Abstract
There is increasing evidence that aspirin initiates biosynthesis of novel antiinflammatory mediators by means of interactions between endothelial cells and leukocytes. These mediators are classified as aspirin-triggered 15-epi-lipoxins. Such compounds may account at least in part for aspirin's clinical benefits, which are distinct from the well appreciated action of aspirin as a platelet inhibitor. Here, we addressed whether aspirin-triggered 15-epilipoxinA4 (ATL) formation is aspirin-dependent in humans and its relationship to aspirin's antiplatelet activity. We conducted a randomized clinical trial among 128 healthy subjects allocated to placebo or to 81-, 325-, or 650-mg daily doses of aspirin for 8 weeks. Plasma thromboxane (TX)B2, an indicator of platelet reactivity, and ATL were assessed from blood collected at baseline and at 8 weeks. Plasma ATL levels significantly increased in the 81-mg aspirin group (0.25 ± 0.63 ng/ml, P = 0.04), with borderline increases in the 325-mg group (0.16 ± 0.71 ng/ml) and no apparent significant changes in the 650-mg group (0.01 ± 0.75 ng/ml, P = 0.96). When ATL and TXB2 were compared, levels changed in a statistically significant and opposite direction (P < 0.01) for all three aspirin doses. These results demonstrated that low-dose aspirin (81 mg daily) initiates production of antiinflammatory ATL opposite to the inhibition of TX. Monitoring ATL may represent a simple clinical parameter to verify an individual's vascular leukocyte antiinflammatory response with low-dose aspirin treatment. These results also emphasize the importance of cell-cell interactions in the modulation of hemostatic, thrombotic, and inflammatory processes.
Aspirin is the most commonly administered nonsteroidal antiinflammatory drug. In addition to its well documented antithrombotic and antiinflammatory actions, low doses of aspirin may evoke beneficial effects that go beyond prevention and treatment of cardiovascular diseases (1), such as possibly decreasing the incidence of lung, colon, and breast cancer and, perhaps, Alzheimer's disease (2-5). Although inhibition of specific cyclooxygenase (COX) products accounts for many of aspirin's therapeutic properties, effects that go beyond inhibition of prostaglandin and thromboxane (TX) are increasingly apparent (6). We recently identified a unique action of aspirin that involves cells containing COX-2, such as vascular endothelium, which are involved in transcellular communication with blood-borne and/or marginating leukocytes (Fig. 1). Briefly, acetylation of vascular COX-2 by aspirin redirects the catalytic activity of COX-2 from generating intermediates of prostaglandins and TX to instead produce intermediates of 15-epimeric lipoxin A4 formation (7). This epimer of lipoxin A4 is termed aspirin-triggered 15-epi-lipoxin A4 (ATL) and carries its carbon 15-hydroxyl group in the R-configuration, which is released by activated human neutrophils in vitro (7). Other widely used nonsteroidal antiinflammatory drugs of general COX inhibitors are unable to generate ATL. Hence, aspirin has the unique ability to generate an endogenous mimetic of natural lipoxin A4, namely ATL.
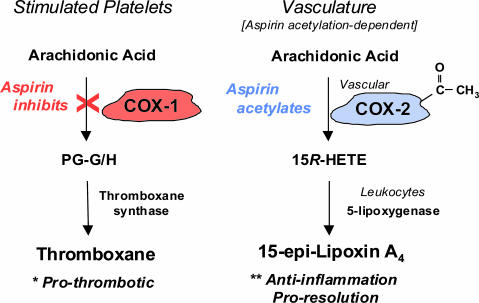
Fig. 1.
Aspirin's acetylation-dependent regulation of TX and ATL. TX is a major eicosanoid from human platelets and a potent platelet activator (1). Acetylation of COX-1 blocks the endoperoxide intermediate for prostaglandins (PG-G/H) and TX. The ATL is generated by the acetylated COX-2 in the vasculature that blocks prostaglandin production and initiates COX-2 to produce 15R-hydroxy eicosatetraenoic acid (15R-HETE). Transcellular biosynthesis of 15-epi-lipoxin A4 is enabled during vascular endothelial and leukocyte interactions. ATL is a local mediator and possesses potent actions in antiinflammation and proresolution (8, 32).
