Abstract
Purpose
Local tumor growth is a major cause of morbidity and mortality in nearly 30% of patients with pancreatic ductal adenocarcinoma (PDAC). Radiotherapy (RT) is commonly used for local disease control in PDAC, but efficacy is limited. We studied the impact of selectively intervening on RT-induced inflammation as an approach to overcome resistance to RT in PDAC.
Experimental Design
PDAC cell lines derived from primary pancreatic tumors arising spontaneously in KrasLSL-G12D/+;Trp53LSL-R172H/+;Pdx-1 Cre (KPC) mice were implanted into syngeneic mice and tumors were focally irradiated using the Small Animal Radiation Research Platform (SARRP). We determined the impact of depleting T cells and Ly6C+ monocytes as well as inhibiting the chemokine CCL2 on RT efficacy. Tumors were analyzed by flow cytometry and immunohistochemistry to detect changes in leukocyte infiltration, tumor viability and vascularity. Assays were performed on tumor tissues to detect cytokines and gene expression.
Results
Ablative RT alone had minimal impact on PDAC growth but led to a significant increase in CCL2 production by tumor cells and recruitment of Ly6C+CCR2+ monocytes. A neutralizing anti-CCL2 antibody selectively inhibited RT-dependent recruitment of monocytes/macrophages and delayed tumor growth but only in combination with RT (p<0.001). This anti-tumor effect was associated with decreased tumor proliferation and vascularity. Genetic deletion of CCL2 in PDAC cells also improved RT efficacy.
Conclusions
PDAC responds to RT by producing CCL2, which recruits Ly6C+CCR2+ monocytes to support tumor proliferation and neovascularization after RT. Disrupting the CCL2-CCR2 axis in combination with RT holds promise for improving RT efficacy in PDAC.
Keywords: Monocyte, CCL2, radiation, SBRT, pancreatic cancer
INTRODUCTION
Clinical outcomes in pancreatic ductal adenocarcinoma (PDAC) are poor with a 5 year overall survival of only 7% (1,2). Although metastasis is the most common cause of mortality, 30% of patients will succumb to disease due to complications of local tumor growth (3). In addition to its effect on mortality, local tumor growth can also cause significant morbidity including obstructive biliary sepsis, duodenal obstruction, and intractable pain related to celiac plexus involvement (4–6).
Approximately 25% of PDAC patients will present with localized but unresectable disease (7); for these patients, radiotherapy (RT) is a major treatment modality for local control. However, local progression despite radiotherapy is common (8–10). Recently, stereotactic ablative body radiotherapy (SBRT or SABR) has garnered significant attention as a means to deliver a higher biologically effective radiation dose (11–16). Ablative RT has also been found to alter the immune response to cancer (17). In particular, for immunologically sensitive models of cancer, ablative RT can increase T cell infiltration leading to durable remissions, as shown in a subcutaneous murine model of colon cancer (18). However, the impact of ablative RT on the immune microenvironment of immunologically resistant tumors, such as PDAC, remains ill-defined.
In PDAC, the tumor microenvironment is characterized by poor infiltration of effector T cells and a predominance of innate inflammatory cells, including macrophages (19,20). This microenvironment is profoundly immunosuppressive and establishes a site of T cell immune privilege (21). In addition, the microenvironment that surrounds PDAC is supportive of cancer growth, invasion and metastasis (22,23). However, the immune reaction to PDAC is inherently pliable such that myeloid cells responding to PDAC can also be induced with anti-tumor properties capable of enhancing the efficacy of standard cytotoxic therapies (19,24).
In this study, we examined the effect of ablative RT on the immune response to PDAC. Unlike immunologically sensitive tumors, RT did not induce T cell infiltration into tumor tissue. In contrast, PDAC cells responded to RT by releasing inflammatory cytokines and chemokines including CCL2. Tumor-derived CCL2 induced an increase in inflammatory monocytes and macrophages within the tumor microenvironment and inhibited anti-tumor activity mediated by RT. In contrast, neutralization of CCL2 blocked inflammatory monocyte recruitment to tumors and resulted in improved tumor control when administered in combination with RT.
MATERIALS AND METHODS
Animals
C57BL/6 mice were purchased from Jackson Laboratories. Animal protocols were reviewed and approved by the Institute of Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Cell Lines
Mouse pancreatic cancer cell lines were derived from spontaneously arising tumors in KrasLSL-G12D/+, Trp53LSL-R172H/+, Pdx1-Cre (KPC) mice as previously described (24,25). Cell lines were authenticated based on histological analysis of the implanted cell line with comparison to the primary tumor from which the cell line was derived as previously described (24). Cell lines were tested for mycoplasma contamination; cultured at 37ºC in DMEM supplemented with 10% FCS, 83μg/mL gentamicin, and 1% L-glutamine; and used in experiments between passage six to eight.
Animal Experiments
PDAC cell lines were implanted subcutaneously at 4.0–5.0x105 cells into syngeneic C57BL/6 mice. For orthotopic implantation of tumor cells, syngeneic C57BL/6 mice were first anesthetized and the abdomen prepared in a sterile fashion. A small (5–10 mm) incision was made over the left upper quadrant of the abdomen and the peritoneal cavity was exposed. The pancreas was then located and exteriorized onto a sterile field. PDAC cell lines (5.0x105 cells) were implanted into the tail of the pancreas. The pancreas was then placed back into the peritoneal cavity, and the peritoneum and skin were closed with suture and wound clips, respectively. Tumors were allowed to develop over 14–17 days to approximately 5 mm in diameter. Established tumors were irradiated in a single fraction (14–20 Gy) using the Small Animal Radiation Research Platform (SARRP). Anti-CCL2 (clone 2H5) neutralizing antibody, anti-Ly6C (clone Monts1) depleting antibody, hamster isotype control (hamster IgG) and rat isotype control (clone 2A3) were administered via intraperitoneal injection on days −1, 0, +1, and +3 of RT. Anti-CD4 (clone GK1.5) and anti-CD8 (clone 2.43) depleting antibodies were administered on day -1. All neutralizing and depleting antibodies were purchased from BioXcell and were endotoxin free. Every 3–4 days, the longest tumor dimension (L) and its perpendicular diameter (W) were measured using calipers; volume was calculated as (L x W2) / 2. For survival studies, mice were euthanized when tumors reached 1000mm3.
