Abstract
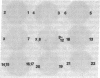
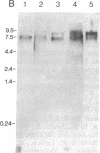
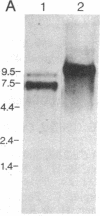
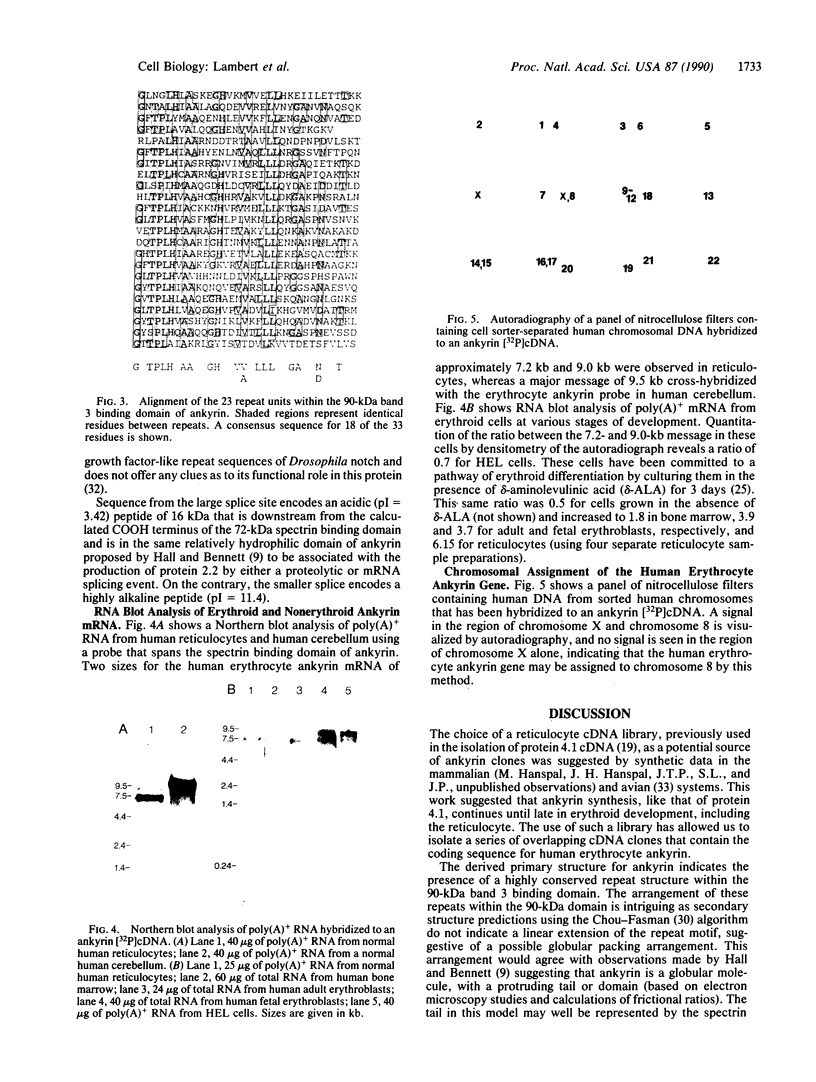
The cDNA for human erythrocyte ankyrin has been isolated from a series of overlapping clones obtained from a reticulocyte cDNA library. The composite cDNA sequence has a large open reading frame of 5636 base pairs (bp) with the complete coding sequence for a polypeptide of 1879 amino acids with a predicted molecular mass of 206 kDa. The derived amino acid sequence contained 194 residues that were identical to those obtained by direct amino acid sequencing of 11 ankyrin proteolytic peptides. The primary sequence contained 23 highly homologous repeat units of 33 amino acids within the 90-kDa band 3 binding domain. Two cDNA clones showed evidence of apparent mRNA processing, resulting in the deletions of 486 bp and 135 bp, respectively. The 486-bp deletion resulted in the removal of a 16-kDa highly acidic peptide, and the smaller deletion had the effect of altering the COOH terminus of the molecule. Radiolabeled ankyrin cDNAs recognized two erythroid message sizes by RNA blot analysis, one of which was predominantly associated with early erythroid cell types. An ankyrin message was also observed in RNA from the human cerebellum by the same method. The ankyrin gene is assigned to chromosome 8 using genomic DNA from a panel of sorted human chromosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Bass E. B., Smith S. W., Jr, Stevenson R. E., Rosse W. F. Further evidence for location of the spherocytosis gene on chromosome 8. Ann Intern Med. 1983 Aug;99(2):192–193. doi: 10.7326/0003-4819-99-2-192. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Human erythrocyte ankyrin. Purification and properties. J Biol Chem. 1980 Mar 25;255(6):2540–2548. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Chilcote R. R., Le Beau M. M., Dampier C., Pergament E., Verlinsky Y., Mohandas N., Frischer H., Rowley J. D. Association of red cell spherocytosis with deletion of the short arm of chromosome 8. Blood. 1987 Jan;69(1):156–159. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Coetzer T. L., Lawler J., Liu S. C., Prchal J. T., Gualtieri R. J., Brain M. C., Dacie J. V., Palek J. Partial ankyrin and spectrin deficiency in severe, atypical hereditary spherocytosis. N Engl J Med. 1988 Jan 28;318(4):230–234. doi: 10.1056/NEJM198801283180407. [DOI] [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. V., Stack J. H., Lazarides E. Erythroid anion transporter assembly is mediated by a developmentally regulated recruitment onto a preassembled membrane cytoskeleton. J Cell Biol. 1987 Sep;105(3):1405–1416. doi: 10.1083/jcb.105.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J Biol Chem. 1984 Nov 10;259(21):13550–13559. [PubMed] [Google Scholar]
- Davis J., Davis L., Bennett V. Diversity in membrane binding sites of ankyrins. Brain ankyrin, erythrocyte ankyrin, and processed erythrocyte ankyrin associate with distinct sites in kidney microsomes. J Biol Chem. 1989 Apr 15;264(11):6417–6426. [PubMed] [Google Scholar]
