Abstract
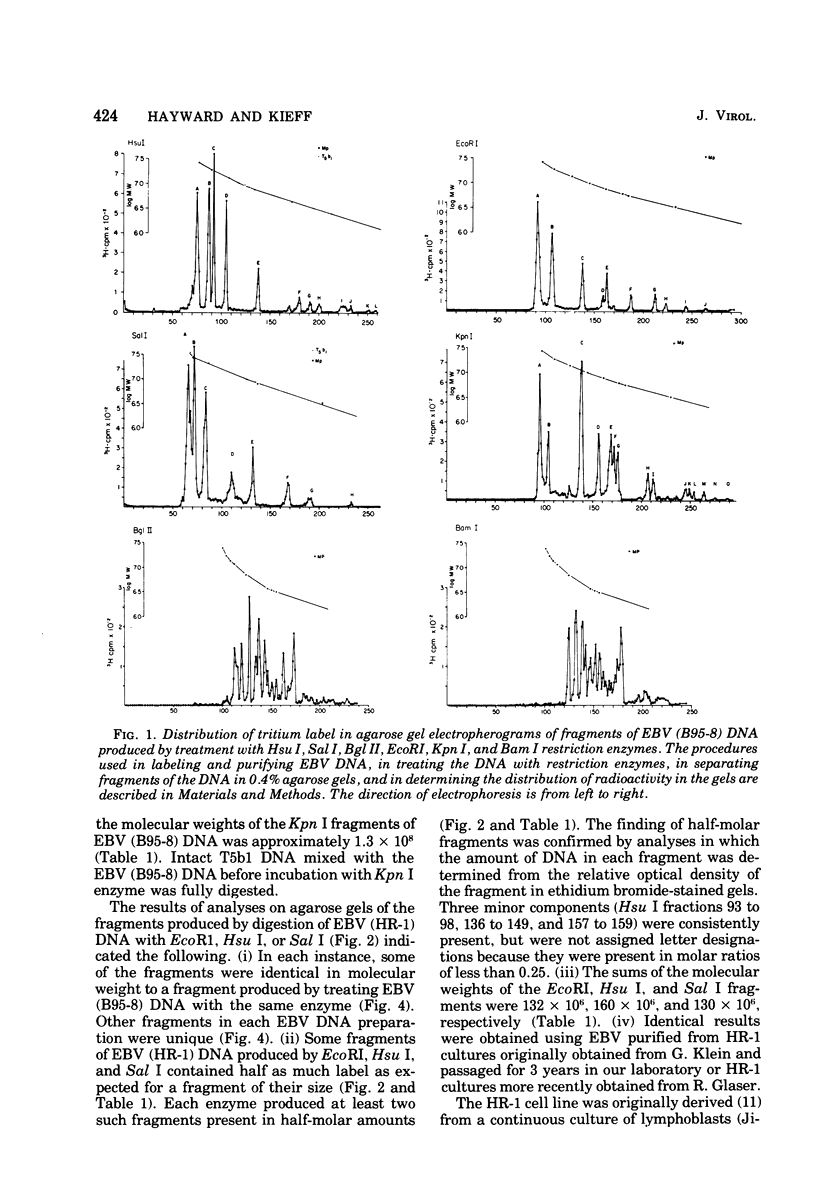
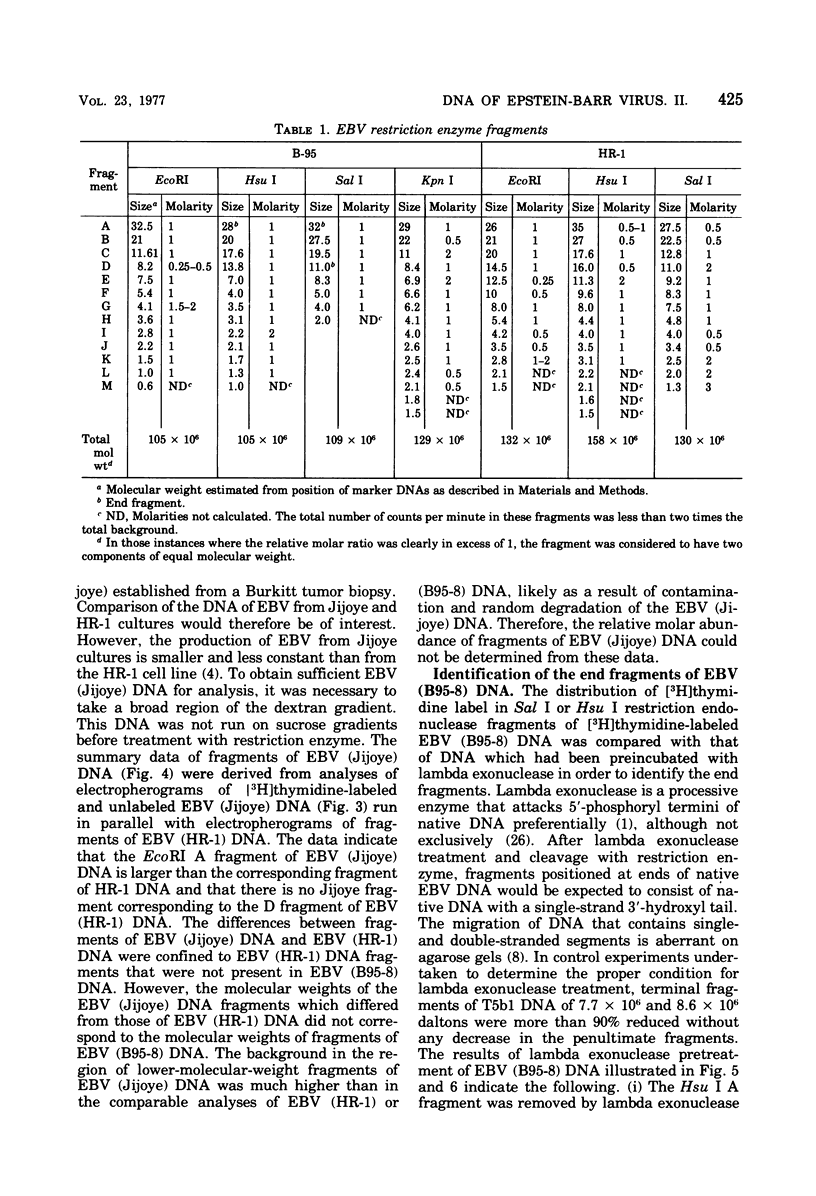
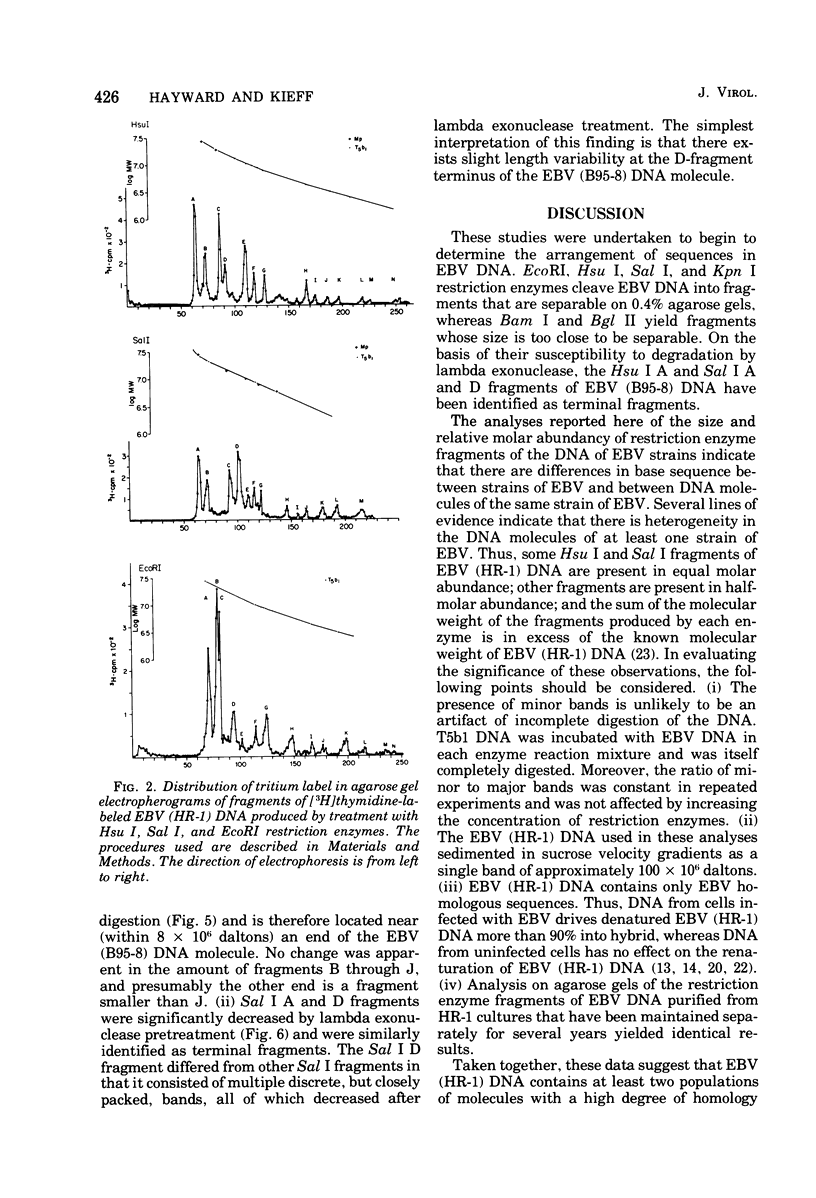
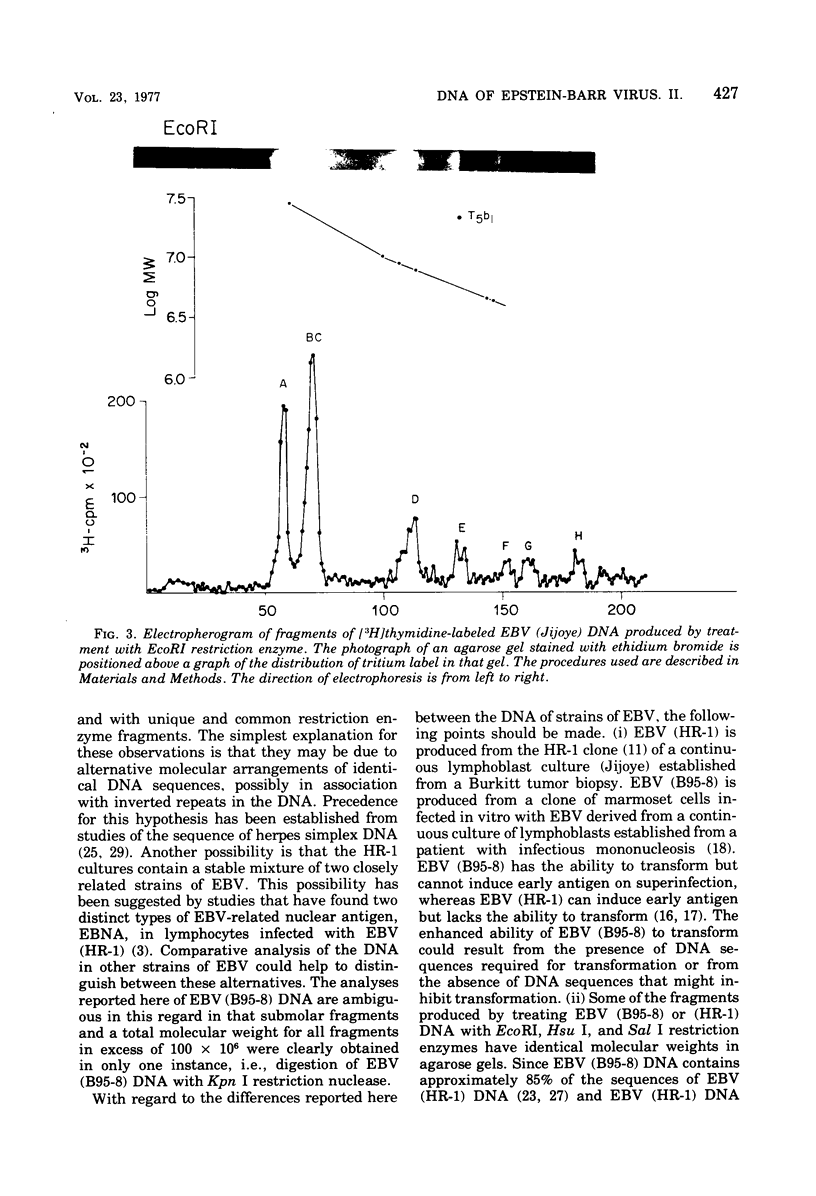
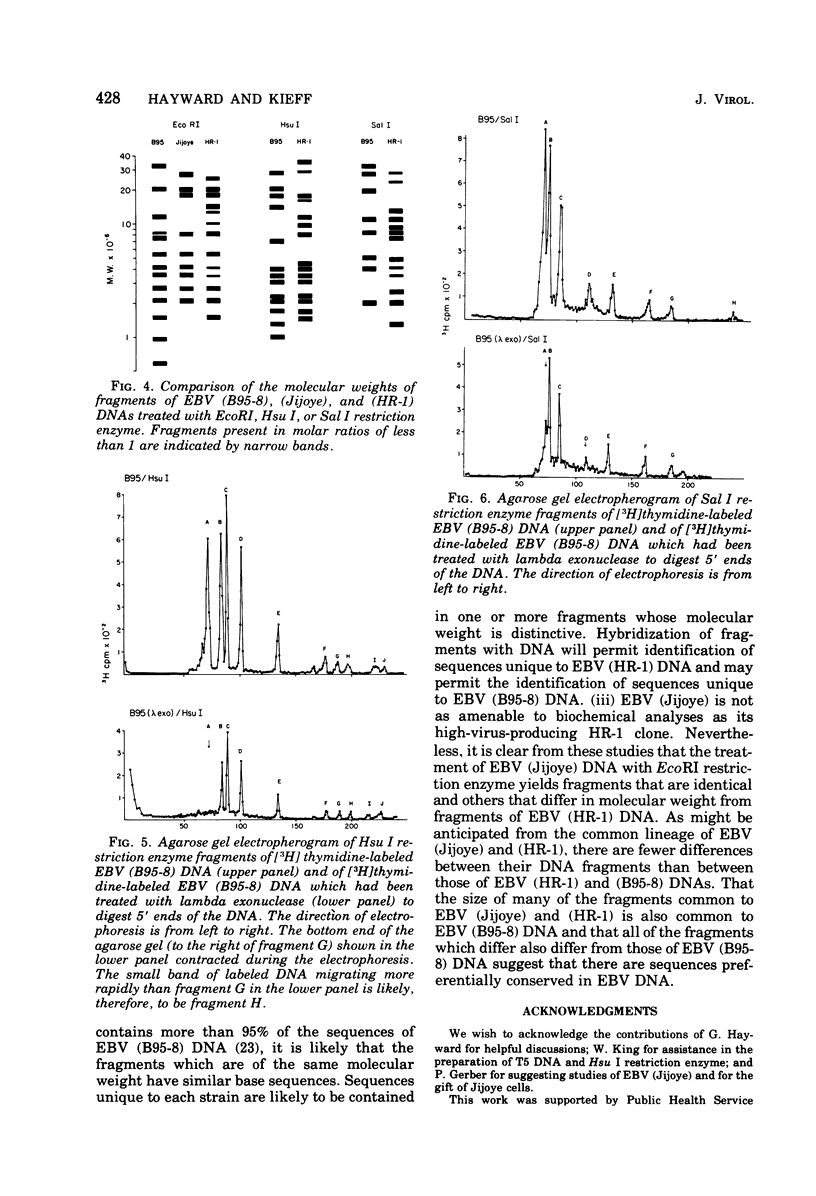
Incubation of the DNA of the B95-8 strain of Epstein-Barr virus [EBV (B95-8) DNA] with EcoRI, Hsu I, Sal I, or Kpn I restriction endonuclease yielded 8 to 15 fragments separable on 0.4% agarose gels and ranging in molecular weight from less than 1 to more than 30 × 106. Bam I and Bgl II yielded fragments smaller than 11 × 106. Preincubation of EBV (B95-8) DNA with lambda exonuclease resulted in a decrease in the Hsu I A and Sal I A and D fragments, indicating that these fragments are positioned near termini. The electrophoretic profiles of the fragments produced by cleavage of the DNA of the B95-8, HR-1, and Jijoye strains of EBV were each distinctive. The molecular weights of some EcoRI, Hsu I, and Sal I fragments