Abstract
The precise cellular function of Arl1 and its effectors, the GRIP domain Golgins, is not resolved, despite our recent understanding that Arl1 regulates the membrane recruitment of these Golgins. In this report, we describe our functional study of Golgin-97. Using a Shiga toxin B fragment (STxB)-based in vitro transport assay, we demonstrated that Golgin-97 plays a role in transport from the endosome to the trans-Golgi network (TGN). The recombinant GRIP domain of Golgin-97 as well as antibodies against Golgin-97 inhibited the transport of STxB in vitro. Membrane-associated Golgin-97, but not its cytosolic pool, was required in the in vitro transport assay. The kinetic characterization of inhibition by anti-Golgin-97 antibody in comparison with anti-Syntaxin 16 antibody established that Golgin-97 acts before Syntaxin 16 in endosome-to-TGN transport. Knock down of Golgin-97 or Arl1 by their respective small interference RNAs (siRNAs) also significantly inhibited the transport of STxB to the Golgi in vivo. In siRNA-treated cells with reduced levels of Arl1, internalized STxB was instead distributed peripherally. Microinjection of Golgin-97 antibody led to the fragmentation of Golgi apparatus and the arrested transport to the Golgi of internalized Cholera toxin B fragment. We suggest that Golgin-97 may function as a tethering molecule in endosome-to-TGN retrograde traffic.
INTRODUCTION
In eukaryotic cells, different types of cargo (solutes, lipids, and membrane proteins, including receptors and their ligands) internalized from the plasma membrane follow several routes upon reaching the early/sorting endosome. They could recycle back to the plasma membrane directly via early/sorting endosome or indirectly via the recycling endosome, or travel further to the lysosome via the late endosome. Recently, there was evidence suggesting the existence of two retrograde transport pathways from endosomes to the trans-Golgi network (TGN) (Ghosh et al., 1998; Mallard et al., 1998; Mallet and Maxfield, 1999). One pathway, used by furin (Mallet and Maxfield, 1999) and mannose-6-phosphate receptor (M6PR) (Diaz and Pfeffer, 1998; Sincock et al., 2003), is from the late endosome to the TGN. The other one, used by TGN38 (Ghosh et al., 1998), Shiga Toxin B fragment (STxB) (Mallard et al., 1998) and likely GLUT4 (Shewan et al., 2003), proceeds via the early endosome (EE) and/or the recycling endosome (RE), without passing through late endosomes. In addition, the large cation-independent M6PR and the small cation-dependent MPR46 are recently shown to also use this EE/RE–TGN pathway, in addition to its well characterized late endosome–TGN route (Medigeshi and Schu, 2003; Lin et al., 2004). These endosome–TGN pathways could be used by other proteins such as P-selectin (Straley and Green, 2000), membrane-type matrix metalloproteinases (Kang et al., 2002; Zucker et al., 2002; Remacle et al., 2003; Wang et al., 2004a, b), copper transporters (Petris and Mercer, 1999; Petris et al., 2002), and VAMP4, a TGN SNARE (unpublished observations). In addition, endosome–TGN transport was exploited by HIV-1 Nef protein to down-regulate cell surface levels of class I major histocompatibility complex (Piguet et al., 2000; Blagoveshchenskaya et al., 2002; Larsen et al., 2004).
Vesicle-mediated transport involves three major steps. The first step, the biogenesis of a vesicle, involves membrane budding and cargo selection that is executed by the coat proteins, such as COPI, COPII, and AP-1 clathrin coats (Scales et al., 2000; Antonny and Schekman, 2001; Boehm and Bonifacino, 2001). In the second step, the vesicles move along the cytoskeleton and anchor to the membrane of a target compartment by its interacting with tethering molecules (or complexes) (Pfeffer, 1999). Rab small GTPases, and their effectors are reported to regulate both processes (Zerial and McBride, 2001). Finally, the vesicle docking and fusion with the membrane of an acceptor compartment is mediated by SNAREs and Sec1/Munc18 proteins (Chen and Scheller, 2001). Some molecular machineries in EE/RE–TGN transport are being revealed through the use of a sulfation based in vitro transport assay, which monitors the sulfation of recombinant STxB or MPR46 as an indication of trafficking to the TGN (Mallard et al., 2002; Mallard and Johannes, 2003; Medigeshi and Schu, 2003). Similar to biosynthetic/secretory traffic, it had been reported that EE/RE–TGN transport may require AP-1, AP-3, Rab proteins (including Rab 6A′ and Rab11), Rab effectors (Rab6IP2), and SNAREs, including VAMP3, VAMP4, Syntaxin 6, Syntaxin 16, and Vti1a (Mallard et al., 1998; Wilcke et al., 2000; Monier et al., 2002; Medigeshi and Schu, 2003). More players involved in this retrograde transport are expected, and among them, the tethering molecules (or complexes) are still elusive. We present data here suggesting that Golgin-97 is likely candidate for a tethering protein functioning in this pathway.
Golgins are Golgi-localized proteins with extensive coiled-coil structure throughout the entire polypeptide, and they include Golgin-45, Golgin-67, Golgin-84, GM130/Golgin-95, Golgin-97, Golgin-160/GCP170, Golgin-245/p230/tGolgin-1, GCC88, GCC185, p115, Giantin, GMAP-210, and CASP in mammalian cells; and Uso1p (similar to p115), Imh1p, Coy1p (similar to CASP), and Grp1p (similar to Golgin-160/GCP170) in yeast (Barr and Short, 2003; Gillingham and Munro, 2003). Most Golgins are autoantigens and were cloned by screening expression libraries with their autoantibodies, such as Golgin-160 (Fritzler et al., 1993), Giantin (Seelig et al., 1994), GMAP-210 (Rios et al., 1994), Golgin-245/p230 (Fritzler et al., 1995), Golgin-97 (Griffith et al., 1997), and Golgin-67 (Eystathioy et al., 2000). Among them, three Golgins, namely, GM130, Giantin, and p115, are the most-well studied. GM130 and p115 form part of the Golgi matrix, which maintains the cisternal stacking architecture of the Golgi apparatus (Nakamura et al., 1995; Seemann et al., 2000). GRASP-65 interacts with GM130 and also functions in the stacking of the Golgi cisternae (Barr et al., 1997). In addition, GM130, p115, and Giantin are tethering molecules. The C-terminal part of p115 can interact with both GM130 and Giantin. p115 is recruited to COPII vesicles by Rab1-GTP (Allan et al., 2000) or on the COPI retrograde vesicles by its interaction with Giantin (Sonnichsen et al., 1998), whereas GM130 is restricted to cis-Golgi membrane (Nakamura et al., 1995). The interaction between p115 and GM130 tethers the incoming COPI or COPII vesicles with the cis-Golgi membrane and leads to the docking and subsequent membrane fusion mediated by SNAREs (Shorter et al., 2002). The functions of other Golgins are still unclear, but the presence of extensive coiled-coil regions suggests that they could also adopt long rod structures and may likely serve as tethering molecules or matrix proteins for the cisternal architecture of Golgi apparatus.
There are four mammalian Golgins, Golgin-97, Golgin-245, GCC88, and GCC185, and one yeast Golgin, Imh1p, forming a subgroup characterized by the presence of a conserved GRIP domain at their extreme C termini (Barr, 1999; Kjer-Nielsen et al., 1999a; Munro and Nichols, 1999; Luke et al., 2003). The GRIP domains of these Golgins are necessary and sufficient for their targeting to the TGN (Barr, 1999; Kjer-Nielsen et al., 1999a; Munro and Nichols, 1999). The functions of the GRIP-domain containing Golgins are still unclear except that Imh1p (Sys3p) was suggested to function in vesicular transport between endosomes and the late Golgi in yeast (Tsukada et al., 1999). Recently, we and others showed that GRIP Golgins are effectors of small GTPase Arl1 as active Arl1 can interact with the GRIP domain and recruit these Golgins to the Golgi membrane (Lu and Hong, 2003; Panic et al., 2003b; Setty et al., 2003). Arl1 is a Golgi-localized member of the ARF/Arl family of small GTPases (Lowe et al., 1996) and is implicated in the regulation of Golgi structure and function (Lu et al., 2001), but its exact cellular function is still obscure. In this study, we tested the role of Golgin-97 and Arl1 in endosome–TGN transport assay utilizing recombinant STxB, which has artificially incorporated sulfation sites. The results demonstrate that both Arl1 and its effector Golgin-97 are required in EE/RE–TGN transport. The in vivo function of Golgin-97 and Arl1 was similarly established by small interference (siRNA)-mediated knock down and microinjection approaches. Our results thus represent the first direct evidence for a possible function of Golgin-97 as a tethering molecule on TGN for retrograde traffic from the early and/or recycling endosomes and highlight that the Arl1-GRIP Golgins pathway could be a key regulatory process for endosome–TGN traffic.
MATERIALS AND METHODS
Antibodies and Reagents
Alexa Fluor 555-conjugated Cholera toxin B fragment (CTxB) and monoclonal antibody (mAb) CDF4 against human Golgin-97 were from Molecular Probes (Eugene, OR). mAb against β-tubulin and control rabbit IgG for transport assay and immunoprecipitation experiments were obtained from Sigma-Aldrich (St. Louis, MO). mAb against STxB, 13C4, was from American Type Culture Collection (Manassas, VA). mAbs against γ-adaptin, GM130 and Golgin-245, were from BD Biosciences (San Jose, CA). Anti-Myc tag polyclonal antibody was from Upstate Biotechnology (Charlottesville, VA). Sheep polyclonal antibody against human TGN46 was purchased from Serotec (Oxford, United Kingdom). Rabbit polyclonal antibody against β-1,4 galacto-syltransferase (GT) was described previously (Subramaniam et al., 1992). Rabbit polyclonal anti rat Arl1 (E6P3), which also recognizes human Arl1, was described previously (Lowe et al., 1996). Rabbit polyclonal antibody against Syntaxin 16 was described previously (Mallard et al., 2002). Preimmune rabbit IgG for microinjection was purified from preimmune rabbit serum using protein A-Sepharose (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom).
Preparation of Recombinant STxB and In Vitro Endosomes to TGN Transport Assay
A plasmid expressing the modified Shiga toxin B fragment, STxB-Sulf2, was generously provided by Dr. Ludger Johannes (Institute Curie, Paris, France) and transformed into Escherichia coli DH5α cells. STxB fragments were extracted from bacterial periplasm and purified as described previously (Johannes et al., 1997).
