Abstract
Organisms respond to heat stress by reprogramming gene expression. In Saccharomyces cerevisiae, heat-induced genes tend to be regulated by factors that belong to the Spt-Ada-Gcn5 acetyltransferase (SAGA) transcription regulatory pathway, whereas heat-repressed genes tend to be regulated by a parallel pathway involving transcription factor IID (TFIID). Here, we examine whether heat stress affects the occupancy of representative factors of each pathway at promoter regions throughout the yeast genome. Representatives of the SAGA pathway include the TATA binding protein, Spt3, and Mot1. Representatives of the TFIID pathway include the TATA binding protein, TAF1, and Bdf1. We find that heat stress causes disassembly of the TFIID pathway at genes that are inhibited by stress. In contrast, heat induces assembly of the SAGA pathway at stress-induced genes, although many also assemble along the TFIID pathway. Other genes were found to assemble almost exclusively along the TFIID pathway. Strikingly, these genes are lowly transcribed and are generally not induced. Thus, heat stress leads to factor assembly along each pathway but with distinct transcriptional outcomes. Further investigation of these pathways reveals that Bdf1 and Mot1 negatively regulate the SAGA pathway in different ways. The findings suggest that Bdf1 blocks assembly, whereas Mot1 promotes disassembly of the transcription machinery.
Keywords: chromatin immunoprecipitation-chip, Spt-Ada-Gcn5 acetyltransferase, transcription factor IID
Acommon theme in eukaryotic gene activation is the binding of sequence-specific transcriptional activators to their cognate promoters. These activators then recruit the TATA binding protein (TBP) to promoters via action of two related multisubunit complexes, transcription factor IID (TFIID) and Spt-Ada-Gcn5 acetyltransferase (SAGA), which enhance TBP delivery in two potential ways. First, as histone acetyltransferases, they have the potential to remodel inhibitory chromatin (1, 2). Second, they directly interact with TBP (3, 4), helping load TBP onto promoters. TFIID and SAGA represent hallmarks of two global transcription regulatory pathways in Saccharomyces cerevisiae (5, 6) and are equivalent to TBP-associated factor (TAF)-dependent and TAF-independent pathways, respectively (7, 8). The SAGA pathway favors TATA-containing genes and typically involves a plethora of positive and negative regulators of chromatin, TBP, and RNA polymerase II (5, 9). The TFIID pathway, on the other hand, predominates at TATA-less genes and appears to involve less regulation than SAGA-dominated genes (5, 9). Most yeast genes are not regulated exclusively by one pathway or the other, but typically involve varying blends of both pathways. Approximately 10% of all genes are dominated by the SAGA pathway, and these genes tend to be stress-induced (5). The remaining 90% are dominated by the TFIID pathway, and these genes tend to play more of a housekeeping role.
Spt3 and Mot1 are connected to the SAGA regulatory pathway (5, 10, 11). Spt3 is a subunit of SAGA that regulates TBP function (3). Mot1 is an ATP-dependent inhibitor of TBP (12). Mot1 occupancy at selected promoters is tightly linked to TBP occupancy, reflecting direct interactions between the two (13, 14). Whereas SAGA-dominated genes tend to be negatively regulated by Mot1 (5), and functional interactions exist between Mot1 and Spt3 (10, 11), it has not been determined whether the two are recruited to the same set of promoters. Moreover, Mot1 has been proposed to form a transcriptionally active complex at stress-induced genes in response to stress (13), but it has not been shown whether Mot1 is a positive or negative regulator of these genes.
TAF1 and Bdf1 are connected to the TFIID regulatory pathway (15–17). TAF1 is a multifunctional essential subunit of TFIID (18). In higher eukaryotes, its carboxyl-terminal domain consists of two bromodomains that bind acetylated histone H4 tails in vitro (19). In yeast, this domain is missing from TAF1 but instead is presumed to be present on a separate protein called Bdf1 (16). Bdf1 interacts with TFIID, possibly tethering it to acetylated nucleosomes (20, 21). However, Bdf1 interacts with other factors (22) and has a number of homologs, making it less clear as to whether it is a physical component of the TFIID pathway.
Little is known about how transcription complex assembly and disassembly fluctuate throughout the genome as genes are reprogrammed in response to environmental changes. Chromatin immunoprecipitation (chIP) assays conducted on a genomewide scale (chIP-chip) provide an unbiased approach to examining changes in physical occupancy of factors at promoter regions during gene activation and repression (23, 24). Here, we use chIP-chip to assess whether genomewide transcriptional reprogramming in response to heat stress is accompanied by assembly and/or disassembly of TBP, Spt3, Mot1, TAF1, and Bdf1 at promoters. We examine whether changes in promoter occupancy of these factors are coupled. Using cluster analysis, we address whether groups of genes recruit factors exclusively along one pathway or the other in response to heat, or whether assembly involves both pathways. These groups are then analyzed further to gain additional insight into TFIID and SAGA pathways. We find that, in general, SAGA assembly at promoters correlates with gene activation, whereas TFIID assembly in the absence of SAGA assembly does not. Thus, heat stress leads to genomewide redistribution of transcription factors, each leading to potentially different outcomes.