As a relatively new class of autacoids identified in 1995, ATL functions as a local endogenous antiinflammatory mediator. ATL acts on neutrophils and possesses protective activities in several target tissues and murine models of disease that include peritonitis, dermal inflammation, reperfusion injury, asthma, and angiogenesis (see ref. 8 for original citations). By using a newly developed specific ELISA along with liquid chromatography-tandem mass spectrometry (8, 9), ex vivo ATL formation was determined with cells from patients with asthma that were both aspirin-tolerant and aspirin-intolerant (10). Formation of ATL also was demonstrated as an endogenous autacoid in several murine models (8). It is of interest to note that COX-2, a critical enzyme in ATL biosynthesis, was initially conceptualized solely as an “inducible” COX. However, in many cell types, including mature megakaryocytes, COX-2 is constitutively expressed (11), and, in vascular endothelial cells, COX-2 is expressed after exposure to laminar shear (12). Whereas ATL clearly has antiinflammatory functions in experimental animal models and in isolated human cells (7), aspirin-dependent production of ATL has not been demonstrated in a randomized human trial. For these reasons, we initiated studies to ascertain whether aspirin administered in standard clinical doses to healthy volunteers would initiate antiinflammatory ATL generation.
Materials and Methods
Randomized Clinical Trial. We conducted a randomized, double-blind, and placebo-controlled clinical trial involving three different doses of aspirin (81, 325, and 650 mg), taken once daily in the morning over an 8-week period. These are the most frequently used doses of aspirin administered in the United States and are clinically recommended for different therapeutic purposes: a low dose (81 mg) for long-term antithrombotic prophylaxis, a medium dose (325 mg) for acute situations, such as myocardial infarction and thrombotic stroke, and a higher dose (650 mg) for analgesic and antipyretic effects that also are classified clinically as antiinflammatory (6, 13, 14).
Between May 2001 and January 2002, 140 healthy subjects aged 40 years and older who provided informed consent were enrolled. Participants were ineligible if they had a prior history of diabetes or any cardiovascular, gastrointestinal, hematologic, renal, hepatic, pulmonary, or chronic inflammatory disorders. Use of aspirin, nonsteroidal antiinf lammatory drugs, aspirin-containing compounds, COX-2 inhibitors, and steroids was not allowed in the 3 weeks before enrollment, and subjects taking medications that may interact adversely with aspirin (e.g., anticoagulants) were excluded. These subjects were randomized at two sites: the Southern Jamaica Plain Health Center in Jamaica Plain, MA, and Advanced Biomedical Research in Hackensack, NJ. Randomization was pre-specified by consecutive subject number and was computer-generated by using block randomization in groups of four without stratification.
Measurement of ATL and TXB2. Blood samples were collected before and after 8 weeks of aspirin or placebo dosing in the fasting state in EDTA tubes and immediately centrifuged, decanted, and stored at -70°C. All collected samples were coded, and the identity of the groups (i.e., placebo versus individual aspirin dose groups) was not revealed until all measurements were made and the results were analyzed. TX production was measured by quantifying its more stable whole-blood metabolite, TXB2. Both TXB2 and ATL were determined by using their respective specific ELISA (Neogen, Lexington, KY). The ATL ELISA is selective for the aspirin-triggered 15R-epimeric form and is <0.1% crossreactive with the lipoxygenase-derived 15S form as validated by liquid chromatography-tandem mass spectrometry (8).
Statistical Analysis. Baseline characteristics were computed as means or proportions among the different treatment groups. Significance for differences in baseline characteristics across treatment groups was determined by using ANOVA for continuous variables and Fisher's exact test for categorical variables. Change in ATL and TXB2 after the 8-week treatment period was computed. The significance of differences for ATL and TXB2 was computed for each treatment group by using a paired t test. Tests for significance in changes of these markers across treatment groups were calculated by using ANOVA. All probabilities were calculated by using a two-tailed alpha set at 0.05 with all confidence intervals computed at the 95% level.
