Abstract
Anti-drug antibodies in hemophilia patients substantially complicate treatment. Their elimination through immune tolerance induction (ITI) protocols poses enormous costs, and ITI is often ineffective for factor IX (FIX) inhibitors. Moreover, there is no prophylactic ITI protocol to prevent anti-drug antibody (ADA) formation. Using general immune suppression is problematic. To address this urgent unmet medical need, we delivered antigen bioencapsulated in plant cells to hemophilia B dogs. Commercial-scale production of CTB-FIX fusion expressed in lettuce chloroplasts was done in a hydroponic facility. CTB-FIX (∼1 mg/g) in lyophilized cells was stable with proper folding, disulfide bonds, and pentamer assembly after 30-month storage at ambient temperature. Robust suppression of immunoglobulin G (IgG)/inhibitor and IgE formation against intravenous FIX was observed in three of four hemophilia B dogs fed with lyophilized lettuce cells expressing CTB-FIX. No side effects were detected after feeding CTB-FIX-lyophilized plant cells for >300 days. Coagulation times were markedly shortened by intravenous FIX in orally tolerized treated dogs, in contrast to control dogs that formed high-titer antibodies to FIX. Commercial-scale production, stability, prolonged storage of lyophilized cells, and efficacy in tolerance induction in a large, non-rodent model of human disease offer a novel concept for oral tolerance and low-cost production and delivery of biopharmaceuticals.
Keywords: oral tolerance, factor IX, hemophilia, inhibitor, transgenic plant
Graphical Abstract
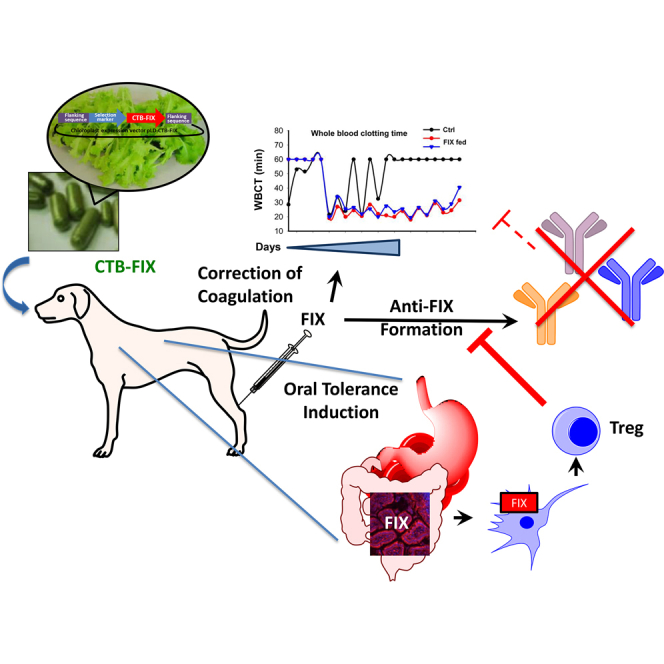
Anti-drug antibodies substantially complicate hemophilia treatment. In this article, authors report an antigen-specific immune tolerance induction protocol based on oral delivery of factor IX expressed in lettuce chloroplasts. Effectiveness and safety of the protocol are demonstrated by successful suppression of inhibitory antibody formation in hemophilia B dogs upon long-term delivery.
Introduction
Currently, protein drugs for the treatment of human diseases are typically administered intravenously (i.v.), such as in enzyme-replacement therapies (ERTs) for genetic diseases or monoclonal antibody therapies. Anti-drug antibody (ADA) formation is a serious complication that not only renders therapy ineffective but can also cause severe immunotoxicities. Unfortunately, no immune tolerance protocols are currently available for patients to prevent ADA formation. General immune suppression can be applied, as for example in treatment of Pompe disease, but is not generally acceptable.1, 2 In the case of the X-linked bleeding disorder hemophilia, immune tolerance induction (ITI) protocols are attempted only after neutralizing antibodies (termed “inhibitors”) have formed. This strategy puts patients at risk of increased morbidity and mortality until the inhibitors are eradicated, which can take months to years and is not effective in all patients.3, 4 Conceptually, oral tolerance offers an elegant solution. Uptake of orally administered protein by the intestinal immune system can induce a state of immune tolerance, in which responses to this specific antigen are systemically suppressed.5 Although proof of principle has been achieved in numerous studies in mice, preclinical studies in large, non-rodent animal models are lacking. Oral tolerance induction has yielded only limited success in clinical trials for autoimmune diseases.5, 6 However, multiple recent studies aimed to desensitize from food allergens suggest that oral tolerance is applicable to humans.7, 8
However, a new emerging system offers potential solutions to aforementioned challenges. Protein drugs are expressed at high levels in plant chloroplasts, and lyophilized plant cells can be stored for several years maintaining their folding, assembly, and efficacy, which facilitate low-cost current good manufacturing practices (cGMP) production.9, 10, 11, 12 Upon oral delivery, the plant cell wall (based on unique structural composition) protects protein drugs from acids and enzymes in the stomach, which are incapable of breaking down all glycosidic bonds. However, when intact plant cells reach the gut, commensal bacteria release cellulases and lyse plant cells, releasing protein drugs into the gut lumen.13, 14 When suitable tags are fused to protein drugs, they cross the gut epithelium and are delivered into circulation or the immune system.13, 15 Therefore, engineered plant cells are exceptionally suitable for oral delivery of protein drugs, preventing protein degradation prior to reaching the small intestine and accomplishing transmucosal delivery to the immune or circulatory system. However, initial studies supporting this concept have been done in small animal models (mice and rats) and are awaiting evaluation in large animal models that could offer valuable information on drug delivery, stability, and potential toxicity.
The challenges for oral tolerance include protection from degradation prior to reaching the small intestine, efficient translocation across the intestinal epithelium and targeting of the gut immune system, and high cost of production of the protein antigen. Current recombinant production systems use fermentation facilities, which cost $500–$900 million,16, 17 and require prohibitively expensive purification of host proteins.18 In addition, purified proteins are highly unstable and require cold storage and transportation. In order to address these concerns, it is important to develop new strategies for production and oral delivery of protein drugs.
