Abstract
Substantial evidence indicates that immunotherapy is a feasible and effective approach for the treatment of numerous types of cancer. Among various immunotherapy options, peptide vaccines to generate antitumor T cells appear as promising candidates, because of their cost effectiveness and ease of implementation. Nevertheless, most peptide vaccines are notorious for being weekly immunogenic and, thus, optimization of the vaccination strategy is essential to achieve therapeutic effectiveness. In addition, effective peptide vaccines must stimulate both CD8 cytotoxic and CD4 helper T lymphocytes. Our group has been successful in designing effective peptide vaccination strategies for inducing CD8 T-cell responses in mouse tumor models. Here we describe a somewhat similar, but distinct, peptide vaccination strategy capable of generating vast CD4 T-cell responses by combining synthetic peptides with TLR agonists and OX40/CD40 costimulation. This vaccination strategy was efficient in overcoming immune tolerance to a self tumor-associated antigen and generated significant antitumor effects in a mouse model of malignant melanoma. The optimized peptide vaccine also allowed the expansion of adoptively transferred CD4 T cells without the need for lymphodepletion and IL2 administration, generating effective anti-melanoma responses through the enhancement of proliferative and anti-apoptotic activities of CD4 T cells. These results have practical implications in the design of more effective T-cell based immunotherapies.
Keywords: CD4 T cells, CD40, OX40, Peptide vaccine, TLR ligands
Introduction
Research in cancer immunology has provided clear evidence that antigen-specific T cells can eliminate tumor and extend survival. Patients treated with genetically engineered T cells in which NY-ESO-1–reactive T-cell receptor (TCR) or anti-CD19 chimeric antigen receptor (CAR) have demonstrated objective clinical responses, including complete remissions (1,2). In addition to the artificially modified T cells, adoptive cell transfer (ACT) of tumor-infiltrating T cells (TILs) has resulted in long-lasting clinical responses, indicating that the natural T-cell repertoire contains effector cells capable of eliciting antitumor responses (3). Although adoptive T cell–based immunotherapy has shown dramatic results, several hurdles limit its practical use in clinic. Because of the complex methodology and the high cost of ACT, at the present time it is not practical to deliver this therapy to the general patient population. Although in some instances TILs are relatively easy to obtain, patients with inoperable tumors or those that have tumors devoid of T cells are not eligible for this treatment. TILs and genetically modified T cells also need to be expanded ex vivo in specialized Good Laboratory Practice facilities, further limiting the accessibility of this therapy. In view of this, development of alternative and cost effective antigen-specific immunotherapies, such as active immunizations, that can be more readily implemented in the clinic, is urgently needed.
A main goal of most therapeutic anticancer vaccines is to generate antigen-specific, tumor reactive T-cell responses. Although CD8 cytotoxic T lymphocytes (CTLs) have been the main focus of antitumor vaccines, accumulating evidence suggests that vaccines targeting CD4 helper T lymphocytes (HTLs) can also be effective in generating antitumor responses. Dual activation of tumor-associated antigen (TAA)-specific CTLs and HTLs by dendritic cell (DC) vaccines induced superior clinical responses than the single CTL vaccine in cancer patients (4). Moreover, tumor suppression by gp100-transfected DC vaccines depended on HTLs, but not on CTLs, in a murine melanoma model (5). Most immunogenic mutation-derived neoepitopes that eradicated tumors were recognized by HTLs (6). Not only do HTLs support CTLs by inhibiting activation-induced cell death and promoting T-cell memory (7), but in many instances HTLs can directly kill tumor cells (8,9). Thus, HTLs-targeted immunotherapy is a feasible strategy to confront malignant diseases. Unfortunately, most research studies and clinical trials have used peptide vaccines administered using strategies developed for generating antibodies, injecting them via a subcutaneous route with inappropriate adjuvants such as complete and incomplete Freund’s adjuvant (CFA, IFA), precluding the generation of substantial responses and limiting the establishment of memory T cells (10). Thus, optimization of peptide vaccination strategies to elicit antitumor T-cell responses is indispensable to ensure clinical efficacy.
The systemic (i.v.) administration of foreign proteins (ovalbumin) or derived synthetic peptides with TLR ligands and agonistic CD40 mAbs can generate large numbers of CD8 T cells in mice (11). Subsequently, although this vaccination approach using a peptide from tyrosinase-related protein 2 (TRP2) generates melanoma-specific T cells, it is inefficient in mediating antitumor effects (12). However, the antitumor effect of TLR ligand/CD40 mAb vaccination was restored by using a modified TRP2 mimetope (13), and the use of neoantigen epitopes has also produced good antitumor responses (14). We helped to refine this potent peptide vaccination strategy (that we called TriVax), which in our hands was successful in eliciting huge numbers of antitumor CD8 T cells capable of eliminating established melanoma tumors in mice (15,16). The purpose of the present study was to develop a comparable vaccination strategy for generating substantial numbers of antigen-specific CD4 HTLs.
The combination of TLR ligands and agonistic CD40 mAb has been reported to expand CTLs, but this strategy has not functioned well with antitumor HTL responses (17,18). Although a TLR3 ligand (poly-IC) is the best adjuvant for CTLs (16), the most suitable TLR ligand for CD4-targeted peptide vaccines remains to be elucidated. We describe here an optimized peptide vaccination strategy for the rapid generation of large numbers of antigen-specific CD4 T cells that displayed substantial antitumor effects in a mouse melanoma model. The results from these studies may pave the way to an effective vaccine strategy to treat human malignant and infectious diseases.
Materials and Methods
Mice and cell lines
Female 6 to 8 weeks old C57BL/6 mice (B6) and B6-Ly5.1 (CD45.1) mice were obtained through the National Cancer Institute/Charles River program. IFNγ deficient (GKO) mice and mice with transgenic T-cell receptor that recognize MHC class II–restricted Trp1 peptide113-127 (TRP1-TCR) were purchased from The Jackson Laboratory. TRP1-TCR mice are deficient for Trp1 and RAG1 (19). IFNαβ receptor–deficient (IFNαβR-KO) on a B6 background were kindly provided by Dr. P. Marrack (National Jewish Medical and Research Center, Denver, CO) and were bred on site. Trp1-KO B6 mice were generated in our facility by crossing TRP1-TCR with B6 mice, subsequently breeding F1 mice with each other and selecting for Trp1− (brown), RAG1+TRP1-TCR−mice. All experimental procedures followed guidelines of the Committee on the Care of Laboratory Animals Resources, Commission of Life Sciences, and National Research Council. The animal facility at Georgia Cancer Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Mouse lymphoma LB27.4, which expresses MHC class II (I-Ab), and mouse melanoma B16F10 (B16) cells were purchased from the American Type Culture Collection on 2015 and maintained as recommended by the vendor for no more than 8 weeks before performing the experiments. The cell lines were banked and have not been tested since their purchase.
Synthetic peptides and reagents
The peptides were purchased from A&A labs and dissolved in DMSO with 0.1% trifluoroacetic acid. The purity (>80%) and identity of peptides were determined by high-performance liquid chromatography and mass spectrometry analysis. The following peptides were used in the present study: TEWTSSNVMEERKIKV (Ova265-280), EAWGALANWAVDSA (2W1S), GVMYAFTTPLISFF (VV H3L273-286), CRPGWRGAACNQKIL (Trp1113-127), TAPDNLGYM (Trp1455-463M), and KVPRNQDWL (hgp10025-33). Monoclonal antibodies (mAbs) for in vivo injections: CD40 (FGK4.5), GITR (DTA-1), 4-1BB (2A), and OX40 (OX86) were purchased from Bio X Cell. TLR ligands used in this study were LPS, CpG (ODN-1826), Gardiquimod (GDQ) and poly-IC (hmw), were all obtained from InvivoGen.