Materials and Methods
Strains. Chromosomal genes encoding TBP, Spt3, Mot1, TAF1, and Bdf1 were hemagglutinin (HA)-tagged at the amino-terminal end in the diploid strain BY4743 (his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) by using the Cre-Lox method of Guldener et al. (25) and verified by PCR. Recombinants were sporulated to create the haploid strains used in this study. The presence of the tagged protein was verified by immunoblot analysis with HA antibodies.
chIP-Chip and Microarray Expression Assays. Tagged and untagged (BY4741) haploid strains were grown at 25°C in 100 ml of YPD (1% yeast extract/2% peptone/2% dextrose) to OD600 of 0.8. Cell growth was unaffected by the presence of the HA tag. Cultures were then either heat-shocked at 37°C for 15 min or mock-treated at 25°C for 15 min by mixing the cultures with equal volumes of appropriately heated media to achieve the final desired temperature. After treatment, cultures were immediately cross-linked at 25°C for 2 h with 1% formaldehyde. chIP-chip experiments were performed on four independent replicates of heat-treated and mock-treated cells, as described (9). In brief, intact chromatin from lysed cells was pelleted, washed, and sonicated to release chromatin fragments. A portion (0.024%) of this material was used as “input” DNA for control hybridizations. The remainder was immunoprecipitated with HA antibodies (Babco, Richmond, CA) to produce enriched DNA. Enriched “chIP” DNA (80–90% of total) from heat-shocked and mock-treated cells was cohybridized to microarrays containing PCR-amplified probes spanning nearly all 5,500 intergenic regions of the yeast genome.
Raw data for 6,329 loci are provided in Table 1, which is published as supporting information on the PNAS web site, and at the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo) under accession numbers GSM22295–GSM22326. Background-subtracted spot intensities that were <1 SD (σ) above background in both the Cy5 and Cy3 channels, or <0 in either channel, were removed. Intensity values were then converted to ratios (37°C/25°C) and log2-transformed. Next, an arithmetic average of the four replicates (three for TBP) was computed and is presented in Table 2, which is published as supporting information on the PNAS web site. Factor occupancy could not be unambiguously assigned to promoter regions of divergently transcribed (head-to-head) genes because the microarray probe for both regions is the same. Where groups of genes were examined (Fig. 1 and see Fig. 3), only data from intergenic regions that were flanked by genes in the same orientation (tail-to-head) were used, although similar results were obtained when all genes were used. P values were calculated by using the chitest function in excel.
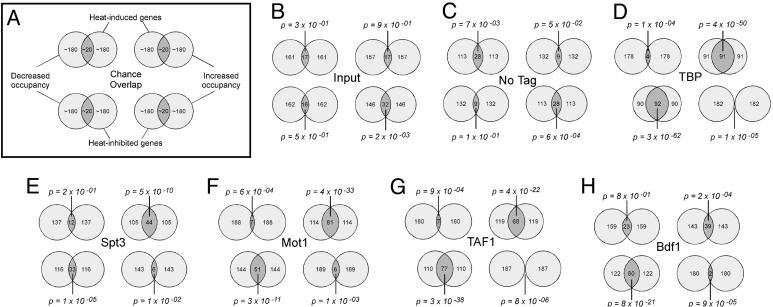
Fig. 1.
Changes in gene expression and factor occupancy during heat stress are linked. (A) Key to the Venn diagrams shown in B–H.(B–H) Inner circles correspond to the 10% of all tail-to-head genes that exhibited the greatest increase (Upper) or decrease (Lower) in gene expression upon heat shock. Tail-to-head refers to those genes that do not share their upstream region with another gene. Outer circles correspond to the 10% of all tail-to-head genes that have intergenic regions with the greatest increase (Right) or decrease (Left) in factor occupancy upon heat shock. We chose a cutoff of 10% because this fraction of the genome tends to be significantly reprogrammed during heat stress (28, 30). Genes narrowly missing this cutoff might nevertheless be regulated by stress. Other types of comparison or cutoffs yielded similar results (Table 3). If no relationship exists, then ≈10% of each data set is expected to overlap by chance. The number of genes meeting each criterion and for which we have data are indicated within each circle. Each factor assayed by chIP is presented separately as indicated.
