sequence alignment of canonical and variant PXVXL motifs, adapted from Lechner et al. (32). The peptides are colored as in A, and conserved interacting residues are highlighted in gray. EMSY_D and EMSY_P represent the ENT-distal and -proximal site peptides of EMSY, respectively (28). C and D, highly conserved binding pockets of position 0 (residue V/P) (C) and position +2 (residue L/I) (D), respectively. CSDβ-Sgo1 complex is taken as a representative of CSDβ-PXVXL complexes. For clarity, only the interaction-associated residues in CSDγ-H3 complex are shown.
As shown in Fig. 3, B–D, the residues at positions 0 and +2 are highly conserved and recognized by equivalent hydrophobic pockets. Despite divergent amino acid types at positions −2, +4, and +5 (Fig. 3B), PXVXL motifs interact with CSDs in a generally conserved mode (Fig. 4, A–C). However, H3G44 (position −2 of H3 peptide) suboptimally interacts with the position −2 binding pocket of the CSD dimer (see above and Figs. 2C and 4A). Thus, CSDγ recognizes the H3 GTVAL sequence using only two of the three hydrophobic pockets that would otherwise recognize other PXVXL motifs. Outside the central motif, recognition of H3I51 by CSDγ resembles position +5 binding between peptides with relaxed PXVXL motifs and CSDs (Fig. 4C). Equivalents of H3E50 (position +4; see above and Fig. 2C) in the EMSY_P (ENT-proximal site) (28) and Sgo1 motifs are both arginine and similarly pack against Trp-170 and hydrogen-bond with Asp-127 in CSDβ (Figs. 3B and 4B) (29). The corresponding residue in CAF1 is aspartate and hydrogen-bonds with Thr-126 in CSDβ (Fig. 3B) (27). Thus, in addition to the hydrophobic residues (25), charged residues at the periphery of the PXVXL motif could also strengthen the interaction. The corresponding residue in the EMSY_D (ENT-distal site) peptide is A118 and lacks these interactions (28), which indicates that the recognition of residues at position +4 is not universally conserved.
Figure 4.
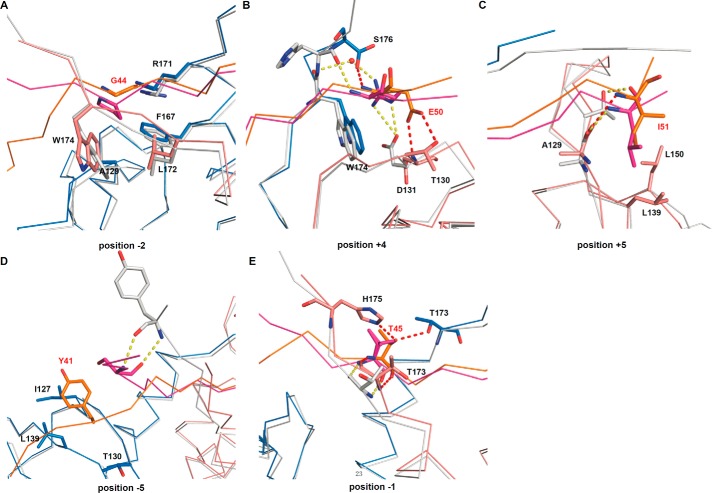
Partially conserved and specific interactions of CSD-PXVXL complexes. A–C, partially conserved interactions of different CSD-PXVXL complexes. D and E, specific interactions of the CSDγ-H3 complex. The CSDβ-Sgo1 complex is taken as a representative of CSDβ-PXVXL complexes and colored in gray. The interaction-associated residues in both complexes are shown, and only the residues of the CSDγ-H3 complex are labeled for clarity. Red and yellow dashed lines represent hydrogen bonds in CSDγ-H3 and CSDβ-Sgo1 complexes, respectively.
In addition to the conserved interactions, some other residues of the histone H3 peptide contact with CSD specifically. As mentioned above, the N termini of H3 and CAF1 peptides bend toward the interaction surface and residue H3Y41 (position −5), similar to F217/I218 (position −7/−6) of CAF1 (27), packs against several hydrophobic residues of CSDγ to stabilize the binding, whereas the residues at position −5 of Sgo1 and EMSY more closely track the respective C termini of a CSD monomer (Fig. 4D) (28, 29). The hydrogen bonds between H3T45 (position −1) and the CSDγ dimer may compensate for the lack of position −2 interactions (Fig. 4E). Thus, each protein ligand has its specific interactions except for the conserved hydrophobic packing to stabilize and specify the binding.