Immunizations
Mice were immunized on day 0 (prime) and on day 12–14 (boost). TriVax consisted of the peptide (200 μg), CD40 mAb (prime: 100 μg; boost: 50 μg), TLR ligands (LPS, 5 μg or 30 μg; CpG, 100 μg; poly-IC, 50 μg; or GDQ, 100 μg), which were mixed and administered intravenously. In the indicated groups, 200 μg of GITR mAb, 4-1BB mAb, or OX40 mAb were administered intraperitoneally. For CFA-IFA protocol, mice were immunized subcutaneously with 200 μg of peptide mixed in PBS and CFA (Sigma; 50% v/v) for prime and in PBS and IFA (Sigma; 50% v/v) for boost. In some experiments, mice were immunized with 200 μg of peptide emulsified in TiterMax® (Sigma; 10 μl) and PBS (90 μl) subcutaneously. In ACT experiments, TRP1-TCR splenocytes (1 x 105 cells/mouse) were injected one day before vaccination. Seven days after each vaccination, blood samples or splenocytes were examined in immunological analysis.
Immunological analysis
The IFNγ EliSpot assays were performed as previously described (20). Briefly, T cells were separated from splenocytes of vaccinated mice using magnetic antibody-coated microbeads (Miltenyi Biotec). The purity of T cells was verified by flow cytometry (> 98%). Effector cells were incubated at different concentrations per well, together with 1 x 105 target cells (LB27.4, LB27.4 pulsed with peptides, DCs, DCs with B16F10 lysate, or B16F10 cells). B16F10 cells were treated with IFNγ (100 ng/ml) overnight to induce MHC class II expression. DCs were established from bone marrow cells cultured with IL4 (10 ng/ml) and murine GM-CSF (10 ng/ml, Peprotech) for seven days. B16F10 lysate was prepared by five freeze and thaw cycles of tumor cells (1 x 106/ml) and 100 μl of lysate was added. After overnight culture, IFNγ–positive spots were developed by using AEC substrate (BD Pharmingen) and spots were counted with ImmunoSpot System (Cellular Technology Ltd, Cleveland, OH). To detect intracellular cytokine production, freshly isolated splenocytes (1 x 106) were left untreated or stimulated with a corresponding peptide (10 μg/ml) overnight at 37 °C in 5% CO2. GolgiPlug (BD Pharmingen) was added during culture period to facilitate intracellular cytokine accumulation. Cell surface staining was performed followed by intracellular staining using the Cytofix/Cytoperm kit (BD Pharmingen) in accordance with the manufacturer’s protocol. PE-conjugated-I-Ab 2W1S tetramer was kindly provided by Dr. M. Jenkins (University of Minnesota) (21). PerCP-conjugated CD4 mAb, Alexa Fluor700-conjugated CD44 mAb, FITC-conjugated CD62L mAb, APC-conjugated CD45.2 mAb, PE-conjugated CD122 mAb, FITC-conjugated MHC class II mAb (I-Ab), APC-conjugated KLRG1 mAb, APC-conjugated PD-1 mAb, APC-conjugated LAG-3 mAb, APC-conjugated Tim-3 mAb, PE-conjugated IFNγ mAb, APC-conjugated TNFα mAb, FITC-conjugated Granzyme B mAb, APC-conjugated IL2 mAb, Af488-conjugated IL10 mAb PE-conjugated IL13 mAb, APC-conjugated IL17 mAb, PE-conjugated Ki-67 mAb, PE-Cy7-conjugated Eomes mAb, FITC-conjugated Annexin V, and 7-AAD viability staining solution were purchased from eBioscience; FITC-conjugated TCR Vβ14 mAb was purchased from BD Biosciences; PE-conjugated IL4 mAb, PE-conjugated IL9 mAb, and PE-Cy7-conjugated T-bet mAb were purchased from Biolegend; and PE-conjugated Bcl-xL mAb was purchased from abcam. The splenocytes from the mice, which received TRP1-TCR ACT and vaccine, were cultured with IL7 (10 ng/ml) for 5 days. After the culture, the surviving TRP1-TCR cells (CD4+Vβ14+7-AAD−Annexin-V−) were calculated. Flow cytometry was performed using an LSRII Cytometer (BD Biosciences). Data analysis was performed using FlowJo software (ver. 8.5, Tree Star).
Cytotoxicity assay
A cytotoxicity assay was performed using fluorescent beads to quantify the number of live tumor cells as previously described (22). Briefly, B16F10 cells were resuspended at 1 x 106 cells/ml and labeled with 1 μM CFSE (Life Technologies) for 10 min at 37 °C. Then, target cells were incubated at 1 x 105 cells/ml with several different concentrations of HTLs in flat-bottom 96-well plates at 37 °C for 18 hours. After culture, cells were harvested and stained with 7-AAD (BD Pharmingen) to exclude dead cells. Flow-Count fluorospheres beads (Coulter Corporation) were added to each sample to calculate the numbers of surviving target cells (CFSE-positive and 7-AAD-negative cells). B16F10 killing rate = (1-Live target cells with HTLs/Live target cells alone) x 100.
Antitumor experiments
Mice (10 mice/group) were injected subcutaneously with 3 x 105 B16F10 cells in a rear flank and 3 or 10 days later, mice received the vaccine. Boost vaccine was administrated 12 days after the first vaccine. In the ACT experiment, TRP1-TCR splenocytes (1 x 105 cells/mouse) were injected one day before vaccination. In the indicated group, 200 μg of PD-L1 mAb (10F.9G2, Bio X cell) or 500 μg of CD8 mAb (2.43, Bio X cell) was injected intraperitoneally. Depletion of CTLs was confirmed by flow cytometry. Tumor growth was monitored every 2 to 4 days by measuring two opposing diameters with a pair of calipers until the termination of the experiment. Mice were euthanized when one of the tumor diameters reached 20 mm.
Statistical analysis
Student t-test was applied at 95% confidence interval to determine the statistical significance of differences between groups (P < 0.05 being considered significant). Mice survival was assessed using log-rank test. All graphics and analysis were done using GraphPad Prism, version 5.0f for Mac OS X (GraphPad Software).