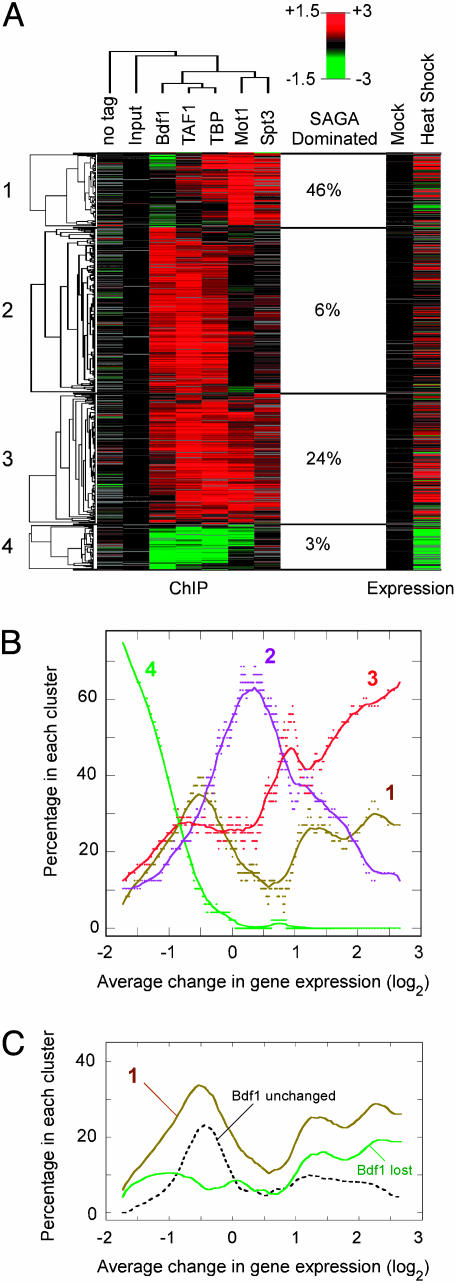
Fig. 3.
Cluster analysis of chIP-chip data. (A) A total of 2,715 tail-to-head intergenic regions were filtered to include only those having an absolute value >0.5 in at least one chIP-chip experiment. A cutoff of 0.5 ensured that the value was >3 SD (3σ) from the median value for the untagged control. Although this cutoff minimized the number of false positives, it also excluded genes with significant but modest changes, resulting in an underestimate of group membership. Filtered intergenic regions (excluding “input” and “no tag”) were next clustered by K-means. Clusters 1–4 are indicated to the left and are represented by 98, 218, 172, and 59 intergenic regions, respectively (see also Table 4, which is published as supporting information on the PNAS web site). Within each cluster, the data were arranged hierarchically. Columns (arrays) were also clustered hierarchically. Similar results were obtained when intergenic regions of divergently transcribed (head-to-head) genes were also included (Fig. 9, which is published as supporting information on the PNAS web site), or when different filtering criteria were used. Increased factor occupancy (Left) or increased expression of the adjacent downstream gene (Right) during heat stress is shown by shades of red, and decreases are shown by shades of green, scaled by the left and right side of the key, respectively. Gray indicates missing data. The percentage of genes in each cluster that are SAGA-dominated is indicated (5). The genomewide distribution is 10%. (B) Genes identified in A were sorted by fold changes in gene expression. Sliding windows (size = 50 genes, step size = 1) through this data set were examined by plotting the average fold change in gene expression in each window against the percentage of genes that fell into clusters 1–4. The curves represent smooth fits. Similar results were obtained by using gene expression profiles published elsewhere (28), ruling out fortuitous cluster-specific expression artifacts. (C) The curve from cluster 1 in B is shown along with curves generated by dividing cluster 1 data in half, representing genes that lost Bdf1 and genes that were generally unchanged.
Data that were subjected to cluster and treeview analysis (26) were first centered to the median value for intergenic regions lacking promoters (i.e., tail-to-tail configured). An assumption is made that these promoterless regions do not bind factors and thus changes in this region should be negligible. DNA detected in these regions is likely to result from nonspecific carryover of genomic DNA. The lack of changes in the centered data for the untagged and input controls supports the validity of this approach.
For expression analysis, BY4741 cultures were treated as described above, except that cells were harvested at the end of the temperature treatment. Sample preparation and microarray assays were performed as described (5, 27). Changes in gene expression matched closely to determinations by Gasch et al. (28) (Fig. 4, which is published as supporting information on the PNAS web site).
Results
Changes in Gene Expression During Heat Stress Are Linked to Changes in Transcription Factor Occupancy at Promoter Regions. Genome-wide changes in factor occupancy during acute heat stress (37°C, 15 min) relative to unstressed cells (25°C) were examined by chIP-chip, in which DNA was cross-linked to proteins in vivo with formaldehyde. Selected proteins were immunopurified via an engineered HA tag on the protein, and the attached cross-linked DNA was detected by hybridization to yeast intergenic microarrays (24, 29). Quality assessment is presented in Figs. 5 and 6, which are published as supporting information on the PNAS web site. In parallel, changes in gene expression were monitored for each gene by using genic microarrays.