Histopathology and Immunohistochemistry Analysis
Histopathology and immunohistochemistry were performed on frozen tissue sections. Frozen sections were air dried and fixed with 3% formaldehyde. Hydrogen peroxide was used to quench endogenous peroxidases. Tissue was blocked with 10% normal goat serum in PBS with 0.1% Tween-20. Primary antibodies against mouse antigens included: rat anti-F4/80 (eBioscience, San Diego, CA), rat anti-Ly6C (Abcam), rat anti-Ki67 clone TEC3 (Dako), rat anti-CD31 (BD Pharmingen), rat anti-CD45 (BD Pharmingen) and rabbit anti-cleaved caspase-3 (R&D Systems). Tissues were incubated in blocking buffer containing primary antibodies overnight at 4ºC. After washing with PBS, tissues were incubated with PBS containing a secondary biotinylated goat anti-rat antibody (BD Pharmingen). Tissues were developed using Vectastain ABC kit and counterstained with hematoxylin. Senescence associated Beta-galactosidase staining was performed using the Senescence β-Galactosidase Staining kit (Cell Signaling Technology). Fluorescent imaging was performed on an IX83 inverted microscope (Olympus) and brightfield images were acquired using a BX43 upright (Olympus) microscope.
Flow Cytometry of Subcutaneous Tumors
Mice were euthanized and subcutaneous tumors were harvested, measured, and weighed. Peritumoral lymph nodes were excluded from analysis. Tumors were minced at 4ºC in digestion media containing collagenase (1mg/mL, Sigma-Aldrich), DNase (150U/mL, Roche) and Dispase (1U/mL, Worthington), and then incubated at 37ºC for 30 minutes with intermittent agitation. After 70μm filtration and washing with PBS, single cell suspensions were stained with antibodies in PBS containing 0.2mM EDTA with 2% FCS at 4ºC for 15 minutes. Anti-mouse antibodies were purchased from BD Biosciences, unless stated otherwise: CD45 (30-F11, PE-Cy7), CD11b (BioLegend, M1/70, APC), F4/80 (eBiosciences, BM8, FITC), Ly6C (AL-21, APC-Cy7), Ly6G (1A8, Percp-Cy5.5), CCR2 (R&D Systems, clone #475301, PE). Antibody-stained single cell suspensions were examined on a FACS Canto II (BD Biosciences) and analyzed using FlowJo version 10 (FlowJo, LLC, Ashland, OR). For gene expression analysis of leukocyte subsets obtained from implanted tumors, antibody-stained single cell suspensions were sorted on a FACSAria II (BD Biosciences) and RNA isolation was performed within 5–6 hours of the initial tumor digest. This protocol required 5 x 104 sorted cells per sample. Gating strategy for inflammatory monocytes (IMs), granulocytes, and tumor-associated macrophages (TAMs) is illustrated in Supplementary Figure 1.
Cytokine Analysis of Tumor Supernatant
For in vivo experiments, tumors were harvested, placed at 4ºC in serum-free DMEM at 1 mg of tissue per 10μL of media, and then minced. Tumor suspensions were centrifuged at 12470 x g for 5 minutes, and supernatant was collected and stored at −20ºC. For in vitro experiments, when tumor cell lines reached 70–80% confluence in 10mm plates, cells were washed and incubated in fresh serum-free DMEM at 37ºC; supernatant was then collected after 24 hours and stored at −20ºC. Cytokines from in vitro and in vivo tumor supernatants were quantified using cytometric bead analysis (CBA, BD Biosciences), using references to recombinant murine standards.
Transwell Migration Assay
Bone marrow-derived cells (2 x 106/mL) from C57BL/6 mice were placed above a transwell-membrane in DMEM containing 1% FCS, which was incubated in tumor supernatant collected in vitro as described above, in the presence or absence of a CCL2 neutralizing antibody (2H5, 10ng/mL). After incubation at 37ºC for 5 hours, transwell membranes were collected, fixed with formaldehyde, stained with crystal violet and dried. Transmigrated cells were counted at 40x magnification using an upright bright-field microscope (Olympus BX43).
In Vitro Irradiation
PDAC cell lines at 70–80% confluence were cultured in DMEM containing 5% FCS at 0.5cm depth and irradiated at a dose rate of 2.8 Gy/min using the X-RAD 320ix (Precision X-ray, Inc). Sham irradiation involved placing cell culture plates at a similar temperature for the length of irradiation.
RNA and Gene Expression Array
Tumor tissue was processed and stored in TRIzol at −80ºC. Tumor lysates were thawed on ice and allowed to equilibrate to room temperature before RNA was isolated using a Qiagen RNeasy Mini kit, according to manufacturer protocol. For in vitro experiments, tumor cells were washed and harvested using TRIzol. Flow sorted samples were collected in TRIzol LS and RNA extraction was performed immediately. RNA was collected in RNase-free water and quantified using a NanoDrop Spectrophotometer. cDNA was synthesized from 1 μg of RNA per sample using MultiScribe Reverse Transcriptase and random hexamers (Applied Biosystems, Foster City, CA). Primers for qRT-PCR were designed using the Primer 3 online program (26,27) and synthesized by Integrated DNA Technologies. SYBR Green chemistry (Applied Biosystems) was used to determine relative quantifications of all products; expression was normalized to beta-actin and calculated using the delta-CT formula. Fold change was calculated relative to the average of the control group. Primer sequences were as follows:
Murine Ccl2: Forward 5’ - AGCAGCAGGTGTCCCAAAGA - 3’
Murine Ccl2: Reverse 5’ – GATCTCATTTGGTTCCGATCCA – 3’
Human Ccl2: Forward 5’ – GAAGAATCACCAGCAGCAAGT-3’
Human Ccl2: Reverse 5’ – TCCTGAACCCACTTCTGCTT – 3’
Microarray Analysis
Microarray services were provided by the UPENN Molecular Profiling Facility, including quality control tests of the total RNA samples by Agilent Bioanalyzer and Nanodrop spectrophotometry. All protocols were conducted as described in the NuGEN Ovation Pico WTA system v2 user guide and the Affymetrix GeneChip Expression Analysis Technical Manual. Briefly, 25ng of total RNA was converted to first-strand cDNA using reverse transcriptase primed by poly(T) and random oligomers that incorporated an RNA priming region. Second-strand cDNA synthesis was followed by ribo-SPIA linear amplification of each transcript using an isothermal reaction with RNase, RNA primer and DNA polymerase, and the resulting ssDNA was assessed by Bioanalyzer, fragmented and biotinylated by terminal transferase end labeling. Labeled cDNA (5.5 μg) were added to Affymetrix hybridization cocktails, heated at 99ºC for 5 min and hybridized for 16 h at 45ºC to Mouse Gene 2.0 ST GeneChips (Affymetrix Inc., Santa Clara CA) using the GeneChip Hybridization oven 645. The microarrays were then washed at low (6X SSPE) and high (100mM MES, 0.1M NaCl) stringency and stained with streptavidin-phycoerythrin. Fluorescence was amplified by adding biotinylated anti-streptavidin and an additional aliquot of streptavidin-phycoerythrin stain. A GeneChip 3000 7G scanner was used to collect fluorescence signal. Affymetrix Command Console and Expression Console were used to quantitate expression levels for targeted genes; default values provided by Affymetrix were applied to all analysis parameters. Heat maps were generated using GENE-E matrix visualization and analysis platform. Gene expression data is available through the NCBI GEO database (accession number GSE82276).
