ABSTRACT
Zika virus (ZIKV; family Flaviviridae, genus Flavivirus) is a rapidly expanding global pathogen that has been associated with severe clinical manifestations, including devastating neurological disease in infants. There are currently no molecular clones of a New World ZIKV available that lack significant attenuation, hindering progress toward understanding determinants of transmission and pathogenesis. Here we report the development and characterization of a novel ZIKV reverse genetics system based on a 2015 isolate from Puerto Rico (PRVABC59). We generated a two-plasmid infectious clone system from which infectious virus was rescued that replicates in human and mosquito cells with growth kinetics representative of wild-type ZIKV. Infectious clone-derived virus initiated infection and transmission rates in Aedes aegypti mosquitoes comparable to those of the primary isolate and displayed similar pathogenesis in AG129 mice. This infectious clone system provides a valuable resource to the research community to explore ZIKV molecular biology, vaccine development, antiviral development, diagnostics, vector competence, and disease pathogenesis.
IMPORTANCE ZIKV is a rapidly spreading mosquito-borne pathogen that has been linked to Guillain-Barré syndrome in adults and congenital microcephaly in developing fetuses and infants. ZIKV can also be sexually transmitted. The viral molecular determinants of any of these phenotypes are not well understood. There is no reverse genetics system available for the current epidemic virus that will allow researchers to study ZIKV immunity, develop novel vaccines, or develop antiviral drugs. Here we provide a novel infectious clone system generated from a recent ZIKV isolated from a patient infected in Puerto Rico. This infectious clone produces virus with in vitro and in vivo characteristics similar to those of the primary isolate, providing a critical tool to study ZIKV infection and disease.
KEYWORDS: Flavivirus, Zika virus, infectious clones
Flaviviruses are the most important arthropod-borne viruses (arboviruses) in the world. Dengue (1–4), West Nile, Japanese encephalitis, tick-borne encephalitis, and yellow fever viruses pose a burden to the health of millions of individuals annually. Zika virus (ZIKV; family Flaviviridae, genus Flavivirus) recently emerged from its historical range in East Africa and SE Asia/Oceania. Primarily transmitted by Aedes species mosquitoes, ZIKV was first detected in humans in 1954 (1). In 2013, an outbreak in French Polynesia resulted in approximately 10,000 human cases (2). ZIKV entered South America in 2013 and was reported to be locally transmitted in 14 states in Brazil in May 2015 (3). In December 2015, the first case of locally transmitted ZIKV was detected in Puerto Rico (4), and on 1 August 2016, local transmission was confirmed in Miami, Florida (5). The ongoing ZIKV epidemic has been accompanied by several severe clinical manifestations, including Guillain-Barré syndrome (6) and congenital malformations in the fetuses of women infected with ZIKV during pregnancy (7). In addition, sexual transmission has been widely documented (8). ZIKV is a rapidly emerging global pathogen with several unique epidemiologic and pathogenic features that are currently poorly understood.
ZIKV clusters phylogenetically into African and Asian genotypes, and the virus implicated in the American outbreak of ZIKV clusters within the Asian lineage (9). Recent reports have shown that Aedes aegypti mosquitoes are highly competent vectors for American isolates of ZIKV (10). In addition, A. aegypti was found to be the primary vector for ZIKV in southern Mexico (11). Mouse models for ZIKV neuropathogenesis and in utero transmission have recently been reported in mice deficient in innate immune responses by using African and Asian genotype isolates (12–15), though there have not been in vivo pathogenesis studies using an isolate from the American outbreak. In addition, infectious clones for Asian genotype ZIKV strains have recently been described, including one from a virus circulating in the American outbreak (16, 17). These clones, however, are attenuated in vitro and in vivo, somewhat limiting their utility for certain types of studies. Differences between strains of the Asian genotype of ZIKV are unclear, and thus clones derived from each virus are important for understanding viral spread in the Americas. The lack of an infectious clone system with phenotypic properties similar to those of wild-type (WT) virus remains a barrier to studies focused on understanding the transmission of, pathogenesis of, and immune responses to currently circulating virus strains.
Therefore, we developed and tested an infectious clone of a ZIKV strain (PRVABC59) recently isolated by the CDC from a human infected in Puerto Rico in 2015 (9) and demonstrated its growth characteristics in cell culture, A. aegypti mosquitoes, and a mouse model of pathogenesis. Virus rescued from this clone is similar in RNA sequence, in vitro replication, mouse pathogenesis, and mosquito transmission to the parental ZIKV isolate. Since the clone reported is derived from a strain widely distributed by the CDC, it represents an important tool to advance ZIKV research.
RESULTS
Construction of PRVABC59 infectious clone.
The parental virus from which the infectious clone was derived, PRVABC59, was isolated from a 2015 serum specimen from Puerto Rico (9) and passaged in Vero cells. The sequence of the virus was determined by next-generation sequencing (RNA-Seq) and supplemented by rapid amplification of cDNA ends (RACE) to generate 5′ and 3′ untranslated sequences. The sequence obtained using this method matched published sequences for PRVABC59, which includes a variable amino acid at E-330. Published phylogenetic analyses of ZIKV strain PRVABC59 place it within the Asian clade of ZIKV (9) and indicate that it is similar to other strains currently circulating in the Americas.