STxB in vitro EE/RE–TGN transport (Mallard et al., 2002) was modified as follows. The characterization of this modified method will be published elsewhere (unpublished data). HeLa cells grown on a 10-cm tissue culture dish were starved for at least 1 h in minimum essential medium without sulfate (Sigma-Aldrich) supplemented with 5% dialyzed fetal bovine serum, 1 mMCa2+, and 10 mM HEPES, pH 7.3. The cells were then incubated in fresh medium containing 1 μM STxB for 1 h at 18°C followed by 30-min chase at 18°C in fresh medium without STxB, allowing its accumulation in the EE/RE. Cells were then incubated for 10 min with 20 μg/ml Streptolysin O (a generous gift from Dr. S. Bhakdi (Johannes-Gutenberg-Universität, Mainz, Germany) in permeabilization buffer [25 mM HEPES, pH 7.3, 125 mM KOAc, 2.5 mM Mg(OAc)2 and 1 mM dithiothreitol (DTT)] on ice. After removing unbound Streptolysin O, cells were perforated at 18°C for 30 min in permeabilization buffer. The cells were then scrapped, pelleted at 1000 × g, and resuspended in 150 μl of buffer containing 25 mM HEPES, pH 7.3, 250 mM sucrose, and 1 mM EDTA. The cells were either used immediately or stored at -80°C until use.
The EE/RE–TGN transport was reconstituted in a 1.5-ml microcentrifuge tube. A standard transport assay contains 10 μl of semiintact cells, 5 μl of HeLa cell cytosol (or rat liver cytosol) at >10 mg/ml in 25/125 buffer (25 mM HEPES, pH 7.2, and 125 mM KOAc), 6 μl of ATP-mix (10 mM ATP, pH 7.0, 150 mM creatine phosphate [Sigma-Aldrich], 210 U/ml creatine phosphokinase [Sigma-Aldrich], and 20 mM MgCl2), 4 μl of reaction buffer [250 mM HEPES, pH 7.3, 250 mM KCl, and 15 mM Mg(OAc)2], 20 μl of 25/125 buffer (used as control) or 25/125 buffer containing the reagents to be tested, 1 mM DTT (final concentration), 0.2 mM GTP (final concentration), 1 mCi/ml Na35SO4 (final concentration) (Amersham Biosciences UK), and H2O to a final volume of 75 μl. After incubation on ice for 1 h, the tubes were transferred to a 37°C water bath to allow the transport proceed for 90 min. Membrane pellets were recovered after centrifugation and were solubilized in SDS sample buffer. Proteins were separated on 15% modified Laemmli peptide separation gels and blotted onto nitrocellulose filters. The 35S sulfated STxB was quantified using a Molecular Imager FX (Bio-Rad, Hercules, CA). During experiments, a negative control, which did not contain cytosol, was always included and the background STxB transport in the negative controls was deducted from each experiment. The STxB transport without cytosol was thus defined as 0%. A positive control also was designed for each experiment, and its relative percentage of STxB transport was set as 100%.
Generation of Golgin-97 Rabbit Polyclonal Antibody
Part of the central coiled-coil region of Golgin-97 (Golgin-97CC, residue 334–667) was cloned into pGEX-4T3 GST fusion vector (Amersham Biosciences UK) using standard polymerase chain reaction (PCR) cloning method. Recombinant GST fusion proteins were produced in E. coli DH5α as described previously (Lowe et al., 1996). Purified glutathione S-transferase (GST)-Golgin-97CC fusion protein (400 μg) was diluted in 1 ml of phosphate-buffered saline (PBS). The protein in PBS was emulsified in Freund's adjuvant (complete for the first time injection and incomplete for the subsequent booster injections) (Pierce Chemical, Rockford, IL). The emulsified antigen was injected subcutaneously into female New Zealand White rabbit (Sem-bawang Laboratory Animals Center, Singapore) once per 10 d. Rabbit serum was collected after fifth injection and subsequently after each boost injection.
To purify the antibody, GST or GST-Golgin-97CC was cross-linked co-valently to glutathione Sepharose 4B beads (Amersham Biosciences UK) by using dimethyl pimelidate (Sigma-Aldrich). Serum was first incubated with GST beads to preabsorb antibody against GST. Then, the unbound serum was subsequently incubated with GST-Golgin-97CC cross-linked glutathione Sepharose beads. After extensive washing, the bound Golgin-97 polyclonal antibody was eluted by 50 mM glycine, pH 2.8, dialyzed in PBS, and concentrated.
Golgin-97 Depletion from HeLa Cytosol
Golgin-97 polyclonal antibody (pAb) or control rabbit IgG was immobilized on the Seize X Protein A Immunoprecipitation beads (2 mg of antibody per 1 ml of equivalent volume of beads) (Pierce Chemical). HeLa cell cytosol (8 mg/ml) was incubated with the immobilized antibody beads (400 μl of cytosol per 200 μl of beads) at 4°C for 1.5 h. The nonbinding fractions representing the Golgin-97 pAb and control IgG-treated cytosols were recovered after spin filtration. The efficiency of the depletion was subsequently examined by Western blot analysis.
Temporal Sensitivity of Golgin-97 and Syntaxin 16 pAbs during In Vitro Transport Assay
Standard transport reactions at 37°C were allowed to proceed for 10, 15, 20, 30, 40, 50, 70, and 90 min, respectively, and stopped by incubation on ice. Either 133 μg/ml anti-Golgin-97 or 20 μg/ml final concentration of anti-Syntaxin 16 pAb was added at these time points. The mixture was left on ice for 50 min. Transport reactions were resumed at 37°C and allowed to proceed until a total transport time of 90 min. The 35S sulfation of STxB was subsequently visualized by autoradiography.
Cell Culture, Transfection, Immunofluorescence Microscopy, and Morphology Statistics
The coding region of Golgin-97CC was PCR cloned into pDMyc-neo to produce an N-terminal double Myc-tagged fusion protein. Golgin-97 GRIP domain and GRIP/Y697A in pGADT7 (Lu and Hong, 2003) were cut by EcoRI/BamHI and ligated to pEGFP-C2 vector (BD Biosciences Clontech, Palo Alto, CA) to make EGFP-GRIP and EGFP-GRIP/Y697A. Arl1-Q71L in pSTAR was described previously (Lu et al., 2001). HeLa cells were cultured in RPMI medium supplemented with 10% fetal bovine serum at 37°C. Transient transfection of Golgin-97CC in pDMyc-neo, Golgin-97 GRIP in pEGFP-C2 (EGFP-GRIP and EGFP-GRIP/Y697A), and Arl1-Q71L pSTAR were conducted using Effectene transfection reagent (QIAGEN, Hilden, Germany) according to manufacturer's protocol. Indirect immunofluorescent microscopy was performed as described previously (Lu et al., 2001). Images were taken using Bio-Rad 1024 (Bio-Rad) or Zeiss LSM510 (Carl Zeiss, Jena, Germany) confocal microscopes. In morphology statistical analysis of Figure 6B, ∼100 cells highly expressing the following chimera were counted for either perinuclear or peripheral staining of STxB: EGFP-GRIP, EGFP-GRIP/Y697A, and Myc-Golgin-97CC. Around 100 nontransfected cells on EGFP-GRIP transfected coverslip were counted as negative controls. For analysis of Figure 9B, those cells with both injection and CTxB internalization were examined for CTxB distribution from confocal images. About 50 cells were counted from cell microinjected with antibodies against Golgin-97 or control IgG.
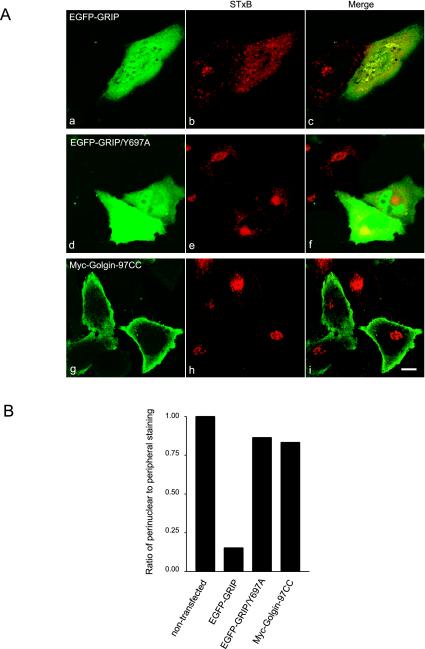
Figure 6.
Overexpression of EGFP-GRIP domain prevented the perinuclear accumulation of STxB in HeLa cells. (A) HeLa cells were transiently transfected to overexpress EGFP-GRIP (a–c), EGFP-GRIP/Y697A (d–f), or Myc-Golgin-97CC (g–i). Cells were subsequently allowed to continuously internalize STxB for 3 h and processed to reveal STxB by IF (b, e, and h). Bars, 10 μm. (B) Statistical analysis of the morphology relating the ratio of cells with perinuclear to cells with only peripheral distribution of STxB under different transfection conditions. The ratio in nontransfected cells is defined as 100%. In each case, ∼100 cells, which contain both the exogenously over expressed protein and internalized STxB, were randomly chosen for scoring the distribution of STxB.
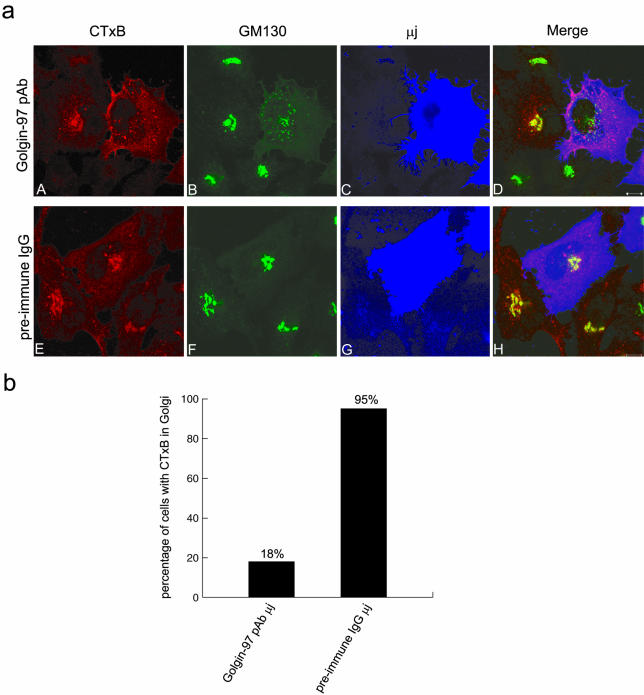
Figure 9.
The retrograde trafficking of CTxB was arrested before the Golgi when Golgin-97's functions were interfered by its microinjected pAb. (a) HeLa cells were microinjected with Golgin-97 pAb (A–D) or preimmune rabbit IgG (E–H) and then allowed to internalize Alexa Fluor 555-conjugated CTxB for 30 min and followed by 60-min chase. Cells were subsequently triple labeled to reveal CTxB (A and E), GM130 (B and F), and injected rabbit IgG (C and G). In D and H, the intensity of blue color was reduced fivefold for clearer observation of red and green signals. Bars, 10 μm. (b) For those injected cells, which also internalized CTxB, the distribution of CTxB was analyzed and the percentage of cells with CTxB in the Golgi region was shown in the bar chart. About 50 cells microinjected with Golgin-97 pAb or control rabbit IgG were examined and scored.