from the DNA of the HR-1 strain of EBV [EBV (HR-1) DNA] and of EcoRI fragments of the DNA of the Jijoye strain of EBV were identical to that of fragments produced by cleavage of EBV (B95-8) DNA with the same enzyme, whereas others were unique to each strain. Some Hsu I, EcoRI, and Sal I fragments of EBV (HR-1) DNA and Kpn I fragments of EBV (B95-8) DNA were present in half-molar abundance relative to the majority of the fragments. In these instances, the sum of the molecular weights of the fragments was in excess of 108, the known molecular weight of EBV (HR-1) and (B95-8) DNA. The simplest interpretation of this finding is that each EBV (HR-1), and possibly also (B95-8), DNA preparation contains two populations of DNA molecules that differ in the arrangement of DNA sequences about a single point, such as has been described for herpes simplex virus DNA. Minor fragments could also be observed if there were more than one difference in primary structure of the DNAs. The data do not exclude more extensive heterogeneity in primary structure of the DNA of the HR-1 strain. However, the observation that the relative molar abundance of major and minor fragments of EBV (HR-1) DNA did not vary between preparations from cultures that had been maintained separately for several years favors the former hypothesis over the latter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter D. M., Radding C. M. The role of exonuclease and beta protein of phage lambda in genetic recombination. II. Substrate specificity and the mode of action of lambda exonuclease. J Biol Chem. 1971 Apr 25;246(8):2502–2512. [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Frenkel N., Roizman B. Anatomy of herpes simplex virus DNA: strain differences and heterogeneity in the locations of restriction endonuclease cleavage sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1768–1772. doi: 10.1073/pnas.72.5.