In previous studies, we documented that repeated oral delivery of frozen transplastomic leaf cells expressing CTB (cholera toxin B subunit) fusions of coagulation factor IX (FIX) prevented inhibitor formation and anaphylaxis to i.v. replacement therapy in hemophilia B mice.19, 20 Similarly, inhibitor formation against factor VIII (FVIII) was suppressed in hemophilia A mice using an analogous approach.21, 22 Early studies utilized transplastomic tobacco, which, however, is not suitable for clinical application due to its nicotine contents. Therefore, we optimized chloroplast gene transfer and expression in lettuce.12 Successful scale up of production and development of a storable edible plant product allowed us to evaluate the approach in a large animal model of hemophilia of similar size as pediatric patients that are likely candidates for oral tolerance.23, 24
Results
Production and Characterization of CTB-FIX Expressed in Lettuce Chloroplasts
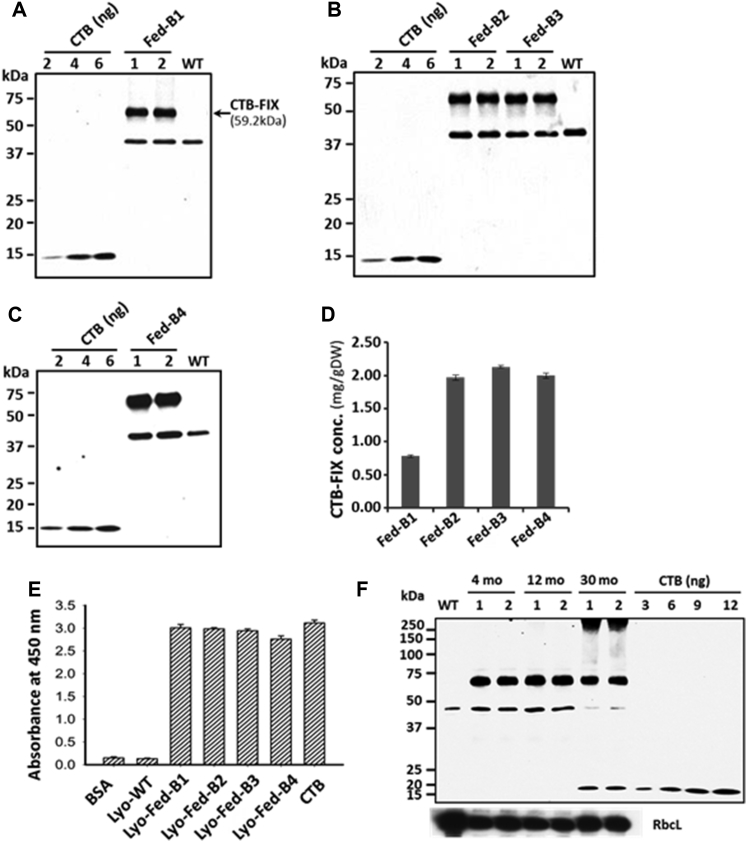
To produce high concentrations of human FIX fused with the transmucosal carrier CTB, we utilized the chloroplast transformation technology in edible plant cells in combination with the unlimited scalability of the plant-production system. Germination of the CTB-FIX lettuce seeds in the selection medium showed maternal inheritance and lack of Mendelian segregation (data not shown). Mature CTB-FIX lettuce leaves were harvested in the H.D. lab greenhouse. Multiple harvests of the mature CTB-FIX leaves were made every ∼3 weeks until transplastomic plants reached the flowering stage. To feed hemophilia B dogs with lettuce-cell-derived CTB-FIX antigen, lyophilized CTB-FIX lettuce dry powder was prepared. A total of ∼700 g (dry weight) of CTB-FIX leaf powder was generated from more than 14 kg of fresh leaves. In this study, approximately 430 g of dry leaf powder derived from four batches of preparations (defined as Fed-B1, Fed-B2, Fed-B3, and Fed-B4, respectively) were used to feed hemophilia B dogs. As determined by western blot quantitation, the CTB-FIX concentrations (mg/g dry weight [DW]) varied in different batches based on age and growth conditions as follows (Figures 1A–1D): Fed-B1 (0.78 ± 0.02); Fed-B2 (1.97 ± 0.04); Fed-B3 (2.13 ± 0.02); and Fed-B4 (2.00 ± 0.04). Fed-B1 was used to feed dogs O07 and O67. Fed-B2 and Fed-B3 were both used to feed dogs S14 and S15. Fed-B3 and Fed-B4 were both used to feed dogs P44 and S12 (Tables S1 and S2). The ∼40-kDa polypeptide was observed in all the plant extracts, including untransformed wild-type control (lane “WT” in Figures 1A–1C). Therefore, the ∼40-kDa polypeptide cross-reacting with CTB antibody is an endogenous protein in lettuce cells and not due to cleavage of CTB-FIX fusion protein. Dry powder weight was adjusted to feed identical doses of CTB-FIX in all batches of dogs. Except for the first batch, during which growth conditions were optimized, all other batches showed similar levels of expression.
Figure 1.
Characterization of CTB-FIX Protein in Different Batches of Lettuce Lyophilized Leaf Powder Used for Dog Studies
(A–C) Western blot analysis and quantification of CTB-FIX lettuce total leaf protein (TLP) (A) Fed-B1: batch 1, 2.0 μg TLP per lane; (B) Fed-B2 and Fed-B3: batches 2 and 3, 1 μg TLP per lane; and (C) Fed-B4: batch 4 2.0 μg TLP per lane. (D) Concentration of CTB-FIX (mg/g DW) in four batches of leaf powder is shown. Data shown are means ± SD of two independent experiments. (E) GM1 binding assay is shown—200 μg total soluble protein (TSP) per assay, 20 ng CTB standard, 1% BSA, and 200 μg TSP from lyophilized untransformed lettuce were used as negative controls. Data shown are means ± SD of triplicates. (F) Western blot analysis of CTB-FIX protein in lyophilized leaves after long-term storage for 4, 12, and 30 months is shown. Equal protein loading is confirmed by re-probing with anti-Rubisco large subunit antibody on the same blot (RbcL). Anti-CTB rabbit polyclonal antibody (titer: 1:10,000) was used to detect CTB-FIX protein in all the western blots.
As pentameric structure of CTB-fusion protein is essential for binding to GM1-ganglioside receptor of the intestinal epithelial cells, GM1-binding-based ELISA assay was used to evaluate stability of folding, disulfide bonds, and pentamer assembly of CTB-FIX in different batches.12, 19, 22, 25 CTB-FIX protein extracts from all four batches of leaf powder showed strong binding affinity to GM1 (Figure 1E), confirming functional stability of the CTB-FIX pentamers in lyophilized leaves after prolonged storage at ambient temperature for 30 months (Fed-B1), 14 months (Fed-B2), 12 months (Fed-B3), and 10 months (Fed-B4). For hemophilia B dog studies, the lyophilized leaves were used within 5–10 months of storage at ambient temperature, which is well within the effective shelf life of bioencapsulated CTB-FIX (Table S2). As shown in Figure 1F, intact CTB-FIX monomer without any cleaved product was detected as the predominant band in all tested samples with equal loading amounts, which was confirmed by re-probing blots with antibody for a major chloroplast protein, Rubisco.