Venn diagram comparisons were made between genes exhibiting the greatest increase or decrease in expression and genes exhibiting the greatest increase or decrease in factor occupancy (Fig. 1). A control comparison using unenriched input DNA revealed the expected random overlap (Fig. 1B). A second control using an untagged strain, which represents ratios from minor DNA contaminants, displayed a weak trend in a direction opposite to the trends observed with the tagged strains (Fig. 1C). If a strong relationship exists in the tagged strains, then the overlap is expected to be substantial. However, because many genes narrowly miss or make the cutoff due in part to experimental variation, complete overlaps of the Venn diagrams are not expected.
Previous studies conducted on a small number of selected genes demonstrated that changes in gene expression in response to heat stress are accompanied by changes in TBP occupancy (7, 14, 30, 31). However, it is not known whether this represents a global trend. As shown in Fig. 1D Upper Right, heat-induced genes overlapped significantly (P = 4 × 10–50) with genes that increased in TBP occupancy. Repressed genes overlapped significantly (P = 3 × 10–62) with genes that lost TBP (Fig. 1D Lower Left). Thus, changes in transcriptional output throughout the genome in response to heat stress appear to be generally linked to changes in TBP occupancy, although as shown below changes in TBP occupancy did not always lead to changes in gene expression. Similar linkages between changes in TBP occupancy and gene expression were observed when SAGA- and TFIID-dominated genes were compared separately (Fig. 7D and Table 3, which are published as supporting information on the PNAS web site), indicating that TBP recruitment and dissociation are central to both regulatory pathways.
Having previously established that stress-induced genes tend to be regulated more by SAGA than the rest of the genome (5), we investigated whether heat-induced changes in gene expression are linked to a corresponding change in Spt3 occupancy. As shown in Figs. 1E and 7F, a significant linkage was observed. The linkage was not as robust as with TBP (P = 10–10 vs. 10–50), possibly because of fewer genes being regulated by SAGA than by TBP. These findings indicate that the connection of SAGA regulation with stress-induced gene expression is likely to be caused by its direct physical assembly at these stress-induced genes.
At selected genes, Mot1 and TBP occupancy are coupled (13, 14), reflecting the fact that Mot1 binds TBP. Moreover, transcriptional activation in response to heat stress is accompanied by an increase in binding of both TBP and Mot1, which has led to the suggestion that Mot1 engages TBP to form a transcriptionally active complex in stressed cells (13). A prediction of these findings is that on a genomewide scale stress-induced gene expression should be accompanied by increased Mot1 occupancy and stress-mediated repression should be accompanied by loss of Mot1. Indeed this linkage was observed (Fig. 1F). However, a central question is whether stress-induced genes that acquire Mot1 are in fact positively regulated by Mot1.
Genomewide dependencies on Mot1 under conditions of heat stress have been previously determined by comparing temperature-sensitive mot1-1 and mot1-14 alleles to WT MOT1 (14, 32, 33). Using the mot1-14 data set, we examined whether stress-induced genes that acquire Mot1 (defined by the overlapping Venn diagram in Fig. 1F) tended to be positively regulated by Mot1. For these 81 genes, loss of Mot1 resulted in a 1.4-fold average increase in expression (Fig. 8, which is published as supporting information on the PNAS web site), which suggests that Mot1 negatively regulates these stress-induced genes. Although counterintuitive, this finding demonstrates that factors that associate with induced genes can inhibit expression rather than promote it.
In addition to using SAGA, many stress-induced genes are regulated by TFIID (5, 7). Consistent with that notion, heat-induced genes significantly overlapped with genes showing maximal increase in TAF1 occupancy (Fig. 1G). Thus, both TFIID and SAGA increase their occupancy at heat-induced promoters in response to heat stress. Reciprocally, genes that are inhibited by heat significantly overlapped with genes that lose TAF1, which is consistent with the notion that transcriptional shutdown from stress involves the dissociation of TFIID.
Although Bdf1 has been associated with TFIID function, it has not previously been implicated in the response to heat stress. As shown in Fig. 1H, the overlap between heat-induced genes and those gaining Bdf1 was modest in comparison to TAF1, possibly reflecting little or no involvement of Bdf1 and raising the question as to whether Bdf1 is linked to TFIID recruitment during gene activation. A more substantial overlap was observed between heat-inhibited genes and those losing Bdf1, suggesting that transcriptional inhibition involves the disassembly of Bdf1 and TAF1, possibly linking Bdf1 with TFIID at these promoters.