Results
A total of 140 subjects were enrolled and randomized (37 in the placebo group, 35 in the 81-mg group, 33 in the 325-mg group, and 35 in the 650-mg group). Six subjects were lost to follow-up (1, 3, 1, and 1 in the placebo, 81-, 325-, and 650-mg groups, respectively), and six did not meet the defined entry criteria during the study period and were therefore excluded from the analysis (3, 0, 2, and 1 in the placebo, 81-, 325-, and 650-mg groups, respectively), leaving a total of 128 subjects that successfully completed the protocol in four randomized groups: 31 in the placebo group and 32, 32, and 33 in the 81-, 325-, and 650-mg daily aspirin groups, respectively. Baseline characteristics of the 128 study subjects evaluated are summarized in Table 1. A total of 36 women (46%) were postmenopausal on entry into the study. No significant differences in traditional vascular risk factors were observed between groups (P > 0.05).
Table 1. Baseline characteristics of study subjects.
| Placebo group (n = 31) | Low-dose group (n = 32) | Medium-dose group (n = 32) | High-dose group (n = 33) | P values | |
|---|---|---|---|---|---|
| Age, years | 53.4 ± 9 | 51.0 ± 7 | 50.5 ± 9 | 52.9 ± 10 | 0.53 |
| Body mass index, kg/m2 | 27.8 ± 6 | 28.3 ± 5 | 28.5 ± 5 | 26.3 ± 5 | 0.12 |
| Women, % | 48.3 | 62.5 | 53.1 | 78.8 | 0.06 |
| Systolic blood pressure, mmHg* | 118.0 ± 13 | 124.0 ± 13 | 118.0 ± 15 | 122.0 ± 13 | 0.26 |
| Diastolic blood pressure, mmHg* | 80 ± 8 | 83 ± 6 | 78 ± 7 | 80 ± 6 | 0.69 |
| Current smoker, % | 12.9 | 12.5 | 15.6 | 15.1 | 0.98 |
| Total cholesterol, mg/dl | 211 ± 40 | 214 ± 35 | 217 ± 35 | 205 ± 40 | 0.42 |
| LDL, mg/dl | 121 ± 36 | 128 ± 35 | 135 ± 32 | 115 ± 36 | 0.06 |
| HDL, mg/dl | 61 ± 18 | 57 ± 13 | 55 ± 13 | 63 ± 14 | 0.07 |
The low-, medium-, and high-dose groups took 81-, 325-, and 650-mg daily doses of aspirin, respectively. All P values for comparisons across groups were >0.05. LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol.
One millimeter Hg = 133 Pa.
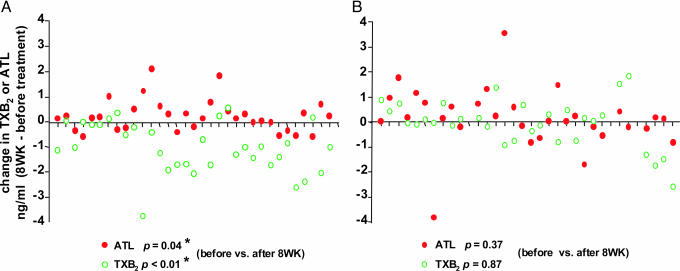
To determine whether ATL generation was aspirin-dependent in vivo, we initially compared plasma ATL levels before and after 8 weeks of treatment. In the 81-mg aspirin group, 22 of the 32 subjects gave increased plasma ATL levels at 8 weeks such that the ATL values at 8 weeks (2.85 ± 0.79 ng/ml) were significantly higher than those before aspirin treatment (2.60 ± 0.74 ng/ml) (P = 0.04, Table 2 and Fig. 2A). In parallel determinations, 26 of the 32 subjects in this 81-mg aspirin group gave decreased TXB2 values, and the difference between before and after 8 weeks also proved significant (P < 0.01) (Table 2 and Fig. 2 A). In sharp contrast, in the placebo group, neither ATL nor TXB2 levels at 8 weeks were significantly different from before treatment (P = 0.37 and 0.87, respectively; Table 2 and Fig. 2B).