Results
TriVax induced more robust antigen-specific CD4 T-cell responses than conventional adjuvants
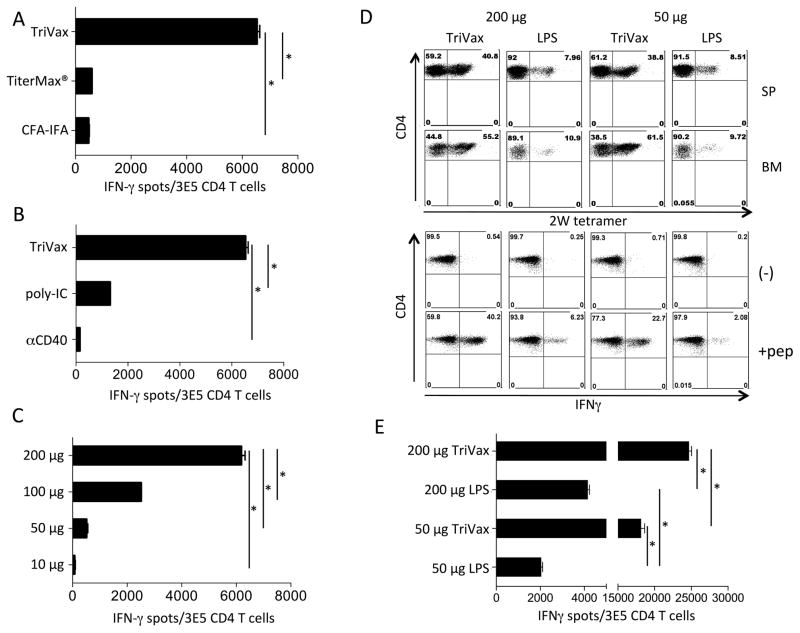
The TriVax vaccine (peptide, TLR ligand, and CD40 mAb, administered i.v.) induces potent CTL responses (15,16,20,22), so to develop an efficient immunization protocol for CD4 T cell responses, we compared this vaccination strategy with two commonly used peptide vaccines formulated as water:oil emulsions (Freund’s and TiterMax) in a prime-boost schedule (14 days apart). TriVax induced substantially higher responses (> 10-fold) to Ova265-280 a well-characterized CD4 T-cell epitope (23), as compared to the two water-in-oil adjuvants (Fig. 1A). The adjuvant activity of TriVax required both poly-IC and CD40 mAb, which contributed to the response in a synergistic manner (Fig. 1B). The magnitude of the T-cell responses highly correlated with the peptide dose, suggesting that a large amount of antigen is necessary to obtain robust T-cell expansions (Fig. 1C). Based on these results, mice were immunized with 200 μg of peptide in all further experiments. To determine that TriVax can be applied to a variety of antigens, we examined the responses to the 2W1S CD4 T-cell epitope, derived from the murine MHC I-Ed α chain (19). TriVax was compared with peptide+LPS, because the latter was reported to function as a strong adjuvant for 2W1S. Nevertheless, TriVax was far more potent than peptide+LPS at eliciting CD4 T-cell responses to 2W1S as measured by tetramer analysis and cytokine release assays (Fig. 1D and E). Moreover, large numbers of antigen-specific HTLs in TriVax immunized mice were observed in the bone marrow (BM) in addition to the spleen (SP), indicating that TriVax is effective at inducing memory T cells, which tend to accumulate in the BM.
Figure 1. Combination of poly-IC and CD40 agonist with peptide vaccine induces robust CD4 T-cell responses.
(A) Mice were immunized with Ova265-280 peptide on days 0 and 14. TriVax (poly-IC and CD40 mAb), TiterMax®, or CFA-IFA were used as adjuvants. TriVax was administered i.v. and TiterMax and CFA-IFA were injected s.c. EliSpot assay was done on day 21 using purified CD4 T cells from spleens (APCs: Ova265-280 peptide-pulsed LB27.4 cells). Less than 100 spots were observed using unpulsed APCs (E. Celis, unpublished observations). (B) Mice were vaccinated i.v. with Ova265-280 peptide with or w/o CD40 mAb, poly-IC, or with the combination of CD40 mAb and poly-IC (TriVax) on days 0 and 14. Mice were sacrificed on day 21, and the responses were measured as described in Fig. 1A. (C) Different doses of Ova265-280 peptide (10 μg, 50 μg, 100 μg, and 200 μg) were injected i.v. with TriVax protocol. EliSpot assay was done on day 21. (D) Mice were injected i.v. with peptide 2W1S (50 μg or 200 μg) combined with LPS or TriVax on days 0 and 14. Mice were sacrificed on day 6 after the boost and splenocytes (SP) and bone marrow cells (BM) were isolated for 2W1S/I-Ab tetramer staining. Splenocytes were incubated with peptide (1 μg/ml) (pep) in an intracellular IFNγ staining assay. Data shown are representative of three experiments with similar results. (E) Purified HTLs from splenocytes were cocultured with 2W1S peptide-pulsed LB27.4 cells for the EliSpot assay. Data represents the average of three experiments including 3–5 mice/experiment. Results are presented as mean ± SD. (*P < 0.05)
Enhancement of T-cell responses by OX40 agonist
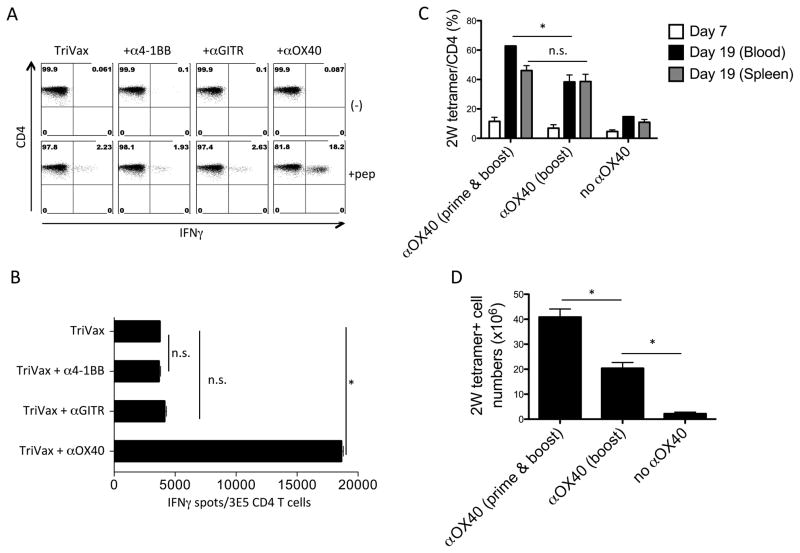
Because recall T cells responses can be further increased by costimulatory molecule activation (24), we examined whether the use of agonistic mAbs to OX40, 4-1BB, and GITR could further augment the Ova265-280–specific CD4 T-cell responses induced by TriVax. The addition of OX40 mAb, but not 4-1BB or GITR mAbs, enhanced the responses generated by TriVax (Fig. 2A and B). OX40 mAb also increased CD4 T-cell responses to TriVax using a vaccinia virus (VV) epitope (25) (Supplementary Fig. S1A and B). To determine whether the main enhancing effect of OX40 stimulation took place during the recall (booster) response as previously reported (24), mice received OX40 mAb either during the boost, or both at the time of prime and booster TriVax immunizations. Although no substantial differences were observed when measuring percentages of antigen-specific CD4 T cells in blood and spleens (Fig. 2C), the total numbers of antigen-specific cells significantly increased (~2-fold) in the mice that received the OX40 mAb both in prime and boost, compared to those animals that received the antibody solely during the boost (Fig. 2D).
Figure 2. OX40 agonist synergizes with TriVax to further expand CD4 T cells.
(A) Mice received TriVax (Ova265-280 peptide, poly-IC: 50 μg, and CD40 mAb) on days 0 and 14 and mice also received α4-1BB, αGITR, or αOX40 mAbs on days 0, 2, 12, and 14. Mice were sacrificed on day 21 and splenocytes were incubated with 1 μg/ml Ova265-280 peptide (+pep) or w/o peptide (−) in an intracellular cytokine-staining assay. The percentages of IFNγ+ CD4 T cells are shown in the upper right quadrants. The dot plots represent the results from one of the three separate experiments with similar results. (B) The number of IFNγ+ spots by CD4 T cells cocultured with Ova265-280 peptide-pulsed LB27.4 cells. Less than 100 spots were observed in the absence of peptide (E. Celis, unpublished observations). (C) Mice were vaccinated with TriVax (2W1S peptide, poly-IC, and CD40 mAb) combined with or w/o OX40 mAb (“prime & boost”: Day 0 and day 12, “boost”: day 12). The percentage of 2W1S tetramer+ cells in CD4 T cells was examined in blood or spleen. (D) The total number of 2W1S tetramer+ CD4 T cells in spleen. (*P < 0.05, n.s.: not significant). These experiments were repeated at least 2 times with similar results.