Knock Down of Endogenous Arl1 or Golgin-97 by siRNA
Arl1-specific and control siRNA duplexes were the same as described previously (Lu and Hong, 2003). The human Golgin-97–specific duplex siRNA oligonucleotides (siRNA1: 5′-AAG AUC ACA GCC CUG GAA CAA-3′, and siRNA2: 5′-AAG UGC UUC UCC AGA AAG AGC-3′) were synthesized at Dharmacon Research (Lafayette, CO). HeLa cells were transfected with siRNA by using Oligofectamine transfection reagent (Invitrogen, Carlsbad, CA) according to protocol provided by Dharmacon Research. Cells were processed for immunofluorescence or immunoblotting after 48 h of incubation. In some experiments, siRNA-treated HeLa cells were allowed to continuously internalize STxB (at 1 μM final concentration) or CTxB-Alexa Fluor 555 (at 5 μg/ml final concentration) for 3 h before fixation.
STxB In Vivo Transport Assay by Using siRNA-treated HeLa Cells
After 48 h of incubation, siRNA-treated HeLa cells were subjected to in vivo STxB transport assay. Cells were first washed twice and then subsequently incubated at 37°C for 1 h with sulfate-free medium (sulfate-free minimal essential medium [Sigma-Aldrich] supplemented with 5% dialyzed fetal bovine serum [Invitrogen]). The cells were then incubated with sulfate-free medium containing 1 μM STxB, 0.5 mCi/ml Na35SO4 (Amersham Biosciences UK) for 90 min at 37°C. After washing three times by cold PBS (containing 1 mM Mg2+ and 1 mM Ca2+), the cells were solubilized by 2× SDS sample buffer and the sulfated STxB was resolved by 15% SDS-PAGE followed by visualization via autoradiography. The levels of endogenous Arl1, Golgin-97, and β-tubulin were assessed by Western blotting.
Microinjection of Golgin-97 pAb into HeLa Cells
Golgin-97 pAb and preimmune IgG were dialyzed in PBS and concentrated to ∼8 mg/ml. HeLa cells grown on the coverslips were microinjected with the antibody by using Eppendorf microinjection system (Hamburg, Germany). Injected HeLa cells were incubated in normal culture medium at 37°C for 150 min before fixation and immunofluorescent labeling. For CTxB morphological transport assay, injected cells were first incubated in normal condition for 50 min before the addition of 5 μg/ml Alexa Fluor 555-conjugated CTxB and subsequent internalization for 30 min. After washing away noninternalized CTxB, cells were incubated in normal condition and the internalized CTxB was chased for 60 min before immunofluorescent labeling.
RESULTS
Recombinant GRIP Domain of Golgin-97 Inhibited In Vitro Transport of STxB from the EE/RE to TGN
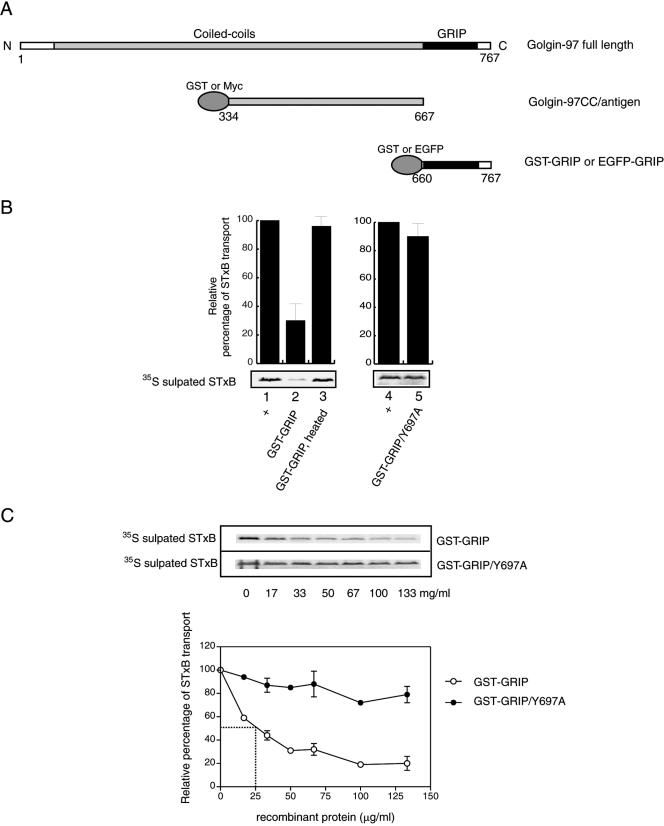
Due to its TGN localization and general structural similarity to other Golgins implicated in tethering processes, Golgin-97 was examined for its involvement in EE/RE–TGN transport by using an established system measuring the transport of STxB (Mallard et al., 2002). The GRIP domain of Golgin-97 is essential and sufficient for its interaction with Arl1-GTP (Lu and Hong, 2003; Panic et al., 2003b; Setty et al., 2003) and subsequent targeting to the Golgi apparatus. Arising from the importance of the GRIP domain, recombinant Golgin-97 GRIP domain GST fusion protein (GST-GRIP; Figure 1A) was first tested in the STxB transport assay. Briefly, sulfate-starved HeLa cells were incubated with STxB at 18°C to continuously accumulate STxB at EE/RE before the plasma membrane of cells was selectively perforated by streptolysin O. The resulting semiintact cells were then incubated at 37°C for 90 min with the transport mix containing: HeLa cytosol, ATP regenerating system, 35S-sulfate, and recombinant GST-GRIP or control proteins. The arrival of recombinant STxB at the TGN was monitored by a TGN-specific tyrosine sulfation reaction (Niehrs and Huttner, 1990). The 35S-sulfated STxB was then resolved through SDS-PAGE and autoradiography (Figure 1B and C). The extent of transport in a complete reaction served as the positive control and was set as 100% (Figure 1B, lane 1), whereas transport occurred in the absence of cytosol was set as 0%. When 133 μg/ml GST-GRIP was added to the assay, the transport of STxB to the TGN was reduced to 30% as shown in lane 2 of Figure 1B. The transport was restored to almost complete level (96%) (lane 3) when the same amount of GST-GRIP was heat-denatured before addition, suggesting that the inhibition requires the intact conformation of the GRIP domain. When the conserved residue Y697, which is essential for interaction with Arl1 and for Golgi targeting (Barr, 1999; Kjer-Nielsen et al., 1999a; Munro and Nichols, 1999; Lu and Hong, 2003; Panic et al., 2003b), was mutated to Ala, the resultant fusion protein, GST-GRIP/Y697A (lane 5), exhibited little inhibition toward STxB transport relative to the control (lane 4). Furthermore, with increasing amounts of GST-GRIP added to the assay, the transport of STxB was correspondingly reduced to a plateau of ∼20% (Figure 1C), indicating that the inhibition is dose dependent. The half-maximal inhibition was achieved at a concentration of 25 μg/ml GST-GRIP. In contrast, with increasing amount of GST-GRIP/Y697A introduced, the transport of STxB was essentially not affected, implying that a functional GRIP is required for the inhibition.
Figure 1.
The recombinant GRIP domain of Golgin-97 inhibited in vitro EE/RE–TGN transport. (A) Schematic representation of the GRIP domain and the central coiled-coil region (Golgin-97CC) used in this study. (B) Relative efficiencies of STxB transport to the TGN as measured by the extents of its sulfation under various conditions as indicated. Lanes 1 and 4 are positive controls, and the transports were set as 100%. In lane 2, the assay contains 133 μg/ml GST-GRIP. In lane 3, GST-GRIP (133 μg/ml) was preboiled before adding to the assay. In lane 5, the reaction includes 133 μg/ml GST-GRIP/Y697A mutant. Error bars represent the standard deviations of two independent experiments. Bottom, typical gel autographic images. (C) Inhibition of EE/RE-TGN transport by GST-GRIP is dose dependent with a half-maximal inhibition at concentration of 25 μg/ml. Top, typical autoradiographs of 35S-sulfated STxB. Bottom shows the correspondent quantification curves. Points with deviation error bars indicate that these experiments were independently repeated twice. Transport in the absence of the recombinant protein is defined as 100%.
The dose-dependent inhibition by the GRIP domain probably arises from the competitive replacement of endogenous GRIP Golgins interacting with a limited amount of cellular binding partners. One likely candidate is active Arl1, which has been found to interact with and recruit GRIP domain Golgins to the membrane (Lu and Hong, 2003; Panic et al., 2003b; Setty et al., 2003). Large amount of GST-GRIP may displace endogenous GRIP Golgins from the Golgi membrane. The loss of its inhibitory property by Y697A mutation in GRIP, which disrupts Arl1 and GRIP interaction (Lu and Hong, 2003; Panic et al., 2003b), is supportive to this interpretation. These results imply that Golgin-97 and/or other GRIP domain Golgins could participate in EE/RE–TGN transport.
Polyclonal Antibody against Golgin-97 Inhibited In Vitro EE/RE–TGN Transport
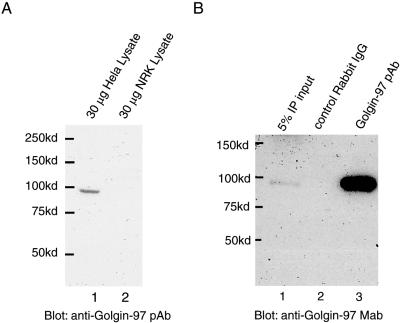
We raised rabbit polyclonal antibodies against human Golgin-97 by using part of its central coiled-coil region (Golgin-97CC; Figure 1A). The purified Golgin-97 polyclonal antibody (Golgin-97 pAb) detected a single endogenous protein of ∼97 kDa in HeLa but not in normal rat kidney (NRK) cell lysate (lane 1 and 2, respectively; Figure 2A), showing that the pAb is specific to human Golgin-97. From 293T cell lysate, this pAb, but not the control rabbit IgG, efficiently immunoprecipitated an endogenous protein of ∼97 kDa, which was detected by immunoblotting with a commercial Golgin-97 mAb (Figure 2B). Furthermore, indirect immuno-fluorescent staining of HeLa cells with the pAb gave a perinuclear signal, which was colocalized with labeling by the mAb against Golgin-97 or Golgin-245 (unpublished data). These experiments thus demonstrated that the generated pAb specifically recognizes endogenous human Golgin-97 and does not cross react with other human Golgins or Golgin-97 in other species.
Figure 2.
Generation and characterization of Golgin-97 pAb. (A) Golgin-97CC (Figure 1A) was used to raise rabbit antibodies and the purified Golgin-97 pAb recognized an endogenous protein of ∼97 kDa in human (lane 1) but not rat (lane 2) cell lysate. HeLa or NRK cell lysate (30 μg) was resolved by SDS-PAGE and analyzed by immunoblotting by using Golgin-97 pAb. (B) Golgin-97 pAb, but not the control rabbit IgG, efficiently immunoprecipitated (IP) endogenous human Golgin-97 from 293T cell lysate, which was detected by a commercial Golgin-97 mAb. Lane 1, 5% IP input; lane 2, IP using control rabbit IgG; lane 3, IP using Golgin-97 pAb.