- Drenckhahn D., Schlüter K., Allen D. P., Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985 Dec 13;230(4731):1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Grasso M., Morelli A., De Flora A. Calcium-induced alterations in the levels and subcellular distribution of proteolytic enzymes in human red blood cells. Biochem Biophys Res Commun. 1986 Jul 16;138(1):87–94. doi: 10.1016/0006-291x(86)90250-0. [DOI] [PubMed] [Google Scholar]
- Hall T. G., Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem. 1987 Aug 5;262(22):10537–10545. [PubMed] [Google Scholar]
- Lebo R. V., Gorin F., Fletterick R. J., Kao F. T., Cheung M. C., Bruce B. D., Kan Y. W. High-resolution chromosome sorting and DNA spot-blot analysis assign McArdle's syndrome to chromosome 11. Science. 1984 Jul 6;225(4657):57–59. doi: 10.1126/science.6587566. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Kidd G. H., Branton D. Identification by peptide analysis of the spectrin-binding protein in human erythrocytes. J Biol Chem. 1979 Apr 10;254(7):2526–2532. [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Goblin (ankyrin) in striated muscle: identification of the potential membrane receptor for erythroid spectrin in muscle cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3292–3296. doi: 10.1073/pnas.81.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. The patterns of expression of two ankyrin isoforms demonstrate distinct steps in the assembly of the membrane skeleton in neuronal morphogenesis. Cell. 1984 Dec;39(2 Pt 1):309–320. doi: 10.1016/0092-8674(84)90009-6. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Kurachi S., Nelson R. Analysis of the erythroid phenotype of HEL cells: clonal variation and the effect of inducers. Blood. 1987 Dec;70(6):1764–1772. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. L., Goodman S. R., Branton D. The effect of endogenous proteases on the spectrin binding proteins of human erythrocytes. Biochim Biophys Acta. 1980 Jun 6;598(3):517–527. doi: 10.1016/0005-2736(80)90032-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Culp E. N., Coleman D. B., Goodman S. R. A structural model of human erythrocyte band 2.1: alignment of chemical and functional domains. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4095–4099. doi: 10.1073/pnas.81.13.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. C., Marchesi V. T. The structural basis of ankyrin function. I. Identification of two structural domains. J Biol Chem. 1984 May 25;259(10):6165–6169. [PubMed] [Google Scholar]
- Weaver D. C., Pasternack G. R., Marchesi V. T. The structural basis of ankyrin function. II. Identification of two functional domains. J Biol Chem. 1984 May 25;259(10):6170–6175. [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Yu J., Goodman S. R. Syndeins: the spectrin-binding protein(s) of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2340–2344. doi: 10.1073/pnas.76.5.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]