Role of TLR ligands and interferons in antigen-specific CD4 responses to TriVax/OX40
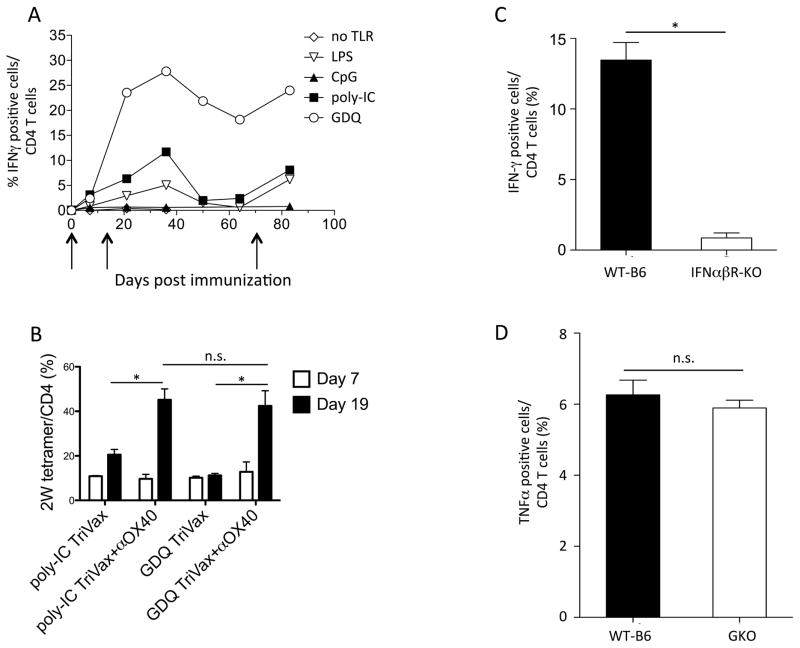
TLR5 and TLR7 ligands stimulate HTL responses more effectively than a TLR3 ligand (26). Thus, we evaluated the use of four TLR agonists, LPS (for TLR4), CpG (TLR9), poly-IC (TLR3) and gardiquimod (TLR7) for their adjuvant effect in TriVax/OX40 immunizations using the VV CD4 T-cell epitope. Under these experimental conditions, gardiquimod (GDQ) induced the strongest CD4 T-cell response, followed by poly-IC and LPS (Fig. 3A). A third immunization of the mice subsequently increased the numbers of antigen-specific T cells in blood, indicating that these vaccines allowed the induction of memory T cells. The TLR9 agonist CpG was not effective as an adjuvant. When the adjuvant activity of GDQ and poly-IC were compared using the strong 2W1S epitope, both TLR ligands were comparably effective when OX40 mAb was included in the vaccination protocol (Fig. 3B). CTL expansions by TriVax rely greatly on type-I interferon (IFN-I), but not in interferon-γ (IFNγ) (16,20), thus we investigated whether this would also apply to TriVax/OX-40–induced CD4 T-cell responses. Wild-type C57BL/6 (WT-B6), IFNαβ receptor–deficient (IFNαβR-KO), or IFNγ-deficient (GKO) mice were immunized with the VV CD4 T-cell epitope using TriVax/OX40. IFNγ or TNFα intracellular staining were used as readout for antigen reactivity in IFNαβR-KO or GKO mice, respectively. Antigen-specific responses induced by TriVax/OX40 were dramatically reduced in IFNαβR-KO mice, but not in GKO mice (Fig. 3C, D), underlining the importance of IFN-I signaling in CD4 T-cell expansion to TriVax/OX40.
Figure 3. TLR7 agonist is a suitable adjuvant for CD4-targeted peptide vaccine, which requires type 1 IFN.
(A) Mice were immunized on days 0, 14, and 65 (arrows) with TriVax (VV H3L273-286 peptide, CD40 mAb, and indicated TLR agonists; LPS, CpG, poly-IC, GDQ or no TLR agonist) combined with OX40 mAb. Antigen-specific CD4 T-cell responses were evaluated in blood using intracellular cytokine staining. (B) Mice received TriVax (2W1S peptide, CD40 mAb, and poly-IC or GDQ), with or w/o OX40 mAb on days 0 and 12. The percentages of 2W1S tetramer + CD4 T cells in blood on days 7 and 19 were examined by flow cytometry. (C) IFNαβ-KO and WT-B6 mice were vaccinated with TriVax (VV H3L273-286 peptide, Gardiquimod, CD40 mAb) and OX40 mAb on days 0 and 14, and responses were measured in blood on day 21. (D) IFNγ KO (GKO) mice and WT-B6 mice were vaccinated on day 0 and 14, and responses were measured in blood on day 21. (*P < 0.05, n.s.: not significant). These results represent example of three independent experiments.
TriVax/OX40 can overcome CD4 T-cell immune tolerance to a self-antigen
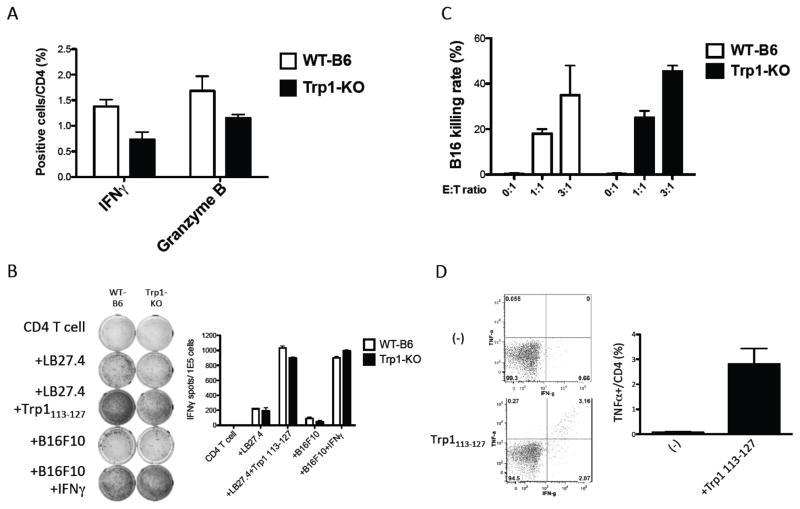
Our goal is to develop peptide vaccines against defined peptide epitopes from TAAs that are recognized by human HTLs (9,27). To test the feasibility of TriVax using a TAA CD4 T-cell epitope, we selected a tyrosinase-related protein-1 (Trp1) peptide (Trp1113-127) that was reported to elicit B16 melanoma antitumor responses in mice (19). However, CD4 T-cell responses to Trp1113-127 generated by recombinant Trp1 vaccinia virus-based immunizations could only be observed in Trp1 deficient (Trp1-KO) mice and not in WT-B6, suggesting the presence of immune tolerance to this epitope (19). Nevertheless, no substantial differences in the CD4 T-cell responses to Trp1113-127 were observed in WT-B6 and Trp1-KO mice receiving TriVax/OX40 (Fig. 4A). Thus, tolerance to this epitope, if it exists, could be overcome by this particular peptide vaccination strategy. More importantly, the HTLs from both WT-B6 and Trp1-KO mice that were generated with TriVax/OX40 using Trp1113-127 produced IFNγ in an antigen-specific manner to peptide-pulsed MHC-II expressing APCs and were also capable of directly recognizing B16 melanoma cells (Fig. 4B). However, B16 recognition by the HTLs necessitated that the tumor cells be previously treated with IFNγ to induce MHC-II expression (Supplementary Fig. S2). Intracellular cytokine staining assays indicated that in addition to IFNγ production, the CD4 T cells also produced granzyme B as the result of antigen-stimulation (Fig. 4A), suggesting the possibility of these cells exhibiting cytotoxic activity. Indeed, the CD4 T cells isolated from both the WT-B6 and Trp1-KO immunized mice exhibited comparably high cytotoxicity against IFNγ-treated B16 cells (Fig. 4C). In addition to producing IFNγ, a large proportion of the HTLs from TriVax/OX40 immunized WT-B6 mice also produced TNFα (Fig. 4D). Comparison of TriVax/OX40 containing poly-IC or GDQ in WT-B6 mice revealed that both TLR agonists generated similar numbers of antigen-specific HTLs, but GDQ produced HTLs that recognized and killed B16 cells slightly better than poly-IC (Supplementary Fig. S3A–C). In addition to directly recognizing IFNγ-treated B16 cells, the CD4 T cells generated with TriVax/OX40 were effective in recognizing dendritic cells (DCs) that were pulsed with B16 cell lysates (non-treated with IFNγ) indicating that these professional APCs could process the Trp1 protein and present the Trp1113-127 epitope (Supplementary Fig. S3D). Collectively, these results demonstrate that TriVax/OX40 was effective in generating cytotoxic HTLs that recognize a self-antigen and effectively kill tumor cells, and that immune tolerance, at least to the Trp1113-127 epitope, was not an obstacle for the induction of these responses.