It has been known for some time that the CSD of HP1 recognizes a common motif with canonical composition of PXVXL (11). At first, valine at the pivotal 0 position of CSD complexes was thought to be invariable, as confirmed by functional, biophysical, and structural studies (11, 12, 14, 27, 31). However, proline can be tolerated at this position (28). It was also believed that prolyl, leucyl, or methionyl was required at +2 and −2 positions for CSD binding (14, 27), but the motif has been relaxed to ΦX(V/P)X(L/M/V), where Φ includes additional hydrophobic residue types (28). The present study shows that Φ may also include glycyl and presumably additional residue types, such as alanyl. Because the CSD dimer, by virtue of its symmetry, recognizes a given peptide ligand's +2 or −2 positions with chemically equivalent binding pockets, we propose to further relax the CSD binding motif to ΦX(V/P)XΦ, where Φ includes α-amino acids without side chain nitrogen or oxygen atoms. Alignment of known relevant peptide sequences appears to restrict at least one of the Φ (±2) residues to the {I, L, F} set (Fig. 3B). This definition is consistent with previous studies of the motifs found in EMSY (EMSY_D) (28), DmHP2 (25), and Sp100A (32), for example (Fig. 3B), with additional refinement of the pattern expected pending future discovery of new CSD-binding peptides. However, not all PXVXL motifs bind to the CSD, such as the PSVSL motif in HP2 (33), the PHVAL motif in STAM2a (32), and the PAVAL motif in Sgo1 (29), which demonstrates that additional factors, such as residues flanking the pivotal pentapeptide or the conformation of the peptide ligand, may also play a role.
Possible interaction model between HP1 and histone H3 within the nucleosome
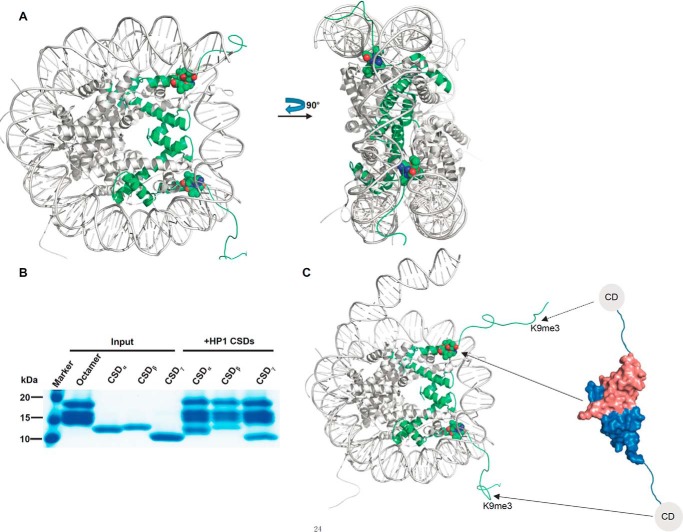
Because the CSD binding motif is located near the DNA entry/exit sites within the nucleosome (Fig. 5A), the binding site is inaccessible in the static nucleosome, which is confirmed by our gel shift assay of interaction between recombinant nucleosome and CSDs of HP1 proteins (data not shown). Whereas the nucleosome structure is dynamic, the interaction site could be exposed during some biological processes, such as DNA replication or transcription, recombination, chromatin remodeling, or histone variant exchange, during which the DNA is unwrapped off the octamer surface (34–36). The previous studies have reported that the de novo assembly of HP1 to chromatin happens in the late S-phage (37), and the remodeling complexes, such as SWI/SNF and ACF1-ISWI, promote the recruitment of HP1 to chromatin (21, 38, 39). In addition, the three HP1 proteins could interact with the tailless nucleosome and specifically bind to the folded histone H3 (40). Most importantly, the study of Cheutin et al. (41) has demonstrated that both the CD and CSD of HP1 proteins could bind to native chromatin in vivo, and these two domains contribute differently to location of HP1 in the nucleus. Consistent with these findings, our pull-down assay confirms the interaction of recombinant octamer and CSDs (Fig. 5B). Based on these studies, a possible model of interaction between HP1 and histone H3 within the nucleosome is proposed (Fig. 5C). When the DNA disassembles from the nucleosome, an open nucleosome structure will benefit the loading of HP1 proteins to nucleosome via the interaction between CSDs and the GTVAL motif, and the CD domains of the HP1 dimer may bind to the H3K9me3 modified histone in the same nucleosome or the nearby nucleosome, which will condense the chromatin structure.