CRISPR Knockout
Oligonucleotides (GCAAGATGATCCCAATGAGT and GGCCTGCTGTTCACAGTTGC, Integrated DNA Technologies) encoding guide RNAs that target murine CCL2 exons were cloned into TOPO-expression vectors (Life Technology). A clonal tumor line, derived from a parental KPC-derived PDAC cell line, was then co-transfected using 5 μg LentiCRISPR V2 plasmid (Addgene) with 5 μg of TOPO-CCL2 gRNA plasmids or a control vector targeting GFP. One day following transfection, transfected cells were selected for 48h with 10 ng/ml puromycin. Isogenic clones of transfected cells were isolated to produce clonal lines, in which CCL2 knockout was confirmed by cytometric bead array (CBA; BD Bioscience) of tumor cell supernatant.
Statistical Analysis
Statistical significance was determined by Student's t test performed with or without Welch's correction for unequal variance using GraphPad Prism Version 6.05 (GraphPad Software, Inc., La Jolla, CA). Significance testing for tumor growth over time was performed using repeated measures two-way Anova with Tukey multiple comparisons of means as a post hoc test to evaluate differences between two groups. In some cases, tumor volumes were compared at each time point using Student’s t test with Holm-Šidák method to correct for multiple comparisons. Significance of overall survival was determined using log-rank analysis.
RESULTS
Ablative RT minimally impacts PDAC tumor growth
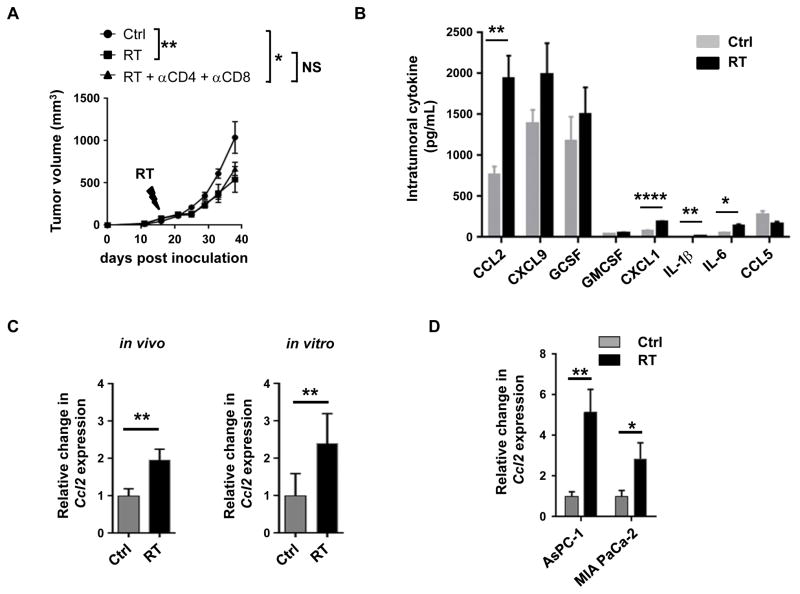
To investigate the impact of ablative RT in an immunologically resistant tumor model (21), we derived PDAC cell lines from tumors arising spontaneously in KPC mice and injected them subcutaneously into syngeneic C57BL/6 mice. Approximately two weeks later, established tumors were treated with 20 Gy ablative RT, a dose that is comparable to that used clinically for human PDAC (13). Similar to results reported in patients with PDAC, we found that a single dose of RT only partially delayed tumor growth (Figure 1A). However, in contrast to findings in immunogenic murine melanoma and colon cancer models (18,28), depletion of CD4+ and CD8+ T-cells did not impact the delay in tumor growth induced by RT (Figure 1A). Further, we observed no significant difference in T cell infiltration into tumors after RT (Supplementary Figure 2). These findings revealed that ablative RT alone is insufficient to invoke a productive anti-tumor immune response against an immunologically resistant tumor such as PDAC.
Figure 1. RT-resistance in PDAC is associated with increased production of tumor-derived CCL2.
(A) Tumor growth curve of a KPC-derived PDAC cell line (152.PDA) implanted subcutaneously and treated on day 16 after implantation with RT (20 Gy) or sham (Ctrl) in combination with CD4 and CD8 T cell depleting antibodies compared to isotype controls. Shown is mean ± s.e.m, n=5 mice per group. Tumor growth was compared using repeated measure two-way Anova (p<0.05) with Tukey multiple comparisons of means to evaluate differences between two groups. (B) Bar graph displaying intratumoral cytokine and chemokine levels (pg/mL) detected 24 hours after sham (Ctrl) or RT (20 Gy). (C) Fold change in Ccl2 expression detected within tumors in vivo and in vitro 3 days after RT (20 Gy) compared to sham (Ctrl). Ccl2 expression was normalized to β-actin. (D) Fold change in CCL2 expression detected in human PDAC cell lines AsPC-1 and MIA PaCa-2 at 3 days after RT compared to sham (Ctrl). For each treatment group, CCL2 expression was normalized to GAPDH. Significance testing was performed using unpaired two-tailed Student’s t test. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p <0.0001.
PDAC tumors respond to ablative RT by releasing chemokines including CCL2
To investigate potential immune mechanisms regulating the efficacy of ablative RT, we next examined the intratumoral cytokine profile induced by RT. At baseline, tumors demonstrated high levels of CCL2, MIG and G-CSF among a panel of cytokines (Figure 1B) which is consistent with the robust inflammatory response seen in PDAC (29). However, after RT, there were small but significant increases in the levels of CXCL1, IL-1β and IL-6. Most notably, though, there was a marked increase in CCL2 (Δ = 1178 ± 294 pg/mL) (Figure 1B). This increase in CCL2 levels in response to RT was also seen at the RNA level (Figure 1C) and was reproducible using a second KPC-derived PDAC cell line in vivo (Supplementary Figure 3).
We next examined the capacity of tumor cells to secrete CCL2 in response to RT. We found that Ccl2 expression increased in vitro in response to RT (Figure 1C), suggesting that tumor cells, not just stromal or immune cells, likely contribute to the increase in CCL2 within the tumor microenvironment. This same RT-induced CCL2 release was also seen with two human PDAC cell lines. Here, RT augmented CCL2 expression in both MIA PaCa-2 and AsPC-1 human PDAC cell lines by 2.8 ± 0.5 and 5.1 ± 0.8 fold, respectively (Figure 1D). Thus, PDAC responds to RT by releasing CCL2.