Figure 4. CD4 T-cell responses to a non-immunogenic melanosomal antigen in wild-type or Trp1 KO mice.
WT-B6 or Trp1-KO mice received TriVax (Trp1113-127 peptide, GDQ, and αCD40 mAb) and αOX40 mAb on days 0 and 12. (A) Splenocytes were harvested on day 19 and were stimulated with Trp1113-127 peptide (1 μg/ml) in intracellular cytokine staining assay. (B) EliSpot assay and (C) flow cytometry-based cytotoxicity assays were performed using purified CD4 T cells from splenocytes. (D) WT-B6 mice received TriVax and αOX40 mAb on days 0 and 12, mice were sacrificed on day 31 and splenocytes were used in intracellular cytokine staining assays; (*P < 0.05). These experiments were repeated 3 times with similar results.
Antitumor therapeutic effect of TriVax/OX40
Next, TriVax/OX40 with Trp1113-127 was evaluated for its antitumor activity in WT-B6 mice bearing 3-day established B16 subcutaneous tumors. This vaccine displayed significant antitumor effects (Fig. 5A and B). A control TriVax/OX40 vaccine (mock Vac, without peptide) delayed tumor growth, suggesting that the adjuvant and costimulatory antibodies could mediate antitumor responses, perhaps by activating endogenous T cells in a similar manner as we observed with the combination of poly-IC and PD-L1 blockade (28). Depletion of CD8 CTLs reduced, but did not eliminate, the antitumor effect of TriVax/OX40 (Fig. 5C and D), suggesting that this vaccine may promote epitope spreading that enhances its effectiveness.
Figure 5. Endogenous CD4 T cells induced by TriVax/OX40 have direct antitumor activity.
(A and B) WT-B6 mice (10 per group) were inoculated subcutaneously on day 0 with 3 x 105 B16F10 melanoma cells. Mice were untreated (No Vac), received a control vaccine (mock Vac: GDQ and CD40 mAb) or TriVax with Trp1113-127 peptide, GDQ and CD40 mAb and OX40 mAb on days 3 and 15 (Vac). Tumor size (A) and the percent survival (time to euthanasia) of mice (B) are shown. Results are presented as mean ± SD. Individual tumor growth curves for each group are also presented. (C and D) WT-B6 mice (10 per group) were inoculated subcutaneously on day 0 with 3 x 105 B16F10 melanoma cells. Mice were untreated, received TriVax with Trp1113-127 peptide, GDQ, and CD40 mAb and OX40 mAb on days 3 and 15 (Vac). One group of vaccinated mice received αCD8 mAb on days 3, 5, 15, and 17 (Vac+CD8). The tumor size (C) and the percent survival of mice (D) are shown. Results are presented as mean ± SD. Arrows denote times of vaccination. These experiments were repeated 2 independent times and similar results were obtained
Use of TriVax/OX40 to enhance the effectiveness of adoptive CD4 T-cell therapy
Adoptive T-cell therapy (ACT) can be very effective against tumors because very large numbers of tumor-reactive T cells that are expanded in vitro are administered into lymphodepleted patients. The use of TriVax for generating CD8 T-cell responses in the setting of ACT results in huge T-cell expansions and substantial antitumor effects in mice without the need for lymphodepletion and high-dose IL2 therapy (29). ACT combined with a potent vaccine can be more effective than vaccinations that rely on the endogenous T-cell repertoire, because the antigen-specific T-cell precursor frequency is substantially increased, allowing treatment of more advanced tumors. Thus, we investigated whether ACT of Trp1113-127 reactive HTLs from TRP1 T-cell receptor (TCR) transgenic mice would increase the antitumor effects of TriVax/OX40.
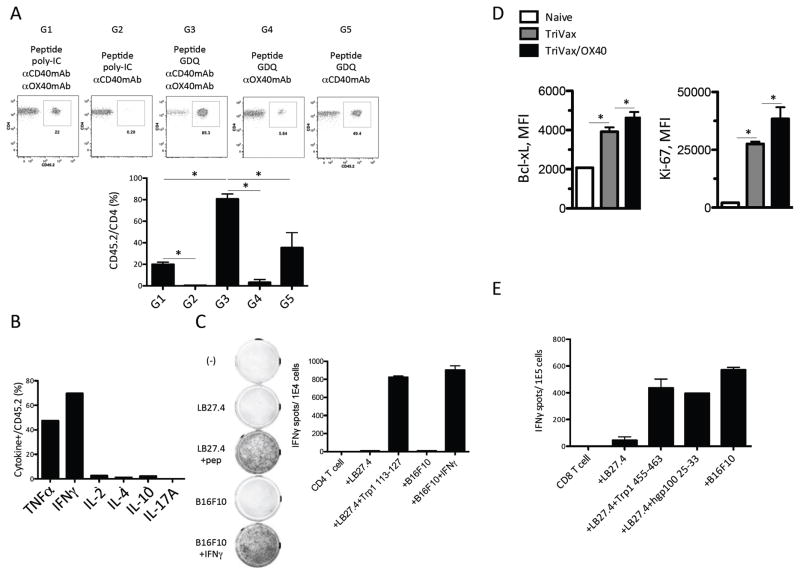
First we compared the effectiveness of TriVax with or without OX40 mAb, using either poly-IC or GDQ adjuvant after ACT of 1 x 105 TRP1-TCR splenocytes (Fig. 6A). Mice that received TriVax/OX40 with GDQ had responses > 80% Trp1113-127 reactive HTLs 7 days after a single immunization (Group 3). On the other hand, TriVax/OX40 with poly-IC generated a weaker response (Group 1). TriVax without OX40 mAb was effective only when GDQ was used as adjuvant, but was approximately 50% less potent that TriVax/OX40 (Group 5 versus Group 3). Peptide administered with GDQ and OX40 mAb generated one tenth of the response compared to peptide plus CD40 mAb and GDQ (Group 4 versus Group 5), indicating that CD40 costimulation is more critical than OX40 costimulation. However, CD40 costimulation alone was completely ineffective when poly-IC was used as the adjuvant (Group 2). The HTLs generated by TriVax/OX40 after ACT produced TNFα and IFNγ but not IL2, IL4, IL10, or IL17 as the result of peptide stimulation (Fig. 6B) and were capable of recognizing IFNγ-treated B16 cells (Fig. 6C).
Figure 6. TriVax/OX40 expands adoptively transferred CD4 T cells without need of lymphodepletion.