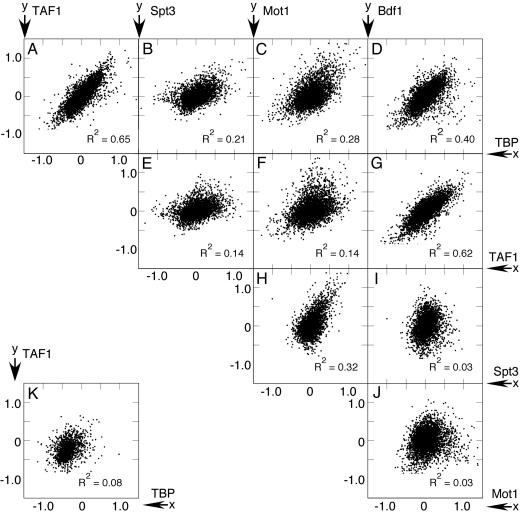
Changes in Occupancy of Functionally Related Factors Are Highly Correlated. Whereas heat induction of genes tends to be connected to increased occupancy by TBP, Spt3, TAF1, and Mot1, the analysis does not reveal relationships between factors. In particular, do factors that are thought to work together actually change promoter occupancy in unison when genes are reprogrammed? To address this question, pairwise comparisons of fold changes in factor occupancy upon heat stress were made by using scatter plots. The strongest relationships were observed among TAF1, Bdf1, and TBP (Fig. 2 A, D, and G), with R2 values ranging between 0.4 and 0.7. These R2 values are similar to what is observed when independent samples of the same protein are compared (Fig. 5B), indicating a very strong linkage. When intergenic regions lacking promoters were examined, no correlation was found (Fig. 2K), indicating that the relationships are specific to promoter-containing intergenic regions. The strength of each correlation suggests that heat-induced changes in TBP, TAF1, and Bdf1 occupancy throughout the genome are generally linked to each other during both transcription complex assembly and disassembly. In all pairwise comparisons the linkage between TAF1 and Bdf1 occupancy was among the strongest, which supports the idea that changes in promoter occupancy of Bdf1 and TAF1 in response to heat stress are generally coordinated. This observation is in apparent contradiction to the findings in Fig. 1, showing that increased occupancy of TAF1 and TBP, but not Bdf1, is strongly linked to heat-induced gene expression. Apparently, the assembly and disassembly of these factors at promoters involves additional complexity that is not reflected by changes in gene expression (examined further below).
Fig. 2.
Correlation of genomewide changes in factor occupancy. (A–J) Median-centered fold changes in factor occupancy are plotted on a log2 scale for all intergenic regions upstream of genes in tail-to-head or head-to-head configurations. Head-to-head refers to divergently transcribed genes, which share their upstream region with another gene. X axis labels are shown to the right, and y axis labels are shown on top. (K) A representative correlation for downstream promoterless intergenic regions (i.e., flanking genes are in a convergent tail-to-tail configuration). Correlation R2 values are indicated.
Heat-induced occupancy changes for TBP, Spt3, and Mot1 were also positively correlated, with R2 values ranging between 0.2 and 0.4 (Fig. 2 B, C, and H). The lower R2 values reflect less linkage than was observed with TBP, TAF1, and Bdf1, possibly caused by Spt3 and Mot1 assembling and disassembling at fewer genes, thereby contributing less to the bulk correlation. In line with previous studies (5, 10, 11), these data provide evidence for physical occupancy of SAGA and Mot1 at the same set of target genes. Conceivably, SAGA might deliver a form of TBP that is accessible to Mot1.
Factor Assembly at Some Genes Involves both the TFIID and SAGA Pathways, Whereas Others Involve Only One Pathway. Having shown that factors within the TFIID regulatory pathway generally assemble and disassemble at the same promoters, and that factors within the SAGA regulatory pathway generally assemble and disassemble at the same promoters, we next asked whether the two pathways operate separately on distinct sets of genes and/or whether they target the same genes. To explore how changes in occupancy of multiple factors are coordinated during heat stress, chIP data were clustered by using a K-means algorithm and compared to changes in gene expression (Fig. 3A). The largest number of visually nonredundant clusters was four. The percentage of genes in each cluster that were previously classified as SAGA-dominated is also shown in Fig. 3A. Values significantly greater than the genomewide average of 9% indicate that the cluster is biased toward SAGA regulation, whereas values significantly <9% indicate a bias toward TFIID regulation.
Cluster 1 generally gained TBP, Mot1, and Spt3, but not Bdf1 or TAF1 upon heat stress (Fig. 3A), suggesting that SAGA but not TFIID assembles at these largely SAGA-dominated stress-induced genes. Cluster 2 genes acquired Bdf1, TAF1, and TBP in response to heat stress. No changes in Mot1 and Spt3 occupancy were detected, suggesting that heat stress promotes assembly of the TFIID pathway but not the SAGA pathway at these genes. Cluster 3 appeared to acquire all of the tested factors, reflecting assembly of both pathways at the same set of target genes. The three clustering patterns reveal that during heat stress many genes assemble factors along both the TFIID and SAGA pathways, whereas other genes use primarily one pathway or the other.
Cluster 4 genes represent heat-inhibited genes that lost Bdf1, TAF1, TBP, and Mot1 upon heat stress, reflecting disassembly of TFIID at these largely TFIID-dominated genes. Interesting, the loss of Mot1 but not Spt3 suggests that Mot1 might function independently of SAGA at many of these genes.