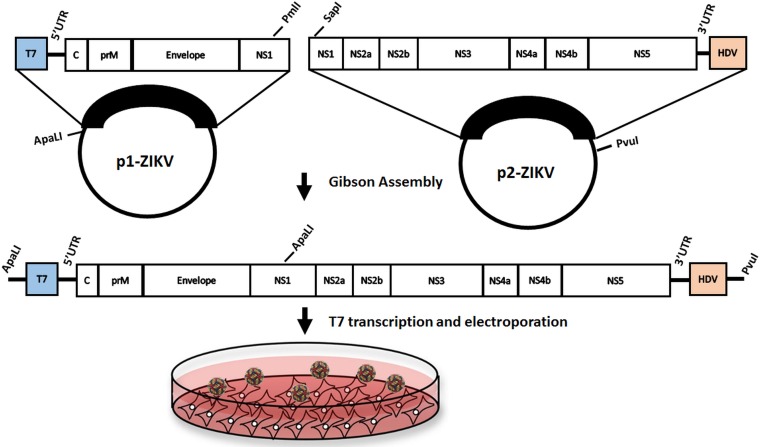
An infectious clone was constructed for the PRVABC59 ZIKV strain. Preliminary studies indicated that full-length infectious clones were unstable in Escherichia coli (data not shown), likely due to cryptic prokaryotic promoters within the E and NS1 coding sequences (18). Therefore, we developed a two-plasmid system that would allow the 5′ and 3′ ends of the viral genome to be joined through unique ApaLI and SapI restriction enzyme sites in NS1 (Fig. 1). Plasmid pJW231 contained the 5′ end (nucleotides [nt] 1 to 3498), and plasmid pJW232 contained the 3′ end (nt 3109 to 10809) of the ZIKV genome. Plasmid pJW231 was engineered to contain a T7 RNA polymerase promoter sequence that initiates transcription at nt 1 of the viral genome. Plasmid pJW232 was generated to contain a hepatitis D virus (HDV) ribozyme to produce an authentic 3′ end of the viral genome. A single synonymous mutation was identified at nucleotide position 8489 (C to T) and retained in order to allow infectious clone-derived virus to be distinguished from parental PRVABC59 (Table 1). To prepare RNA from the two-plasmid system, pJW231 (ApaLI and PmlI) and pJW232 (SapI and PvuI) were digested, PCR purified, and ligated using Gibson assembly. From the assembled fragments, capped T7 RNA transcripts were generated, and the resulting RNA was electroporated into Vero cells. Eight days after electroporation, 1.25 × 107 PFU/ml were obtained in the culture supernatant. Viral RNA was isolated from recovered virus and sequenced using RNA-Seq. The expected synonymous mutation at position 8489 was observed, indicating that the virus was infectious clone derived.
FIG 1.
Infectious Zika virus (ZIKV) generated from a two-part cDNA clone system. The ZIKV genome was cloned in two separate pieces into the pACYC177 vector using Gibson assembly. Plasmids were amplified, digested, and ligated using Gibson assembly to create a plasmid containing the full-length virus sequence with a T7 promoter and hepatitis D virus ribozyme sequence, which was then transcribed into RNA using T7 polymerase. The resulting RNA was then electroporated into Vero cells, producing significant CPE 8 days later.
TABLE 1.
Sequence difference between a ZIKV infectious clone and parental strain PRVABC59
| Nucleotide position | Nucleic acid in sequence for: |
Amino acid change | Gene location | |
|---|---|---|---|---|
| Parental ZIKV | Clone ZIKV | |||
| 8489 | C | T | None | NS5 |
In vitro growth properties of primary and infectious clone-derived PRVABC59.
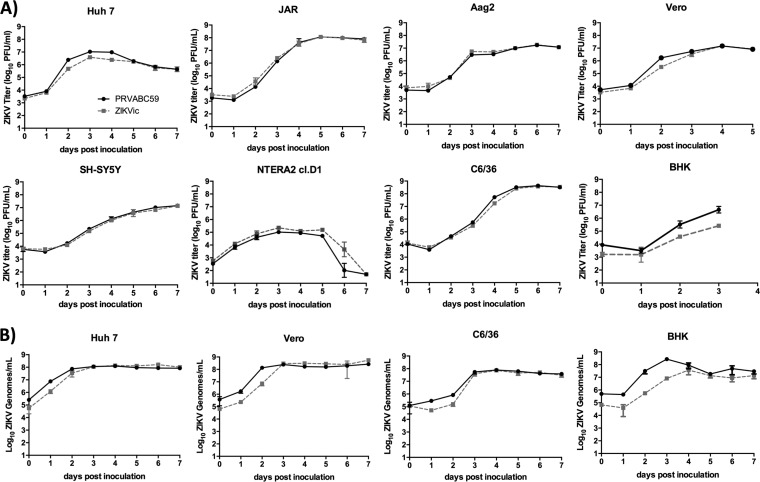
In order to compare the in vitro replication kinetics of the PRVABC59 isolate and the ZIKV infectious clone-derived virus, we inoculated multiple mammalian and mosquito cell lines and measured infectious viral titers by plaque assay (Fig. 2A) and reverse transcription-quantitative PCR (qRT-PCR) of viral RNA in the culture supernatant (Fig. 2B). The replication profiles of the isolate and the infectious clone-derived virus were essentially indistinguishable. Peak viral titers occurred on day 3 to 4 postinoculation in mammalian cell lines, including Vero cells and multiple human cell lines, i.e., placental cells (JAR), testicular cells (NTERA2 cl.D1), neuroblastoma cells (SH-SY5Y), and Huh7 cells (liver), and were not statistically different between the parental strain and the infectious clone-derived virus. A difference in infectious titers between parental and infectious clone-derived virus was observed in BHK cells at early time points but resolved at later times, as indicated by qRT-PCR. Titers of infectious virus in the tissue culture supernatant peaked on day 5 to 6 in two Aedes mosquito cell lines, C6/36 and Aag2.