PDAC-derived CCL2 regulates migration of bone marrow-derived cells
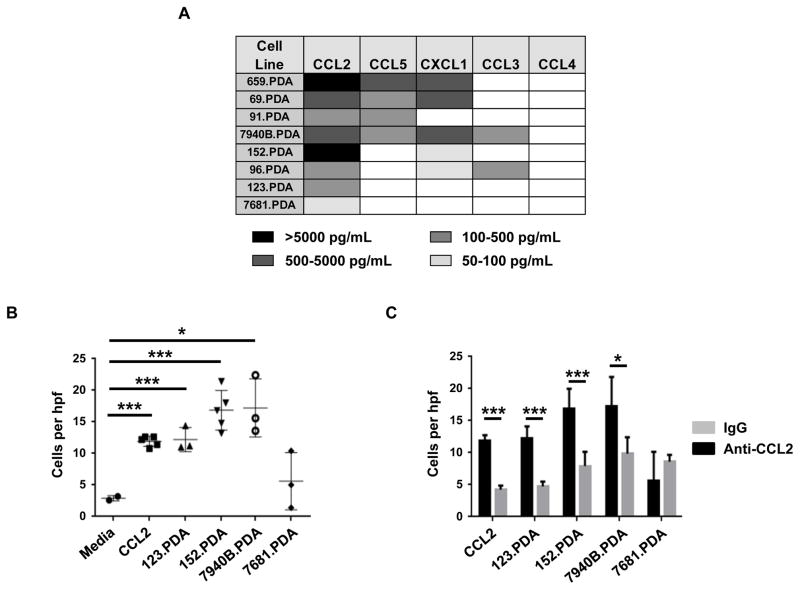
Consistent with clinical data suggesting that CCL2 expression is increased in human PDAC (30), we detected high levels of CCL2 produced in vitro by an array of KPC-derived PDAC cell lines. In contrast, other chemokines important for the recruitment of myeloid cells, such as CCL5, CXCL1, CCL3 and CCL4, were not uniformly detected (Figure 2A).
Figure 2. PDAC-derived CCL2 induces myeloid cell migration.
(A) Heat map displaying chemokine production by KPC-derived PDAC cell lines grown in vitro. (B) Migration of bone marrow-derived cells in response to tumor-derived soluble factors from indicated cell lines with comparison to CCL2 (10 ng/mL) and media alone. (C) Shown is the impact of anti-CCL2 neutralizing antibodies versus isotype control (IgG) on bone marrow-derived cell migration induced by tumor-derived soluble factors from indicated cell lines or CCL2 (10 ng/mL). For B and C, migrated cells were quantified at 40x magnification (hpf). Significance testing was performed using unpaired 2-tailed Student’s t test. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p <0.0001.
We next used an in vitro chemotaxis assay to understand the significance of tumor-derived factors in recruiting myeloid cells to the tumor microenvironment. We found that soluble factors produced by PDAC cell lines induced the migration of bone marrow-derived cells across a transwell membrane (Figure 2B). The magnitude of cell migration induced by PDAC was comparable to myeloid cell migration toward 10 ng/mL CCL2 (Figure 2B). Further, bone marrow derived cells that migrated across the transwell membrane were enriched for CCR2 expression (Supplementary Figure 4). To determine the role of tumor-derived CCL2 in directing myeloid cell chemotaxis, we neutralized CCL2 in vitro using a CCL2 specific antibody and found a significant reduction in the migration of bone marrow-derived cells (Figure 2C) demonstrating a major role for PDAC-derived CCL2 for myeloid cell chemotaxis.
Ablative RT induces tumor recruitment of CCR2-expressing inflammatory monocytes/macrophages in vivo
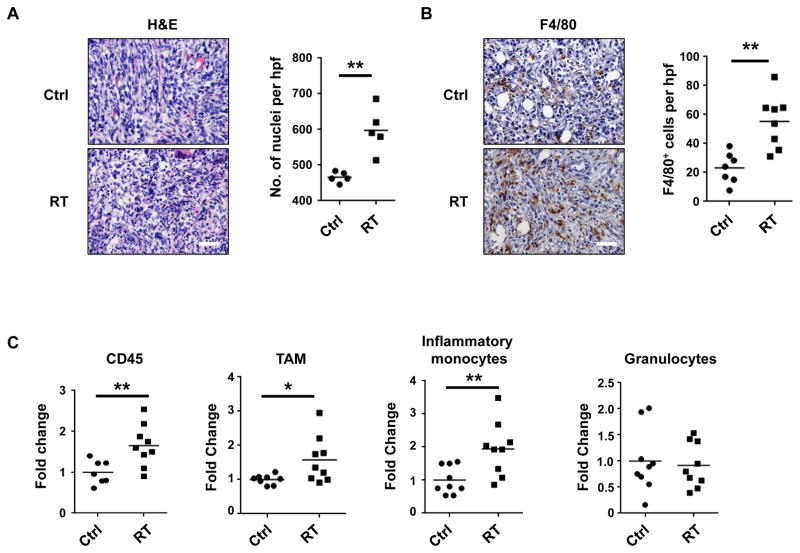
Given the effect of ablative RT on intratumoral chemokine levels, we examined the dynamics of myeloid cell composition within the tumor microenvironment after RT. By H&E staining, we detected an increase in cellular content three days after RT, suggesting an acute inflammatory response (Figure 3A). On more detailed examination of the immune microenvironment, we detected an increase in the frequency of F4/80+ monocytes/macrophages within tumors (Figure 3B). Using flow cytometry, we also found an increase in leukocytes as a percentage of live cells within tumors after RT (Figure 3C). Specifically, we observed an increase in tumor-associated macrophages (TAM, CD45+CD11b+Ly6CloF4/80+) and inflammatory monocytes/macrophages (CD45+CD11b+Ly6Chi). In contrast, we detected no change in granulocyte (CD45+CD11b+Ly6G+) recruitment. Similar findings were observed in an orthotopic model of PDAC, where ablative RT induced an increase in inflammatory monocytes/macrophages without a change in granulocyte infiltration (Supplementary Figure 5).
Figure 3. RT enhances myeloid cell recruitment to the tumor microenvironment in PDAC.
(A) Representative images of H&E staining of PDAC tumors (152.PDA) at 3 days after treatment with RT (20 Gy) versus sham. Nuclei are quantified as a measure of cellularity. (B) Representative images of F4/80 immunostaining of PDAC tumors (152.PDA) at 3 days after treatment with RT (20 Gy) versus sham (Ctrl). F4/80+ cells are quantified. For A and B, images are taken at 40X magnification (hpf) and scale bar represents 50 microns. (C) Leukocyte subsets within tumors at 3 days after RT (20 Gy) versus sham (Ctrl). Shown are the fold changes of total CD45+ cells, tumor-associated macrophages (TAM, CD45+CD11b+F480+Ly6Clo), inflammatory monocytes (CD45+CD11b+F4/80+Ly6Chi), and granulocytes (CD45+CD11b+F4/80negLy6G+) as a percentage of total live cells. Fold change is calculated relative to the average of the control group. Each data point represents a single mouse. Significance testing was performed using unpaired two-tailed Student’s t test. *, p<0.05; **, p<0.01.