Table 2. Change and net change in plasma TXB2 and ATL levels after 8 weeks of aspirin or placebo.
| Placebo group (n = 31) | Low-dose group (n = 32) | Medium-dose group (n = 32) | High-dose group (n = 33) | |
|---|---|---|---|---|
| TXB2 | ||||
| BL | 1.27 ± 1.15 | 1.40 ± 0.88 | 1.39 ± 1.06 | 1.33 ± 1.02 |
| Eight weeks | 1.25 ± 0.96 | 0.39 ± 0.55 | 0.28 ± 0.57 | 0.12 ± 0.23 |
| Δ TXB2, ng/ml | −0.02 ± 0.93 | −1.01 ± 0.99 | −1.10 ± 0.97 | −1.21 ± 0.96 |
| Δ, pg per 106 platelets | −0.1 | −3.7 | −4.1 | −4.4 |
| P values for 8 weeks vs. BL | 0.87 | <0.0001 | <0.0001 | <0.0001 |
| ATL | ||||
| BL | 2.45 ± 0.96 | 2.60 ± 0.74 | 2.42 ± 0.97 | 2.38 ± 0.99 |
| Eight weeks | 2.65 ± 0.96 | 2.85 ± 0.79 | 2.58 ± 1.10 | 2.39 ± 0.93 |
| ΔATL, ng/ml | 0.20 ± 1.18 | 0.25 ± 0.63 | 0.16 ± 0.71 | 0.01 ± 0.75 |
| Δ, pg per 106 leukocytes | 26 | 33 | 21 | 2 |
| P values for 8 weeks vs. BL | 0.37 | 0.04 | 0.22 | 0.96 |
| Net change | ||||
| ΔATL - ΔTXB2 | 0.22 ± 1.54 | 1.25 ± 1.18 | 1.26 ± 1.36 | 1.22 ± 1.02 |
| C.I. (95%) for ΔATL - ΔTXB2 | (−0.34, 0.79) | (0.83, 1.68) | (0.77, 1.76) | (0.86, 1.58) |
| P values for ΔATL vs. ΔTXB2 | 0.42 | <0.0001 | <0.0001 | <0.0001 |
| P values for aspirin vs. placebo | N/A | 0.006 | 0.005 | 0.007 |
The low-, medium-, and high-dose groups took 81-, 325-, and 650-mg daily doses of aspirin, respectively. Data are expressed in nanograms per milliliter (mean ± SD). Δ represents TXB2 or ATL levels after 8 weeks of treatment minus levels at baseline (BL). Δ values are expressed in either nanograms per milliliter or picograms per 106 cells. Sample sizes did not vary from those indicated at the top of the columns. P values for the net change were as follows: all groups, P = 0.02 (linear) and P = 0.03 (quadratic); aspirin groups, P = 0.91 (linear) and P = 0.93 (quadratic). N/A, not applicable; C.I., confidence interval.
Fig. 2.
Changes in TXB2 and ATL for each subject in the 81-mg daily aspirin and placebo groups. Changes in the amounts of TXB2 (○) and ATL (•) are expressed in nanograms per milliliter (from before treatment to 8 weeks after treatment) in the 81-mg daily aspirin dose (A) or placebo group (B). In the 81-mg aspirin dose group (A), statistical differences were obtained for both TXB2 (P < 0.01) and ATL (P = 0.04) when comparing values before versus after 8 weeks. All P values were calculated by using a two-tailed Student t test.
It is important to note that the magnitude of this effect at higher doses (325 or 650 mg of aspirin daily) was not further enhanced above that observed with the 81-mg daily dose of aspirin. For example, when all of the data points from 32 subjects taking 325 mg of aspirin were analyzed, the values of ATL at 8 weeks were not significantly higher than those before aspirin treatment (0.16 ± 0.71 ng/ml, P = 0.21). Nevertheless, it should be noted that, in the 325-mg group, five subjects showed increased values of TXB2 after the aspirin regimen. It is likely that these subjects did not respond to the assigned aspirin dose or that they failed to follow medication instructions. For these reasons, statistical analysis excluding those five subjects was also carried out and gave significant increase of ATL values at 8 weeks in this group (0.26 ± 0.65 ng/ml, P = 0.05; data not shown). No significantly increasing or decreasing linear trend was obtained among the three nonplacebo groups (P = 0.40). Thus, whereas a net increase in ATL and a net decrease in TXB2 was observed for all study participants allocated aspirin (P < 0.01), the magnitude of these differences did not further increase with 325- and 650-mg doses of aspirin.