FIG 2.
Growth kinetics of PRVABC59 infectious clone virus. (A) The growth kinetics of wild-type ZIKV PRVABC59 and infectious clone-derived ZIKV (ZIKVic) on human (Huh7, Jar, SH-SY5Y, and NTERA2 cI.D1), mosquito (C6/63 and Aag2), nonhuman primate (Vero), and hamster (BHK) cells was assessed by plaque assay. n = 3. Error bars represent standard deviations from the mean. (B) The growth kinetics of wild-type PRVABC59 and infectious clone-derived virus on Huh7, Vero, C6/36, and BHK cells was assessed by qRT-PCR. n = 3. Error bars represent standard deviations from the mean.
Transmission of PRVABC59 WT and clone-derived virus by Aedes aegypti mosquitoes.
In order to be maintained in nature, arboviruses must infect and replicate within the midguts of mosquito vectors, disseminate to secondary tissues, and ultimately be expectorated in the saliva during bloodfeeding. Therefore, we tested the ability of the infectious clone-derived virus to be transmitted by relevant vector mosquitoes relative to that of the parental virus. Female Aedes aegypti mosquitoes derived from Poza Rica, Mexico, were exposed to 8 × 106 PFU of each virus in an artificial blood meal. Fourteen days post-blood feed, mosquitoes were examined for the presence of virus in bodies, dissemination of virus into legs, and presence of virus in expectorated saliva. These A. aegypti mosquitoes were highly susceptible to both cloned and parental ZIKV by the oral route, with 75 to 100% of individuals becoming infected (Table 2). Dissemination from infected midguts was also highly efficient, with nearly 100% of infected mosquitoes exhibiting dissemination after 14 days of extrinsic incubation. Furthermore, approximately 50% of infected mosquitoes transmitted both parental and clone-derived virus.
TABLE 2.
Rates of infection, dissemination, and transmission potential in A. aegypti mosquitoes 14 days post-blood meal
| Virus | Biological replicate 1 |
Biological replicate 2 |
||||
|---|---|---|---|---|---|---|
| No. with infection/total no. (%) | No. with disseminated infection/total no. infected (%) | No. with transmission/total no. infected (%) | No. with infection/total no. (%) | No. with disseminated infection/total no. infected (%) | No. with transmission/total no. infected (%) | |
| Parental ZIKV | 43/48 (89.6) | 42/43 (97.7) | 25/43 (58.1) | 43/46 (93.5) | 42/43 (97.7) | 26/43 (60.5) |
| Infectious clone | 36/48 (75) | 35/36 (97.2) | 19/36 (52.8) | 48/48 (100) | 48/48 (100) | 20/48 (41.7) |
| P value | 0.11 | 1 | 0.66 | 0.11 | 0.47 | 0.09 |
Pathogenesis of PRVABC59 primary isolate and infectious clone-derived virus in mice.
To evaluate the pathogenesis of the PRVABC59 isolate and recombinant ZIKV, we inoculated AG129 mice (alpha/beta and gamma interferon receptor deficient), which have previously been shown to be a model for ZIKV pathogenesis (12, 13). Four-week-old AG129 mice (11 per group; 7 males and 4 females) were inoculated with 1,000 PFU of either PRVABC59 or infectious clone virus via intraperitoneal injection. Weight and survival were followed after infection. Disease symptoms, including ruffled fur, hunched posture, tremors, conjunctivitis, ataxia, hind limb paralysis, and recumbency, were observed starting 8 to 9 days postinoculation (dpi). Mortality was observed starting on day 10 postinoculation for both the parental isolate and the recombinant virus, with 100% mortality by dpi 14 (Fig. 3A). Median survival times were 11.5 and 11 days for the parental and infectious clone-derived viruses, respectively, and survival curves did not differ significantly. Both groups of mice showed weight loss starting at 7 dpi, with differences not statistically significant (Fig. 3B).
FIG 3.
