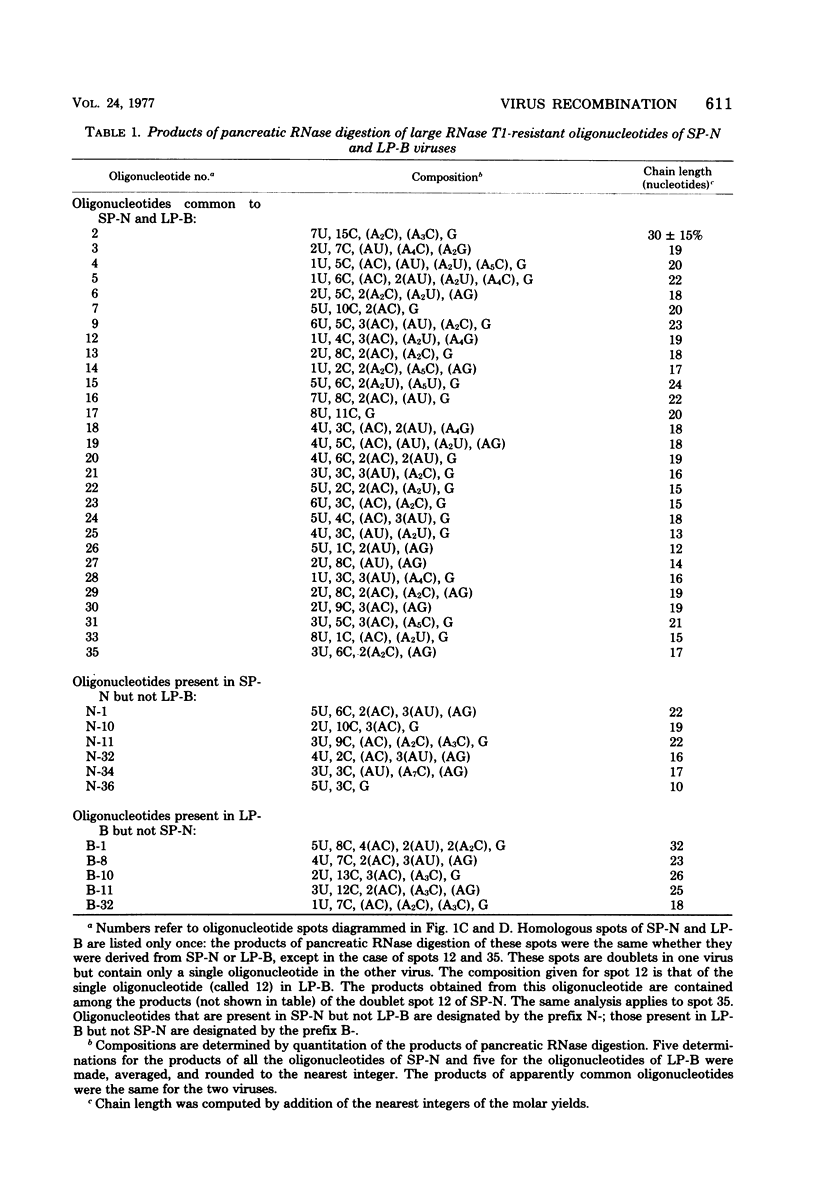
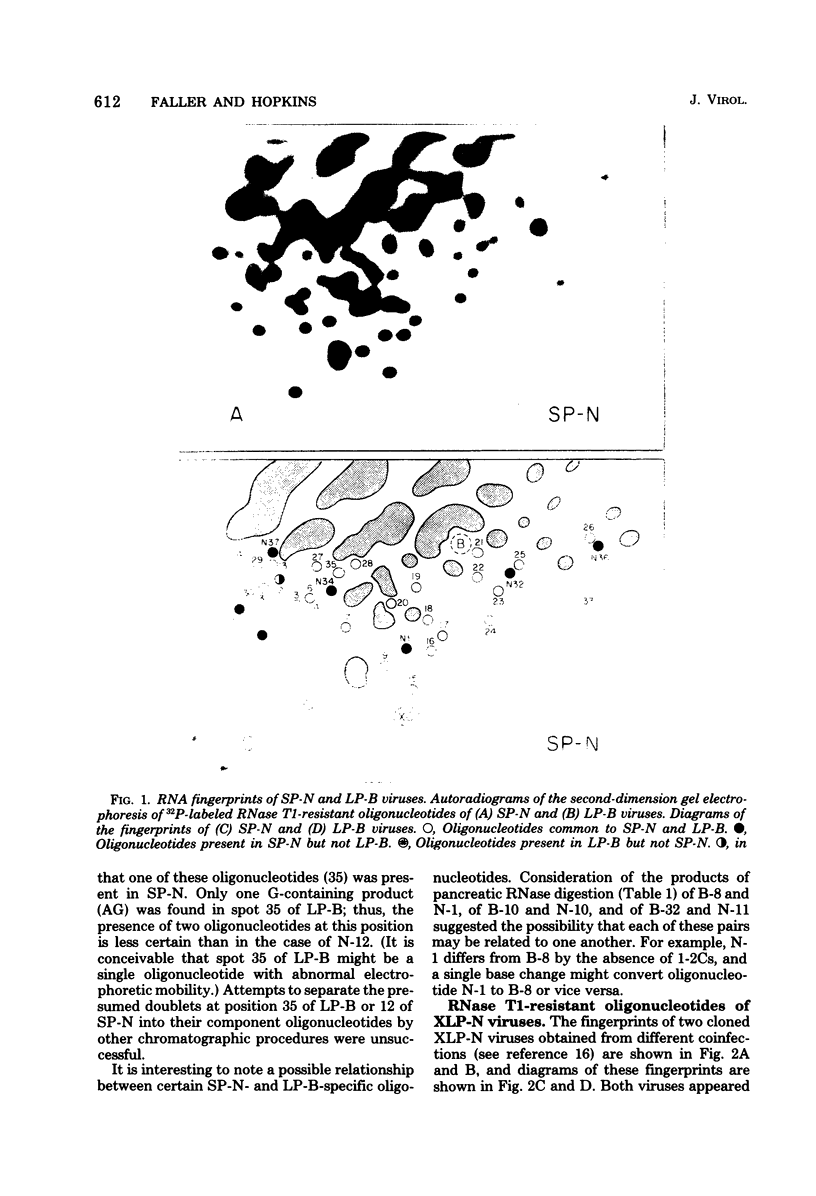
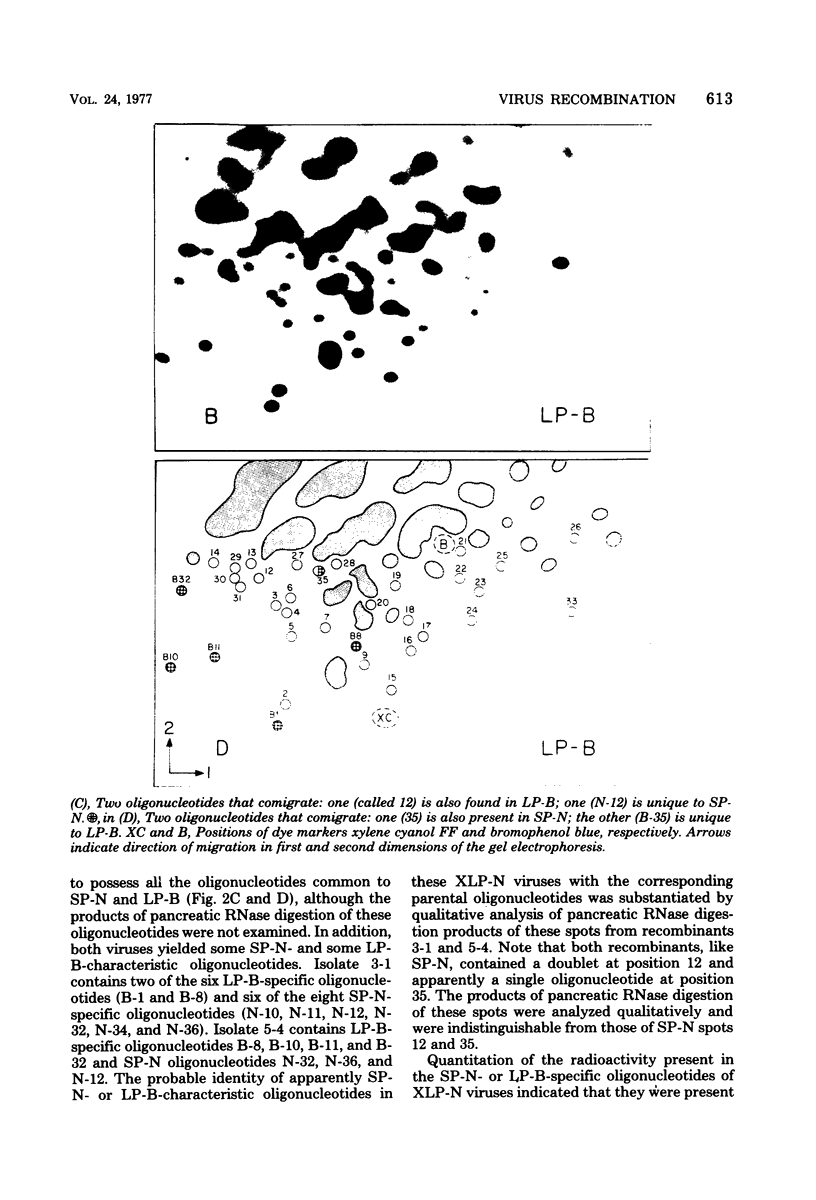
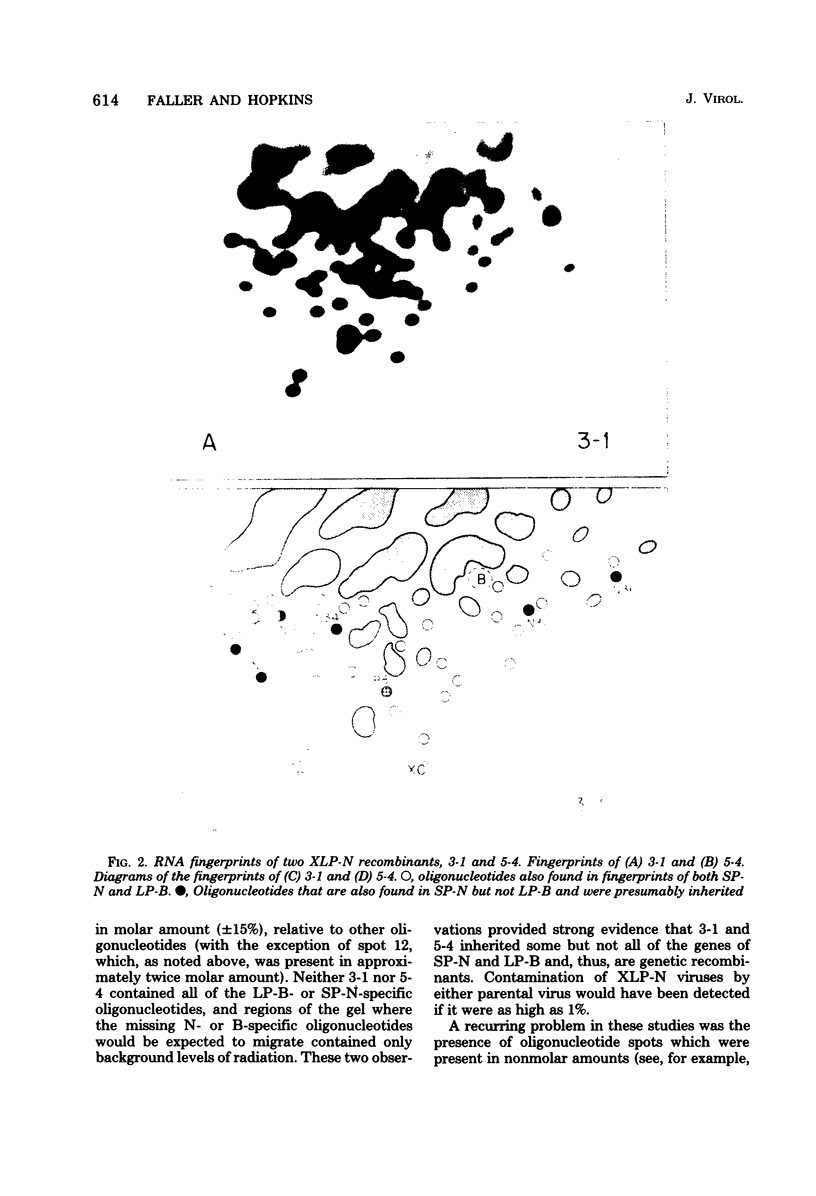
Abstract
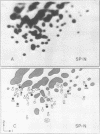
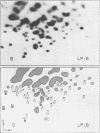
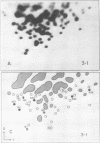
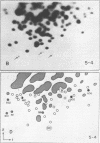
We used two-dimensional gel electrophoresis to obtain fingerprints of 32P-labeled RNase T1-resistant oligonucleotides derived from the genomes of an N- and a B-tropic murine leukemia virus of BALB/c. These viruses share approximately 30 large T1-resistant oligonucleotides. In addition, there are eight large oligonucleotides unique to the N-tropic virus, and there are six B-trophic virus-specific oligonucleotides. Viruses, designated XLP-N, which appear by biological criteria and analysis of virion proteins to be recombinants between these N- and B-tropic viruses, possess some but not all of the N or B virus-specific oligonucleotides.