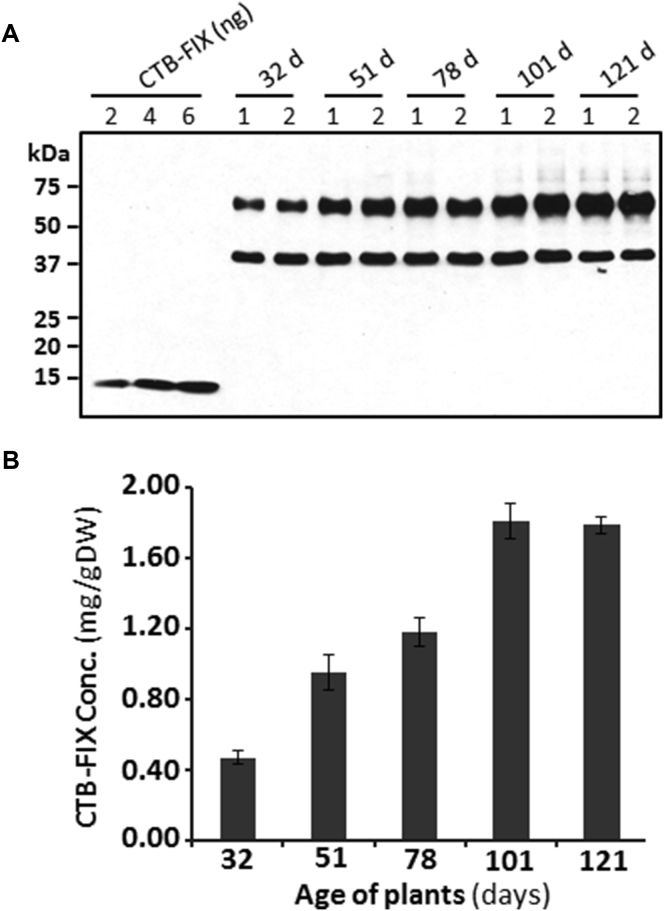
To facilitate translational studies of hemophilia B, we also used the Fraunhofer cGMP hydroponic system to generate clinical-grade CTB-FIX lettuce biomass. In order to find an optimal growth condition in this hydroponic system, a time course study was performed via harvesting different batches of leaves from the same population of plants at different plant age. The collected fresh leaves were lyophilized and ground into dry powder. We observed that the CTB-FIX expression levels increased as plants grew older. The CTB-FIX concentrations (mg/g DW) in five tested plant ages (days) were 0.47 ± 0.04 (32 days), 0.95 ± 0.10 (51 days), 1.18 ± 0.08 (78 days), 1.81 ± 0.10 (101 days), and 1.79 ± 0.05 (121 days), which were equivalent to 0.15% (±0.01%), 0.32% (±0.03%), 0.37% (±0.03%), 0.48% (±0.03%), and 0.57% (±0.01%) of total leaf proteins, respectively (Figure 2B). These results showed that high-level expression of CTB-FIX was obtained between 101 days and 121 days of plant growth, suggesting that the CTB-FIX lettuce leaves should be harvested at later stages of plant growth in the hydroponic system, balancing this with biomass yield. The fresh weight (g) of the leaves harvested from ∼500 plants at five different ages were 1,304 (32 days), 1,068 (51 days), 1,368 (78 days), 926 (101 days), and 656 (121 days), respectively. Irrespective of the harvest time, CTB-FIX was highly stable during storage and no cleaved product was detected (Figure 2A). It should be pointed out that the CTB-FIX fusion lacks the pre-pro-peptide of FIX and thus is not subject to γ-carboxylation, resulting in a complete lack of coagulation activity.
Figure 2.
cGMP Hydroponic Production of CTB-FIX
Transplastomic lettuce plants expressing CTB-FIX were grown in Fraunhofer cGMP hydroponic system. Leaves were harvested after 32, 51, 78, 101, and 121 days of plant growth. (A) Western blot quantification of CTB-FIX amounts in lettuce leaves harvested at different ages is shown. (B) Concentrations of CTB-FIX in lyophilized lettuce leaves at different stages of growth are shown. Data shown are means ± SD of two independent experiments.
Antibody Formation against Human FIX in Hemophilia B Dogs upon Repeated i.v. Delivery
Outbred hemophilia B dogs (20–24 kg) of the UNC-Chapel Hill strain were used. These dogs have a F9 missense mutation, undetectable circulating FIX antigen and activity, and phenotypically severe hemophilia B.26, 27 This strain of dog was chosen because it has a track record of high degree of predictive accuracy for translational studies.23 First, a pilot study was performed to document safety of CTB-FIX in dogs and to try to identify a suitable protocol for i.v. FIX challenges for a follow-on study (Table S1). Bioencapsulated CTB-FIX was fed to two female hemophilia B dogs (O07 and O67) 2× per week for 10 months in a dose that ranged from 0.05 to 0.1 mg/kg. CTB-FIX-expressing lyophilized plant cells were mixed with canned dog food, and the dogs usually completely consumed the mixture in <5 min. The dogs were fed dry dog food on the other days. No adverse events were detected clinically, nor were there any significant changes in serum liver enzymes, serum creatinine, or total protein and albumin (Table 1).
Table 1.
Serum Chemistry in CTB-FIX-Fed Dogs
| Marker (Normal Range) | S13 (Control) |
S14 |
S15 |
O07 |
O67 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D 0 | D 98 | D 0 | D 98 | D 0 | D 98 | D 0 | D 176 | D 302 | D 0 | D 176 | D 302 | |
| ALP (5–131 U/L) | 46 | 75 | 28 | 27 | 37 | 35 | 17 | 18 | 18 | 33 | 23 | 24 |
| ALT (12–118 U/L) | 63 | 48 | 97 | 91 | 90 | 80 | 45 | 43 | 51 | 34 | 38 | 33 |
| AST (15–66 U/L) | 22 | 21 | 25 | 21 | 23 | 28 | 26 | 18 | 25 | 23 | 22 | 20 |
| GGT (1.0–12 U/L) | 3 | 3 | 4 | 3 | 2 | 4 | 2 | 2 | 1 | 3 | 3 | 1 |
| TBIL (0.1–0.3 mg/dL) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| BUN (6–25 mg/dL) | 15 | 18 | 14 | 18 | 19 | 19 | 14 | 14 | 14 | 13 | 14 | 19 |
| CREA (0.5–1.6 mg/dL) | 0.5 | 0.3a | 0.6 | 0.8 | 0.9 | 1 | 0.6 | 0.7 | 0.6 | 0.7 | 0.7 | 0.6 |
| BUN/CREA (4–27) | 21 | 60b | 23 | 23 | 21 | 19 | 23 | 20 | 23 | 19 | 20 | 32b |
| TP (5–7.4 g/L) | 5.5 | 6 | 5.3 | 5.8 | 5.6 | 5.8 | 6.2 | 6.3 | 6.1 | 5.9 | 5.8 | 5.8 |
| ALB (2.7–4.4 g/dL) | 3.3 | 3 | 3.5 | 3.7 | 3.4 | 3.5 | 3.4 | 3.2 | 3.4 | 3.4 | 3.5 | 3.4 |
| GLOB (1.6–3.6 g/dL) | 2.2 | 3 | 1.7 | 2.1 | 2.2 | 2.3 | 2.8 | 3.1 | 2.7 | 2.5 | 2.3 | 2.4 |
| ALB/GLOB (0.8–2) | 1.5 | 1 | 2.1b | 1.8 | 1.5 | 1.5 | 1.2 | 1 | 1.3 | 1.4 | 1.5 | 1.4 |
| TRIG (29–291 mg/dL) | 27a | 21a | 26a | 27a | 37 | 45 | 31 | 37 | 40 | 30 | 24a | 65 |
| CHOL (92–324 mg/dL) | 161 | 99 | 117 | 123 | 152 | 149 | 222 | 241 | 166 | 161 | 168 | 261 |
| Ca2+ (8.9–11.4 mg/dL) | 9 | 9.9 | 8.9 | 10.2 | 10 | 10.1 | 10 | 9 | 10 | 10.2 | 10 | 9.8 |
| P (2.5–6 mg/dL) | 4.3 | 3.9 | 4.7 | 4 | 4.5 | 4.2 | 4.3 | 3.7 | 4 | 3.8 | 3.4 | 4.1 |
| Na+ (139–154 mEq/dL) | 145 | 157b | 146 | 147 | 146 | 148 | 145 | 146 | 148 | 148 | 147 | 148 |
| K+ (3.6–5.5 mEq/dL) | 4 | 4.2 | 3.8 | 3.8 | 4.2 | 4.3 | 4.1 | 3.6 | 3.8 | 4.1 | 3.7 | 4.3 |
| Cl− (102–120 mEq/dL) | 110 | 113 | 112 | 114 | 113 | 114 | 111 | 113 | 111 | 111 | 113 | 114 |
| Mg2+ (1.5–2.5 mEq/dL) | 1.5 | 3.6b | 1.5 | 1.6 | 1.5 | 1.6 | 1.7 | 1.5 | 1.6 | 1.6 | 1.5 | 1.6 |
| GLU (70–136 mg/dL) | 94 | 78 | 94 | 99 | 102 | 91 | 85 | 85 | 83 | 100 | 92 | 96 |
| AMYL (290–1,125 U/L) | 583 | 549 | 442 | 492 | 620 | 545 | 425 | 456 | 512 | 583 | 456 | 353 |
TBIL, total bilirubin; TP, total protein; ALB, albumin; GLOB, globulin; TRIG, triglycerides; CHOL, cholecterol; GLU, glucose; AMYL, amylase.