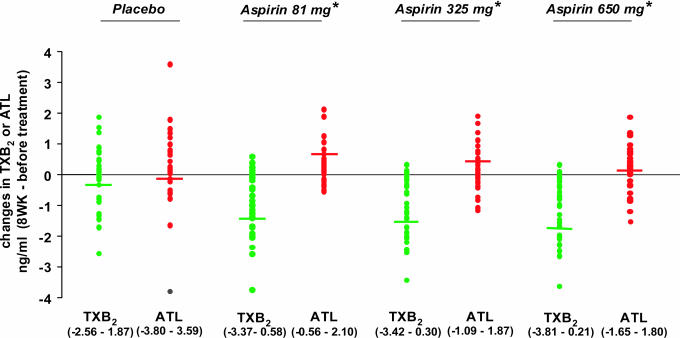
As shown in Fig. 3, differences between changes in TXB2 and ATL were significant for all aspirin doses (P < 0.01), and no differences were observed in the placebo group (P = 0.42). Given the antiinflammatory action of 15-epi-lipoxinA4 (8) and the prothrombotic action of TX (15), we assessed aspirin's impact by determining the net changes in 15-epi-lipoxinA4 and TXB2 levels (i.e., ΔATL - ΔTXB2) for each randomized dose group (Table 2). The pairwise contrasts of each aspirin dose against placebo were significant even after adjusting for multiple comparisons (P < 0.01). There was a significant increasing trend in net change across four dose groups (P = 0.02), and, more importantly, the quadratic trend was significant (P = 0.03), reflecting the leveling off after 81 mg. This leveling after 81 mg was further implicated in nonsignificant linear and quadratic trends when considering the three aspirin groups only (P > 0.90). Thus, whereas aspirin showed an inverse action on ATL and TX and increased net changes for all study participants allocated aspirin, the magnitude of these differences did not increase further with the 650-mg group.
Fig. 3.
Changes in TXB2 and ATL with all aspirin dose groups. Change in the amounts of TXB2 or ATL are expressed in nanograms per milliliter (from before treatment to after 8 weeks of treatment). The horizontal lines represent median values. Statistical differences were obtained for all three aspirin groups when comparing changes in TXB2 versus ATL values (*, P < 0.01).
Discussion
In this 8-week, randomized, placebo-controlled study, clinically relevant doses of aspirin were found to increase antiinflammatory ATL that was in addition to the aspirin's known antiplatelet action with inhibition of TX. This increase was aspirin-dependent and apparent with 81-mg, low-dose daily aspirin. These observations are of interest because several recent reports suggest that reductions in the incidence of vascular events among patients treated with high-dose aspirin (500-1500 mg daily) do not exceed that already attained with lower doses (75-150 mg daily) (14, 16). Also, in the Antiplatelet Trialist's Collaboration, which reviewed 145 randomized trials involving nearly 70,000 high-risk and 30,000 low-risk participants using a full range of antiplatelet dosing, neither a higher aspirin dose nor any other antiplatelet regimen was shown to be more effective than 75-325 mg/day aspirin in preventing vascular events (13). Thus, given the important contribution of inflammation in cardiovascular disease (17-19) and the antiinflammatory and proresolving properties of ATL (8), it is likely that the local 15-epi-lipoxin A4 biosynthetic circuit within the vasculature is relevant in considering cardioprotective actions of aspirin documented in many clinical studies (1-5, 17-19). Because the low-dose aspirin (81 mg daily) is clinically recommended for long-term antithrombotic prophylaxis and in the present study it is the most effective dose for ATL generation, our results implicate that ATL formation in vivo contributes to aspirin's beneficial action in preventing chronic atherothrombotic diseases. In comparison, medium doses of aspirin (325 mg daily), which are required for acute situations, such as myocardial infarction and thrombotic stroke, only gave marginal increase of ATL, suggesting that additional mediators/pathways may be responsible for aspirin's beneficial action in preventing acute coronary syndromes.