Figure 5.
A proposed working model of the interaction between HP1 and histone H3 within the nucleosome. A, location of CSD binding site within the nucleosome (PDB code 1KX5). Histone H3 is colored in green, and the GTVAL motif is shown in a sphere model. B, CSDs bind to the histone H3 in the context of the octamer. Tag-removed HP1 CSDs are pulled down by His-tagged octamer. C, possible model of the interaction between HP1 and histone H3 within the nucleosome.
In conclusion, a combination of structural analyses and quantitative binding assays reveals that human HP1α/β/γ can bind to histone H3 in the narrow H3P38–H3R52 region, although an expanded H3 peptide binds more tightly. Our crystal structure suggests that H3 recognition by CSDγ resembles CSD-PXVXL interaction, but that the proline residues of the PXVXL and PXXVXL motifs, respectively, are functionally distinct. Consequently, and in refinement of work published by others, we propose the expanded pseudopalindromic ΦX(V/P)XΦ pattern to describe both histone and non-histone sequence motifs that interact with CSD homodimers. The monomers of the CSD homodimer cooperatively bind to peptides that, due to their unidirectionality, cannot perfectly complement the homodimer's symmetry. The enabling plastic capacity of the homodimer suggests that additional protein ligands of CSDs may remain to be discovered.
Experimental procedures
Protein expression and purification
The CSDs of HP1α (residues 109–191, 107–179, and 107–173), HP1β (residues 106–185, 104–174, and 104–169), and HP1γ (residues 109–183, 110–176, and 110–173) were subcloned into a modified pET28-MHL vector. The encoded N-terminal His-tagged fusion protein was overexpressed in Escherichia coli BL21 (DE3) codon plus RIL (Stratagene) cells at 15 °C and purified by affinity chromatography on nickel-nitrilotriacetate resin (Qiagen), followed by tobacco etch virus protease treatment to remove the tag. Protein was further purified by Superdex75 gel filtration (GE Healthcare). For crystallization experiments, purified protein was concentrated to 20 mg/ml in a buffer containing 20 mm Tris, pH 7.5, 150 mm NaCl, and 1 mm DTT. The molecular weights of purified proteins were determined by mass spectrometry.
ITC
All histone peptides were synthesized by Peptide 2.0 Inc. For ITC measurements, the concentrated proteins were diluted in 20 mm Tris, pH 7.5, and 150 mm NaCl. Lyophilized peptides were dissolved in the same buffer, and pH was adjusted by adding NaOH. Because all of the used peptides contained tyrosine, the concentrations were estimated with absorbance spectroscopy using the extinction coefficient, ϵ280 = 1280 m−1 cm−1. All measurements were performed at 25 °C, using a VP-ITC microcalorimeter. Protein with a concentration of 50–100 μm was placed in the chamber, and peptide with a concentration of 0.5–1 mm was injected in 25 successive steps with a spacing of 180 s and a reference power of 13 μcal/s. Control experiments were performed under identical conditions to determine the heat signals that arise from injection of the peptides into the buffer. Data were fitted using the single-site binding model within the Origin software package (MicroCal, Inc.).
Pull-down assays
The purified His-tagged fusion octamer (30 μg) was bound to nickel-nitrilotriacetate resin (Qiagen) and mixed with the purified, tag-removed CSDs (100 μg) in a buffer containing 20 mm Tris, pH 8.0, 150 mm NaCl, 1 mm DTT for 1 h at 25 °C. After washing five times with the buffer described above, recovered proteins were loaded to SDS-polyacrylamide gels and stained by Coomassie Brilliant Blue.
Crystallization
CSDγ (residues 110–176) was mixed with H3(38–52) at a 1:3 stoichiometric ratio and crystallized using the sitting drop vapor diffusion method at 20 °C by mixing 0.5 μl of protein solution with 0.5 μl of reservoir solution with 25% PEG 3350, 0.2 m NaCl, 0.1 m Hepes, pH 7.5, 5% ethylene glycol. Before flash-freezing in liquid nitrogen, the crystal was soaked in a cryoprotectant consisting of 85% reservoir solution and 15% ethylene glycol.