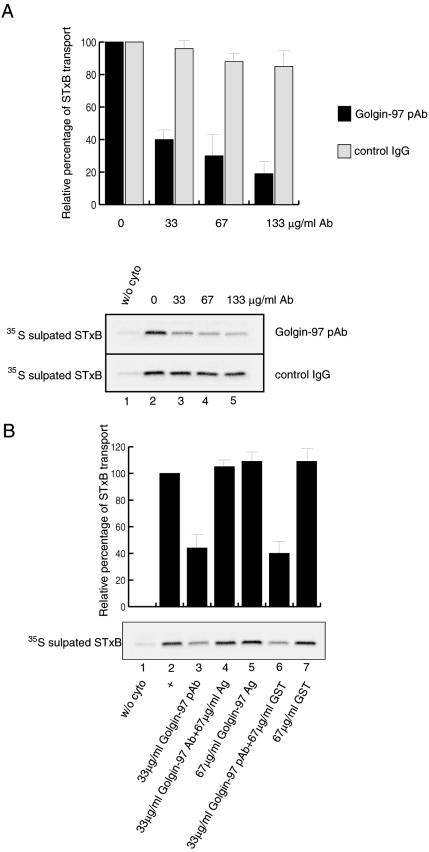
The Golgin-97 pAb was applied to the in vitro EE/RE–TGN transport assay and found to inhibit STxB transport in a dose-dependent manner relative to controls, in which the same amount of nonspecific rabbit IgG was used (Figure 3A). With a final concentration of 133 μg/ml Golgin-97 pAb in the assay, STxB transport was reduced to 19%. This degree of inhibition is similar to that exhibited by GST-GRIP, because both have a half-maximal inhibitory concentration at around 25 μg/ml. Considering the antibody has a much larger molecular weight, the pAb is probably a more potent inhibitor.
Figure 3.
Golgin-97 pAB inhibited in vitro EE/RE–TGN transport of STxB. Top, quantification of bottom panels, which are typical autoradiographs of 35S-sulfated STxB. (A) Golgin-97 pAb, but not control rabbit IgG, inhibited STxB transport in a dose-dependent manner. The STxB transport was reduced to 19% with the increasing amount of Golgin-97 pAb added to a concentration of 133 μg/ml (black bars), whereas the transport was still 85% when control rabbit IgG was increased to the same concentration (gray bars). Error bars represent the standard deviations of two independent experiments. Bottom, typical autoradiograph of 35S-sulfated STxB in the presence of increasing amount of Golgin-97 pAb or control rabbit IgG. Lane 1, transport assay in the absence of cytosol, serving as a negative control. The transport assays conducted in lanes 2–5 contained the indicated amount of Golgin-97 pAb or control rabbit IgG. (B) Inhibition of Golgin-97 pAb in STxB transport was specific as it could be relieved by preincubation with antigen. Lane 1, transport reaction in the absence of cytosol serving as a negative control. Lane 2, complete reaction, as positive control and the STxB transport was set as 100%. Lane 3, 33 μg/ml Golgin-97 pAb was added. Lane 4, 33 μg/ml Golgin-97 pAb was first neutralized by 67 μg/ml antigen (Ag, GST-Golgin-97CC) before addition to the assay. In lane 5, 67 μg/ml Ag was added to the reaction. Lane 6 contains 33 μg/ml Golgin-97 pAb preincubated with 67 μg/ml GST protein. In lane 7, 67 μg/ml GST protein was added to the reaction. Error bars represent the standard deviations of two independent experiments.
The specificity of inhibition by Golgin-97 pAb was revealed by antigen neutralization experiments as shown in Figure 3B. The 60% inhibition of the STxB transport by 33 μg/ml Golgin-97 pAb (Figure 3B, lane 3) could be relieved by preincubation of pAb with a noninhibitory amount (lane 5) of its antigen GST-Golgin-97CC (lane 4). This inhibition was not neutralized by preincubation with just GST protein (lane 6). Furthermore, the Fab fragment of Golgi-97 also exhibited inhibition in the transport assay (unpublished data). Together, these experiments corroborate and further support the above findings and suggest that Golgin-97 is important in EE/RE–TGN transport.
The Membrane Pool of Golgin-97 Is Responsible for In Vitro EE/RE–TGN Transport
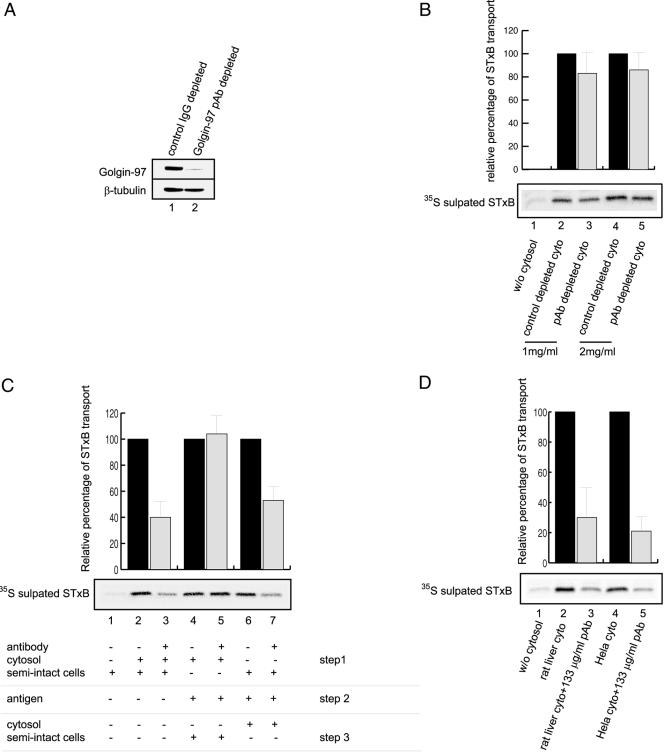
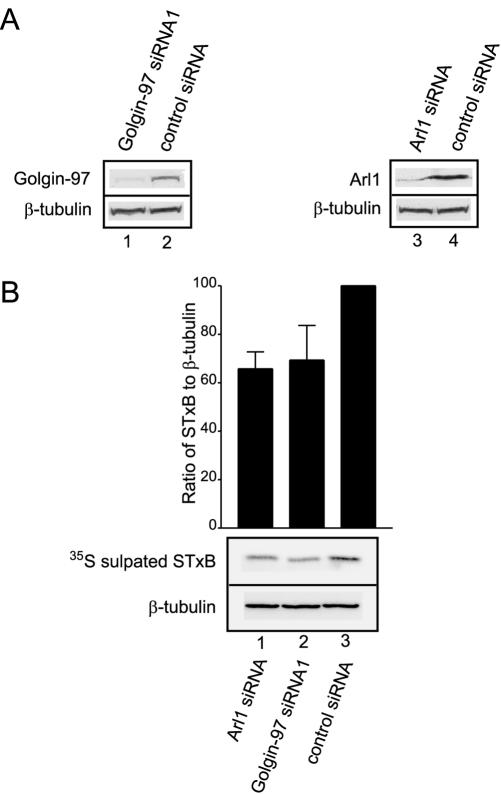
As a peripheral Golgi membrane protein, Golgin-97 has both cytosolic and membrane-associated pools. In HeLa cells, >80% of Golgin-97 is in the cytosol as assessed by subcellular fractionation (unpublished data). When the HeLa cytosol used in the STxB transport assay was incubated with Golgin-97 pAb and protein A-Sepharose, ∼90% of Golgin-97 was depleted (Figure 4A, lane 2) compared with the mock-depleted cytosol by using control rabbit IgG (Figure 4A, lane 1). Both Golgin-97–depleted and mock-depleted cytosols were tested for STxB transport in vitro (Figure 4B). It was found that transport events observed with Golgin-97–depleted cytosol were only slightly reduced (∼80–90%, lanes 3 and 5) compared with levels with equal amounts of mock-depleted cytosol (defined as 100%, lanes 2 and 4). This reduction was marginal compared with the inhibitory effects of GST-GRIP and Golgin-97 pAb on the transport. This prompted us to examine the in vivo effect of reducing Golgin-97 level. When Golgin-97 was reduced by 70% by using the siRNA knock-down approach (Figure 7A), in vivo transport of STxB to the TGN was reduced to 69% (Figure 7B), suggesting an essential role of Golgin-97 in vivo.
Figure 4.
The membrane pool of Golgin-97 was responsible for in vitro EE/RE-TGN transport of STxB. (A) HeLa cytosol used in the STxB transport assay were mock depleted by control rabbit IgG (lane 1) or Golgin-97 pAb (lane 2). Top, Golgin-97 levels in two treated cytosols. Bottom, equal loading of two cytosols as assessed by blotting with antibodies against β-tubulin. About 90% of Golgin-97 was depleted in Golgin-97 pAb-treated cytosol. In B–D, top shows the relative percentage of STxB transport in bar charts. A typical set of autoradiographs of 35S-sulfated STxB is shown in the bottom panels for each experiment. Black bars are positive controls for adjacent gray bars and their STxB transports are set as 100%. Error bars represent standard deviations of two independent experiments. (B) Transport of STxB was reduced only slightly using Golgin-97 depleted HeLa cytosol. Lane 1, reaction without cytosol. Lanes 2 and 4, assays with 1 and 2 mg/ml control depleted cytosol, respectively. Lanes 3 and 5, assays with 1 and 2 mg/ml Golgin-97 pAb-depleted cytosol, respectively. (C) Separate treatment of membrane and cytosolic pools of Golgin-97 affected STxB transport differently. Lane 1, reaction without cytosol, as negative control. Lane 2, complete reaction (cytosol and semiintact cells), as positive control, and the transport is set as 100%. Lane 3, Golgin-97 pAb was mixed with cytosol and semiintact cells. In lanes 4 and 5, the cytosol was incubated without (lane 4, control for lane 5) or with Golgin-97 pAb (lane 5) for 1 h on ice (step 1), the excess amount of Golgin-97 pAb was then neutralized by twice amount of antigen (step 2). The resulting cytosol in lane 5 supported the transport of STxB with efficiency similar to control in lane 4 after addition of semiintact cells (step 3). In lanes 6 and 7, semiintact cells (containing membrane pool of Golgin-97) were incubated without (lane 6, as positive control for lane 7) or with Golgin-97 pAb (lane 7) on ice for 1 h (step 1). After antigen neutralization (step 2), the resulting semiintact cells were mixed with cytosol to complete the reaction (step 3). In lane 7, the STxB transport was reduced to ∼50% compared with lane 6. (D) Transport of STxB in HeLa semiintact cells was equally inhibited by human Golgin-97 specific pAb in the presence of either rat liver (which provided rat Golgin-97 that is not reactive with the antibody) or HeLa cytosol. Lane 1, without cytosol, as negative control. Lane 2, rat liver cytosol as positive control for lane 3. Lane 3, rat liver cytosol with 133 μg/ml Golgin-97 pAb. Lane 4, HeLa cell cytosol as positive control for lane 5. Lane 6, HeLa cell cytosol with 133 μg/ml Golgin-97 pAb.