In an aspirin-free system, platelets will use much of the arachidonic acid for conversion to TX within a few seconds (Fig. 1). With aspirin treatment, TX production is inhibited presumably by means of platelet COX-1, and unused arachidonic acid can be processed by platelet-derived 12-lipoxygenase, with large quantities of platelet 12-hydroxy eicosatetraenoic acid produced until arachidonic acid is depleted (15). During platelet-neutrophil interactions, free arachidonate and leukotriene A4 are released and converted to lipoxins. In the vasculature, aspirin acetylates COX-2 and, during endothelial-neutrophil interactions, switches to ATL generation (7-9). These events are likely to contribute to the increase in ATL and decrease in TX values as observed in Fig. 3. Along these lines, we noted that ATL was detected in subjects who have not received aspirin (i.e., the placebo group). In this scenario, it is possible that COX-2 is acetylated by the endogenous acetylating agents to produce 15R-hydroxy eicosatetraenoic acid and/or ATL can be generated by means of a P450 pathway to produce 15R-hydroxy eicosatetraenoic acid (20), which could also account for the levels of ATL observed in the healthy individuals who claimed not to take aspirin.
Both lipoxin A4 and ATL interact with the same cell-surface receptor and display similar bioactions as local counterregulatory mediators of leukocyte adhesion and diapedesis in the low nanomolar to subnanomolar range (8). In this manner, these compounds regulate important initial steps in tissue inflammation (20, 21) and its resolution (22). In the present study, we estimated ATL levels in these healthy subjects. For example, in the 81-mg aspirin group, the detectable amounts of ATL increased from 2.6 to 2.85 ng/ml (baseline versus 8 weeks), which correlate to ≈7.4 nM and 8.1 nM, respectively. Thus, the amounts of ATL generated in these healthy subjects are indeed sufficient (i.e., nanomolar range) to evoke and account for the antiinflammatory actions of ATL (cf. 7, 20, 29, 30).
It is noteworthy that native lipoxins are formed in vivo not only during the resolution of inflammation (22) but also in several physiological circumstances. Lipoxin A4 is excreted in human urine, for example, and is significantly elevated after strenuous exercise in healthy volunteers (23). Exercise also results in platelet activation and consequent TXB2 production. It also activates leukocytes and promotes platelet-leukocyte aggregate formation (24, 25). Hence, local eicosanoid production is associated with strenuous physical activities in healthy individuals and these autocoids may serve in homeostatic mechanism(s) to limit exercise-induced responses. In the present study of healthy subjects, it is likely that COX-2 is constitutively expressed in the vasculature (12) and promotes local ATL production by means of endothelial-leukocyte interactions that, along with native lipoxin A4, may constitute a homeostatic system. The large number of platelets per unit of area (150-400 × 106 platelets per ml of whole blood) versus leukocytes (4-11 × 106 leukocytes per ml of whole blood) that can interact directly with endothelial cells on their luminal surface to initiate transcellular biosynthesis (see Fig. 1) likely reflects the different levels of these two autacoids generated in healthy subjects. In this regard, we normalized the changes of TXB2 with the average platelet (275 × 106 platelets per ml of whole blood) and the changes of ATL with the average leukocyte (7.5 × 106 leukocytes per ml of whole blood). The magnitude of ATL changes in the 81-mg aspirin group dramatically increased with this normalization (see Table 2). Only a relatively small percentage of leukocytes (compared with the large numbers of platelets) are likely to directly interact with endothelial cells on their luminal surface and therefore have the capacity to produce ATL in response to aspirin treatment; hence, the actual levels of aspirin-dependent ATL increase per leukocyte could be even higher in vivo than calculated from the present results.
COX-2 inhibitors block prostacyclin production in the vasculature. Prostacyclin is a vasodilator and interferes with platelet reactivity (26). Hence, an individual could be more “prothrombotic” (1, 27) because platelet TX production continues in patients being treated with COX-2 inhibitors (7). Of interest, COX-2 inhibitors prevent formation of ATL during human leukocyte-endothelial cell interactions (28) and by the gastrointestinal tract of rats (29, 30). This blockage in ATL production and actions may contribute to the leukocyte-driven gastrointestinal ulcer formation (29, 30). Whether the assessment of ATL production might also be useful to monitor potential prothrombotic effects of recent concern (1, 27, 31) in regimens with COX-2 inhibitors remains to be determined.