The increase in inflammatory monocyte/macrophage infiltration seen after RT was consistent with the increase in intratumoral CCL2 levels induced by RT (Figure 1). Inflammatory monocytes/macrophages express CCR2, the receptor for CCL2, which can direct both the egress of immature inflammatory monocytes from the bone marrow into the peripheral blood and their subsequent infiltration into tissues (24,31). Therefore, we hypothesized that inflammatory monocytes/macrophages may respond to radiation-induced injury by acquiring an altered phenotype that regulates the efficacy of ablative RT. To test this hypothesis, we examined the gene expression profile of flow-sorted inflammatory monocytes/macrophages isolated from tumors one day after ablative RT compared to control tumors. This analysis revealed only 8 of 34,365 transcripts that reached a pre-determined threshold of two-fold change in expression with a <25% false-discovery rate (Supplementary Figure 6). Four of these, including Phlda3, Ccna1, Ddias, and Trp53inp1 are recognized as cell cycle or stress response genes. However, none of these genes are known as major determinants of cellular phenotype to suggest a role in redirecting the biology of tumor-infiltrating monocytes/macrophages. Therefore, the minimal impact of ablative RT on the gene expression profile of inflammatory monocytes/macrophages supports a role for RT primarily in altering the quantity rather than the quality of inflammatory monocytes/macrophages recruited to the tumor microenvironment.
CCL2 neutralization delays tumor regrowth after RT by impeding inflammatory monocyte recruitment
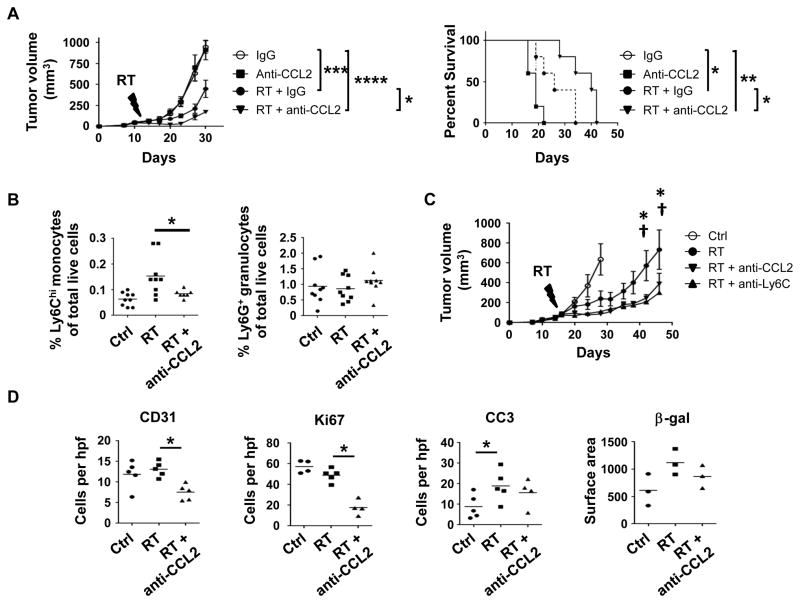
CCL2 expression and inflammatory monocyte recruitment have been implicated as poor prognostic factors in a variety of malignancies (30,32,33). We hypothesized that RT induced expression of CCL2 within the tumor microenvironment may recruit inflammatory monocytes/macrophages and TAMs to promote resistance to ablative RT. To test this hypothesis, mice bearing established PDAC tumors injected subcutaneously were treated with a neutralizing antibody to CCL2 (or control) on days −1, 0, +1 and +3 relative to RT versus sham treatment. Compared to RT alone, the combination of RT and a CCL2 neutralizing antibody significantly impacted tumor growth and prolonged survival (Figure 4A). This effect was reproduced using a second KPC-derived PDAC cell line (Supplementary Figure 7).
Figure 4. CCL2 neutralization inhibits RT-induced recruitment of inflammatory monocytes to PDAC and enhances RT efficacy.
Established PDAC tumors (152.PDA) implanted into syngeneic mice were treated with RT (14 Gy) or sham. Anti-CCL2 neutralizing antibodies, anti-Ly6C depleting antibodies or isotype control were administered on days -1, 0, 1, and 3 of RT. (A) Tumor growth curve and Kaplan Meier survival plot showing impact of CCL2 neutralizing antibodies on RT efficacy. Significance testing for tumor growth over time was performed using repeated measures two-way Anova (p<0.0001) with Tukey multiple comparisons of means as a post hoc test to evaluate differences between two groups. Survival significance was tested using log-rank analysis. (B) Shown are percentages of Ly6Chi inflammatory monocytes and Ly6G+ granulocytes of total live cells detected within tumors at 3 days after RT (20 Gy), versus sham (Ctrl), with or without anti-CCL2 neutralizing antibodies. Each data point represents a single mouse. (C) Tumor growth curve showing impact of anti-CCL2 and anti-Ly6C antibodies on RT efficacy. Significance testing was performed at each time point using Student’s t test with Holm-Šidák method to correct for multiple comparisons. *, p<0.05 for RT vs RT + anti-CCL2; †, p<0.05 for RT versus RT + anti-Ly6C. Data from panels A and C are representative of at least five and two independent experiments, respectively. (D) Quantification of immunohistochemical staining for vascularity (CD31), proliferation (Ki67), apoptosis (Cleaved Caspase 3), and senescence (β-galactosidase). Immunostains were quantified at 40x magnification (hpf). Each data point represents a single mouse. For panels B and D, significance testing was performed using unpaired 2-tailed Student’s t test. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p <0.0001.
While some tumor cells have been reported to express CCR2, we found that CCR2 expression within PDAC tumors was limited to a subset of CD45+ leukocytes (Supplementary Figure 8). In addition, CCL2 neutralization alone did not impact tumor growth (Figure 4A). Therefore, to understand the impact of CCL2 neutralization on the efficacy of RT, we examined the myeloid compartment of tumors three days after RT. We found that the acute increase in inflammatory monocytes induced by RT was abrogated by CCL2 neutralization in vivo (Figure 4B). In contrast, the presence of granulocytes within tumors was not impacted by RT or the combination of RT and CCL2 neutralization (Figure 4B).