Value lower than the normal range.
Value higher than the normal range.
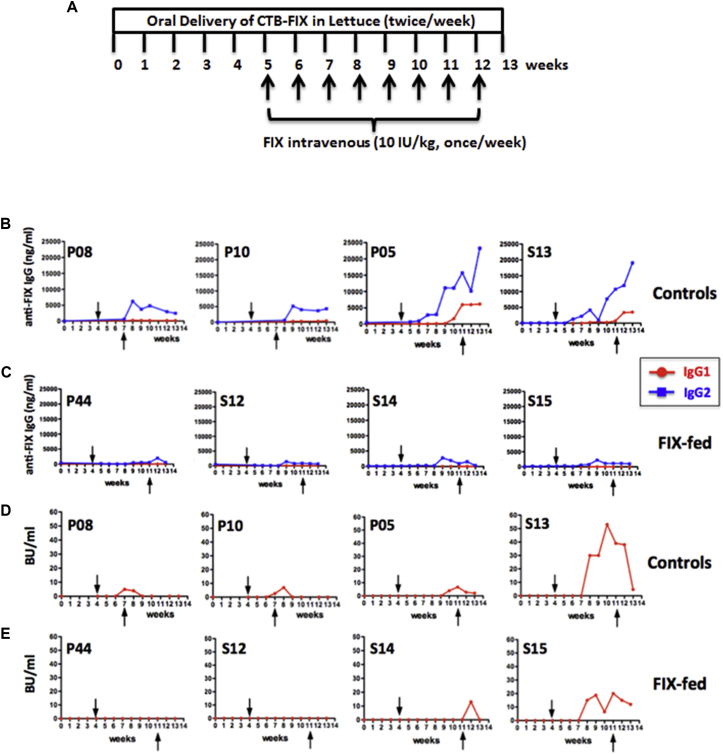
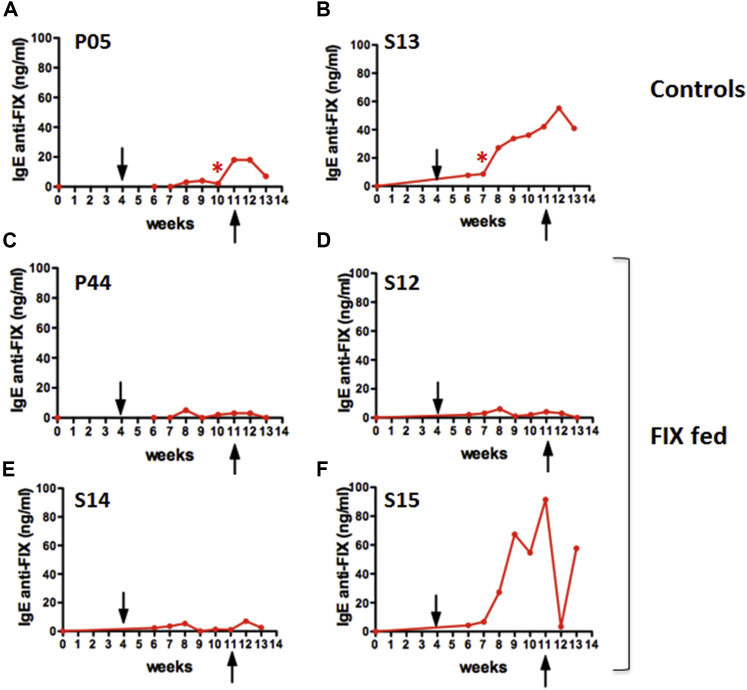
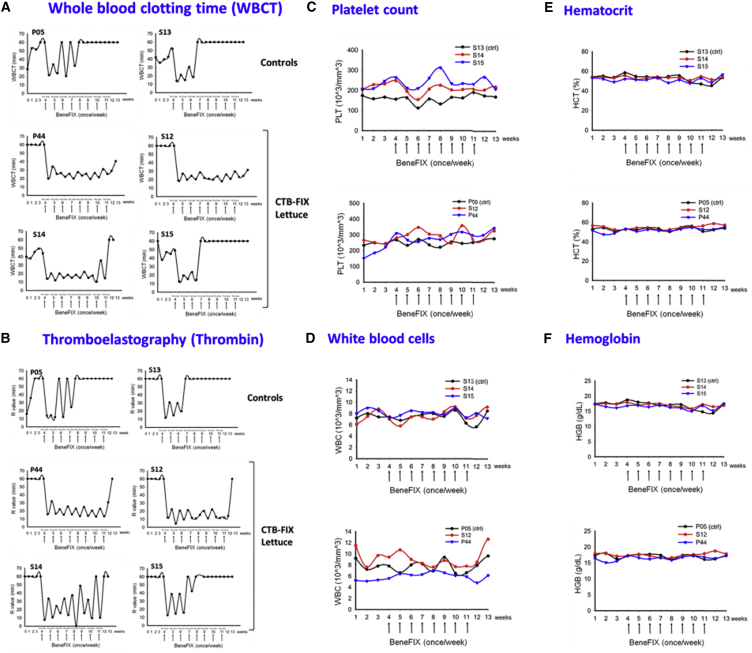
Two other female hemophilia B dogs (P08 and P10) received weekly i.v. injections of recombinant human FIX (10 IU/kg) for 4 weeks (Figure 3A). As a result, both animals formed human FIX-specific immunoglobulin G2 (IgG2) (the equivalent to murine IgG1 and human IgG4) at ∼5 μg/mL (Figure 3B). Inhibitory antibodies were also detected at 5–7 BU/mL at week 5, which however declined to undetectable within 1 week (Figure 3D). Therefore, we chose an extended treatment of eight weekly i.v. injections of FIX for subsequent experiments. Furthermore, to “standardize” the comparison of the humoral immune response to FIX in fed versus control dogs, we sought to establish a challenge protocol that consistently raised IgG2 anti-FIX levels to >10 μg/mL. Two male dogs (P05 and S13) again formed IgG2 against FIX at ∼5 μg/mL after four injections (Figure 3B; hence, IgG2 anti-FIX formation is highly reproducible at a predictable titer and is independent of gender or age, which ranged from 15 months to 5 years). By eight injections in these two animals, IgG2 titers rose to 19–23 μg/mL. Furthermore, both dogs (P05 and S13) additionally formed IgG1 at later time points (Figure 3B). One dog (S13) developed an inhibitory antibody by 1 week after the fourth i.v. injection, which peaked at 53 BU/mL and lasted for 6 weeks (Figure 3D). The other dog (P05) formed an inhibitor after the sixth injection, which peaked at 7 BU/mL and lasted for 4 weeks (Figure 3D). In addition, both dogs had visible anaphylactic reactions (including a transient drop in blood pressure and general unresponsiveness) at the fourth or seventh i.v. injection and developed circulating IgE against FIX (Figures 4A and 4B). To counter these anaphylactic reactions, antihistamine was given. Antihistamine was administered with all subsequent FIX injections to these control dogs out of precaution (orally tolerized dogs also received antihistamine with these late FIX injections in order to keep the treatment protocols identical). WBCT and thromboelastography showed complete correction of coagulation after each of the first three injections and partial correction upon the fourth injection (Figures 5A and 5B), correlating with the onset of anti-FIX formation. Coagulation times entirely failed to correct upon injections 5–8. Allergic reactions were also reflected in hematological parameters, characterized by a transient (<1 hr) drop in platelet and white blood cell (WBC) counts (Figures 5C and 5D).