Genes That Recruit TFIID but Not Components of the SAGA Pathway During Heat Stress Are Not Induced. The data in Fig. 3A indicate that in response to heat stress factors assemble on some promoters primarily through the SAGA pathway (cluster 1), others primarily through the TFIID pathway (cluster 2), and others use both pathways (cluster 3). Because we have shown that factor assembly is generally coupled to increased gene expression (Fig. 1), clusters 1–3 should represent highly induced genes. Alternatively, the TFIID and SAGA assembly pathways might be tailored for different levels of induction, as suggested from expression data (5). To distinguish between these possibilities, we assessed to what extent each cluster was represented among groups of similarly induced genes. Genes in clusters 1–4 were sorted according their change in gene expression upon heat stress. Next, sliding 50-gene windows through this sorted data set were examined for the percentage of genes belonging to each of the four clusters. These percentages are plotted in Fig. 3B as a function of the average fold change in gene expression in each 50-gene window.
There were clear tendencies of clusters to dominate at different positions along the induction/repression spectrum. Transcriptional repression (Fig. 3B, left side of the spectrum) was dominated by cluster 4, as expected. Transcriptional induction (Fig. 3B, right side of the spectrum) was dominated by cluster 3, which represents genes that assemble along both the TFIID and SAGA pathways. Surprisingly, the middle of the spectrum, reflecting little or no change in gene expression, was dominated by cluster 2. Cluster 2 genes are relatively quiescent, having the lowest average mRNA level of all four clusters (data not shown). Thus, heat shock promotes the assembly of TFIID at many lowly expressed genes but fails to substantially activate them.
Induction of SAGA-Dominated Genes Is Linked to Dissociation of Bdf1. Cluster 1 has many genes that assemble predominantly through the SAGA pathway and are highly transcribed under nonstress conditions (data not shown). This cluster displayed a strikingly bimodal induction profile (Fig. 3B). One subset of genes appeared to be highly induced, and another subset was not. A distinctive feature of many of the genes in cluster 1 is a tendency to lose Bdf1 upon heat shock (Fig. 3A). We wondered whether this tendency was in some way related to the bimodal induction behavior of cluster 1. To address this idea, we separately plotted the induction profile of those cluster 1 genes that lost Bdf1 and those that did not (Fig. 3C). Strikingly, the subset of genes that lost Bdf1 predominated at the high end of the induction spectrum, suggesting that dissociation of Bdf1 from promoter regions is linked to transcriptional activation of these genes. Consistent with this, comparisons with published microarray expression data revealed that these genes are negatively regulated by Bdf1 and factors that function with Bdf1 such as histone H4 tails (data not shown). Thus, Bdf1 might block assembly of the SAGA pathway at these promoters.
Cluster 1 genes that tended not to lose Bdf1 in response heat stress peaked toward the left side of the spectrum in Fig. 3C, reflecting a lack of induction or slight inhibition. Expression of these genes was also largely unaffected by deletion of Bdf1, and these genes had relatively low levels of Bdf1 occupancy (data not shown), indicating that these genes are Bdf1-independent. They were also among the most highly expressed genes in the genome before and after heat stress, having an average mRNA level that was in the top 10% genomewide (data not shown). Thus, heat stress leads to more assembly along the SAGA pathway at many constitutively active genes but without a net increase in mRNA levels.
Discussion
Heat-Induced Changes in Gene Expression Involve Mobilization of the TFIID and SAGA Pathways in Different Ways. Transcriptional regulation of the yeast genome is regulated by two major pathways. Approximately 10% of all genes tend to be stress-induced and highly regulated through the SAGA pathway (5, 9). The remaining 80–90% tend to be housekeeping genes that appear to involve less regulation than the stress-induced genes. These genes use primarily the TFIID pathway, and many are inhibited by stress. Both pathways are used to varying extent at all genes. The existence of these pathways is supported by the data presented here, which examine the change in promoter occupancy of factors in each pathway in response to heat stress. During heat stress components of the SAGA pathway assemble at heat-induced genes, and components of the TFIID pathway dissociate from heat-repressed genes. TFIID also contributes to the expression of many SAGA-regulated heat-induced genes, and their promoter regions recruit TFIID in response to heat.
Assembly via the TFIID and SAGA Pathways Results in Distinct Transcriptional Outcomes. The most surprising find is that TFIID is recruited to a large number of lowly transcribed genes in response to heat, but fails to substantially activate them. These genes are functionally diverse, displaying no particular penchant for stress-related processes, and as a group display no “signature” dependence on particular sequence-specific or general transcriptional regulators (data not shown). Their ability to assemble TFIID in the absence of substantial gene activation suggests that postassembly transcriptional controls in the TFIID pathway are widespread in yeast.