Data collection and structure determination
Diffraction data were collected under cooling to 100 K at beam line 19-ID of the Advanced Photon Source and reduced with DENZO/SCALEPACK (42) or, for later steps of refinement, with XDS (43)/POINTLESS/AIMLESS (44) software. Molecular replacement was performed with PHASER (45) and coordinates from PDB entry 3KUP. Geometric restraints for terminally protected peptide residues were prepared with GRADE (46, 47). The current model was obtained through iterative manual rebuilding with COOT (48), restrained refinement with REFMAC (49), and model validation with MOLPROBITY (50). Anisotropic displacement parameters were analyzed on the PARVATI server (51). CCP4 (52), PHENIX (53), and PDB_EXTRACT (54) programs and the IOTBX library (55) were used in preparing the model summary (Table 1) and PDB deposition.
Author contributions
Y. Liu purified and crystallized the protein; Y. Liu, S. Q., and Y. Z. conducted the ITC assays; M. L. conducted the pull-down assays; W. T. determined the crystal structure; P. L. and Y. Li cloned the constructs; J. M. conceived and designed the study; Y. Liu, W. T., and J. M. wrote the paper. All authors contributed to data analysis and approved the final version of the manuscript.
Acknowledgments
We thank Masoud Vedadi and Irene Chau for the generous gift of nucleosome. Results shown in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the United States Department of Energy, Office of Biological and Environmental Research under Contract DE-AC02-06CH11357.
This work was supported by National Natural Science Foundation of China Grant 31500613. The Structural Genomics Consortium (SGC) is a registered charity (number 1097737) that receives funds from AbbVie, Boehringer Ingelheim, the Canadian Institutes of Health Research (CIHR), Genome Canada, the Ontario Genomics Institute (Grant OGI-055), GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda, and the Wellcome Trust (Grant 092809/Z/10/Z). The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (code 5T1I) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- HP1
- heterochromatin protein 1
- CSD
- chromoshadow domain
- CD
- chromodomain
- HR
- hinge region
- CTE
- C-terminal extension
- ITC
- isothermal titration calorimetry
- aa
- amino acids
- PDB
- Protein Data Bank.
References
- 1. Will H., and Bautz E. K. F. (1980) Immunological identification of a chromocenter-associated protein in polytene chromosomes of Drosophila. Exp. Cell Res. 125, 401–410 [DOI] [PubMed] [Google Scholar]
- 2. Chandra T., and Narita M. (2013) High-order chromatin structure and the epigenome in SAHFs. Nucleus 4, 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y., Kirschmann D. A., and Wallrath L. L. (2002) Does heterochromatin protein 1 always follow code? Proc. Natl. Acad. Sci. U.S.A. 99, 16462–16469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eissenberg J. C., and Elgin S. C. (2000) The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10, 204–210 [DOI] [PubMed] [Google Scholar]
- 5. Maison C., and Almouzni G. (2004) HP1 and the dynamics of heterochromatin maintenance. Nat. Rev. Mol. Cell Biol. 5, 296–304 [DOI] [PubMed] [Google Scholar]
- 6. Kellum R., and Alberts B. M. (1995) Heterochromatin protein 1 is required for correct chromosome segregation in Drosophila embryos. J. Cell Sci. 108, 1419–1431 [DOI] [PubMed] [Google Scholar]
- 7. Aucott R., Bullwinkel J., Yu Y., Shi W., Billur M., Brown J. P., Menzel U., Kioussis D., Wang G., Reisert I., Weimer J., Pandita R. K., Sharma G. G., Pandita T. K., Fundele R., and Singh P. B. (2008) HP1-β is required for development of the cerebral neocortex and neuromuscular junctions. J. Cell Biol. 183, 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Assland R., Stewart A. F. (1995) The chromo shadow domain, a second chromo domain in heterochromatin-binding protein 1, HP1. Nucleic Acids Res. 23, 3168–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bannister A. J., Zegerman P., Partridge J. F., Miska E. A., Thomas J. O., Allshire R. C., and Kouzarides T. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 [DOI] [PubMed] [Google Scholar]
- 10. Lachner M., O'Carroll D., Rea S., Mechtler K., and Jenuwein T. (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 [DOI] [PubMed] [Google Scholar]