Figure 3.
Antibody Formation against Human FIX as a Function of Time
(A) Experimental timeline. (B) FIX-specific IgG1 and IgG2 in control dogs are shown. (C) FIX-specific IgG1 and IgG2 in dogs that received oral CTB-FIX in lettuce twice per week for 13 weeks (FIX-fed) are shown. (D) Inhibitor formation (in BU/mL) in control dogs is shown. (E) Inhibitor formation in orally fed dogs (FIX-fed) is shown. Arrows indicate first and last of weekly intravenous injections of recombinant FIX (Benefix; 10 IU/kg; once/week). These i.v. challenges were performed for 8 weeks (except for P08 and P10: these two control dogs received only four weekly injections).
Figure 4.
Specific IgE Formation against Human FIX as a Function of Time in Dogs that Received Eight Weekly i.v. Challenges with Recombinant FIX
(A and B) Control dogs. (C–F) Dogs orally fed with CTB-FIX lettuce twice per week for 13 weeks (FIX fed) are shown. Asterisk indicates visible anaphylactic reaction to FIX i.v. delivery. Arrows indicate the first and the last intravenous challenge of recombinant FIX (Benefix; 10 IU/kg; once/week).
Figure 5.
Coagulation Times and Hematological Evaluations after Intravenous Recombinant Human FIX Injection into Control and CTB-FIX-Fed Hemophilia B Dogs
(A) Whole blood clotting time (WBCT) and (B) thromboelastography were measured weekly over the entire duration (13 weeks) of this study. (C) Weekly platelet counts are shown. (D) Weekly measurements of white blood cell counts are shown. (E) Percentage of hematocrit is shown. (F) Hemoglobin content is shown. (A–F) Arrows indicate i.v. challenges of recombinant human FIX (10 IU/kg of BeneFIX).
Prevention of Antibody Formation by Oral Delivery of CTB-FIX in Lettuce
In contrast to the observation in the control dogs, three dogs (P44, S12, and S14) that received oral doses of lyophilized lettuce (mixed into their chow twice per week for 13 weeks to deliver 0.3 mg CTB-FIX/kg) showed robust suppression of IgG2 formation and entirely failed to form IgG1 or IgE (Figures 3C and 4C–4E). No anaphylactic reactions were observed. In these animals, eight weekly i.v. injections of FIX were given during weeks 4–11 of the oral tolerance regimen (Figure 3A). Importantly, because of a lack of anti-FIX formation, coagulation times were corrected upon all eight i.v. injections of FIX (Figures 5A and 5B). These orally tolerized dogs had no detectable inhibitor throughout the experiment (P44 and S12) or showed a BU titer only at a single time point (S14; Figure 3E). A fourth dog (S15) fed with CTB-FIX responded only partially to the oral tolerance protocol. Whereas IgG2 formation was still substantially suppressed and no IgG1 was detected, an inhibitor still formed (Figures 3C and 3E) and coagulation times only transiently corrected (Figures 5A and 5B). In addition, the dog formed IgE and showed a transient decrease in platelet counts and WBC after the sixth i.v. injection (Figures 5C and 5D). In contrast to the other three orally treated dogs (P44, S12, and S14), this animal (S15) had a pre-existing antibody to human FIX (Figure S1) of unknown origin (the animal had no prior exposure to human FIX). However, even when this animal is factored in, average IgG2 titers 1 week after the last FIX i.v. injection were 32 times lower in the four orally treated compared to the control dogs that also received eight i.v. injections. When comparing the four experimental with all four control dogs, IgG2 peak titers were significantly lower for orally tolerized dogs (p < 0.05), even though two of the control dogs received only four i.v. injections (Figures 3B and 3C).
Lack of Toxicity following Oral Delivery of Bioencapsulated FIX
Consistent with our prior observations in hemophilic mice, none of the dogs that were fed with bioencapsulated CTB-FIX (0.3 mg/kg twice weekly for 3 months) formed antibodies against FIX prior to challenge with i.v. FIX, and no allergic reactions against the fed lettuce material were noted, indicating that oral delivery is not immunogenic. All dogs maintained good general health and normal eating habits and had no loss in weight (data not shown). During the entire feeding period, values of different liver enzymes, including alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT), remained in the normal range, indicating lack of liver toxicity after repeated administration of CTB-FIX lettuce for 302 days (∼10 months; Table 1). Long-term (302 days) feeding of CTB-FIX lettuce did not impair the renal function as creatinine (CREA) and blood urea nitrogen (BUN) were maintained at normal levels. More intriguingly, consumption of extra carbohydrates derived from plant cells did not affect blood glucose levels in dogs fed with CTB-FIX lettuce. Hematological parameters also remained normal in CTB-FIX-fed dogs (Figures 5C–5F), except for the animal that was not fully orally tolerized and thus developed allergic reactions to i.v.-infused FIX. Finally, none of the CTB-FIX-fed dogs developed circulating antibodies against CTB (data not shown).
Discussion
Our study demonstrates successful induction of oral tolerance, preventing pathogenic antibody formation in protein-replacement therapy in a large, non-rodent animal model of human disease. These results establish that both the concept of oral tolerance for therapeutic protein antigens and the approach of oral delivery of plant cells containing chloroplast-made proteins are not limited to mice. More specifically, the data support clinical development of oral administration of transplastomic lettuce as a means of prevention of antibody formation and anaphylaxis in patients with hemophilia.