Figure 7.
Knockdown of Arl1 or Golgin-97 inhibited in vivo trafficking of STxB to the TGN. (A) HeLa cells were transfected with indicated siRNAs. After 48 h, the total cell lysate was subjected to immunoblotting analysis to detect Golgin-97 (lanes 1 and 2 in the top panel), Arl1 (lanes 3 and 4 of the top panel), and β-tubulin (bottom). (B) The relative transport of STxB to Golgi in siRNA-treated HeLa cells. siRNA-treated HeLa cells were allowed to internalize STxB for 90 min in the presence of 35S-sulfate. The 35S-sulfated STxB was revealed by autoradiograph (middle panel shows a typical result). The loading of each lane was normalized by immunoblotting with antibody against β-tubulin (bottom). For each lane, the relative ratio of sulfated STxB to β-tubulin densities was quantified as bar chart in the top panel. Error bars represent standard deviations of four independent experiments. Knock down of Golgin-97 or Arl1 significantly reduced the sulfation of STxB compared with controls (p <7E-5 and 0.006, respectively).
The exchange of Golgin-97 between the membrane and cytosolic pools could be so slow that the equilibrium and thus the reduction of Golgin-97 in membrane pool would be achieved only in long time (days) siRNA treatment, but not in short time (hours) incubation during in vitro transport assay. The limited transport effect obtained from Golgin-97–depleted cytosol could be explained by the possibility that the membrane-bound Golgin-97, but not its cytosolic pool, is the active participant in the in vitro EE/RE–TGN transport. This possibility seems likely as only the membrane pool of another Golgin, p115, is responsible for in vitro ER–Golgi transport and cytosol depletion of p115 only results in partial inhibition of transport (Allan et al., 2000).
To examine this possibility, we treated the cytosol and/or semiintact cells separately with the antibody against Golgin-97 and allowed the transport to finish in three steps. In step 1, either semiintact cells (Figure 4C, lanes 6 and 7) or HeLa cell cytosol (lanes 4 and 5), or both (lanes 2 and 3) were incubated with (lanes 3, 5, and 7) or without (lanes 2, 4, and 6) Golgin-97 pAb on ice for 1 h (step 1). In step 2, the excess amount of Golgin-97 pAb was neutralized with double amount of antigen (lanes 4–7) before the assay was completed with the supplementation of semiintact cells (lanes 4 and 5) or HeLa cytosol (lanes 6 and 7) (step 3). The transport of STxB in control assays was set as 100% (lanes 2, 4, and 6). When the cytosol alone was treated by antibody, there was little inhibition of STxB transport (compare lanes 5 and 4). In contrast, when the semiintact cells alone were treated with Golgin-97 pAb, the transport of STxB was reduced to ∼50% (compare lanes 7 and 6), similar to the 40% inhibition obtained when both semiintact cells and cytosol were treated (compare lanes 3–2). Unlike antibody neutralization experiment in Figure 3B, the antigen addition described (Figure 4C, lane 7) did not neutralize the inhibition of this antibody in EE/RE–TGN transport. In lane 4 of Figure 3B, the antibody was first incubated with twice amount of antigen before its contact with semiintact cells. The antigen-occupied antibody would not interact with membrane-bound Golgin-97. However, in lane 7 of Figure 4C the antibody was first allowed to bind membrane Golgin-97. The excess free antibody was subsequently neutralized by the antigen. We think that once bound to membrane Golgin-97, the antibody could not be displaced by its recombinant antigen under our experimental condition.
Because our Golgin-97 pAb reacts only with human Golgin-97 but not its homolog from rat, we have reconstituted in vitro transport assay by using rat liver cytosol in the presence of Golgin-97 pAb. Under such a setting, human Golgin-97 associated with semiintact HeLa cells (but not cytosolic rat Golgin-97) was recognized by the antibody. Transport of STxB under this condition was reduced to 30% by Golgin-97 pAb (Figure 4D, lane 3), suggesting that the majority of cytosolic rat Golgin-97 could not rescue the inhibitory effect of Golgin-97 antibody acted on membrane pool of human Golgin-97. As a control, when HeLa cytosol was used (Figure 4D, lane 5), transport was reduced to 25% by Golgin-97 pAb. This experiment suggests that neutralization of the membrane-bound Golgin-97 by the antibody is sufficient to execute an inhibition on the transport, although the majority of Golgin-97 in the assay (the cytosolic pool of rat Golgin-97) was not recognized by the antibody. These experiments, together, clearly established that the membrane pool of Golgin-97, rather than the cytosolic pool, is responsible for EE/RE–TGN transport.
Golgin-97 Is Required before the SNARE-mediated Docking/Fusion Step
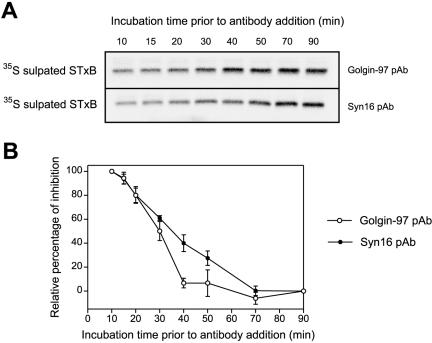
Syntaxin 16 is a key component of a SNARE complex involved in EE/RE-TGN transport (Mallard et al., 2002). We have examined the kinetics of inhibition exhibited by anti-Golgin-97 in relation to anti-Syntaxin 16 pAb to resolve the temporal requirement of these two molecules during EE/RE-TGN transport (Figure 5). The complete in vitro transport cocktail was assembled and the transport was allowed to proceed as per normal. The Golgin-97 pAb (Figure 5A, top) or Syntaxin 16 pAb (Figure 5A, bottom) was added at various time points, and the transport reactions were resumed for a total time of 90 min. Sulfation of STxB at the TGN for each assay is shown in Figure 5A, and the relative inhibition is quantified in Figure 5B. The 90-min lane in Figure 5A shows the complete reaction without the addition of antibodies and the inhibition was set as 0% and the 10 min lane was set as 100% inhibition for each time point (Figure 5B). At early time points (≤30 min), the transport of STxB was sensitive to both Golgin-97 and Syntaxin 16 pAbs. However, the inhibition by Golgin-97 pAb quickly declined when the prereaction time increased. After 40 min of prereaction, STxB trafficking to TGN became almost insensitive to Golgin-97 pAb as the relative inhibition curve reached its minimum platform; in contrast, the trafficking of STxB was still sensitive to Syntaxin 16 pAb up to the 70-min time point, when inhibition of transport was reduced to the minimum level.
Figure 5.
Golgin-97 was required before SNARE-mediated docking/fusion step in EE/RE–TGN transport in vitro. (A) Set of typical autoradiographs of 35S-sulfated STxB derived from the in vitro transport assays showed the temporal sensitivities to antibodies against Golgin-97 and Syntaxin 16. The complete transport assays were assembled and conducted for various time points as per normal. The Golgin-97 (top) or Syntaxin 16 (bottom) pAb was subsequently added at these time points, and the transport reactions were allowed to proceed in the presence of antibody for a total of 90 min. (B) Quantification of relative percentage of inhibition. The inhibition of STxB transport in which Golgin-97 and Syntaxin16 was added at 10-min time point is set as 100% and 90-min time point is defined as 0%. Error bars represent the standard deviations of two independent experiments.
Syntaxin 16 functions as part of the t-SNARE complex together with Syntaxin 6 and Vti1a in EE/RE–TGN transport (Mallard et al., 2002). The finding that Golgin-97 is likely required in a step earlier than Syntaxin 16 implicates Golgin-97 in tethering events. It suggests that one of Golgin-97's functions is to serves as a tethering molecule, similar to p115, GM130 and Giantin, to mediate long-range “landing” of transport vesicles and tether them to the target membranes to facilitate the subsequent docking and fusion mediated by SNAREs.
Overexpression of EGFP-Golgin-97 GRIP Prevented the Perinuclear TGN Accumulation of STxB in HeLa Cells
To test the in vivo effect of the Golgin-97 GRIP domain on retrograde trafficking of STxB to the TGN, EGFP-Golgin-97 GRIP domain (EGFP-GRIP; Figure 1A) was overexpressed in HeLa cells by transient transfection followed by 3 h of continuous internalization of STxB. When expressed at high level, EGFP-GRIP was mainly cytosolic (Figure 6A, a) instead of Golgi localization, consistent with our understanding that the GRIP domain-mediated Golgi targeting is a saturable process (Kjer-Nielsen et al., 1999a,b; Yoshino et al., 2003). In these cells, internalization of STxB was still observed, but the distribution of internalized STxB was peripheral (b), instead of perinuclear TGN as seen in the control (h). When the conserved residue Y697 of Golgin-97 GRIP domain was mutated to Ala, the inhibition of STxB transport to the Golgi apparatus was not observed and internalized STxB was localized to the perinuclear TGN, in line with our in vitro result that the inhibition requires a functional GRIP domain. As a negative control, over expression of Myc tagged Golgin-97 central coiled-coil region (Golgin-97CC; Figure 1A), which is cytosol distributed regardless of expression levels (Figure 6A, g), did not prevent the perinuclear accumulation of internalized STxB (h). The peripheral spotty localization of STxB in b is suggestive of endosomes, a potential site where the STxB transport is arrested. Statistical analysis of the ratio of transfected cells with perinuclear to peripheral STxB in each case is shown in Figure 6B.