Our findings with CCL2 neutralization suggested a key role for inflammatory monocytes/macrophages as an immune resistance mechanism to ablative RT in PDAC. To further address this possibility, we depleted Ly6Chi inflammatory monocytes/macrophages using a Ly6C depleting antibody (24). Using this approach, we found that depletion of inflammatory monocytes significantly delayed tumor growth after RT, and mirrored the impact of CCL2 neutralization (Figure 4C). To understand the mechanism by which inflammatory monocytes promote tumor growth after RT, we examined tumor specimens by immunohistochemistry. After RT alone there was no change in vascularity or proliferation, but increased apoptosis (cleaved caspase-3) and a trend toward increased senescence (Figure 4D). Compared to RT alone, we found that the combination of RT and CCL2 neutralization significantly reduced tumor vascularity (decrease in CD31+ cells per high-power field) and proliferation (decreased Ki67+ cells per high-power field) without further impacting apoptosis (cleaved caspase-3) or senescence (β-galactosidase) (Figure 4D). Further, in contrast to immunogenic cancer models where CCL2 neutralization can induce T cell infiltration, we found that CCL2 neutralization with or without RT failed to lead to recruitment of CD3+ T cells to the tumor microenvironment (Supplementary Figure 9). Thus, PDAC responds to ablative RT by releasing CCL2 which then recruits inflammatory monocytes/macrophages to support tumor proliferation and vascularization.
Tumor-derived CCL2 limits efficacy of ablative RT in PDAC
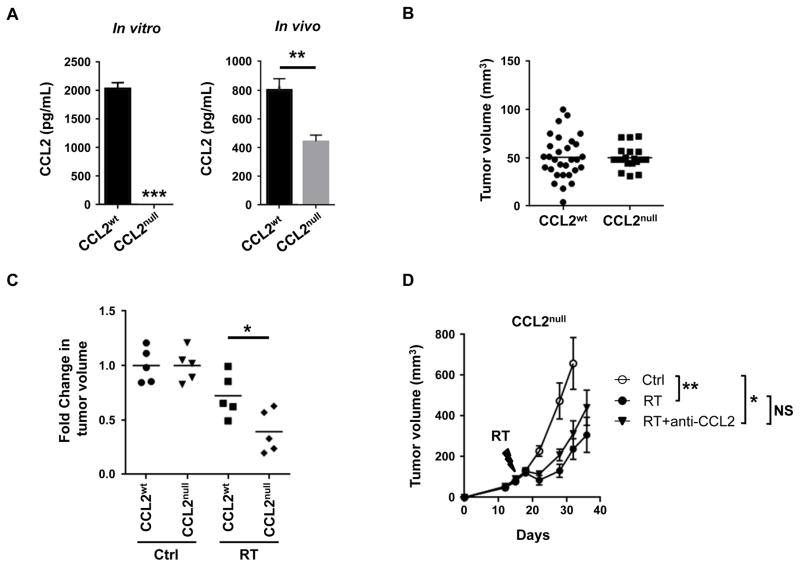
Both malignant and non-malignant cells can secrete CCL2 within the tumor microenvironment. In a breast cancer metastasis model, CCL2 produced by both malignant and non-malignant cells was found to be important for recruiting inflammatory monocytes/macrophages to promote metastasis (32). However, our findings in models of PDAC demonstrate that malignant cells respond to ablative RT by significantly increasing CCL2 production. This raised the possibility that CCL2 produced by malignant cells may be the main mediator of immune resistance to RT. Therefore, we next examined the role of tumor-derived CCL2 in promoting resistance to RT. To do this, we genetically eliminated CCL2 from a clonal KPC-derived PDAC cell line using CRISPR technology. As expected, compared to the wild type clonal cell line (CCL2wt), the CCL2null cell line did not produce CCL2 (Figure 5A). We next examined the biology of CCL2null versus CCL2wt tumors implanted into syngeneic mice. We found that levels of CCL2 were significantly decreased in CCL2null PDAC tumors, although not completely eliminated (Figure 5A), which likely represents non-malignant sources of CCL2. In contrast, we did not observe any difference in the engraftment or outgrowth of CCL2null compared to CCL2wt tumors (Figure 5B). Finally, we determined the capacity of RT to impact the in vivo growth of CCL2null versus CCL2wt tumors in vivo. We found that the responsiveness of CCL2null tumors to RT was significantly increased compared to CCL2wt tumors as seen by deeper tumor regressions (Figure 5C). In addition, consistent with the importance of tumor-derived CCL2 in regulating the efficacy of ablative RT, CCL2 neutralization did not improve RT efficacy in CCL2null tumors (Figure 5D), as had been seen in CCL2 expressing tumors (Figure 4A). Together, our findings support a role for tumor-derived CCL2 as a key mechanism of resistance to ablative RT in PDAC.
Figure 5. Tumor-derived CCL2 regulates RT efficacy in PDAC.
CRISPR technology was used to knockout CCL2 in PDAC cells. (A) Shown is CCL2 (pg/mL) produced by wild-type PDAC cells (CCL2wt) and CCL2-knockout PDAC cells (CCL2null) in vitro and in vivo. (B) Tumor volume of CCL2wt and CCL2null PDAC cell lines at 12 days after implantation. Each data point represents a single mouse. (C) Fold change in tumor volume 4 days after ablative RT (14 Gy) in control (CCL2wt) and CCL2null clonal PDAC cell lines, relative to baseline. Data were normalized to untreated controls. (D) Tumor growth curve for CCL2null PDAC cells treated with RT (14 Gy), versus sham (Ctrl), with or without anti-CCL2 neutralizing antibodies. Shown is mean ± s.e.m; n=5 mice per group. Data are representative of 2 independent experiments. For panels A-C, significance testing was performed using unpaired two-tailed Student’s t test. For panel D, significance testing for tumor growth over time was determined using repeated measures two-way Anova (p<0.0001) with Tukey multiple comparisons of means as a post hoc test to evaluate differences between two groups. *, p<0.05; **, p <0.01, ***, p<0.001.