The logic for assembling TFIID in response to a stimulus without increasing net transcriptional output is not obvious. Perhaps these genes provide a delayed response to heat stress, being poised to respond to products of the initial response. This possibility seems unlikely in that these genes are not expressed at higher levels later on in the stress response (data not shown). A second possibility is that the TFIID pathway assembles transcription complexes inefficiently or with low transcriptional activity in comparison to the SAGA pathway. Although it is clear that SAGA is linked to greater inducibility of genes, ribosomal genes that operate almost exclusively through the TFIID pathway are nonetheless highly expressed (34). Thus, the TFIID pathway is capable of efficiently assembling transcription complexes with high output.
Two Modes of Negative Regulation: Blocking vs. Dismantling. The TFIID and SAGA pathways involve more than just TFIID and SAGA. In the TFIID pathway, Bdf1 associates with TFIID but also has functions apart from TFIID (20–22). Here, we find that changes in promoter occupancy throughout the genome by the TAF1 component of TFIID is highly coupled to changes in occupancy of Bdf1. This places Bdf1 firmly in the TFIID pathway.
We find one striking example of Bdf1 operating outside of TFIID. At stress-induced genes that recruit factors predominantly along the SAGA pathway, Bdf1 plays an inhibitory role. Factor recruitment in response to heat stress is linked to the dissociation of Bdf1 at these genes. Mechanistically, how Bdf1 might block the SAGA pathway remains to be determined. Although alleviation of Bdf1 inhibition might or might not be the basis for inducibility of these genes, it is interesting that many constitutively active SAGA-regulated genes are not regulated by Bdf1 (Fig. 3C).
In the SAGA pathway, SAGA-regulated genes tend to be negatively regulated by Mot1. During heat stress, we find that Mot1 is recruited to stress-induced genes along with SAGA. Although Mot1 has been proposed to play a positive role at stress-induced genes, microarray expression data reveal that it is a negative regulator. Mot1 might be involved in dismantling stress-activated complexes as they are being assembled, thereby attenuating transcription. This activity is consistent with its well defined biochemical activity and might explain the transient induction that is typical of stress-induced genes (28, 35).
The analyses presented here reveal two distinct modes of negative regulation, each with potentially different outcomes. One involves Bdf1 whose dissociation from promoter regions is linked to assembly of the SAGA pathway, and the other involves Mot1 whose association to promoter regions is linked to assembly of the SAGA pathway. The former might prevent assembly. Alleviating this block would lead to transcriptional induction. The latter targets an assembled complex, possibly dismantling it, and thus would be found only at actively transcribed genes.
It is intriguing that both Bdf1 and Mot1 negatively regulate the SAGA pathway and positively regulate the TFIID pathway, despite each belonging to different pathways. This cross-talk might serve to coordinate the activities of the two pathways.
Supplementary Material
Acknowledgments
We thank John Robinson for the construction of the HA-tagged strains; Kathryn Huisinga, Joe Reese, Dave Gilmour, and Song Tan for many helpful discussions; and Jerry Workman and the late Bob Simpson for initiating production of intergenic arrays. This work was supported by National Institutes of Health Grant GM059055.
Author contributions: B.F.P. designed research; S.J.Z. performed research; S.J.Z. and B.F.P. analyzed data; and B.F.P. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: chIP, chromatin immunoprecipitation; TBP, TATA binding protein; TAF, TBP-associated factor; TFIID, transcription factor IID; SAGA, Spt-Ada-Gcn5 acetyltransferase; HA, hemagglutinin.
Data deposition: The sequences reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (accession nos. GSM22295–GSM22326).
References
- 1.Brownell, J. E., Zhou, J., Ranalli, T., Kobayashi, R., Edmondson, D. G., Roth, S. Y. & Allis, C. D. (1996) Cell 84, 843–851. [DOI] [PubMed] [Google Scholar]
- 2.Mizzen, C. A., Yang, X. J., Kokubo, T., Brownell, J. E., Bannister, A. J., Owen-Hughes, T., Workman, J., Wang, L., Berger, S. L., Kouzarides, T., et al. (1996) Cell 87, 1261–1270. [DOI] [PubMed] [Google Scholar]