Knock Down of Arl1 or Golgin-97 Inhibited In Vivo Trafficking of STxB to TGN
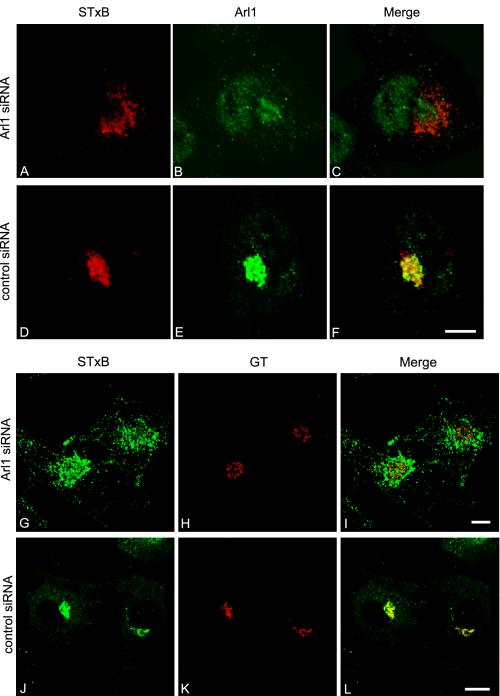
Our previous study showed that Arl1-GTP interacts with the GRIP domain and this interaction regulates the Golgi recruitment of Golgin-97 (Lu and Hong, 2003). Because Golgin-97 is important for EE/RE–TGN transport, Arl1 also might function in this pathway. Polyclonal antibody against rat Arl1 did not show any inhibition in our EE/RE–TGN in vitro transport assay (unpublished data), probably because the epitopes are located at nonessential region of Arl1 (Lu and Hong, 2003). We have thus developed an in vivo STxB transport assay by using siRNAs to selectively knock down the endogenous level of Arl1 or Golgin-97. In a typical experiment (Figure 7A), endogenous levels of both Golgin-97 (lane 1) or Arl1 (lane 3) were reduced to <30% of controls (lanes 2 and 4) by their respective siRNAs. The knock down of both Arl1 (Figures 10 and 12) and Golgin-97 (unpublished data) did not impair the internalization of STxB in immunofluorescent experiments. Transport of STxB to the TGN was reduced to 66% in Arl1 siRNA-treated (Figure 7B, lane 1) and 69% in Golgin-97 siRNA1-treated HeLa cells (Figure 7B, lane 2) relative to HeLa cells under control siRNA treatment (Figure 7B, lane 3). A similar result was obtained using another Golgin-97–specific siRNA (Golgin-97 siRNA2; unpublished data). Although the assay measures transport of STxB from the plasma membrane to TGN via endosomes, the results, together with the trans-Golgi localization of both Arl1 and Golgin-97, suggest that both Arl1 and Golgin-97 are involved in the in vivo STxB retrograde transport to TGN.
Figure 10.
Internalized STxB was arrested at a stage before the Golgi in Arl1 knocked down HeLa cells. Arl1 siRNA (A–C and G–I) and control siRNA (D–F and J–L)-treated HeLa cells were allowed to internalized STxB for 3 h and subsequently double labeled to reveal internalized STxB (A, D, G, and J) and Ar1l (B and E) or GT (H and K). Bars, 10 μm.
Figure 12.
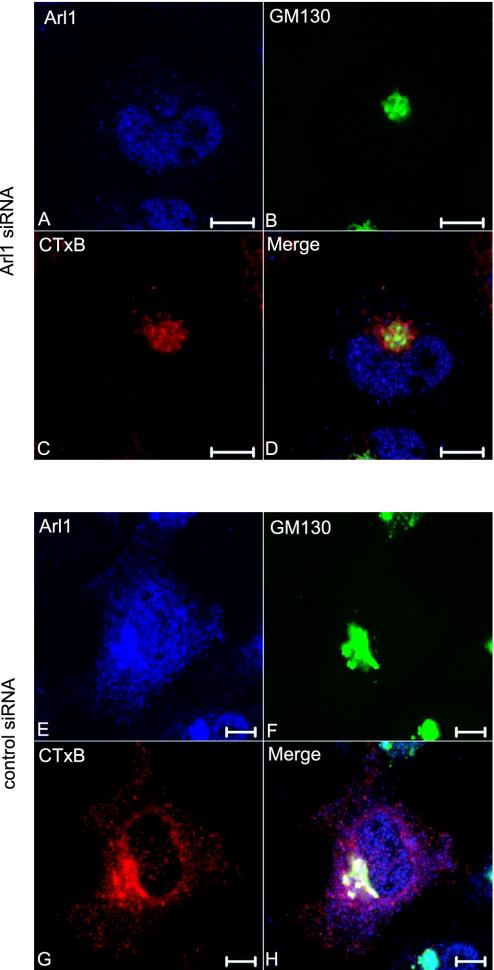
Internalized CTxB was similarly arrested at a stage before the Golgi in Ar1l knocked-down HeLa cells. Arl1 siRNA (A–D) and control siRNA (E–H)-treated HeLa cells were allowed to internalize Alexa Fluor 555-conjugated CTxB for 3 h and subsequently triple labeled to reveal Arl1 (A and E), GM130 (B and F) and internalized CTxB (C and G). Bars, 10 μm.
Interference of Endogenous Golgin-97 Function by Antibody Microinjection Fragmented the Golgi Apparatus and Arrested the EE/RE-to-TGN Transport of CTxB
Our siRNA knock-down approach to reduce endogenous Golgin-97 did not give a morphologically observable phenotype, such as the change of Golgi organization or STxB retrograde trafficking (unpublished data), probably due to the incomplete knock down of cellular Golgin-97 in our experiments. Residual amount of Golgin-97 could account for maintaining the morphology of Golgi structure and EE/RE-to-TGN trafficking pathway. Although the amount of STxB transported from endosome to TGN is reduced, STxB is still present in the Golgi region in these Golgin-97 knock-down cells.
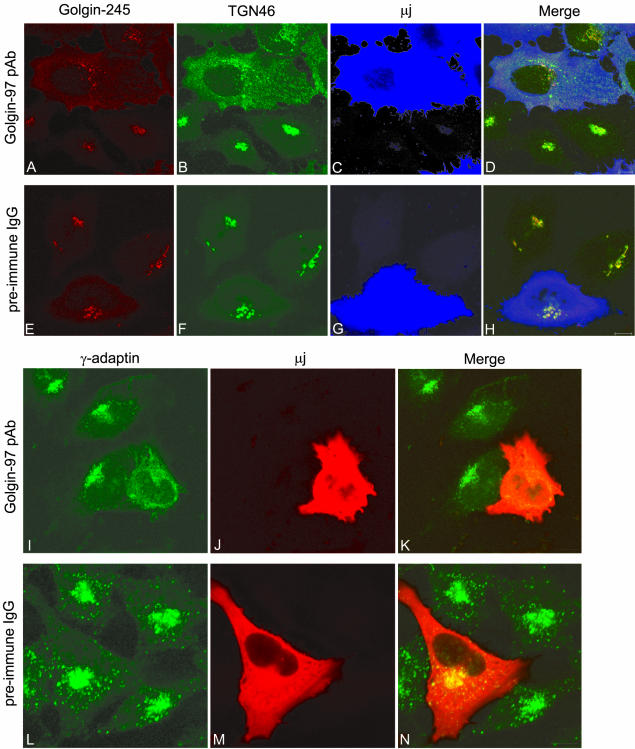
To explore the in vivo function of Golgin-97 by an independent approach, HeLa cells were microinjected with Golgin-97 pAb to interfere with the function of Golgin-97. When the function of endogenous Golgin-97 was disturbed by its antibody, the morphology of Golgi was changed dramatically compared with correspondent preimmune IgG-injected cells (Figures 8 and 9). First, the Golgi apparatus was fragmented as shown by trans-Golgi/TGN markers Golgin-245 (Figure 8A) and TGN46 (Figure 8B), and cis-Golgi marker GM130 (Figure 9a, B), compared with their compact perinuclear distributions in control cells (Figures 8, E and F, and 9a, F). Especially, in Golgin-97 pAb-injected cells, TGN46 only partially colocalized with Golgin-245. A significant amount of TGN46 was separated from Golgin-245 and showed a peripheral vesicular profile (Figure 8B). The rat homolog of TGN46, TGN38, was reported to cycle from the plasma membrane to the TGN through EE/RE (Mallet and Maxfield, 1999), so the morphological separation of TGN46 from Golgin-245 could be explained by suggesting that the EE/RE-to-TGN transport of TGN46 was inhibited when the normal function of Golgin-97 was disturbed. Accordingly, the vesicular profile could represent arrested TGN46 in EE/RE en route to TGN. Second, the majority of AP-1 (γ-adaptin)-positive staining became a peripherally distributed and diffused vesicular profile (Figure 8I) compared with the control cells, in which AP-1 nicely concentrated to the TGN with some peripheral distribution (Figure 8L). AP-1 clathrin-coated vesicles were reported to be responsible for EE/RE-to-TGN retrograde trafficking (Mallard et al., 1998). The diffuse localization of AP-1 clathrin coat probably is a reflection that these AP-1 clathrin vesicles failed to target or tether to the fragmented TGN when the function of Golgin-97 was interfered by injected antibody.
Figure 8.
Microinjection Golgin-97 pAb-fragmented Golgi apparatus. HeLa cells were microinjected with Golgin-97 pAb (A–D and I–K) or preimmune rabbit IgG (E–H and L–N) and subsequently triple labeled to reveal Golgin-245 (A and E), TGN46 (B and F) and injected rabbit IgG (C and G) or double labeled to reveal γ-adaptin (I and L) and injected rabbit IgG (J and M). In D and H, the intensity of blue color was reduced fivefold for clearer observation of red and green signals. Bars, 10 μm.
A commercially available CTxB conjugated with Alexa Fluor 555 was used to study the retrograde trafficking in microinjected cells, because CTxB uses a similar trafficking itinerary as STxB (Sandvig and van Deurs, 2002a,b). The EE/RE–TGN traffic of CTxB also was inhibited in Golgin-97 pAb-injected HeLa cells (Figure 9). In control IgG-injected cells, the internalized CTxB was colocalized with GM130 (Figure 9a, E–H). In the presence of Golgin-97 pAb, the internalized CTxB distributed peripherally and did not colocalize with GM130, whose labeling was fragmented under this condition (Figure 9a, A–D). A morphological statistical analysis indicated that CTxB reached fragmented Golgi marked by GM130 in only 18% internalization positive and Golgin-97 pAb-injected cells, whereas, among control IgG-injected cells, 95% internalization-positive cells had CTxB in the Golgi apparatus (Figure 9b), corroborating Golgin-97 is essential for EE/RE-to-TGN trafficking in vivo.
Internalized STxB Was Arrested at a Stage before Golgi in Arl1 Knocked-Down HeLa Cells
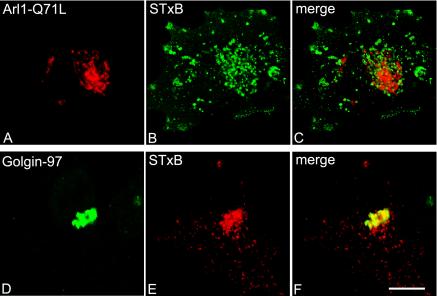
We next investigated the distribution of internalized STxB in Arl1 knocked-down HeLa cells. After siRNA treatment to knock down endogenous Arl1, HeLa cells were allowed to continuously internalize STxB for 3 h. As reported previously (Lu and Hong, 2003), the majority of cells showed only trace amounts of Arl1 that were barely visible through immunofluorescent confocal microscope (Figure 10B). In cells with undetectable levels of Arl1, the internalization of STxB and the morphology of trans-Golgi apparatus, as marked by GT, looked normal (unpublished data). The STxB was localized to perinuclear spotty structures in these Arl1 knocked-down cells (Figure 10A), and it did not overlap with the staining of reduced endogenous Arl1, which was barely detectable (Figure 10C). Instead, the perinuclear STxB was often found to surround the Golgi labeled by the remaining Arl1, indicating that STxB did not reach the Golgi apparatus. On the other hand, in control siRNA-treated cells, the internalized STxB showed extensive colocalization with the endogenous Arl1 (Figure 10, D–F). The failure of STxB to reach Golgi apparatus in Arl1 siRNA-treated cells is also evident in G–I of Figure 10, where STxB did not colocalize with GT, in contrast to a very good colocalization in control siRNA-treated cells (J–L). Different from the knock down of Golgin-97, the knock down of Arl1 could reduce the Golgi association of all its effectors, including GRIP domain Golgins, such as Golgin-97 and Golgin-245 (Lu and Hong, 2003), and other unidentified effectors, thus producing more pronounced morphological effect on STxB transport than knock down of Golgin-97 alone. Unlike the Golgin-97 pAb microinjection experiment, in which the Golgi apparatus was fragmented, the remaining Golgin-97 level in these Arl1 knock-down cells could still be high enough to maintain the integrity of Golgi structure. Corroborating with the knock-down experiments, internalized STxB also failed to reach the Golgi apparatus in cells expressing dominant active Arl1 (Arl1-Q71L) (Figure 11). The results, together, suggest that Arl1 is important for in vivo EE/RE–TGN transport of STxB.