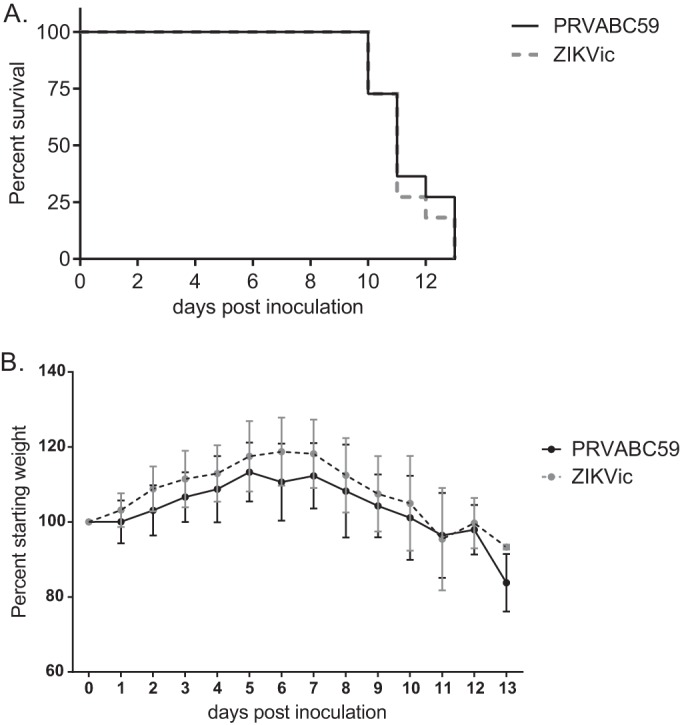
Pathogenesis of infectious clone-derived ZIKV (ZIKVic) in AG129 mice. AG129 mice were inoculated with PRVABC59 or the infectious clone-derived virus and monitored over time (n = 11). (A) Mortality of AG129 mice postinoculation; (B) weight of AG129 mice postinoculation, represented as a percentage of starting weight.
To examine serum viremia and tissue distribution of infection for the PRVABC59 isolate and the infectious clone-derived virus, serum samples and tissues (brain and testes) were collected from three 4-week-old male AG129 mice on days 1, 3, and 5 postinoculation. Serum titers for both the parental PRVABC59 strain and the infectious clone-derived virus were detectable on dpi 1, peaked at 6 to 7 log10 PFU/ml sera on dpi 3, and dropped to 4 log10 PFU/ml sera by dpi 5 (Fig. 4A). Additionally, both the parental and infectious clone-derived viruses were observed in the testes and brains on dpi 3 and 5, with titers of 4 to 5 log10 PFU/g in the brain (Fig. 4B) and 5 to 6 log10 PFU/g in the testes (Fig. 4C). Differences in mean titers between the parental strain and the infectious clone-derived virus were not statistically significant.
FIG 4.
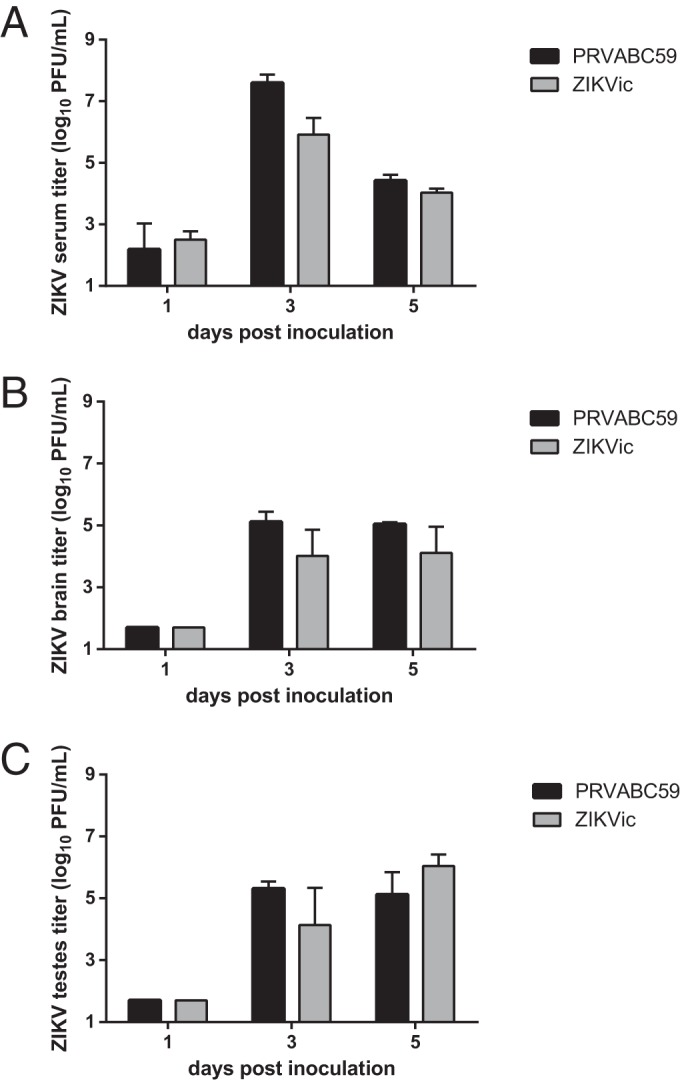
Tissue distribution of infectious clone-derived ZIKV (ZIKVic) in male AG129 mice. Male AG129 mice were inoculated with PRVABC59 or the infectious clone-derived virus, and tissues were collected at days 1, 3, and 5 postinoculation (n = 3 per time point). (A) Viral titer in serum; (B) viral titer in brain tissue; (C) viral titer in testes. The lower limit for this assay was 1.7 log10 PFU/ml.