Clinical Challenges for Tolerance Induction in Hemophilia
During FVIII replacement therapy, 20%–30% of severe hemophilia A patients develop inhibitors that severely complicate therapy. These mostly form in <50 days of exposure. Titers >5 BU/mL render FVIII administration completely ineffective in improving coagulation. ITI protocols to eliminate inhibitors are based on frequent high-dose FVIII injections, which usually require implantation of a port to facilitate i.v. access. Elimination of the inhibitor by this method can take months to years, cost >$1,000,000, and is not always effective, so that immune suppression may be considered.4, 28 Inhibitor formation against FIX occurs less frequently (in 2%–5% of patients, with an elevated risk for those with F9 gene deletions). However, ITI often has to be stopped because of anaphylaxis or nephrotic syndrome.3 Moreover, there are currently no prophylactic ITI protocols to prevent ADA formation to FVIII or FIX. Such a protocol would have to be acceptable in pediatric patients, with a favorable risk/benefit ratio, in part also because prediction of inhibitor formation for a specific patient is not yet very accurate. Use of immune-suppressive drugs or genetic manipulations is therefore viewed with caution. However, an oral tolerance based on delivery of the antigen in an edible crop plant would be attractive. Multiple clinical trials on oral tolerance induction in patients with autoimmune disease have largely failed to show efficacy despite excellent preclinical data in mice.6 Autoimmune diseases are complex, often involving multiple antigens and immune dysregulation. However, other factors likely also contributed, such as insufficient protection of the antigen from degradation and ineffective targeting to the gut immune system.29
Assessing the Potential of Plant-Based Oral Tolerance for Hemophilia in Different Species
Our new data on oral delivery of lettuce cells demonstrate effectiveness in a non-rodent model of genetic disease. Success in two very diverse species (mice and dogs) suggests that our approach is broadly applicable. Ultimately, we envision that pediatric patients with elevated risk for inhibitor formation to FIX (such as those with F9 gene deletion) will be considered for a prophylactic oral immune tolerance protocol. In mice, CTB-FIX made in lettuce was effective over a broad dose range of 0.06–0.6 mg/kg.12 Because delivery to the dogs was less controlled (compared to oral gavages in mice), and because their diet is less plant based, we chose a dose (0.3 mg/kg) closer to the highest tested dose in mice. Our prior studies in mice indicate that tolerance is sustained for some time after oral delivery is stopped, which remains to be studied in the canine model.12, 19 It would also be of interest to know whether the oral tolerance induction regimen and subsequent i.v. injections of FIX could be done sequentially rather than overlapping. Thus far, our protocol always included a period when antigen was administered both orally (in form of bioencapsulated CTB fusion) and i.v. (in form of traditional recombinant factor). Mechanistic studies in mice showed that this combination of oral and systemic administration of antigen amplified immune regulatory responses and thus enhanced the tolerance response.20
Whereas hemophilia B mice and dogs showed similarities in their responses to i.v. delivery of FIX, there were also differences. In mice, inhibitors persisted after injections were stopped,19, 20 whereas in the dogs, inhibitor titers declined rapidly afterward. However, IgG2 formation against FIX in dogs persisted for several weeks with minimal decline and was highly reproducible, both with regard to timing and titers. Also highly reproducible was the resulting lack of correction of coagulation by the fourth to fifth injection. Inhibitor formation was much more variable and did not correlate well with IgG2 titers. Interestingly, there was good correlation between the onset of the inhibitor formation and of anaphylaxis or IgE formation. In hemophilia B mice, inhibitor formation was more suppressed than IgG formation. Non-neutralizing antibodies persisted at considerable titers, although these did not enhance clearance of FIX or interfere with correction of coagulation.12 In contrast, we observed robust suppression of binding antibodies in the canine model. Further studies are needed to characterize the severe allergic reactions to FIX in dogs and determine how they are similar or may differ from those seen in inhibitor patients or in the murine model. For example, the changes in hematological parameters are less typical in FIX inhibitor patients. Both the dogs and mice form IgE, which is why we refer to these as anaphylactic (as opposed to IgG-dependent “anaphylactoid” reactions). Antihistamine was useful to counter the reactions, whereas mice required antihistamine combined with anti-platelet activating factor.19, 30, 31 Abrupt reductions in blood pressure immediately following the i.v. administration of human FIX were consistent with cardiovascular complications in the dogs and similar to human anaphylactic reactions.
Oral tolerance was successful in three hemophilia B dogs. A fourth dog showed only a partial response. Whereas there could be natural variability in an outbred population, we noticed that this dog (in contrast to the other three) had a relatively low-titer antibody that reacted to human FIX by ELISA prior to the onset of the study that may have confounded the results. Because this dog (S15) had not been exposed to this antigen before, the natural history of the antibody is unknown. S15 only had two prior exposures during bleeding events to normal canine plasma that contains ∼5 μg/mL of canine FIX (spaced several months apart), making it unlikely, but not impossible, that there was a cross-reacting antibody against canine FIX. However, S12, S13, and S14 had higher exposure to normal canine plasma for bleeding events and had no pre-existing antibody that bound human FIX. Within this litter, S12 had the best response to oral tolerance induction but also had the highest prior exposure to normal canine plasma. One could therefore speculate that repeated treatment with normal canine plasma actually reduced the response to FIX, even when given in the context of a bleeding event. However, control dog P05 had similar high and frequent exposure to normal canine plasma for bleeding events and still formed a strong immune response. Therefore, we conclude that modifying effects, derived from genetic factors or exposure to normal canine plasma, have minimal impact. As discussed above, all control dogs, regardless of litter, age, or gender, formed very similar levels of IgG2 against human FIX, which was substantially suppressed in orally tolerized animals.
Interestingly, oral delivery of bioencapsulated CTB-FIX reversed pre-existing inhibitors from >10 BU/mL to 1 or 2 BU/mL and prevented anaphylactic reactions in hemophilia B mice (correlating with a reversal of IgE formation) as long as oral delivery was continued when i.v. replacement therapy resumed.20 Therefore, the approach has the potential to reverse the pathogenic antibody response to FIX. Future studies can address this question in the canine model, including a potential requirement to adjust antigen doses or frequency and duration of oral delivery when switching from a preventive application to reversal. In other studies, we induced oral tolerance to B-domain-deleted (BDD) FVIII.22 Whereas detailed dose responses to oral CTB-FVIII are still forthcoming, initial data suggest that the required antigen doses will likely be similar to those for CTB-FIX.22 Ongoing work will determine whether a single BDD-FVIII antigen can be produced at adequate levels or whether a mixture of two transgenic lettuce plants (each expressing a portion of FVIII as published) will be superior.22 Importantly, recent advances in chloroplast genomics and codon-optimization technology results in substantially increased transgenic protein expression.13, 18 In general, FVIII is considered more immunogenic than FIX. Future translation work will show the extent to which the current oral tolerance approach reduces the risk and magnitude of inhibitor formation in hemophilia A or whether adjunct therapeutic approaches may be needed.
One potential concern with our protocol is use of CTB for repeated antigen delivery. CTB is clinically approved as a component of some oral vaccines, and oral delivery of a CTB fusion protein was successfully tested for prevention of relapse of autoimmune uveitis in patients with Behçet’s disease.32 Here, repeated oral administration to dogs over several months did not cause detectible toxicities or negatively affect the animals’ health. Equally important, CTB fusions contained in plant cells fail to prime an antibody response against FIX, which has now been shown for a large number of antigens.9, 33 Given the large number of “foreign” antigens contained in food, the immune system of the gut has evolved a complex regulatory network to maintain tolerance to dietary antigens while still providing mucosal tolerance against pathogens.34 Recent studies have shown that regulatory T cells (T-reg) are a key component of this mechanism, of which our approach takes advantage.20, 35 Upon CTB-mediated binding to the GM1 receptor on the epithelium of the small intestine and translocation from the gut lumen, the FIX antigen is released via proteolytic cleavage and delivered to dendritic cells (DCs), including CD103+ DCs that are critical for transport of antigen to the mesenteric lymph nodes and T-reg cell induction. Antigen presentation to MHC-II-restricted CD4+ T cells results in induction of several subsets of T-reg cells. CD4+CD25+FoxP3+ T-reg cells and interleukin-10 (IL)-10-expressing LAP+CD4+CD25−FoxP3− T-reg cells systemically enforce suppression of antibody formation, which we found to be antigen specific.20, 36 Immunological reagents in the canine model are limited (for example, an antibody to canine LAP is lacking). Nonetheless, we measured frequencies of FoxP3+ T-reg cells in peripheral blood of those dogs that received eight i.v. FIX challenges. Antigen-specific FoxP3+ T-reg cells suppress at low numbers so that total FoxP3+ T-reg cell frequencies may not significantly change. However, it is intriguing that, in one of the orally tolerized dogs (S12), their frequency doubled over time during oral CTB-FIX delivery, whereas it declined in one of the control dogs (P05; Figure S2).
