Abstract
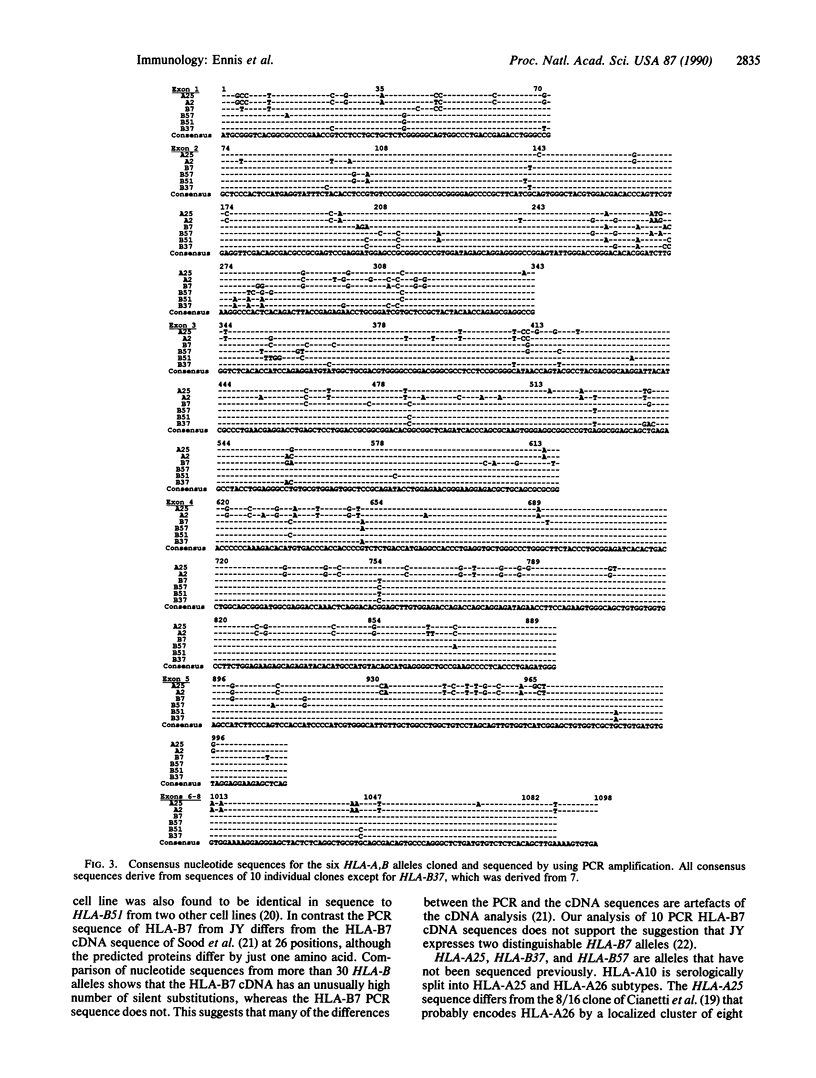
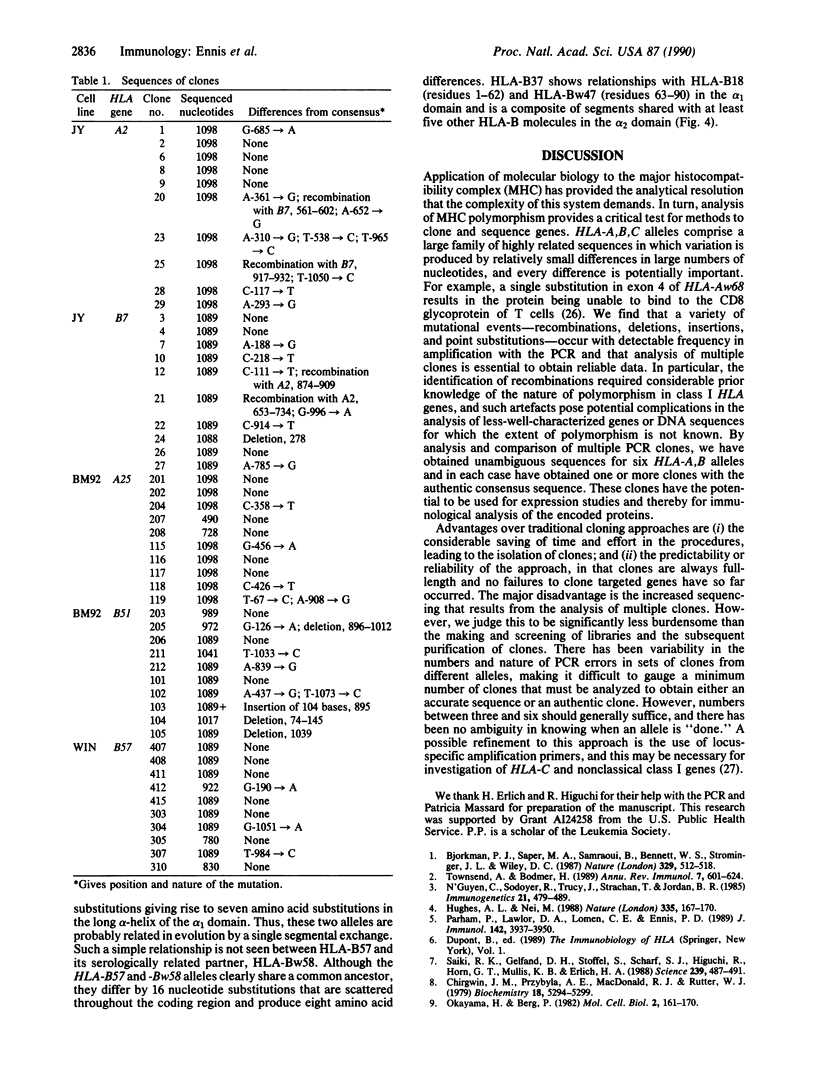
A method for cloning full-length HLA-A,B cDNA (1.1 kilobases) by using the polymerase chain reaction (PCR) is described. Six HLA-A,B alleles (HLA-A2, -A25, -B7, -B37, -B51, and -B57) were cloned, and their structures were determined. Multiple PCR clones for each allele were sequenced to obtain both an accurate consensus sequence and an "authentic" clone having that sequence. Sequences from 50 clones encoding five different alleles permit assessment of the frequency and nature of PCR-produced errors. These include recombinations, deletions, and insertions in addition to point substitutions. Authentic clones were obtained at a frequency of between 30% and 70%, and analysis of three or four clones generally should be sufficient for characterization of an allele.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cianetti L., Testa U., Scotto L., La Valle R., Simeone A., Boccoli G., Giannella G., Peschle C., Boncinelli E. Three new class I HLA alleles: structure of mRNAs and alternative mechanisms of processing. Immunogenetics. 1989;29(2):80–91. doi: 10.1007/BF00395855. [DOI] [PubMed] [Google Scholar]
- Ennis P. D., Jackson A. P., Parham P. Molecular cloning of bovine class I MHC cDNA. J Immunol. 1988 Jul 15;141(2):642–651. [PubMed] [Google Scholar]
- Güssow D., Rein R. S., Meijer I., de Hoog W., Seemann G. H., Hochstenbach F. M., Ploegh H. L. Isolation, expression, and the primary structure of HLA-Cw1 and HLA-Cw2 genes: evolutionary aspects. Immunogenetics. 1987;25(5):313–322. doi: 10.1007/BF00404424. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Ennis P. D., Ariga H., Salter R. D., Parham P., Kano K., Takiguchi M. HLA-B51 and HLA-Bw52 differ by only two amino acids which are in the helical region of the alpha 1 domain. J Immunol. 1989 Jan 1;142(1):306–311. [PubMed] [Google Scholar]
- Holmes N., Ennis P., Wan A. M., Denney D. W., Parham P. Multiple genetic mechanisms have contributed to the generation of the HLA-A2/A28 family of class I MHC molecules. J Immunol. 1987 Aug 1;139(3):936–941. [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988 Sep 8;335(6186):167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Kottmann A. H., Seemann G. H., Guessow H. D., Roos M. H. DNA sequence of the coding region of the HLA-B44 gene. Immunogenetics. 1986;23(6):396–400. doi: 10.1007/BF00372673. [DOI] [PubMed] [Google Scholar]