In summary, this paper documents antiinflammatory ATL generation in a randomized human trial of healthy volunteers. These findings indicate that ATL production in humans depends on a low daily dose of aspirin (81 mg). The low-dose aspirin treatment may exert an antiinflammatory action by means of this local production of ATL. Other events may be operative at the higher doses. The pattern of association between aspirin treatment and net changes in TXB2-ATL levels were consistent and statistically significant with each of the three popularly used dose groups (e.g., 81, 325, and 650 mg, daily, of aspirin). Hence, local ATL production may not only account for aspirin's antiinflammatory and antineutrophil actions in vivo, but could be a sensitive new means to directly assess aspirin as well as other acetylation-based antiinflammatory treatment.
Acknowledgments
We thank Mary Halm Small for skillful assistance in manuscript preparation and Dannie Chang for technical assistance. This work was supported in part by National Institutes of Health Grants GM 38765 and P01-DE13499 (to C.N.S.) and by grants from the Doris Duke Charitable Foundation and the Donald W. Reynolds Foundation (to P.M.R.). Statistical analyses were supported in part by the Biostatistics Consulting Service, Center for Clinical Investigation, Brigham and Women's Hospital.
Author contributions: N.C. and C.N.S. designed research; N.C., E.A.B., and C.N.S. performed research; N.C. and C.N.S. contributed new reagents/analytical tools; N.C., P.M.R., S.H., and C.N.S. analyzed data; and N.C., P.M.R., and C.N.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ATL, aspirin-triggered-15-epi-lipoxin A4; COX, cyclooxygenase; TX, thromboxane.
References
- 1.Marcus, A. J., Broekman, M. J. & Pinsky, D. J. (2002) N. Engl. J. Med. 347, 1025-1026. [DOI] [PubMed] [Google Scholar]
- 2.Flower, R. (2003) Br. Med. J. 327, 572-573.12969898 [Google Scholar]
- 3.Moysich, K. B., Menezes, R. J., Ronsani, A, Swede, H., Reid, M. E., Cummings, K. M., Falkner, K. L., Loewen, G. M. & Bepler, G. (2002) BMC Cancer 2, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imperiale, T. F. (2003) N. Engl. J. Med. 348, 879-880. [DOI] [PubMed] [Google Scholar]
- 5.in t' Veld, B. A., Ruitenberg, A., Hofman, A., Launer, L. J., van Duijn, C. M., Stijnen, T., Breteler, M. M. & Stricker, B. H. (2001) N. Engl. J. Med. 345, 1515-1521. [DOI] [PubMed] [Google Scholar]
- 6.Weissmann, G. (1991) Sci. Am. 264, 84-90. [DOI] [PubMed] [Google Scholar]
- 7.Clària, J. & Serhan, C. N. (1995) Proc. Natl. Acad. Sci. USA 92, 9475-9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan, C. N. (2002) Curr. Med. Chem.: Anti-Inflammatory Anti-Allergy Agents 1, 177-192. [Google Scholar]
- 9.Chiang, N., Takano, T., Clish, C. B., Petasis, N. A., Tai, H. H. & Serhan, C. N. (1998) J. Pharmacol. Exp. Ther. 287, 779-790. [PubMed] [Google Scholar]
- 10.Sanak, M., Levy, B. D., Clish, C. B., Chiang, N., Gronert, K., Mastalerz, L., Serhan, C. N. & Szczeklik, A. (2000) Eur. Respir. J. 16, 44-49. [DOI] [PubMed] [Google Scholar]
- 11.Rocca, B., Secchiero, P., Ciabattoni, G., Ranelletti, F. O., Catani, L., Guidotti, L., Melloni, E., Maggiano, N., Zauli, G. & Patrono, C. (2002) Proc. Natl. Acad. Sci. USA 99, 7634-7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topper, J. N., Cai, J., Falb, D. & Gimbrone, M. A., Jr. (1996) Proc. Natl. Acad. Sci. USA 93, 10417-10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cronstein, B. N. & Weissmann, G. (1995) Annu. Rev. Pharmacol. Toxicol. 35, 449-462. [DOI] [PubMed] [Google Scholar]
- 14.Antiplatelet Trialists' Collaboration. (1994) Br. Med. J. 308, 81-106. [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus, A. J. (1999) in Basic Principles and Clinical Correlates, eds. Gallin, J. I., Snyderman, R., Fearon, D. T., Haynes, B. F. & Nathan, C. (Lippincott, Philadelphia), 3rd Ed., pp. 77-95.