Distinct Advantages of Producing Antigens in Chloroplasts
In this novel protein drug production and delivery concept using transplastomic plants, genes encoding human blood proteins are stably integrated into the chloroplast genome of edible plants. When all copies of plastid genomes in each cell are modified, protein drugs can be expressed at high levels.37, 38 Lyophilized plant cells can be stored for several months or years at room temperature without protein drugs losing their efficacy.12, 39, 40 Low-cost, commercial-scale production in a cGMP facility has also been recently achieved, facilitating further clinical development.12 Plant cells offer natural bioencapsulation through their cell wall. Thus, the expressed protein is protected from stomach acids and digestive juices but is released in the small intestine, following degradation by enzymes produced by commensal bacteria. Although these basic concepts have been demonstrated in mice or rats, this is the first demonstration of feasibility of this concept in a non-rodent animal model, thereby advancing this concept further toward human clinical studies. The combination of bioencapsulation and efficient targeting reduces required antigen doses and are unique features of this low-cost drug delivery concept. This study shows that these features are similar in dogs and most likely in the human system.
However, there are always lingering questions on long-term, repetitive delivery of CTB fusion proteins. None of the dogs that were fed with bioencapsulated CTB-FIX for 98 days or 176 days formed antibodies against FIX prior to challenge with i.v. FIX, and no allergic reactions against the fed lettuce material were noted, indicating that oral antigen delivery is not immunogenic. Good health and lack of liver or kidney toxicity after prolonged (>300 days) and repetitive feeding was observed. More importantly, consumption of additional carbohydrates derived from plant cells did not affect blood glucose levels in dogs fed with CTB-FIX lettuce. These studies provide solid foundation for advancing this approach toward human clinical studies.
Ability to store CTB-FIX for more than 30 months at ambient temperature is a major breakthrough that addresses the relatively short shelf life of many current protein drugs. This study further establishes conditions to deliver effective doses of CTB-FIX to a large animal model of hemophilia of similar size as pediatric patients that are likely candidates for oral tolerance. Our ability to produce cGMP-grade material in a US Food and Drug Administration (FDA)-approved facility and recent approval of plant cells for production of protein drugs similar to other cell culture systems argue well for further clinical advancement of this technology.12, 41
Materials and Methods
Generation and Characterization of Lettuce-Cell-Derived CTB-FIX Antigen
Transformation of lettuce (Lactuca sativa) cv. Simpson Elite chloroplasts, analysis of CTB-FIX transgene integration, and homoplasmic transplastomic lines were performed as previously reported.12 To generate a large scale of lettuce biomass with high concentration of antigen, hundreds of CTB-FIX lettuce seeds were germinated on MS medium for 3 weeks in the growth room. The 3-week-old seedlings were then transferred to the greenhouse and were grown up to 4 months. Lettuce leaves were harvested every ∼3 weeks starting from ∼2.5-month-old plants. Fresh lettuce leaves were stored at −80°C for several weeks or months. Frozen leaves were then subjected to lyophilization as previously described.12 The freeze-dried leaves were ground into fine powder with coffee grinders at maximum speed for three times (5 s pulse/90 s off, each time). The dry leaf powder was packed in 500 mL moisture-free plastic bottles for shipping or storage at room temperature. Production of CTB-FIX lettuce leaves in Fraunhofer hydroponic system was carried out as described by Su et al.12 Western blot analysis, GM1-ganglioside ELISA assay, and protein stability detection of the CTB-FIX fusion proteins in lyophilized leaf samples were performed by following the protocols in previous publications.12, 19
Animals and Treatment Schedule
All procedures in dogs were approved by the University of North Carolina at Chapel Hill’s Institutional Animal Care and Use Committee. Outbred hemophilia B dogs (20–24 kg) of the UNC-Chapel Hill strain were used. These dogs have a F9 missense mutation, resulting in a lack of circulating antigen and severe hemophilia B.26, 27 To induce oral tolerance, lyophilized lettuce cells were mixed into dog chow and fed twice per week for 13 weeks. Each dose was adjusted to deliver CTB-FIX antigen at 0.3 mg/kg. Starting at 4 weeks into the experiment, recombinant human FIX (Benefix; Pfizer) was given i.v. at 10 IU/kg (once per week for 8 weeks). The four dogs undergoing oral tolerance studies were S12 (male; 2 years old), S14 and S15 (males; 15 months old), and P44 (male; 4 years old). Control dogs received FIX i.v. but no oral antigen: P08 and P10 (females; 3 years old) were injected weekly at 10 IU/kg for 4 weeks and S13 (male; 15 months old) and P05 (male; 5 years old) were injected weekly at 10 IU/kg for 8 weeks. Finally, two additional dogs (O07 and O67; female; 3 years old) received oral CTB-FIX antigen at 0.03–0.12 mg/kg twice per week for ∼6 months.
Coagulation, Antibody, and Blood Chemistry Measurements
Coagulation times (whole blood clotting time [WBCT] and thromboelastography [TEG]) as well as inhibitory antibody/Bethesda titers against human FIX were performed as published.42 Measurement of FIX-specific IgG1 and IgG2 titers in sera or plasma by ELISA were also as published.25 An analogous ELISA to measure FIX-specific IgE in sera was established using reagents from Bethyl Laboratories. Briefly, wells were coated with serially diluted canine immunoglobulin containing known amounts of IgE to establish a standard curve. Other wells were coated with human FIX and then incubated with serum samples diluted 1:3. Horseradish peroxidase (HRP)-conjugated goat anti-dog IgE was used for detection. Complete blood counts were performed on a SCIL VetABC calibrated for canine cells, and serum chemistries were performed by Antech Diagnostics.
Author Contributions
J.S., T.C.N., A.S., E.P.M., R.R., and B.Z. performed experiments. J.S., T.C.N., R.W.H., A.S., M.H., B.W., and H.D. analyzed the data. T.C.N., R.W.H., B.W., and H.D. wrote the manuscript. T.C.N., R.W.H., B.W., and H.D. designed and coordinated this project.
Conflicts of Interest
M.H. and B.W. are employees of Novo Nordisk. R.W.H. and H.D. received a grant from Novo Nordisk. H.D. has several patents granted in the US or globally on biopharmaceuticals made in plant chloroplasts. H.D. and R.W.H. have filed patent applications on induction of oral tolerance induction in hemophilia.
Acknowledgments
This work was supported by NIH grants R01 HL107904, R01 HL109442, and HL63098 and grants by Novo Nordisk to H.D., R.W.H., and T.C.N. The authors thank Dr. Xiaomei Wang for assistance with IgE ELISA. The authors thank Drs. Steven Streatfield and Joey Norikane for help in production of CTB-FIX lettuce at the Fruauhofer USA hydroponic facility.
Footnotes
Supplemental Information includes two figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.11.009.