Full text
PDF

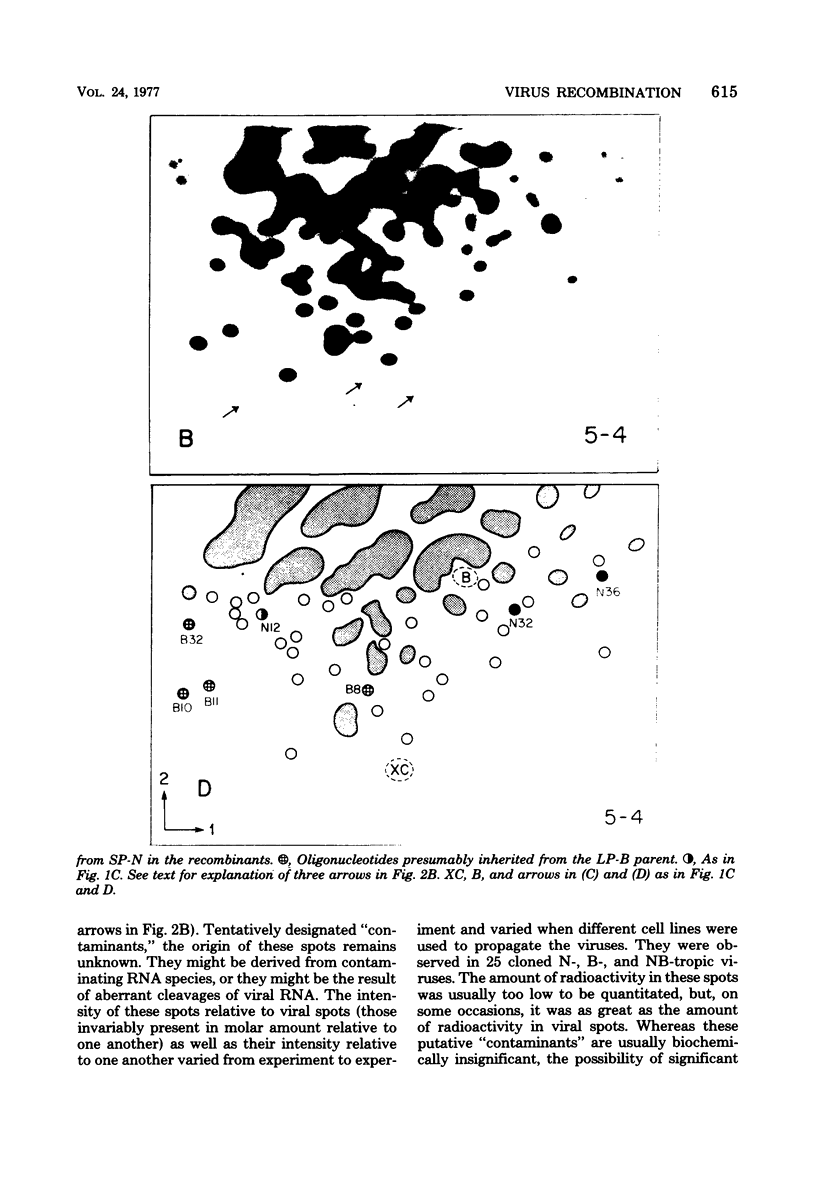


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. RNase T1-resistant oligonucleotides of B-tropic murine leukemia virus from BALB/c and five of its NB-tropic derivatives. J Virol. 1977 Jul;23(1):188–195. doi: 10.1128/jvi.23.1.188-195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Jolicoeur P. Variants of N-tropic leukemia virus derived from BALB/c mice. J Virol. 1975 Oct;16(4):991–999. doi: 10.1128/jvi.16.4.991-999.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Gottlieb P. D. Evidence for recombination between N- and B-tropic murine leukemia viruses. J Virol. 1977 Mar;21(3):1074–1078. doi: 10.1128/jvi.21.3.1074-1078.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Traktman P., Whalen K. N-Tropic variants obtained after co-infection with N- and B-tropic murine leukemia viruses. J Virol. 1976 Apr;18(1):324–331. doi: 10.1128/jvi.18.1.324-331.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on synthesis of N-tropic and B-tropic murine leukemia viral RNA. Cell. 1976 Jan;7(1):33–39. doi: 10.1016/0092-8674(76)90252-x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E. Induction of GIX antigen and gross cell surface antigen after infection by ecotropic and xenotropic murine leukemia viruses in vitro. J Virol. 1976 Dec;20(3):545–554. doi: 10.1128/jvi.20.3.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. L., Spahn G. J., Rabstein L. S., Kelloff G. J., Huebner R. J. Murine C-type RNA virus from spontaneous neoplasms: in vitro host range and oncogenic potential. Science. 1973 Aug 17;181(4100):665–667. doi: 10.1126/science.181.4100.665. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler J., Hynes R., Hopkins N. Evidence for recombination between N- and B-tropic murine leukemia viruses: analysis of three virion proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1977 Sep;23(3):700–700. doi: 10.1128/jvi.23.3.700-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Galehouse D., Mellon P., Duesberg P., Mason W. S., Vogt P. K. Mapping oligonucleotides of Rous sarcoma virus RNA that segregate with polymerase and group-specific antigen markers in recombinants. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3952–3956. doi: 10.1073/pnas.73.11.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]