- 16.FitzGerald, G. A. (2003) Lancet. 361, 542-543. [DOI] [PubMed] [Google Scholar]
- 17.Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. (1997) N. Engl. J. Med. 336, 973-979. [DOI] [PubMed] [Google Scholar]
- 18.Ridker, P. M., Rifai, N., Rose, L., Buring, J. E. & Coor, N. R. (2002) N. Engl. J. Med. 347, 1557-1565. [DOI] [PubMed] [Google Scholar]
- 19.Libby, P. (2002) Nature. 420, 868-874. [DOI] [PubMed] [Google Scholar]
- 20.Claria, J., Lee, M. H. & Serhan, C. N. (1996) Mol. Med. 2, 583-596. [PMC free article] [PubMed] [Google Scholar]
- 21.Cotran, R. S., Kumar, V. & Robbins, S. L., eds. (1999) Robbins Pathologic Basis of Disease (Saunders, Philadelphia), 6th Ed.
- 22.Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K. & Serhan, C. N. (2001) Nat. Immunol. 2, 612-619. [DOI] [PubMed] [Google Scholar]
- 23.Gangemi, S., Luciotti, G., D'Urbano, E., Mallamace, A., Santoro, D., Bellinghieri, G., Davi, G. & Romano, M. (2003) J. Appl. Physiol. 94, 2237-2240. [DOI] [PubMed] [Google Scholar]
- 24.Li, N., Wallen, N. H. & Hjemdahl, P. (1999) Circulation 100, 1374-1379. [DOI] [PubMed] [Google Scholar]
- 25.Todd, M. K., Goldfarb, A. H. & Boyer, B. T. (1992) Thromb. Res. 65, 487-493. [DOI] [PubMed] [Google Scholar]
- 26.Vane, J. R. (1982) in Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures (Almqvist & Wiksell, Stockholm), 181-206.
- 27.Cheng, Y., Austin, S. C., Rocca, B., Koller, B. H., Coffman, T. M., Grosser, T., Lawson, J. A. & FitzGerald, G. A. (2002) Science 296, 539-541. [DOI] [PubMed] [Google Scholar]
- 28.Serhan, C. N., Clish, C. B., Brannon, J., Colgan, S. P., Chiang, N. & Gronert, K. (2000) J. Exp. Med. 192, 1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiorucci, S., Distrutti, E., De Lima, O. M., Romano, M., Mencarelli, A., Barbanti, M., Palazzini, E., Morelli, A. & Wallace, J. L. (2003) FASEB J. 17, 1171-1173. [DOI] [PubMed] [Google Scholar]
- 30.Fiorucci, S., de Lima, O. M., Jr., Mencarelli, A., Palazzetti, B., Distrutti, E., McKnight, W., Dicay, M., Ma, L., Romano, M., Morelli, A. & Wallace, J. L. (2002) Gastroenterology 123, 1598-1606. [DOI] [PubMed] [Google Scholar]
- 31.Bombardier, C., Laine, L., Reicin, A., Shapiro, D., Burgos-Vargas, R., Davis, B., Day, R., Ferraz, M. B., Hawkey, C. J., Hochberg, M. C., et al. (2000) N. Engl. J. Med. 343, 1520-1528. [DOI] [PubMed] [Google Scholar]
- 32.Goh, J., Godson, C., Brady, H. R. & Macmathuna, P. (2003) Gastroenterology 124, 1043-1054. [DOI] [PubMed] [Google Scholar]