Supplemental Information
References
- 1.Doerfler P.A., Nayak S., Corti M., Morel L., Herzog R.W., Byrne B.J. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol. Ther. Methods Clin. Dev. 2016;3:15053. doi: 10.1038/mtm.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su J., Sherman A., Doerfler P.A., Byrne B.J., Herzog R.W., Daniell H. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol. J. 2015;13:1023–1032. doi: 10.1111/pbi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMichele D.M. Immune tolerance in haemophilia: the long journey to the fork in the road. Br. J. Haematol. 2012;159:123–134. doi: 10.1111/bjh.12028. [DOI] [PubMed] [Google Scholar]
- 4.Leissinger C.A. Advances in the clinical management of inhibitors in hemophilia A and B. Semin. Hematol. 2016;53:20–27. doi: 10.1053/j.seminhematol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Sherman A., Liao G., Leong K.W., Daniell H., Terhorst C., Herzog R.W. Mechanism of oral tolerance induction to therapeutic proteins. Adv. Drug Deliv. Rev. 2013;65:759–773. doi: 10.1016/j.addr.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner H.L., da Cunha A.P., Quintana F., Wu H. Oral tolerance. Immunol. Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbank A.J., Sood P., Vickery B.P., Wood R.A. Oral immunotherapy for food allergy. Immunol. Allergy Clin. North Am. 2016;36:55–69. doi: 10.1016/j.iac.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Vickery B.P., Scurlock A.M., Kulis M., Steele P.H., Kamilaris J., Berglund J.P., Burk C., Hiegel A., Carlisle S., Christie L. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J. Allergy Clin. Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniell H., Chan H.-T., Pasoreck E.K. Vaccination via chloroplast genetics: affordable protein drugs for the prevention and treatment of inherited or infectious human diseases. Annu. Rev. Genet. 2016;50:595–618. doi: 10.1146/annurev-genet-120215-035349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniell H., Lin C.S., Yu M., Chang W.J. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin S., Daniell H. The engineered chloroplast genome just got smarter. Trends Plant Sci. 2015;20:622–640. doi: 10.1016/j.tplants.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su J., Zhu L., Sherman A., Wang X., Lin S., Kamesh A., Norikane J.H., Streatfield S.J., Herzog R.W., Daniell H. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015;70:84–93. doi: 10.1016/j.biomaterials.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon K.C., Daniell H. Oral delivery of protein drugs bioencapsulated in plant cells. Mol. Ther. 2016;24:1342–1350. doi: 10.1038/mt.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens E.C., Lowe E.C., Chiang H., Pudlo N.A., Wu M., McNulty N.P., Abbott D.W., Henrissat B., Gilbert H.J., Bolam D.N., Gordon J.I. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Y., Kwon K.C., Hoffman B.E., Kamesh A., Jones N.T., Herzog R.W., Daniell H. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials. 2016;80:68–79. doi: 10.1016/j.biomaterials.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabowski H., Cockburn I., Long G. The market for follow-on biologics: how will it evolve? Health Aff. (Millwood) 2006;25:1291–1301. doi: 10.1377/hlthaff.25.5.1291. [DOI] [PubMed] [Google Scholar]
- 17.Spök A., Karner S. JRC Scientific and Technical Reports; 2008. Plant Molecular Farming: Opportunities and Challenges (European Communities) [Google Scholar]
- 18.Kwon K.C., Chan H.T., León I.R., Williams-Carrier R., Barkan A., Daniell H. Codon optimization to enhance expression yields insights into chloroplast translation. Plant Physiol. 2016;172:62–77. doi: 10.1104/pp.16.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma D., Moghimi B., LoDuca P.A., Singh H.D., Hoffman B.E., Herzog R.W., Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. USA. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Su J., Sherman A., Rogers G.L., Liao G., Hoffman B.E., Leong K.W., Terhorst C., Daniell H., Herzog R.W. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood. 2015;125:2418–2427. doi: 10.1182/blood-2014-08-597070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolgin E. Immunology: oral solutions. Nature. 2014;515:S166–S167. doi: 10.1038/515S166a. [DOI] [PubMed] [Google Scholar]
- 22.Sherman A., Su J., Lin S., Wang X., Herzog R.W., Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–1668. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols T.C., Hough C., Agersø H., Ezban M., Lillicrap D. Canine models of inherited bleeding disorders in the development of coagulation assays, novel protein replacement and gene therapies. J. Thromb. Haemost. 2016;14:894–905. doi: 10.1111/jth.13301. [DOI] [PubMed] [Google Scholar]
- 24.Sabatino D.E., Nichols T.C., Merricks E., Bellinger D.A., Herzog R.W., Monahan P.E. Animal models of hemophilia. Prog. Mol. Biol. Transl. Sci. 2012;105:151–209. doi: 10.1016/B978-0-12-394596-9.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog R.W., Fields P.A., Arruda V.R., Brubaker J.O., Armstrong E., McClintock D., Bellinger D.A., Couto L.B., Nichols T.C., High K.A. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum. Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 26.Evans J.P., Brinkhous K.M., Brayer G.D., Reisner H.M., High K.A. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc. Natl. Acad. Sci. USA. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog R.W., Arruda V.R., Fisher T.H., Read M.S., Nichols T.C., High K.A. Absence of circulating factor IX antigen in hemophilia B dogs of the UNC-Chapel Hill colony. Thromb. Haemost. 2000;84:352–354. [PMC free article] [PubMed] [Google Scholar]
- 28.Peyvandi F., Garagiola I., Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187–197. doi: 10.1016/S0140-6736(15)01123-X. [DOI] [PubMed] [Google Scholar]
- 29.Rawle F.E., Pratt K.P., Labelle A., Weiner H.L., Hough C., Lillicrap D. Induction of partial immune tolerance to factor VIII through prior mucosal exposure to the factor VIII C2 domain. J. Thromb. Haemost. 2006;4:2172–2179. doi: 10.1111/j.1538-7836.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 30.Markusic D.M., Hoffman B.E., Perrin G.Q., Nayak S., Wang X., LoDuca P.A., High K.A., Herzog R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sack B.K., Wang X., Sherman A., Rogers G.L., Markusic D.M. Immune responses to human factor IX in haemophilia B mice of different genetic backgrounds are distinct and modified by TLR4. Haemophilia. 2015;21:133–139. doi: 10.1111/hae.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanford M., Whittall T., Bergmeier L.A., Lindblad M., Lundin S., Shinnick T., Mizushima Y., Holmgren J., Lehner T. Oral tolerization with peptide 336-351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease. Clin. Exp. Immunol. 2004;137:201–208. doi: 10.1111/j.1365-2249.2004.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan H.T., Xiao Y., Weldon W.C., Oberste S.M., Chumakov K., Daniell H. Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes. Plant Biotechnol. J. 2016;14:2190–2200. doi: 10.1111/pbi.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn C., Weiner H.L. Immunology. How does the immune system tolerate food? Science. 2016;351:810–811. doi: 10.1126/science.aaf2167. [DOI] [PubMed] [Google Scholar]
- 35.Kim K.S., Hong S.W., Han D., Yi J., Jung J., Yang B.G., Lee J.Y., Lee M., Surh C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858–863. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Terhorst C., Herzog R.W. In vivo induction of regulatory T cells for immune tolerance in hemophilia. Cell. Immunol. 2016;301:18–29. doi: 10.1016/j.cellimm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyhan D., Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol. J. 2011;9:585–598. doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruhlman T., Verma D., Samson N., Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon K.C., Daniell H. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol. J. 2015;13:1017–1022. doi: 10.1111/pbi.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon K.C., Verma D., Singh N.D., Herzog R., Daniell H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv. Drug Deliv. Rev. 2013;65:782–799. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaaltiel Y., Gingis-Velitski S., Tzaban S., Fiks N., Tekoah Y., Aviezer D. Plant-based oral delivery of β-glucocerebrosidase as an enzyme replacement therapy for Gaucher’s disease. Plant Biotechnol. J. 2015;13:1033–1040. doi: 10.1111/pbi.12366. [DOI] [PubMed] [Google Scholar]
- 42.Nichols T.C., Franck H.W., Franck C.T., De Friess N., Raymer R.A., Merricks E.P. Sensitivity of whole blood clotting time and activated partial thromboplastin time for factor IX: relevance to gene therapy and determination of post-transfusion elimination time of canine factor IX in hemophilia B dogs. J. Thromb. Haemost. 2012;10:474–476. doi: 10.1111/j.1538-7836.2011.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.