Sequence diversity of clone-derived virus versus parental ZIKV PRVABC59.
RNA-Seq was performed in order to compare the genetic diversities of clone-derived virus and wild-type PRVABC59. Clone-derived virus was less diverse by all measures tested (Fig. 5A). Most of the variants found in the two viruses were present at relatively low frequencies across the genome (Fig. 5B and C). A single synonymous variant at nt 7200 in the clone-derived virus (but not in parental virus) was detected at 28.6%, while all other variants were detected at less than 8% of the population and most were under 2%.
FIG 5.
Genetic diversity of infectious clone-derived ZIKV (ZIKVic) compared to that of wild-type ZIKV PRVABC59. RNA-Seq was performed to assess the genetic diversity of infectious clone-derived virus compared to that of wild-type PRVABC59 stock. (A) Summary of sequencing across the ZIKV genome: total ZIKV reads, the number of quality filtered reads that mapped to the ZIKV PRVABC59 genome; average depth, the average number of reads representing a given nucleotide across the genome; distance, the sum of all variant frequencies; complexity, the uncertainty of randomly sampling a variant at each site (Shannon entropy); pi (nucleotide diversity), the average number of nucleotide differences per site. (B and C) Mutational profiles across the genomes of wild-type PRVABC59 (A) and clone-derived virus (B).
DISCUSSION
As ZIKV continues to emerge as a global pathogen, the need for a wide array of molecular tools to study important aspects of its replication, transmission, and pathogenesis and to define the viral molecular determinants of its surprising pathogenic and transmission phenotypes has become abundantly clear. Toward that end, several reverse genetics systems have been developed to support these critical research needs. Interestingly, virus rescued from these systems has been found to be fairly significantly attenuated in cell cultures, mice, and other systems (16, 17). Although the reasons for this rather severe attenuation are not fully clear at present, an important characteristic of infectious clones is the degree to which they mimic critical phenotypes of wild-type uncloned virus. We therefore sought to develop a reverse genetics system for a New World ZIKV strain that was highly similar or identical to the parental virus strain. Further, we sought to meet the emerging need for an infectious clone of the PRVABC59 strain of ZIKV, which has been widely distributed among the flavivirus research community.
After initial failures to generate stable full-length infectious clone-derived ZIKV, we developed a two-plasmid system that is relatively straightforward to use. The cloned virus is genetically identical to the consensus sequence of the ZIKV WT virus except for a single synonymous difference at nt 8489 and produces high-titer ZIKV 8 days after electroporation, eliminating the need for subsequent passage, during which potentially adaptive or phenotypically significant mutations could be acquired. Additionally, through next-generation sequencing (NGS) analysis, clone-derived virus was found to be less diverse than parental virus and no high-frequency nonsynonymous variants were found, indicating a lack of adaptation. These observations indicate that RNA from the PRVABC59 ZIKV two-plasmid infectious clone is infectious and reaches high titers after electroporation.
We found that replication of parental and clone-derived ZIKV was the same or nearly the same in a wide array of cell types, including cells of human, monkey, rodent, and mosquito origin. These data indicate that the viruses circulating in the Americas have a wide tropism for cell types potentially involved in neuropathogenesis, sexual transmission, in utero transmission, and mosquito transmission. Further, they suggest that the virus derived from the clone reported here is likely to accurately model various aspects of transmission and pathogenesis in vivo.
An important characteristic of arbovirus epidemiology is transmission by mosquitoes. We therefore evaluated transmission of parental and clone-derived ZIKV by a recently colonized Mexican strain of A. aegypti. Parental ZIKV and clone-derived ZIKV were transmitted with equal efficiency by these mosquitoes. These results support the observation that clone-derived ZIKV and parental ZIKV are similar in key phenotypes, including vector transmissibility, and indicate that this tool may be useful in subsequent studies of virus-vector interactions.
Finally, we assessed pathogenesis in a mouse model of ZIKV infection. We report identical mortalities between mice infected with parental or clone-derived virus and highly similar weight losses; differences between viruses were not statistically significant. Further, we found similar virus loads in mouse serum, brain, and testes at a range of days postinoculation. Moreover, we demonstrate here that the virus derived from our infectious clone is not attenuated in mice, further indicating the use of AG129 mice as models of ZIKV disease.
In sum, our results show that the PRVABC59 ZIKV strain is highly virulent in immunodeficient mice and is transmissible by A. aegypti mosquitoes. In addition, mortality in AG129 mice and transmission by A. aegypti were nearly identical between the primary isolate PRVABC59 and the infectious clone-derived virus. Clone-derived virus replicated well in human placental, testicular, and neuronal cells, and in AG129 mice, both clone-derived and wild-type virus rapidly disseminated to the brain and testes of male mice. These data suggest that the clone-derived virus can be used in studies of ZIKV sexual transmission, in utero transmission, and neuropathogenesis. The reverse genetics system reported here will therefore facilitate downstream studies of viral genetic determinants of ZIKV transmission and pathogenesis.