(A) B6 CD45.1 mice received 1 x 105 TRP1-TCR (CD45.2) splenocytes (~3 x 104 CD4 T cells) one day before the vaccination. The components of the vaccine (Trp1113-127 peptide with adjuvants) are indicated in the figure. The percentages of TRP1-TCR cells T cells (CD45.2+/CD4+) were measured in blood on day 7 after vaccination. Images of the representative results are shown. (B) Peptide-induced intracellular cytokine profiles of Trp1-TCR cells after vaccination. (C) Splenocytes were harvested from mice, which received 1 x 105 Trp1-TCR cells ACT (1 day before vaccination) and TriVax (Trp1113-127 peptide, GDQ, and CD40 mAb) and OX40 mAb on day 7 after vaccination. CD4 T cells were purified from the splenocytes and used in EliSpot assay with various target cells. (D) B6 mice received 1 x 105 TRP1-TCR (CD45.2) splenocytes (~3 x 104 CD4 T cells) one day before the TriVax with or without OX40 mAb agonist. Seven days later, the expression of Bcl-xL or Ki-67 in TRP1-TCR CD4 T cells from naïve TRP1-TCR or vaccinated mice were examined. (E) Splenocytes were harvested from mice, which received 1 x 105 Trp1-TCR cells ACT (1 day before vaccination) and TriVax (Trp1113-127 peptide, GDQ, and CD40 mAb) and OX40 mAb on day 7 after vaccination. CD4 T cells were purified from the splenocytes and used in EliSpot assay with various target cells; (*P < 0.05).
Next, we examined how OX40 stimulation supported the expansion of CD4 T cells. The addition of OX40 agonist augmented the proliferative capacity (Ki-67) and anti-apoptotic activity (Bcl-xL) of Trp1113-127 reactive CD4 T cells (Fig. 6D). The expression of CD122, CD127, KLRG1, T-bet, or Eomes was not affected by the addition of OX40 stimulation (Supplementary Fig. S4A). In addition, most of Trp1113-127 reactive CD4 T cells became effector memory T cells after vaccination regardless of OX40 stimulation (Supplementary Fig. S4B). In accordance with Ki-67 and Bcl-xL expression, TriVax/OX40 endowed Trp1113-127 reactive CD4 T cells with a clear survival advantage (Supplementary Fig. S4C).
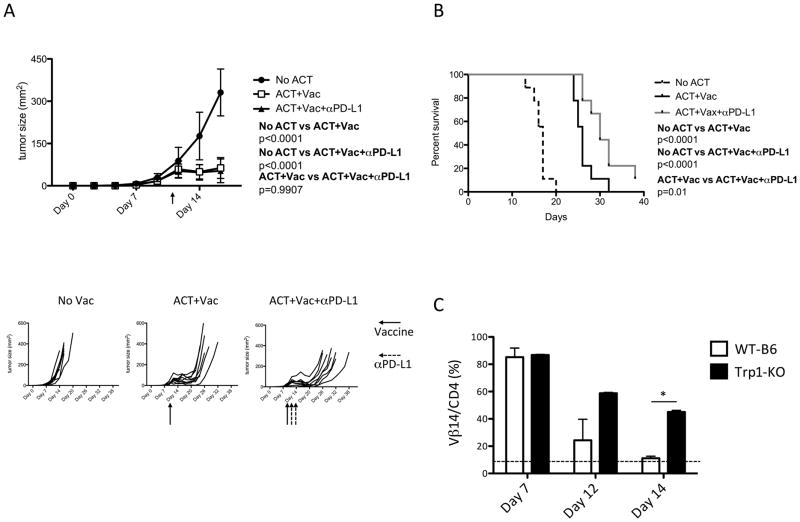
To investigate whether ACT with TriVax/OX40 results in epitope spreading, we examined CD8 T-cell responses to unrelated tumor antigens/epitopes after vaccination. Epitope spreading to induce a new CD8 T cell response would enhance the therapeutic effects of a CD4 vaccine. CD8 T-cell responses were observed to 2 immunodominant MHC-I epitopes (gp10025-33 and Trp1455-463) and against B16 tumor cells (not treated with IFNγ) in mice receiving Trp1-TCR cells, followed by TriVax/OX40 (Fig. 6E). On the other hand, CD8 T-cell responses of this type were not observed in mice receiving TriVax/OX40 without ACT (E. Celis, unpublished observations). Comparing the magnitude of the responses obtained with ACT to those observed without ACT, where only ~1–2% antigen-specific CD4 T cells could be generated (Fig. 4A), it suggests that the precursor frequency of T cells for this epitope in WT-B6 and Trp1-KO mice may be very low and therefore one would predict that ACT should dramatically increase the antitumor efficacy of TriVax/OX40. Thus, we tested the therapeutic effect of ACT followed by TriVax/OX40 (with GDQ adjuvant) against more established B16 tumors (day 10 versus day 3 used w/o ACT, Fig. 5A–D). Because Trp1113-127 reactive HTLs expressed PD-1 (in addition to Tim-3 and LAG-3) after vaccination (Supplementary Fig. S4D), one group of mice received concurrent PD-L1 blockade with the prospect of further increasing the therapeutic efficacy. The combination of ACT with TriVax/OX40 resulted in the control of tumor growth for approximately 7–10 days, and the administration of αPD-L1 mAb slightly prolonged the median survival resulting from this therapy (Fig. 7A and B). Because we were not able to detect Trp1113-127 reactive HTLs in blood 7 days post-immunization (E. Celis, unpublished observations), we assumed that disease progression observed after day 20 was the result of a short-lived T-cell response. It is possible that the continuous presence of the self-antigen Trp1 in melanocytes and in B16 could contribute to the disappearance of the antigen-reactive T cells. Indeed, TRP1-TCR cells disappeared 2 weeks after TriVax/OX40 in WT-B6 mice, whereas ~50% of the cells remained in Trp1-KO mice (Fig. 7C), indicating that the presence of self-antigen has a deleterious effect in the persistence of the Trp1113-127 reactive CD4 T cells, reducing the antitumor efficacy.
Figure 7. TriVax/OX40 potentiates the therapeutic antitumor effect of adoptively transferred CD4 T cells.
(A and B) WT-B6 mice (10 per group) were inoculated subcutaneously on day 0 with 3 x 105 B16F10 melanoma cells. Mice received 1 x 105 TRP1-TCR cells on day 10 and TriVax (Trp1113-127 peptide, GDQ, and CD40 mAb) and OX40 mAb on day 11 (red arrows). Some mice received 2 injections of αPD-L1 following vaccination (black arrows). The size of tumor (A) and the percent survival of mice (B) are shown. Individual tumor growth curves for each group are also presented. (C) The percentages of TRP1-TCR cells TCR Vβ14+/CD4+) in blood after vaccine were measured in WT-B6 and Trp1-KO mice. The dotted line indicates the normal percentage of Vβ14+ CD4 T cells in WT-B6 mice. The blood was withdrawn on days 7, 12, and 14 after vaccination. Results are presented as mean ± SD; (*P < 0.05).
Discussion
Our group has been involved in the identification of tumor-specific CD4 epitopes and in the enhancement of T-cell immune responses over a decade (8,27). Although HTLs have a strong antitumor ability (19,30) and peptide vaccines could be a promising way of generating these cells, the use of suboptimal adjuvants has hindered the development of effective HTLs-targeted therapeutic peptide vaccines. Taking advantages of our prior knowledge on how to induce huge antitumor CTL responses by combining synthetic peptides, TLR ligands, and costimulatory molecules (11–16), we have demonstrated here that the use of some TLR ligands (GDQ or poly-IC), together with CD40 and OX40 agonists were effective in inducing robust and long-lasting memory HTLs responses with various synthetic peptides. Since vaccination using tumor-derived or virus-derived epitopes was successful, TriVax/OX40 has the potential to treat both tumor and infectious diseases.