DISCUSSION
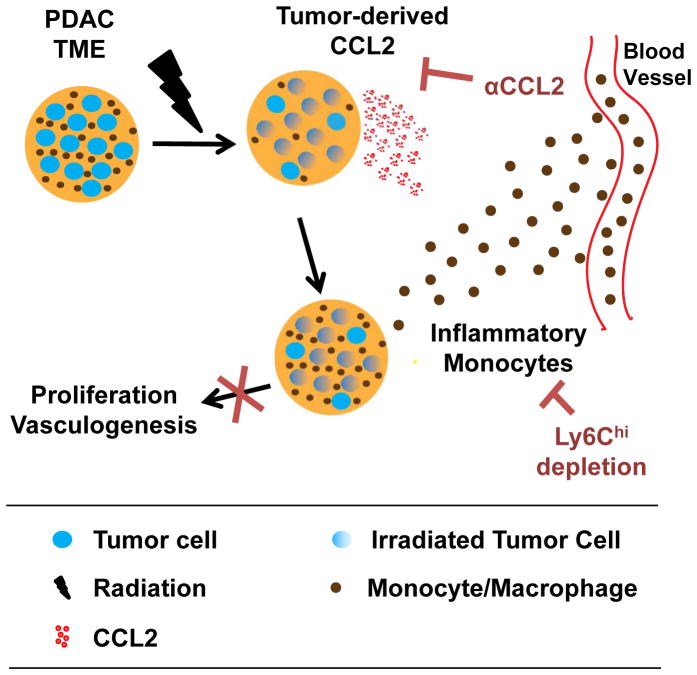
Local disease progression can be a major complication of unresectable PDAC with patients developing (i) sepsis secondary to biliary obstruction, (ii) hemorrhage and bowel obstruction related to duodenal invasion, and (iii) intractable pain from celiac plexus involvement. Overall, nearly one third of patients with PDAC will die of uncontrolled local disease (3). While RT remains a mainstay for disease control in the locally advanced setting, human PDAC has demonstrated significant resistance to RT. This resistance has been attributed to an inherent radioresistance of tumor cells, increased hypoxia, poor vascularity, and dense fibrosis (34–37). Our findings now identify a novel mechanism of RT resistance wherein PDAC cells appear inherently programmed to respond to RT-induced stress by releasing high levels of CCL2 that recruit inflammatory monocytes to promote tumor proliferation and vascularity (Figure 6). Thus, our data support a role for CCL2-dependent inflammation as a major checkpoint to the efficacy of RT in PDAC.
Figure 6. Conceptual model describing a role for monocytes and CCL2 in PDAC resistance to RT.
The PDAC tumor microenvironment (TME) consists of tumor cells (blue), leukocytes (brown) including macrophages and their precursor monocytes, and other non-malignant stromal cells including fibroblasts and endothelial cells (not shown). Ablative RT results in cell death in a fraction of tumor cells and leukocytes within the TME. PDAC cells respond to RT by increasing their production of chemokines, including CCL2, which acts to recruit CCR2+ inflammatory monocytes to the TME. Tumor-infiltrating monocytes/macrophages then support tumor proliferation and neovascularization and therein establish PDAC resistance to RT. Neutralization of CCL2 or depletion of Ly6Chi inflammatory monocytes blocks RT-induced monocyte/macrophage recruitment to tumors and their subsequent pro-tumor effects leading to enhanced RT efficacy.
In this study, we found that ablative RT, while limited in its anti-tumor efficacy, induced the accumulation of chemokines and cytokines within the tumor microenvironment – in particular, CCL2. While chemokine production within the tumor microenvironment can be driven by both malignant and non-malignant cells, in vitro studies of murine and human PDAC cell lines showed that tumor cells respond to the stress of ablative RT by augmenting CCL2 expression. Tumor-derived CCL2 was found to be critical for inducing chemotaxis of inflammatory monocytes and directed the recruitment of inflammatory monocytes/macrophages and tumor-associated macrophages to the tumor microenvironment. We found that selective blockade of inflammatory monocyte recruitment, while ineffective alone, improved the anti-tumor effect of ablative RT and prolonged survival in a murine model of PDAC. This approach was contingent on tumor-derived CCL2 expression. Using cell lines in which CCL2 was genetically deleted, we observed that tumor-derived CCL2 was necessary for the anti-tumor effect of CCL2 neutralization in combination with RT, despite continued expression of CCL2 by non-malignant cells within the tumor microenvironment. This finding suggests that the proximity of inflammatory monocytes/macrophages to tumor cells which may be directed by tumor-derived CCL2 is critical for monocyte/macrophage pro-tumor activity in the setting of RT (38).
Using a poorly immunogenic model of PDAC, we found that T cells were not required for CCL2 neutralization to improve the efficacy of ablative RT. Rather, our findings demonstrate that CCL2 neutralization inhibits inflammatory monocyte recruitment to tumors and their subsequent role in supporting tumor recovery from the cytotoxic effects of RT. This finding contrasts the use of CCL2-CCR2 blockade in other more immunogenic tumors, where it has been shown to induce T cell dependent anti-tumor immunity when administered in combination with cytotoxic chemotherapy (30,33). In addition, our findings contrast recent reports in immunogenic cancer models describing a role for T cells in the anti-tumor effects of ablative RT (18,28). These distinct outcomes observed with ablative RT across immunogenic and poorly immunogenic models suggest a role for baseline tumor immunogenicity in defining rational therapeutic combinations with RT. As the PDAC tumor microenvironment commonly excludes effector T cells, therapeutic strategies that combine ablative RT with T cell based therapies such as immune checkpoint blockade may be unlikely to be effective without first restoring productive T cell immunosurveillance, such as with vaccines (39). Our findings in a poorly immunogenic model of PDAC demonstrate that ablative RT alone is insufficient to overcome T cell exclusion and, thus, is unlikely to potentiate the activity of immunotherapies that rely on a pre-existing T cell response.
Inflammatory monocytes and the CCL2-CCR2 axis are commonly associated with a poor prognosis in several malignancies, including PDAC, hepatocellular carcinoma and breast cancer (30,32,33). Our findings suggest that RT does not alter the phenotype of tumor-infiltrating monocytes/macrophages but rather enhances their recruitment to the tumor microenvironment. We performed this analysis on tumor-infiltrating monocytes one day after RT reasoning that an early time point would be necessary to capture changes in gene expression prior to their differentiation within the tumor microenvironment. Under basal conditions, inflammatory monocytes/macrophages appear to comprise a small subset of leukocytes in the PDAC microenvironment, but unlike granulocytes and tumor-associated macrophages, this subset expresses the chemokine receptor CCR2. Inflammatory monocytes recruited to the tumor microenvironment in a CCL2-CCR2 dependent manner can undergo rapid differentiation into tumor-associated macrophages and in doing so, lose Ly6C expression. This is consistent with inflammatory monocytes/macrophages as precursors of tumor-associated macrophages and implies that their recruitment to the tumor microenvironment in response to ablative RT may help reconstitute the myeloid compartment. However, the transient nature of Ly6C expression may lead to an underestimation of the magnitude of this recruitment when examined on a quantitative basis.
We have shown a therapeutic role for inhibiting monocyte infiltration into tumors after ablative RT. However, the robust innate immune response that is induced by RT within tumors also raises the possibility that this immune reaction may be redirected with anti-tumor activity. For example, IFN-γ released systemically in response to a CD40 agonist can redirect tumor-infiltrating monocytes with anti-fibrotic and anti-tumor properties (19,24). Thus, we propose that combining RT with immune adjuvants may be one way to harness the inflammatory response invoked by ablative RT.
The myeloid response to cancer is inherently pliable with its phenotype dependent on local signals received within the tumor microenvironment. Our findings show that this myeloid response is a key determinant of tumor cell survival and resilience to RT. In our study, we demonstrate that modulating ablative RT-induced inflammation using CCL2 neutralizing antibodies can enhance the efficacy of RT. These findings support targeting the CCL2-CCR2 axis in combination with ablative RT to improve local disease control in patients with locally advanced PDAC. This translational approach may have particular promise in patients with genetic signatures that suggest a more locally invasive disease phenotype (40).