MATERIALS AND METHODS
ZIKV isolate.
The PRVABC59 ZIKV strain was originally isolated by passage on Vero cells of sera from an individual returning from travel to Puerto Rico collected in December of 2015 (GenBank accession number KU501215) (9). A third Vero cell-passaged stock was utilized for the generation of amplicons for clone construction as well as for all in vivo and in vitro comparative assessments. The sequence of the isolate was confirmed by amplifying the genome in 8 overlapping fragments by RT-PCR, followed by direct sequencing of amplicons. 5′ and 3′ RACE reactions (Invitrogen) were performed to generate amplification products of the terminal untranslated regions and sequenced directly. Primer sequences are available upon request.
Construction of ZIKV infectious clone.
Briefly, viral RNA was extracted using the DirectZOL RNA extraction kit (Zymo, Irvine, CA) with on-column DNase treatment. cDNA was then produced using random nonamers using the Superscript IV first-strand synthesis system (Invitrogen, Carlsbad, CA). ZIKV fragments were amplified with Q5 high-fidelity polymerase (New England BioLabs) and cloned into the pACYC177 vector using Gibson assembly or circular polymerase extension cloning (CPEC) to generate two plasmids. Plasmid pJW231 containing the sequences for the 5′ untranslated region (UTR) to nt 3498 and plasmid pJW232 containing the sequence from nt 3109 to the end of the 3′ UTR were generated. An overlapping region of the genome is found in both plasmids, allowing the 5′ and 3′ portions of the viral genome to be conjoined following digestion with ApaLI and SapI. The sequence for the T7 promoter and hepatitis D virus ribozyme were previously cloned into a West Nile virus infectious clone (19). Clones with the correct restriction pattern were verified by sequencing, and correct clones were selected for virus rescue.
In vitro transcription and transfection.
Virus was rescued by RNA transcription from ligated full-length infectious clone DNA. pJW231 was prepared by digestion with ApaLI and PmlI and pJW232 was prepared by digestion with SapI and PvuI, leaving a 52-bp overlapping region that could be joined using Gibson assembly. Digested products were PCR purified (Macherey-Nagel, Germany) and ligated using the NEBuilder HiFi DNA Assembly master mix (New England BioLabs, Ipswich, MA). After a 1-h incubation at 50°C, the reaction mixture was purified and used in a T7 RNA transcription reaction using the ARCA 2× T7 master mix (NEB). RNA was electroporated into Vero cells (170 V, 0 resistance, and 950 μF; 1 pulse with the BTX ECM 630 electroporation system), and cells were subsequently monitored for cytopathic effect (CPE) daily. On day 8 postelectroporation, significant CPE was observed and supernatant was harvested, centrifuged to remove cellular debris, and supplemented to a final concentration of 20% fetal bovine serum (FBS) and 10 mM HEPES before being frozen in single-use aliquots. The titer was measured using the plaque assay on Vero cells.
Recovered infectious clone virus was sequenced using amplicon-based RNA sequencing with next-generation sequencing (NGS). For NGS, RNA was isolated from recovered virus, cDNA was produced, and PCR was performed to amplify amplicons for sequencing. DNA was then fragmented with double-stranded DNA (dsDNA) fragmentase (NEB) for 15 min at 37°C. Fragmented DNA was then purified with AMPure XP beads (1.2 bead/DNA ratio) before library preparation with the NEBNext Ultra II DNA library prep kit for Illumina sequencing in accordance with the manufacturer's protocol. Sequencing was subsequently performed using the Illumina NextSeq system. After demultiplexing and trimming of adapters and removal of low-quality reads, fastq files were aligned to the PRVABC59 reference file using Mosaik2 (20). Variants were then called using LoFreq with standard settings (21). Genetic diversity was assessed using distance (i.e., sum of the variant frequencies), complexity (i.e., Shannon entropy, the uncertainty of randomly sampling a variant at each site), and nucleotide diversity (i.e., the sum of the variant frequencies per genome length).
Cell culture and in vitro virus studies.
Vertebrate cell lines were maintained at 37°C with 5% CO2. Mosquito cell lines were maintained at 28°C and 5% CO2. The JAR human placental cell line (ATCC HTB-144) was grown in RPMI medium supplemented with 10% FBS and 1% penicillin-streptomycin (Pen-Strep). The SH-SY5Y human neuroblastoma cell line (ATCC CRL-2266) was a gift from D. Beckham and was maintained in DMEM (Dulbecco's modified Eagle's medium)/F12 medium supplemented with 10% FBS and 1% Pen-Strep. The NTERA-2 cl.D1 human testicular cell line (ATCC CRL-1973), Vero cells, and C6/36 mosquito cells (all obtained from ATCC) were grown in DMEM supplemented with 10% FBS and 1% Pen-Strep. Huh7 cells (a gift from R. Kuhn) were grown in DMEM supplemented with 10% FBS and 1% Pen-Strep. Aag2 mosquito cells were grown in Schneider's medium supplemented with 10% FBS and 1% Pen-Strep.