Whereas the mechanism of how to develop memory CTLs has been extensively demonstrated, the factors involved in the generation of memory HTLs are not yet fully understood. Here, we showed that TriVax/OX40 successfully induced recall responses of HTLs reactive with non-self antigen, but that HTL responses to self-antigens may be short-lived. In accordance with the previous finding that OX40 signaling is essential in boosting the HTLs responses (31), we found that OX40 signaling is indeed important for recall responses, but including OX40 costimulation in the priming vaccination doubled the yield of antigen-specific CD4 T cells generated by TriVax. Although OX40 signaling was known to be important in the activation of HTLs (32), we also show that OX40 stimulation has a synergistic effect with TLR and CD40 stimulation in the expansion of tumor-reactive HTLs. TriVax alone could induce HTLs that react to exogenous antigens, but this strategy was not sufficient to elicit tumor-reactive HTLs (17,18), suggesting that the additional OX40 stimulation is necessary to expand rare tumor-reactive HTLs. Involvement of the Bcl-2 family, which inhibits the apoptosis of HTLs, or IL12/STAT4 activation could be reasons for the increased survival of HTLs after the booster vaccine (33,34), but the exact downstream factors underlying the OX40 pathway in the recall responses remain to be elucidated. We found that the effect of OX40 at least relies on the enhanced survival and anti-apoptotic activity of CD4 T cells, but not on the increase of Th1 lineage commitment/cytotoxicity (T-bet, Eomes, KLRG1), on memory phenotypes, or on the reduction of inhibitory checkpoints. Because OX40-dependent proliferation of memory HTLs requires MHC class II, but the expansion of expanding memory HTLs by IL7 is antigen-independent (35), OX40 and IL7 may use different mechanisms for expanding HTLs (36).
It is also interesting to clarify why only OX40, but not other tumor necrosis factor receptor superfamily members, like 4-1BB or GITR agonists, was effective in the induction of peptide-specific HTLs. One possible explanation might be that 4-1BB signaling mainly affects CTLs, whereas OX40 stimulation is superior in HTLs (24). Although 4-1BB or GITR agonists reprogram regulatory CD4 T cells (Treg) to cytotoxic HTLs (37), our results show that TriVax/OX40 was effective in generating Th1 and cytotoxic CD4 T-cell responses. The effect of TriVax/OX40 to polarize Th1 might be partly due to GDQ, which induces IL12 production from macrophages and DCs that strongly skews HTLs to a Th1 subset (38). Accordingly, although the influence of OX40 over the Treg development is still a matter of debate (39–41), the induction of Treg responses may not occur in the context of TriVax/OX40. Because only activated HTLs express OX40 (34), the proper timing of OX40 agonist administration might be a critical issue to expand T cells. OX40 is also known to enhance CD8 responses (42). Therefore, it would be of interest to test TriVax/OX40 with CD8 epitopes and indeed, we have observed that the combination of αOX40 mAb with TriVax increased the expansion of Trp1-specific CTLs (T. Kumai, unpublished) suggesting that TriVax/OX40 can be a potent vaccine strategy for both HTLs and CTLs.
Comparing several TLR ligands, we previously found that the TLR3 ligand poly-IC is an ideal adjuvant for CTLs. Although poly-IC is a prominent inducer of IFN-I that we found was required for the expansions of both CTLs and HTLs by peptide vaccines (16,20), we were surprised to find that TLR7 ligand GDQ performed better than other TLR ligands, including TLR3 ligand, in stimulating HTL responses. One potential reason for this difference is the direct stimulation of HTLs by TLR ligands. TLR7 ligand stimulates HTLs better than poly-IC, at least in humans (26). Although TLR7 agonists are as good as poly-IC at increasing expression of CD40, CD80, and CD86 on APCs, downstream chemokine or cytokine production from APCs differs between TLR3 and TLR7 stimulation: poly-IC induces RANTES and TLR7 ligand resiquimod induces IL6 from DCs (43). Because RANTES upregulates CTL responses, whereas IL6 is an anti-apoptotic factor for HTLs (44,45), it is plausible that poly-IC or GDQ acts as a CTL- or an HTL-preferential adjuvant, respectively. Although IL6 has been considered a Th2-skewing cytokine, this effect may be countered by IL12 signaling via CD40 agonist or GDQ (38).
Our results revealed that a HTL-targeted peptide vaccine had a therapeutic impact with ACT therapy. Since Th1 responses are required to obtain antitumor effects with ACT therapy using HTLs (18), the advantage of TriVax/OX40 for ACT therapy is that we can induce Th1 HTLs in vivo, thereby bypassing the time-consuming and complicated process of in vitro cultures. Although in vitro-polarized Th17 HTLs have a longer half-life than do in vitro-polarized Th1 HTLs, the antitumor effects of Th17 HTLs still depend on Th1 cytokine IFNγ (19). Thus, the enormous expansion of IFNγ-producing Th1 HTLs by TriVax/OX40 may be sufficient to inhibit tumor growth. Even though we here found that TriVax/OX40 in the context of ACT could generate huge CD4 T-cell responses, the antitumor effect was short-lived. The absence of antigen-specific HTLs in blood in tumor-bearing mice 7 days after TriVax/OX40 (E. Celis, unpublished observations) suggests that either these cells rapidly disappear or that the vaccine is less efficient in the presence of tumor. We favor the first explanation because: (i) there was a clear antitumor effect that lasted ~2 weeks and (ii) even in tumor-free mice the presence of self-antigen impaired the long-term survival of the Trp1113-127 reactive HTLs. Thus, it is possible that the antitumor effect of ACT TriVax/OX40 therapy could be substantially improved by repeating the therapy for more than one cycle.
One important aspect of the combination of ACT therapy and peptide vaccines is that the patients do not need to receive lymphoablative therapies, such as irradiation or chemotherapy, to reduce the competition between the transferred tumor-reactive T cells and irrelevant endogenous T cells (46). CTL elimination by lymphodepletion could decrease the benefit of ACT therapy with HTLs because these helper cells could promote CD8 priming by epitope spreading and may also upregulate the antitumor activity of heterospecific CTLs through CD27/CD70 signaling (47,48). Indeed, we observed here that CTL depletion reduced the therapeutic effect of TriVax/OX40 and that CD8 responses to several MHC-I epitopes and to B16 cells were generated with ACT followed by Trivax/OX40. Collectively, TriVax/OX40 is a promising alternative to lymphoablative therapies to potentiate the antitumor activity of adoptive transferred HTLs by preserving CTLs that have potential to acquire the antitumor activity.
CD4 T cells that react to self-antigens are supposedly depleted during thymic education to prevent autoimmunity. It has been suggested that the self-reactive T cells that egress to periphery are low avidity cells that are difficult to expand in periphery and may not be able to recognize tumor cells that express low density of peptide/MHC complexes (49). Because most TAA are expressed in healthy tissues as well as in the tumor, the disruption of immune tolerance to these self-antigens is a crucial step to induce antitumor T cells. Although the use of Trp1-KO mice was a prerequisite to obtain Trp1-specific immune responses with a recombinant VV vaccine (19,50), we found that TriVax/OX40 was effective in generating endogenous HTL responses to the same epitope in WT-B6 mice, and these responses were similar as those observed in Trp1-KO mice. More importantly, the ability of the HTLs from WT-B6 mice to recognize IFNγ-treated B16 cells was comparable with the response of the HTLs from Trp1-KO mice. Our results suggest that either TriVax/OX40 was capable of breaking the immune tolerance to this self-antigen or that there is no central tolerance to the Trp1113-127 epitope. Thus, it is possible that recombinant VV Trp1 vaccines could induce CD4 T-cell responses in WT-B6 mice but were difficult to detect, because the presence of self-antigen in the periphery resulted in a rapid disappearance of the vaccine activated T cells, as we observed here.