- N'Guyen C., Sodoyer R., Trucy J., Strachan T., Jordan B. R. The HLA-AW24 gene: sequence, surroundings and comparison with the HLA-A2 and HLA-A3 genes. Immunogenetics. 1985;21(5):479–489. doi: 10.1007/BF00430931. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Lawlor D. A., Lomen C. E., Ennis P. D. Diversity and diversification of HLA-A,B,C alleles. J Immunol. 1989 Jun 1;142(11):3937–3950. [PubMed] [Google Scholar]
- Parham P., Lomen C. E., Lawlor D. A., Ways J. P., Holmes N., Coppin H. L., Salter R. D., Wan A. M., Ennis P. D. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4005–4009. doi: 10.1073/pnas.85.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Norment A. M., Chen B. P., Clayberger C., Krensky A. M., Littman D. R., Parham P. Polymorphism in the alpha 3 domain of HLA-A molecules affects binding to CD8. Nature. 1989 Mar 23;338(6213):345–347. doi: 10.1038/338345a0. [DOI] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pan J., Biro P. A., Pereira D., Srivastava R., Reddy V. B., Duceman B. W., Weissman S. M. Structure and polymorphism of class I MHC antigen mRNA. Immunogenetics. 1985;22(2):101–121. doi: 10.1007/BF00563508. [DOI] [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Ways J. P., Coppin H. L., Parham P. The complete primary structure of HLA-Bw58. J Biol Chem. 1985 Oct 5;260(22):11924–11933. [PubMed] [Google Scholar]
- Zemmour J., Ennis P. D., Parham P., Dupont B. Comparison of the structure of HLA-Bw47 to HLA-B13 and its relationship to 21-hydroxylase deficiency. Immunogenetics. 1988;27(4):281–287. doi: 10.1007/BF00376123. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Spits H., Yssel H., Melief C. J., Ivanyi P. Differential recognition by human cytotoxic T cell clones of human M1 fibroblasts transfected with an HLA-B7 gene (JY150) suggests the existence of two different HLA-B7 alleles in the cell line JY (HLA-A2,2;B7,7;Cw-,-;DR4,w6). J Immunol. 1988 Jul 15;141(2):417–422. [PubMed] [Google Scholar]