- 11. Le Douarin B., Nielsen A. L., Garnier J. M., Ichinose H., Jeanmougin F., Losson R., and Chambon P. (1996) A possible involvement of TIF1 α and TIF1 β in the epigenetic control of transcription by nuclear receptors. EMBO J. 15, 6701–6715 [PMC free article] [PubMed] [Google Scholar]
- 12. Murzina N., Verreault A., Laue E., and Stillman B. (1999) Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4, 529–540 [DOI] [PubMed] [Google Scholar]
- 13. Nielsen A. L., Ortiz J. A., You J., Oulad-Abdelghani M., Khechumian R., Gansmuller A., Chambon P., and Losson R. (1999) Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18, 6385–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smothers J. F., and Henikoff S. (2000) The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol. 10, 27–30 [DOI] [PubMed] [Google Scholar]
- 15. Muchardt C., Guilleme M., Seeler J. S., Trouche D., Dejean A., and Yaniv M. (2002) Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1α. EMBO Rep. 3, 975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meehan R. R., Kao C. F., and Pennings S. (2003) HP1 binding to native chromatin in vitro is determined by the hinge region and not by the chromodomain. EMBO J. 22, 3164–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danzer J. R., and Wallrath L. L. (2004) Mechanisms of HP1-mediated gene silencing in Drosophila. Development 131, 3571–3580 [DOI] [PubMed] [Google Scholar]
- 18. Smothers J. F., and Henikoff S. (2001) The hinge and chromo shadow domain impart distinct targeting of HP1-like proteins. Mol. Cell. Biol. 21, 2555–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Platero J. S., Hartnett T., and Eissenberg J. (1995) Functional analysis of the chromo domain of HP1. EMBO J. 14, 3977–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dawson M. A., Bannister A. J., Göttgens B., Foster S. D., Bartke T., Green A. R., and Kouzarides T. (2009) JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature 461, 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavigne M., Eskeland R., Azebi S., Saint-André V., Jang S. M., Batsché E., Fan H.-Y., Kingston R. E., Imhof A., and Muchardt C. (2009) Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression. PLoS Genet. 5, e1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richart A. N., Brunner C. I., Stott K., Murzina N. V., and Thomas J. O. (2012) Characterization of chromoshadow domain-mediated binding of heterochromatin protein 1α (HP1α) to histone H3. J. Biol. Chem. 287, 18730–18737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jang S. M., Azebi S., Soubigou G., and Muchardt C. (2014) DYRK1A phosphorylates histone H3 to differentially regulate the binding of HP1 isoforms and antagonize HP1-mediated transcriptional repression. EMBO Rep. 15, 686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vermaak D., Henikoff S., and Malik H. S. (2005) Positive selection drives the evolution of rhino, a member of the heterochromatin protein 1 family in Drosophila. PLoS Genet. 10.1371/journal.pgen.0010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendez D. L., Kim D., Chruszcz M., Stephens G. E., Minor W., Khorasanizadeh S., and Elgin S. C. (2011) The HP1a disordered C terminus and chromo shadow domain cooperate to select target peptide partners. Chembiochem 12, 1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brasher S. V., Smith B. O., Fogh R. H., Nietlispach D., Thiru A., Nielsen P. R., Broadhurst R. W., Ball L. J., Murzina N. V., and Laue E. D. (2000) The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 19, 1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thiru A., Nietlispach D., Mott H. R., Okuwaki M., Lyon D., Nielsen P. R., Hirshberg M., Verreault A., Murzina N. V., and Laue E. D. (2004) Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 23, 489–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Y., Myers M. P., and Xu R.-M. (2006) Crystal structure of the HP1-EMSY complex reveals an unusual mode of HP1 binding. Structure 14, 703–712 [DOI] [PubMed] [Google Scholar]
- 29. Kang J., Chaudhary J., Dong H., Kim S., Brautigam C. A., and Yu H. (2011) Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol. Biol. Cell 22, 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krissinel E., and Henrick K. (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 [DOI] [PubMed] [Google Scholar]