It has become evident that immune checkpoint inhibitors are quite powerful weapons to fight cancer. Several studies have demonstrated that the effect of these therapies may rely on the activation of already existing tumor-reactive HTLs (51). Because we and others (52) have found that TCR signaling or cytokine induces PD-1 expression on HTLs, it was reasonable to combine PD-L1/PD-1 blockade with TriVax/OX40. Our findings that PD-L1 mAb increased the therapeutic effects of TriVax/OX40 support the rationale for the combination of a checkpoint inhibitor and a peptide vaccine for HTLs. It could be of interest to assess whether a more sustained administration of the PD-L1 mAb or a combination with Tim-3 and LAG-3 mAbs would delay disease progression by preventing the rapid disappearance of the vaccine generated T cells.
Supplementary Material
Acknowledgments
This work was supported by grants R01CA136828 and R01CA157303 from the National Cancer Institute of the National Institutes of Health and by start-up funds from Augusta University, Georgia Cancer Center, and the Georgia Research Alliance (GRA).
Footnotes
Disclosure of potential conflicts of interest
Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). All other authors declare no conflict of interest.
References
- 1.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation eithout causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–27. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL-2 regimen. Clin Cancer Res. 2016;22:3734–45. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 4.Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228–39. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Li W, Xia Z, Mao Y. CD4 T cell dependent tumor immunity stimulated by dendritic cell based vaccine. Biochem Biophys Res Commun. 2011;413:294–8. doi: 10.1016/j.bbrc.2011.08.089. [DOI] [PubMed] [Google Scholar]
- 6.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy R, Celis E. T helper lymphocytes rescue CTL from activation-induced cell death. J Immunol. 2006;177:2862–72. doi: 10.4049/jimmunol.177.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumai T, Matsuda Y, Ohkuri T, Oikawa K, Ishibashi K, Aoki N, et al. c-Met is a novel tumor associated antigen for T-cell based immunotherapy against NK/T cell lymphoma. Oncoimmunology. 2015;4:e976077. doi: 10.4161/2162402X.2014.976077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, et al. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109:2155–66. doi: 10.1038/bjc.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–84. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McWilliams JA, McGurran SM, Dow SW, Slansky JE, Kedl RM. A modified tyrosinase-related protein 2 epitope generates high-affinity tumor-specific T cells but does not mediate therapeutic efficacy in an intradermal tumor model. J Immunol. 2006 Jul 1;177(1):155–61. doi: 10.4049/jimmunol.177.1.155. [DOI] [PubMed] [Google Scholar]
- 13.Ahonen CL, Wasiuk A, Fuse S, Turk MJ, Ernstoff MS, Suriawinata AA, et al. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111:3116–25. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 15.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68:9892–9. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182:5217–24. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 18.Dovedi SJ, Lipowska-Bhalla G, Beers SA, Cheadle EJ, Mu L, Glennie MJ, et al. Antitumor Efficacy of Radiation plus Immunotherapy Depends upon Dendritic Cell Activation of Effector CD8+ T Cells. Cancer Immunol Res. 2016;4:621–30. doi: 10.1158/2326-6066.CIR-15-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61:1307–17. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–13. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Celis E. STING activator c-di-GMP enhances the anti-tumor effects of peptide vaccines in melanoma-bearing mice. Cancer Immunol Immunother. 2015;64:1057–66. doi: 10.1007/s00262-015-1713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maecker HT, Umetsu DT, DeKruyff RH, Levy S. Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC. J Immunol. 1998;161:6532–6. [PubMed] [Google Scholar]
- 24.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–51. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 25.Moutaftsi M, Bui HH, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, et al. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J Immunol. 2007;178:6814–20. doi: 10.4049/jimmunol.178.11.6814. [DOI] [PubMed] [Google Scholar]
- 26.Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–7. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, Celis E. Peptide epitope identification for tumor-reactive CD4 T cells. Curr Opin Immunol. 2008;20:221–7. doi: 10.1016/j.coi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res. 2014;20:1223–34. doi: 10.1158/1078-0432.CCR-13-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho HI, Reyes-Vargas E, Delgado JC, Celis E. A potent vaccination strategy that circumvents lymphodepletion for effective antitumor adoptive T-cell therapy. Cancer Res. 2012;72:1986–95. doi: 10.1158/0008-5472.CAN-11-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 32.Kurche JS, Burchill MA, Sanchez PJ, Haluszczak C, Kedl RM. Comparison of OX40 ligand and CD70 in the promotion of CD4+ T cell responses. J Immunol. 2010;185:2106–15. doi: 10.4049/jimmunol.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–8. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 34.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 35.Caserta S, Alessi P, Basso V, Mondino A. IL-7 is superior to IL-2 for ex vivo expansion of tumour-specific CD4(+) T cells. Eur J Immunol. 2010;40:470–9. doi: 10.1002/eji.200939801. [DOI] [PubMed] [Google Scholar]
- 36.Yamaki S, Ine S, Kawabe T, Okuyama Y, Suzuki N, Soroosh P, et al. OX40 and IL-7 play synergistic roles in the homeostatic proliferation of effector memory CD4(+) T cells. Eur J Immunol. 2014;44:3015–25. doi: 10.1002/eji.201444701. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Shin SM, Choi BK, Oh HS, Kim CH, Lee SJ, et al. Authentic GITR Signaling Fails To Induce Tumor Regression unless Foxp3+ Regulatory T Cells Are Depleted. J Immunol. 2015;195:4721–9. doi: 10.4049/jimmunol.1403076. [DOI] [PubMed] [Google Scholar]
- 38.Kato T, Hakamada R, Yamane H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J Immunol. 1996;156:3932–8. [PubMed] [Google Scholar]
- 39.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–9. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 40.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 41.Kim IK, Kim BS, Koh CH, Seok JW, Park JS, Shin KS, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med. 2015;21:1010–7. doi: 10.1038/nm.3922. [DOI] [PubMed] [Google Scholar]
- 42.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–66. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, et al. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 44.Kim JJ, Nottingham LK, Sin JI, Tsai A, Morrison L, Oh J, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–24. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–6. [PubMed] [Google Scholar]
- 46.Cozza EM, Cooper TK, Budgeon LR, Christensen ND, Schell TD. Protection from tumor recurrence following adoptive immunotherapy varies with host conditioning regimen despite initial regression of autochthonous murine brain tumors. Cancer Immunol Immunother. 2015;64:325–36. doi: 10.1007/s00262-014-1635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Goer de Herve MG, Cariou A, Simonetta F, Taoufik Y. Heterospecific CD4 help to rescue CD8 T cell killers. J Immunol. 2008;181:5974–80. doi: 10.4049/jimmunol.181.9.5974. [DOI] [PubMed] [Google Scholar]
- 48.Ahrends T, Babala N, Xiao Y, Yagita H, van Eenennaam H, Borst J. CD27 Agonism plus PD-1 blockade recapitulates CD4+ T-cell help in therapeutic anticancer vaccination. Cancer Res. 2016;76:2921–31. doi: 10.1158/0008-5472.CAN-15-3130. [DOI] [PubMed] [Google Scholar]
- 49.Gebe JA, Falk BA, Rock KA, Kochik SA, Heninger AK, Reijonen H, et al. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur J Immunol. 2003;33:1409–17. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 50.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:2982–7. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;7:10582. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.