Our study was mainly conducted in a subcutaneous model of PDAC using KPC-derived cell lines. While there may differences in the efficacy of ablative RT in PDAC depending on the anatomical location of the treated tumor, we have found that ablative RT can induce inflammatory monocyte recruitment into PDAC tumors irrespective of whether they were implanted subcutaneously or orthotopically. Nonetheless, it is possible that PDAC tumors arising spontaneously may establish a microenvironment that is distinct from tumors implanted subcutaneously or orthotopically. Our study does not address this possibility given current technical limitations in delivering focal image-guided RT to abdominal tumors in mice. However, this is not a limitation to treating patients with locally invasive pancreatic tumors where conformal techniques for delivering ablative doses of RT are well-established.
Multiple CCL2 and CCR2 antagonists have been developed and their clinical investigation is ongoing (33,41–43). However, efforts to combine these agents with radiotherapy have not yet been explored. In contrast, agents targeting the CCL2/CCR2 axis in PDAC are actively under investigation in combination with cytotoxic chemotherapy and early results appear promising (44). The efficacy of chemotherapy in PDAC, though, is limited by the dense fibrotic reaction that surrounds PDAC, whereas ablative doses of radiotherapy can be effectively delivered to produce potent cell injury that is associated with a robust inflammatory response. Thus, combining CCL2/CCR2 antagonists with ablative RT may be particularly effective at enhancing the efficacy of RT and warrants clinical investigation based on our findings.
In summary, CCL2 plays an important role in the myeloid response to ablative RT in PDAC, a tumor that is inherently poorly immunogenic. Our data demonstrate that ablative RT-induces tumor-derived CCL2 expression which drives recruitment of inflammatory monocytes and macrophages into the tumor microenvironment to promote resistance to radiotherapy (Figure 6). We propose that inhibition of inflammatory monocyte recruitment, by antagonizing the CCL2-CCR2 chemokine axis in combination with ablative RT, is a novel therapeutic approach for improving disease control in PDAC, where local tumor growth is responsible for significant morbidity and mortality.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Pancreatic ductal adenocarcinoma has demonstrated substantial resistance to radiotherapy (RT). Here, we report that pancreatic ductal adenocarcinoma responds to RT by releasing inflammatory molecules, including the chemokine CCL2. By recruiting inflammatory monocytes/macrophages to the tumor microenvironment to promote tumor proliferation and vascularization, tumor-derived CCL2 inhibited the efficacy of ablative RT in a mouse model of pancreatic ductal adenocarcinoma. In contrast, selective blockade of CCL2 using neutralizing antibodies blocked monocyte/macrophage recruitment to tumors and, in combination with RT, produced anti-tumor activity with enhanced survival. As primary tumor growth in pancreatic ductal adenocarcinoma is responsible for up to 30% of patient mortality, our findings highlight a potential role for targeting the inflammatory response to RT for improving local control and patient outcomes.
Acknowledgments
Financial support: This work was supported by a Conquer Cancer Foundation of ASCO Young Investigator Award (A. Kalbasi); National Institutes of Health grants K08 CA138907 (G. L. Beatty)., F30 CA196106 (J.W. Lee), and F30 CA196124 (M. Liu); Abramson Cancer Center-Radiation Oncology Joint Pilot Award (A. Kalbasi, E. Ben-Josef and G. L. Beatty); and Damon Runyon Cancer Research Foundation for which G. L. Beatty. is Nadia’s Gift Foundation Innovator of the Damon Runyon-Rachleff Innovation Award (DRR-15-12).
We thank Kristen B. Long and Ioannis I. Verginadis for technical assistance and helpful discussions; W. Tim Jenkins and Cameron J. Koch for assistance with the Small Animal Radiation Research Platform; and Adam Bedenbaugh for assistance with frozen sectioning of tumors.
Footnotes
Conflict of interest statement: The authors have no competing financial interests.
References
- 1.Ryan D, Hong T, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–49. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: Am. Cancer Soc; 2016. [Google Scholar]
- 3.Iacobuzio-Donahue Ca, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–6. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 5.Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006:CD004200. doi: 10.1002/14651858.CD004200.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CHJ, Schwartz MP, Vleggaar FP, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. American Society for Gastrointestinal Endoscopy. 2010;71:490–9. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Fernandez-del Castillo C, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–6. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JD, Adusumilli S, Griffith KA, Ray ME, Zalupski MM, Lawrence TS, et al. Full-Dose Gemcitabine and Concurrent Radiotherapy for Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–8. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 9.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–12. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huguet F, Hammel P, Vernerey D, Goldstein D, Van Laethem JL, Glimelius B, et al. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. J Clin Oncol. 2014;(suppl 5s) abstr 4001. [Google Scholar]
- 11.Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–21. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Schellenberg D, Goodman Ka, Lee F, Chang S, Kuo T, Ford JM, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–86. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Chang DT, Schellenberg D, Shen J, Kim J, Goodman Ka, Fisher Ga, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–72. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 14.Goodman Ka, Wiegner Ea, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–93. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Mahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735–42. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 16.Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–37. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz ER, Wu Aa, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–31. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, et al. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6Clow F4/80+ Extratumoral Macrophages. Gastroenterology. 2015;149:201–10. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Quail D, Joyce J. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. IFN-gamma and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 2016;6:400–13. doi: 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3 - new capabilities and interfaces. Nucleic Acids Research. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–91. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells_: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson Da, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 30.Sanford DE, Belt Ba, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–7. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 32.Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2015 doi: 10.1136/gutjnl-2015-310514. in press. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DL, Bankson JA, Lemos R, Lai SY, Arun K, He Y, et al. Radiosensitization and Stromal Imaging Response Correlates for the HIF-1 Inhibitor PX-478 Given with or without Chemotherapy in Pancreatic Cancer. Mol Cancer Ther. 2011;9:2057–67. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011;71:3453–8. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meirovitz A, Hermano E, Lerner I, Zcharia E, Pisano C, Peretz T, et al. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res. 2011;71:2772–80. doi: 10.1158/0008-5472.CAN-10-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, Bommer GT, et al. GSK3β and β-Catenin Modulate Radiation Cytotoxicity in Pancreatic Cancer. Neoplasia. 2010;12:357–65. doi: 10.1593/neo.92112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green CE, Liu T, Montel V, Hsiao G, Lester RD, Subramaniam S, et al. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One. 2009;4:e6713. doi: 10.1371/journal.pone.0006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–33. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: Correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–43. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–8. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol. 2011;107:906–11. doi: 10.1016/j.amjcard.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 43.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3:687–96. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 44.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. The Lancet. 2016;17:651–62. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.