- 31. Vassallo M. F., and Tanese N. (2002) Isoform-specific interaction of HP1 with human TAFII130. Proc. Natl. Acad. Sci. U.S.A. 99, 5919–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lechner M. S., Schultz D. C., Negorev D., Maul G. G., and Rauscher F. J. 3rd (2005) The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem. Biophys. Res. Commun. 331, 929–937 [DOI] [PubMed] [Google Scholar]
- 33. Stephens G. E., Slawson E. E., Craig C. A., and Elgin S. C. (2005) Interaction of heterochromatin protein 2 with HP1 defines a novel HP1-binding domain. Biochemistry 44, 13394–13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flaus A., and Owen-Hughes T. (2004) Mechanisms for ATP-dependent chromatin remodelling: farewell to the tuna-can octamer? Curr. Opin. Genet. Dev. 14, 165–173 [DOI] [PubMed] [Google Scholar]
- 35. Li G., and Widom J. (2004) Nucleosomes facilitate their own invasion. Nat. Struct. Mol. Biol. 11, 763–769 [DOI] [PubMed] [Google Scholar]
- 36. Poirier M. G., Bussiek M., Langowski J., and Widom J. (2008) Spontaneous access to DNA target sites in folded chromatin fibers. J. Mol. Biol. 379, 772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dialynas G. K., Makatsori D., Kourmouli N., Theodoropoulos P. A., McLean K., Terjung S., Singh P. B., and Georgatos S. D. (2006) Methylation-independent binding to histone H3 and cell cycle-dependent incorporation of HP1β into heterochromatin. J. Biol. Chem. 281, 14350–14360 [DOI] [PubMed] [Google Scholar]
- 38. Collins N., Poot R. A., Kukimoto I., García-Jiménez C., Dellaire G., and Varga-Weisz P. D. (2002) An ACF1–ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat. Genet. 32, 627–632 [DOI] [PubMed] [Google Scholar]
- 39. Eskeland R., Eberharter A., and Imhof A. (2007) HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol. Cell. Biol. 27, 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nielsen A. L., Oulad-Abdelghani M., Ortiz J. A., Remboutsika E., Chambon P., and Losson R. (2001) Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol. Cell 7, 729–739 [DOI] [PubMed] [Google Scholar]
- 41. Cheutin T., McNairn A. J., Jenuwein T., Gilbert D. M., Singh P. B., and Misteli T. (2003) Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299, 721–725 [DOI] [PubMed] [Google Scholar]
- 42. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 43. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Evans P. R., and Murshudov G. N. (2013) How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phasercrystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bruno I. J., Cole J. C., Kessler M., Luo J., Motherwell W. D., Purkis L. H., Smith B. R., Taylor R., Cooper R. I., Harris S. E., and Orpen A. G. (2004) Retrieval of crystallographically-derived molecular geometry information. J. Chem. Inform. Comput. Sci. 44, 2133–2144 [DOI] [PubMed] [Google Scholar]
- 47. Smart O., Womack T., Sharff A., Flensburg C., Keller P., Paciorek W., Vonrhein C., and Bricogne G. (2014) Grade, version 1.2.9. Global Phasing Ltd., Cambridge, UK [Google Scholar]
- 48. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murshudov G. N., Skubák P., Lebedev A. A., Pannu N. S., Steiner R. A., Nicholls R. A., Winn M. D., Long F., and Vagin A. A. (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen V. B., Arendall W. B. 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zucker F., Champ P. C., and Merritt E. A. (2010) Validation of crystallographic models containing TLS or other descriptions of anisotropy. Acta Crystallogr. D Biol. Crystallogr. 66, 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G. W., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang H., Guranovic V., Dutta S., Feng Z., Berman H. M., and Westbrook J. D. (2004) Automated and accurate deposition of structures solved by X-ray diffraction to the Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 60, 1833–1839 [DOI] [PubMed] [Google Scholar]
- 55. Gildea R. J., Bourhis L. J., Dolomanov O. V., Grosse-Kunstleve R. W., Puschmann H., Adams P. D., and Howard J. A. K. (2011) iotbx.cif: a comprehensive CIF toolbox. J. Appl. Crystallogr. 44, 1259–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thompson J. D., Higgins D. G., and Gibson T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Robert X., and Gouet P. (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baker N. A., Sept D., Joseph S., Holst M. J., and McCammon J. A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dolinsky T. J., Nielsen J. E., McCammon J. A., and Baker N. A. (2004) PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 [DOI] [PMC free article] [PubMed] [Google Scholar]