Figure 11.
The trafficking of STxB to Golgi was inhibited in Arl1-Q71L expressing cells. HeLa cells were transiently transfected with Arl1-Q71L and then allowed to continuously internalize STxB for 3 h. The coverslips were then processed for immunofluorescence microscopy to reveal over expressed Arl1-Q71L (by a limited amount of Arl1 antibody; A), Golgin-97 (in nontransfected cells; D), and STxB (B and E). Bar, 10 μm.
Transport to the TGN of CTxB also was examined in the Arl1 knocked-down cells. In contrast to the control siRNA-treated cells (Figure 12, E–H), in which Arl1 (E), GM130 (F) and CTxB-Alexa Fluor 555 (G) colocalized very well. However, internalized CTxB (C) in Arl1 knocked-down cell (A) did not colocalize with GM130 (B), suggesting that Arl1 could be important for general traffic from the EE/RE to the TGN in vivo.
DISCUSSION
In this study, we provide biochemical and cell biological evidence that Arl1 and its effector Golgin-97 play a role in EE/RE–TGN transport. To our knowledge, our report presents the first direct evidence that supports a role of a specific GRIP Golgin (Golgin-97) in a defined intracellular transport event. Golgin-97 may likely represent the first tethering molecule revealed to participate in EE/RE–TGN transport in mammalian cells. Several lines of evidences support these conclusions. The first arises from our demonstration that the recombinant GST-Golgin-97 GRIP domain (GST-GRIP) but not its Y697A mutant (GST-GRIP/Y697A), is a potent inhibitor in the in vitro EE/RE–TGN transport assay. Consistent with this observation, overexpression of EGFP-GRIP but not its Y697A mutant in HeLa cells prevents the trafficking of STxB from the peripheral to the perinuclear Golgi region, implying that STxB does not reach the Golgi apparatus in cells overexpressing EGFP-GRIP. This observation can be explained in that excess amounts of GRIP domain in cells could compete with endogenous GRIP Golgins for limited amounts of common membrane receptor(s), effectively abolishing the membrane association of all GRIP Golgins (Kjer-Nielsen et al., 1999a,b). At the same time, the excess GRIP domain probably displaces membrane-bound Golgin-97, reducing the membrane pool of Golgin-97 and/or other GRIP domain proteins. These results suggest that Golgin-97 and/or other GRIP domain proteins are involved in EE/RE–TGN transport.
The second line of evidence is based on our study to establish a specific and direct role for Golgin-97 in this retrograde trafficking pathway. We made a specific pAb against human Golgin-97. The antibody is a potent and specific inhibitor in EE/RE–TGN transport of STxB. Because the inhibition exhibited by the antibody can be neutralized by noninhibitory amounts of the antigen, the inhibition must thus be mediated by a specific interaction of the antibody with Golgin-97 in the in vitro transport assay.
Importantly, a role of Golgin-97 in vivo also was revealed. Effective reduction of cellular levels of Golgin-97 by its siRNA significantly reduces the sulfation of STxB, a reaction characteristic of the TGN compartment (Niehrs and Huttner, 1990). When functions of endogenous Golgin-97 were disturbed by microinjected Golgin-97 pAb, the Golgi apparatus was fragmented as assessed by cis- and trans-Golgi markers, thus implying that Golgin-97 is essential for the integrity of Golgi structure. The retrograde trafficking of CTxB to the fragmented Golgi also is disrupted in these microinjected cells. Additional support comes from our observation that small GTPase Arl1, which regulates Golgi recruitment of GRIP Golgins, also plays a role in EE/RE–TGN transport in vivo. Selective knock down of endogenous Arl1 prevents STxB trafficking to TGN. First, the knock down of Arl1 reduces the sulfation of STxB in the entire cell population, providing biochemical evidence. Second, in cells with undetectable/reduced Arl1 levels, the internalized STxB is distributed in spotty structures and fails to reach the Golgi apparatus, because it does not colocalize with GT or GM130. Furthermore, transport of another toxin, CTxB, to the Golgi apparatus is arrested at endosome-like structures. Together, these results suggest that Arl1 and Golgin-97 are essential for EE/RE–TGN traffic.
The involvement of Arl1 in endosome to TGN retrograde trafficking is consistent with studies on the yeast Arl1 homolog Arl1p. Deletion of Arl1p results in missorting of carboxypeptidase Y (CPY), probably due to the impaired retrograde trafficking of the CPY receptor Vps10p to the late Golgi (Bonangelino et al., 2002). Arl1p is also reported to genetically or biochemically interact with component(s) of protein machineries that function in endsome-late Golgi trafficking pathway, such as the Ric1p/Rgp1p complex, Ypt6p, and the GARP/VFT/Vps51/52/53/54 complex (Bensen et al., 2001; Panic et al., 2003b). The function of Arl1 seems to be conserved from the yeast to the mammal.
Our data suggest that Golgin-97 is involved in EE/RE–TGN retrograde transport, but its exact function in this process is still obscure. According to several roles recently proposed for Golgins (Gillingham and Munro, 2003), Golgin-97 could be matrix protein for the integrity of trans-Golgi/TGN. Supporting that role, upon interference of its endogenous function by microinjection of Golgin-97 pAb, the trans-Golgi/TGN is fragmented. The fragmentation of cis-Golgi further implies that Golgin-97 might have a global structural role on the Golgi apparatus. Golgin-97 could be a scaffold protein for assembling fusion machinery at the trans-Golgi/TGN membrane. Most importantly, Golgin-97 also could be tethering molecule in this event. It could tether incoming vesicles derived from endosomes-to-Golgi membrane for heterotypic fusion with the TGN. The demonstration that the membrane-bound Golgin-97, but not its cytosolic pool, is directly required for EE/RE–TGN transport suggests that Golgin-97 functions on the membrane. In addition, Golgin-97 is required at a step earlier than Syntaxin 16, implying that Golgin-97 functions before the action of SNAREs. This temporal requirement and its spatial requirement at the membrane present Golgin-97 as a possible tethering molecule. Imh1p (Sys3p), the sole GRIP Golgin in Saccharomyces cerevisiae, is suggested to function before the fusion step in endosome to late Golgi retrograde trafficking (Tsukada et al., 1999), supporting a role of GRIP Golgins as tethering molecules. From these observations, in conjunction with its structural similarities (extensive coiled-coil regions) to other tethering molecules such as GM130, Giantin, p115 and EEA1, we propose that Golgin-97 acts as a tethering molecule for EE/RE–TGN transport and Arl1 regulates the membrane recruitment of this tethering protein as well as other GRIP Golgins.
We and others recently demonstrated that GRIP Golgins form homodimers through both their GRIP and coiled-coil regions (Panic et al., 2003a; Wu et al., 2004). Similar to p115, GM130 and Giantin, the homodimer of Golgin-97 probably adopt a long rod structure, with its C-terminal GRIP domain anchoring to the TGN membrane through interaction with two Arl1-GTP, whereas its N-terminal globular regions extend into the cytosol to mediate the tethering of retrograde transport vesicles derived from endosomes. The tethering function of GRIP Golgins is likely regulated by Arl1. The exchange of GDP for GTP on Arl1 mediated by its cognate yet to be identified GEF initiates the targeting of GRIP Golgins to the TGN membrane. Then, the N-terminal region of GRIP Golgins likely tethers the incoming vesicles by interacting with molecule(s) on the surface of the vesicles. For the tethered vesicles to proceed to the docking/fusion step, a process that is mediated by proteins very proximal to the surface of the TGN membrane (Pfeffer, 1999), the long arm of Golgins is probably a great hindrance and thus it would be necessary to disassemble the Golgins (Barr and Short, 2003). The unique arrangement of the Arl1-GRIP Golgin complex provides a solution to this through sequential hydrolysis of GTPs in two Arl1 molecules in the Arl1/GRIP complex by the unsynchronized action of its GAP(s). The hydrolysis of GTP in either one of two Arl1 molecules, which anchor the Golgin dimer, would abolish the perpendicular angle of Golgin dimer to membrane. The Golgin dimer would then collapse, and thus deliver the tethered vesicle into proximity of the membrane. The v-SNAREs on the vesicle, including VAMP3 and VAMP4, then interact with the t-SNARE complex syntaxin 6/syntaxin 16/Vti1a (Mallard et al., 2002) for short-range docking and fusion. At the same time, hydrolysis of GTP in the last Arl1 molecule by its GAP would finally disassemble the membrane anchoring of the Golgin dimer, leaving the vesicle free for fusion.
Our demonstration that Golgin-97 probably functions as a tethering molecule also indicates that other GRIP domain proteins such as Golgin-245, GCC88, and GCC185 are likely to be involved in tethering process of incoming traffic to the TGN. This working model is consistent with the results presented here as well as published studies. Our preliminary characterizations indicate that Golgin-97, Golgin-245, GCC88, and GCC185 do not interact with one another to form heteroligomeric complexes (unpublished data), suggesting that these four Golgins may participate in distinct incoming traffic or distinct events of a similar traffic. Further studies along these lines will provide greater understanding of the underlying mechanism at the interface between the endocytic pathway and the biosynthetic pathway at the TGN.
Acknowledgments
We thank Drs. Tang Bor Luen and Singh Paramjeet for critical reading of this manuscript. This work was supported by a grant from Agency for Science, Technology, and Research (A*STAR), Singapore (to W.H.). W.H. is also a faculty member of the Department of Biochemistry, National University of Singapore.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–12–0872. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–12–0872.
Abbreviations used: CTxB, Cholera toxin B fragment; EE, early endosome; pAb, polyclonal antibody; RE, recycling endosome; siRNA, small interference RNA; STxB, Shiga toxin B fragment; TGN, trans-Golgi network.