All viral inoculations were performed in 12-well plates. Cells were grown to 70 to 90% confluence before inoculation, and infections were performed at a multiplicity of infection (MOI) of 0.1 in triplicate. Culture supernatant was harvested every 24 h, and virus was titrated on Vero cells by plaque assay as previously described (22), with the modification that the second overlay was added 4 days postinoculation. The lower limit for this assay was 1.7 log10 PFU/ml. Significance was determined by an unpaired t test with a Holm-Sidak correction for multiple comparisons (Prism).
Real-time RT-PCR.
Viral RNA was extracted from cellular supernatant using the Qiagen viral RNA minikit. Quantification of viral RNA was performed by a real-time RT-PCR assay using previously described ZIKV primers and probe (23). To make a standard curve, a fragment of ZIKV from nt 859 to 1278 was amplified by RT-PCR, and the fragment was TOPO cloned into pcDNA3.1 (Invitrogen). The plasmid was linearized with BglII (NEB), in vitro transcribed using the Ampliscribe T7 transcription kit (Epicentre), and DNase treated.
Mosquito infections and analysis.
Infectivity of the primary PRVABC59 isolate and infectious clone-derived virus was determined in A. aegypti mosquitoes (Poza Rica). The mosquitoes used were originally collected from wild populations in Mexico (24). Mosquitoes were maintained on calf blood and given 10% sucrose ad libitum. Larvae were reared and adults were maintained under controlled conditions of temperature (28°C), humidity (70% relative humidity), and light (14-h light–10-h dark diurnal cycle). Experiments involving infectious ZIKV in mosquitoes were performed under biosafety level 3 (BSL3) conditions. Generation F13 female mosquitoes were given an infectious blood meal containing 8 × 106 PFU of either primary PRVABC59 or infectious clone-derived virus in defibrinated calf blood. Following each infectious blood meal, back-titration was performed to ensure that the virus titers to which mosquitoes were exposed during each experimental replicate were comparable. At 14 days postinfection, mosquitoes were incapacitated using triethylamine. The legs were removed and transferred into a tube containing 500 μl mosquito diluent (1× phosphate-buffered saline [PBS] supplemented with 20% FBS [heat-inactivated], 50 μg/ml Pen-Strep, 50 μg/ml gentamicin, and 2.5 μg/ml amphotericin B [Fungizone]) and a stainless steel bead for homogenization. The mosquito proboscis was inserted into a capillary tube containing immersion oil (∼5 μl) and allowed to salivate for 20 min. Following salivation, mosquito bodies were placed in a separate tube containing mosquito diluent and a bead. The ends of capillary tubes containing oil and saliva were broken off into microcentrifuge tubes containing 250 μl mosquito diluent. Mosquito tissues (bodies and legs) were homogenized at 25 cycles/s for 1 min using a Retsch mixer mill MM400 (Germany) and centrifuged at 15,000 × g for 5 min at 4°C. Clarified supernatant was titrated by Vero cell plaque assay to determine the amount of infectious virus present in mosquito bodies. Saliva titers were confirmed by qRT-PCR. The presence of infectious virus in mosquito bodies was defined as infection positive. Dissemination is reported as the percentage of mosquitoes that became infected and contained infectious virus in their legs. The transmission rate is reported as the number of infected mosquitoes that contained infectious virus in the saliva.
In vivo growth and pathogenesis in mice.
Groups of 20 4-week-old alpha/beta and gamma interferon receptor-deficient AG129 mice were inoculated with 1,000 PFU of parental or infectious clone-derived ZIKV in a 0.1-ml inoculum by the intraperitoneal route. Mice were weighed and monitored daily for signs of disease. On days 1, 3, and 5 postinoculation, 3 male mice per group were deeply anesthetized, a blood sample was obtained by cardiac puncture, and mice were perfused with PBS prior to harvesting of tissues. The remaining 11 mice (7 males and 4 females per group) were euthanized when evidence of neurological disease was observed, and blood and tissues were obtained. Euthanasia criteria included hind limb paralysis or weakness, incoordination, and tremors. Additional euthanasia criteria included recumbency or conjunctivitis that precluded vision. Survival curves were compared using a log rank (Mantel-Cox) test (Prism). Serum was separated by centrifugation of whole blood. Tissues were homogenized in BA-1 medium (M199-H, 0.05 M Tris buffer, 1% bovine serum albumin, 2 mM l-glutamine, 4.2 mM sodium bicarbonate, 1% Pen-Strep, 1 μg/ml amphotericin B) by using a pestle and clarified by centrifugation. Serum and tissues were titrated by Vero cell plaque assay. Significance was determined by an unpaired t test with a Holm-Sidak correction for multiple comparisons (Prism). All animal studies were conducted under CDC-approved IACUC protocols.
ACKNOWLEDGMENTS
We gratefully thank the members of the Arthropod-Borne and Infectious Diseases Laboratory for valuable discussions and Sean Masters, John Liddell, Jason Velez, Karen Boroughs, and Janae Stovall at the CDC for animal care, cell culture, and sequencing support.
This work was supported in part by the National Institute of Allergy and Infectious Diseases, NIH, under grants AI114675 (B.J.G.) and AI067380 (G.D.E.).