- 3.Grant, P. A., Duggan, L., Cote, J., Roberts, S. M., Brownell, J. E., Candau, R., Ohba, R., Owen-Hughes, T., Allis, C. D., Winston, F., et al. (1997) Genes Dev. 11, 1640–1650. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa, J., Kokubo, T., Horikoshi, M., Roeder, R. G. & Nakatani, Y. (1997) Proc. Natl. Acad. Sci. USA 94, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huisinga, K. L. & Pugh, B. F. (2004) Mol. Cell 13, 573–585. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, J. X., Floer, M., Ononaji, P., Bryant, G. & Ptashne, M. (2002) Curr. Biol. 12, 1828–1832. [DOI] [PubMed] [Google Scholar]
- 7.Kuras, L., Kosa, P., Mencia, M. & Struhl, K. (2000) Science 288, 1244–1248. [DOI] [PubMed] [Google Scholar]
- 8.Li, X. Y., Bhaumik, S. R. & Green, M. R. (2000) Science 288, 1242–1244. [DOI] [PubMed] [Google Scholar]
- 9.Basehoar, A. D., Zanton, S. J. & Pugh, B. F. (2004) Cell 116, 699–709. [DOI] [PubMed] [Google Scholar]
- 10.Madison, J. M. & Winston, F. (1997) Mol. Cell. Biol. 17, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalidou, I., Papamichos-Chronakis, M., Thireos, G. & Tzamarias, D. (2004) EMBO J. 23, 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira, L. A., Klejman, M. P. & Timmers, H. T. (2003) Gene 315, 1–13. [DOI] [PubMed] [Google Scholar]
- 13.Geisberg, J. V. & Struhl, K. (2004) Mol. Cell 14, 479–489. [DOI] [PubMed] [Google Scholar]
- 14.Geisberg, J. V., Moqtaderi, Z., Kuras, L. & Struhl, K. (2002) Mol. Cell. Biol. 22, 8122–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon, D., Bai, Y., Campbell, A. M., Bjorklund, S., Kim, Y. J., Zhou, S., Kornberg, R. D. & Weil, P. A. (1995) Proc. Natl. Acad. Sci. USA 92, 8224–8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matangkasombut, O., Buratowski, R. M., Swilling, N. W. & Buratowski, S. (2000) Genes Dev. 14, 951–962. [PMC free article] [PubMed] [Google Scholar]
- 17.Reese, J. C., Apone, L., Walker, S. S., Griffin, L. A. & Green, M. R. (1994) Nature 371, 523–527. [DOI] [PubMed] [Google Scholar]
- 18.Wassarman, D. A. & Sauer, F. (2001) J. Cell Sci. 114, 2895–2902. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson, R. H., Ladurner, A. G., King, D. S. & Tjian, R. (2000) Science 288, 1422–1425. [DOI] [PubMed] [Google Scholar]
- 20.Ladurner, A. G., Inouye, C., Jain, R. & Tjian, R. (2003) Mol. Cell 11, 365–376. [DOI] [PubMed] [Google Scholar]
- 21.Matangkasombut, O. & Buratowski, S. (2003) Mol. Cell 11, 353–363. [DOI] [PubMed] [Google Scholar]
- 22.Krogan, N. J., Keogh, M. C., Datta, N., Sawa, C., Ryan, O. W., Ding, H., Haw, R. A., Pootoolal, J., Tong, A., Canadien, V., et al. (2003) Mol. Cell 12, 1565–1576. [DOI] [PubMed] [Google Scholar]
- 23.Lieb, J. D., Liu, X., Botstein, D. & Brown, P. O. (2001) Nat. Genet. 28, 327–334. [DOI] [PubMed] [Google Scholar]
- 24.Ren, B., Robert, F., Wyrick, J. J., Aparicio, O., Jennings, E. G., Simon, I., Zeitlinger, J., Schreiber, J., Hannett, N., Kanin, E., et al. (2000) Science 290, 2306–2309. [DOI] [PubMed] [Google Scholar]
- 25.Guldener, U., Heck, S., Fielder, T., Beinhauer, J. & Hegemann, J. H. (1996) Nucleic Acids Res. 24, 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. (1998) Proc. Natl. Acad. Sci. USA 95, 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitikila, C., Huisinga, K. L., Irvin, J. D., Basehoar, A. D. & Pugh, B. F. (2002) Mol. Cell 10, 871–882. [DOI] [PubMed] [Google Scholar]
- 28.Gasch, A. P., Spellman, P. T., Kao, C. M., Carmel-Harel, O., Eisen, M. B., Storz, G., Botstein, D. & Brown, P. O. (2000) Mol. Biol. Cell 11, 4241–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieb, J. D. (2003) Methods Mol. Biol. 224, 99–109. [DOI] [PubMed] [Google Scholar]
- 30.Kuras, L. & Struhl, K. (1999) Nature 399, 609–613. [DOI] [PubMed] [Google Scholar]
- 31.Li, X. Y., Virbasius, A., Zhu, X. & Green, M. R. (1999) Nature 399, 605–609. [DOI] [PubMed] [Google Scholar]
- 32.Andrau, J. C., Van Oevelen, C. J., Van Teeffelen, H. A., Weil, P. A., Holstege, F. C. & Timmers, H. T. (2002) EMBO J. 21, 5173–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasgupta, A., Darst, R. P., Martin, K. J., Afshari, C. A. & Auble, D. T. (2002) Proc. Natl. Acad. Sci. USA 99, 2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mencia, M., Moqtaderi, Z., Geisberg, J. V., Kuras, L. & Struhl, K. (2002) Mol. Cell 9, 823–833. [DOI] [PubMed] [Google Scholar]
- 35.Causton, H. C., Ren, B., Koh, S. S., Harbison, C. T., Kanin, E., Jennings, E. G., Lee, T. I., True, H. L., Lander, E. S. & Young, R. A. (2001) Mol. Biol. Cell 12, 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.