References
- Allan, B.B., Moyer, B.D., and Balch, W.E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444-448. [DOI] [PubMed] [Google Scholar]
- Antonny, B., and Schekman, R. (2001). ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 13, 438-443. [DOI] [PubMed] [Google Scholar]
- Barr, F.A. (1999). A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol. 9, 381-384. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., Puype, M., Vandekerckhove, J., and Warren, G. (1997). GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91, 253-262. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., and Short, B. (2003). Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15, 405-413. [DOI] [PubMed] [Google Scholar]
- Bensen, E.S., Yeung, B.G., and Payne, G.S. (2001). Ric1p and the Ypt6p GTPase function in a common pathway required for localization of trans-Golgi network membrane proteins. Mol. Biol. Cell 12, 13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya, A.D., Thomas, L., Feliciangeli, S.F., Hung, C.H., and Thomas, G. (2002). HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111, 853-866. [DOI] [PubMed] [Google Scholar]
- Boehm, M., and Bonifacino, J.S. (2001). Adaptins: the final recount. Mol. Biol. Cell 12, 2907-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino, C.J., Chavez, E.M., and Bonifacino, J.S. (2002). Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2486-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.A., and Scheller, R.H. (2001). SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2, 98-106. [DOI] [PubMed] [Google Scholar]
- Diaz, E., and Pfeffer, S.R. (1998). TIP 47, a cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93, 433-443. [DOI] [PubMed] [Google Scholar]
- Eystathioy, T., Jakymiw, A., Fujita, D.J., Fritzler, M.J., and Chan, E.K. (2000). Human autoantibodies to a novel Golgi protein Golgin-67, high similarity with golgin-95/gm 130 autoantigen. J. Autoimmun. 14, 179-187. [DOI] [PubMed] [Google Scholar]
- Fritzler, M.J., Hamel, J.C., Ochs, R.L., and Chan, E.K. (1993). Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J. Exp. Med. 178, 49-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler, M.J., Lung, C.C., Hamel, J.C., Griffith, K.J., and Chan, E.K. (1995). Molecular characterization of Golgin-245, a novel Golgi complex protein containing a granin signature. J. Biol. Chem. 270, 31262-31268. [DOI] [PubMed] [Google Scholar]
- Ghosh, R.N., Mallet, W.G., Soe, T.T., McGraw, T.E., and Maxfield, F.R. (1998). An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142, 923-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham, A.K., and Munro, S. (2003). Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta 1641, 71-85. [DOI] [PubMed] [Google Scholar]
- Griffith, K.J., Chan, E.K., Lung, C.C., Hamel, J.C., Guo, X., Miyachi, K., and Fritzler, M.J. (1997). Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjogren's syndrome. Arthritis Rheum. 40, 1693-1702. [DOI] [PubMed] [Google Scholar]
- Johannes, L., Tenza, D., Antony, C., and Goud, B. (1997). Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272, 19554-19561. [DOI] [PubMed] [Google Scholar]
- Kang, T., Nagase, H., and Pei, D. (2002). Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network. Cancer Res. 62, 675-681. [PubMed] [Google Scholar]
- Kjer-Nielsen, L., Teasdale, R.D., van Vliet, C., and Gleeson, P.A. (1999a). A novel Golgi-localisation domain shared by a class of coiled-coil peripheral membrane proteins. Curr. Biol. 9, 385-388. [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen, L., van Vliet, C., Erlich, R., Toh, B.H., and Gleeson, P.A. (1999b). The Golgi-targeting sequence of the peripheral membrane protein p230. J. Cell Sci. 112, 1645-1654. [DOI] [PubMed] [Google Scholar]
- Larsen, J.E., Massol, R.H., Nieland, T.J., and Kirchhausen, T. (2004). HIV Nef-mediated major histocompatibility complex class I down-modulation is independent of Arf6 activity. Mol. Biol. Cell 15, 323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S.X., Mallet, W.G., Huang, A.Y., and Maxfield, F.R. (2004). Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol. Biol. Cell 15, 721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, S.L., Wong, S.H., and Hong, W.J. (1996). The mammalian ARF-like protein 1 (ArI1) is associated with the Golgi complex. J. Cell Sci. 109, 209-220. [DOI] [PubMed] [Google Scholar]
- Lu, L., and Hong, W. (2003). Interaction of Arl1-GTP with GRIP Domains Recruits Autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol. Biol. Cell 14, 3767-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L., Horstmann, H., Ng, C., and Hong, W.J. (2001). Regulation of Golgi structure and function by ARF-like protein 1 (Arl1). J. Cell Sci. 114, 4543-4555. [DOI] [PubMed] [Google Scholar]
- Luke, M.R., Kjer-Nielsen, L., Brown, D.L., Stow, J.L., and Gleeson, P.A. (2003). GRIP domain-mediated targeting of two new coiled-coil proteins, GCC88 and GCC185, to subcompartments of the trans-Golgi network. J. Biol. Chem. 278, 4216-4226. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B., and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 143, 973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., and Johannes, L. (2003). Shiga toxin B-subunit as a tool to study retrograde transport. Methods Mol. Med. 73, 209-220. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Tang, B.L., Galli, T., Tenza, D., Saint-Pol, A., Yue, X., Antony, C., Hong, W., Goud, B., and Johannes, L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, W.G., and Maxfield, F.R. (1999). Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146, 345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medigeshi, G.R., and Schu, P. (2003). Characterization of the in vitro retrograde transport of MPR46. Traffic 4, 802-811. [DOI] [PubMed] [Google Scholar]
- Monier, S., Jollivet, F., Janoueix-Lerosey, I., Johannes, L., and Goud, B. (2002). Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic 3, 289-297. [DOI] [PubMed] [Google Scholar]
- Munro, S., and Nichols, B.J. (1999). The GRIP domain - a novel Golgi-targeting domain found in several coiled-coil proteins. Curr. Biol. 9, 377-380. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., Rabouille, C., Watson, R., Nilsson, T., Hui, N., Slusarewicz, P., Kreis, T.E., and Warren, G. (1995). Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131, 1715-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs, C., and Huttner, W.B. (1990). Purification and characterization of tyrosylprotein sulfotransferase. EMBO J. 9, 35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic, B., Perisic, O., Veprintsev, D.B., Williams, R.L., and Munro, S. (2003a). Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol. Cell 12, 863-874. [DOI] [PubMed] [Google Scholar]
- Panic, B., Whyte, J.R., and Munro, S. (2003b). The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr. Biol. 13, 405-410. [DOI] [PubMed] [Google Scholar]
- Petris, M.J., and Mercer, J.F. (1999). The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Hum. Mol. Genet. 8, 2107-2115. [DOI] [PubMed] [Google Scholar]
- Petris, M.J., Voskoboinik, I., Cater, M., Smith, K., Kim, B.E., Llanos, R.M., Strausak, D., Camakaris, J., and Mercer, J.F. (2002). Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J. Biol. Chem. 277, 46736-46742. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S.R. (1999). Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1, E17-E22. [DOI] [PubMed] [Google Scholar]
- Piguet, V., Wan, L., Borel, C., Mangasarian, A., Demaurex, N., Thomas, G., and Trono, D. (2000). HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2, 163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle, A., Murphy, G., and Roghi, C. (2003). Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J. Cell Sci. 116, 3905-3916. [DOI] [PubMed] [Google Scholar]
- Rios, R.M., Tassin, A.M., Celati, C., Antony, C., Boissier, M.C., Homberg, J.C., and Bornens, M. (1994). A peripheral protein associated with the cis-Golgi network redistributes in the intermediate compartment upon brefeldin A treatment. J. Cell Biol. 125, 997-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002a). Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18, 1-24. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002b). Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 529, 49-53. [DOI] [PubMed] [Google Scholar]
- Scales, S.J., Gomez, M., and Kreis, T.E. (2000). Coat proteins regulating membrane traffic. Int. Rev. Cytol. 195, 67-144. [DOI] [PubMed] [Google Scholar]
- Seelig, H.P., Schranz, P., Schroter, H., Wiemann, C., Griffiths, G., and Renz, M. (1994). Molecular genetic analyses of a 376-kilodalton Golgi complex membrane protein (giantin). Mol. Cell. Biol. 14, 2564-2576. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Seemann, J., Jokitalo, E., Pypaert, M., and Warren, G. (2000). Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature 407, 1022-1026. [DOI] [PubMed] [Google Scholar]
- Setty, S.R., Shin, M.E., Yoshino, A., Marks, M.S., and Burd, C.G. (2003). Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr. Biol. 13, 401-404. [DOI] [PubMed] [Google Scholar]
- Shewan, A.M., Van Dam, E.M., Martin, S., Luen, T.B., Hong, W., Bryant, N.J., and James, D.E. (2003). GLUT4 Recycles via a trans-Golgi Network (TGN) Subdomain enriched in syntaxins 6 and 16 but not TGN 38, involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., Beard, M.B., Seemann, J., Dirac-Svejstrup, A.B., and Warren, G. (2002). Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincock, P.M., Ganley, I.G., Krise, J.P., Diederichs, S., Sivars, U., O'Connor, B., Ding, L., and Pfeffer, S.R. (2003). Self-assembly is important for TIP47 function in mannose 6-phosphate receptor transport. Traffic 4, 18-25. [DOI] [PubMed] [Google Scholar]
- Sonnichsen, B., Lowe, M., Levine, T., Jamsa, E., Dirac-Svejstrup, B., and Warren, G. (1998). A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140, 1013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley, K.S. and Green, S.A. (2000). Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules. J. Cell Biol. 151, 107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam, V.N., bin Mohd Yusoff, A.R., Wong, S.H., Lim, G.B., Chew, M., and Hong, W. (1992). Biochemical fractionation and characterization of proteins from Golgi-enriched membranes. J. Biol. Chem. 267, 12016-12021. [PubMed] [Google Scholar]
- Tsukada, M., Will, E., and Gallwitz, D. (1999). Structural and functional analysis of a novel coiled-coil protein involved in Ypt6 GTPase-regulated protein transport in yeast. Mol. Biol. Cell 10, 63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Ma, D., Keski-Oja, J., and Pei, D. (2004a). Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal. J. Biol. Chem. 279, 9331-9336. [DOI] [PubMed] [Google Scholar]
- Wang, P., Wang, X., and Pei, D. (2004b). Mint-3 regulates the retrieval of internalized MT5-MMP to plasma membrane by binding to its carboxyl end motif EWV. J. Biol. Chem. [Epub ahead of print]. [DOI] [PubMed]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M., Lu, L., Hong, W., and Song, H. (2004). Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat. Struct. Mol. Biol. 11, 86-94. [DOI] [PubMed] [Google Scholar]
- Yoshino, A., Bieler, B.M., Harper, D.C., Cowan, D.A., Sutterwala, S., Gay, D.M., Cole, N.B., McCaffery, J.M., and Marks, M.S. (2003). A role for GRIP domain proteins and/or their ligands in structure and function of the trans Golgi network. J. Cell Sci. 116, 4441-4454. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
- Zucker, S., Hymowitz, M., Conner, C.E., DiYanni, E.A., and Cao, J. (2002). Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab. Investig. 82, 1673-1684. [DOI] [PubMed] [Google Scholar]