J.W.-L., N.K.D., A.C.B., B.J.G., and G.D.E. designed the research, J.W.-L., N.K.D., K.B.-F., M.V., R.A.B., A.C.B., M.S., H.R., C.N., and C.R. performed the research, J.W.-L., N.K.D., and M.S. analyzed the data, and J.W.-L., A.C.B., N.K.D., B.J.G., and G.D.E. wrote the paper.
REFERENCES
- 1.Macnamara FN. 1954. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 2.Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, Parra B, Mora J, Becerra N, Lagos N, Vera L, Olivares B, Vilches M, Fernandez J. 2016. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol 161:665–668. doi: 10.1007/s00705-015-2695-5. [DOI] [PubMed] [Google Scholar]
- 3.Kindhauser MK AT, Frank V, Santhana RS, Dye C. 9 February 2016. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DL, Sharp TM, Torres J, Armstrong PA, Munoz-Jordan J, Ryff KR, Martinez-Quinones A, Arias-Berrios J, Mayshack M, Garayalde GJ, Saavedra S, Luciano CA, Valencia-Prado M, Waterman S, Rivera-Garcia B. 2016. Local transmission of Zika virus–Puerto Rico, November 23, 2015–January 28, 2016. MMWR Morb Mortal Wkly Rep 65:154–158. doi: 10.15585/mmwr.mm6506e2. [DOI] [PubMed] [Google Scholar]
- 5.Boulet SL, D'Angelo DV, Morrow B, Zapata L, Berry-Bibee E, Rivera M, Ellington S, Romero L, Lathrop E, Frey M, Williams T, Goldberg H, Warner L, Harrison L, Cox S, Pazol K, Barfield W, Jamieson DJ, Honein MA, Kroelinger CD. 2016. Contraceptive use among nonpregnant and postpartum women at risk for unintended pregnancy, and female high school students, in the context of Zika preparedness—United States, 2011-2013 and 2015. MMWR Morb Mortal Wkly Rep 65:780–787. doi: 10.15585/mmwr.mm6530e2. [DOI] [PubMed] [Google Scholar]
- 6.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. 2014. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill 19:pii=20720. [DOI] [PubMed] [Google Scholar]
- 7.Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, Jamieson DJ. 2016. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 65:30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- 8.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, Signor Ldel C. 2016. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg Infect Dis 22:933–935. doi: 10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. 2016. Culex pipiens and Aedes triseriatus mosquito susceptibility to Zika virus. Emerg Infect Dis 22:1857–1859. doi: 10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerbois M, Fernandez-Salas I, Azar SR, Danis-Lozano R, Alpuche-Aranda CM, Leal G, Garcia-Malo IR, Diaz-Gonzalez EE, Casas-Martinez M, Rossi SL, Del Rio-Galvan SL, Sanchez-Casas RM, Roundy CM, Wood TG, Widen SG, Vasilakis N, Weaver SC. 19 July 2016. Outbreak of Zika virus infection, Chiapas State, Mexico, 2015, and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. J Infect Dis. doi: 10.1093/infdis/jiw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. 2016. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg 94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 2016. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. 2016. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi SL, Vasilakis N. 2016. Modeling Zika virus infection in mice. Cell Stem Cell 19:4–6. doi: 10.1016/j.stem.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, Laassri M, Chumakov K, Pletnev AG. 2016. A full-length infectious cDNA clone of Zika virus from the 2015 epidemic in Brazil as a genetic platform for studies of virus-host interactions and vaccine development. mBio 7:e01114-16. doi: 10.1128/mBio.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, Yang Y, Tesh RB, Bourne N, Barrett AD, Vasilakis N, Weaver SC, Shi PY. 2016. An infectious cDNA clone of Zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 19:891–900. doi: 10.1016/j.chom.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu SY, Wu RH, Yang CC, Jao TM, Tsai MH, Wang JC, Lin HM, Chao YS, Yueh A. 2011. Successful propagation of flavivirus infectious cDNAs by a novel method to reduce the cryptic bacterial promoter activity of virus genomes. J Virol 85:2927–2941. doi: 10.1128/JVI.01986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubaugh ND, Weger-Lucarelli J, Murrieta RA, Fauver JR, Garcia-Luna SM, Prasad AN, Black WC IV, Ebel GD. 2016. Genetic drift during systemic arbovirus infection of mosquito vectors leads to decreased relative fitness during host switching. Cell Host Microbe 19:481–492. doi: 10.1016/j.chom.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WP, Stromberg MP, Ward A, Stewart C, Garrison EP, Marth GT. 2014. MOSAIK: a hash-based algorithm for accurate next-generation sequencing short-read mapping. PLoS One 9:e90581. doi: 10.1371/journal.pone.0090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilm A, Aw PP, Bertrand D, Yeo GH, Ong SH, Wong CH, Khor CC, Petric R, Hibberd ML, Nagarajan N. 2012. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res 40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. 2004. Differential virulence of West Nile strains for American crows. Emerg Infect Dis 10:2161–2168. doi: 10.3201/eid1012.040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black WC IV. 2015. Coevolution of the Ile1,016 and Cys1,534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis 9:e0004263. doi: 10.1371/journal.pntd.0004263. [DOI] [PMC free article] [PubMed] [Google Scholar]