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. II. Arrangement of the single-stranded DNA fragments in the T5 + and T5st(O) chromosomes. J Mol Biol. 1972 Feb 14;63(3):397–407. doi: 10.1016/0022-2836(72)90436-6. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Unique double-stranded fragments of bacteriophage T5 DNA resulting from preferential shear-induced breakage at nicks. Proc Natl Acad Sci U S A. 1974 May;71(5):2108–2112. doi: 10.1073/pnas.71.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. D. Epstein-Barr virus-specific RNA. I. Analysis of viral RNA in cellular extracts and in the polyribosomal fraction of permissive and nonpermissive lymphoblastoid cell lines. J Virol. 1976 May;18(2):518–525. doi: 10.1128/jvi.18.2.518-525.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn U., Lindahl T., Klein C. Fate of virus DNA in the abortive infection of human lymphoid cell lines by Epstein-Barr virus. J Gen Virol. 1972 Sep;16(3):409–412. doi: 10.1099/0022-1317-16-3-409. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Nonoyama M., Pagano J. S. Reassociation kinetics for Epstein-Barr virus DNA: nonhomology to mammalian DNA and homology of viral DNA in various diseases. J Virol. 1973 Nov;12(5):1006–1012. doi: 10.1128/jvi.12.5.1006-1012.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Levine J. Homology between Burkitt herpes viral DNA and DNA in continuous lymphoblastoid cells from patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):355–358. doi: 10.1073/pnas.71.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R., Pendersen M., Kieff E. Complexity of EBV homologous DNA in continous lymphoblastoid cell lines. Virology. 1976 Oct 1;74(1):227–231. [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Sriprakash K. S., Lundh N., Huh MM-O, Radding C. M. The specificity of lambda exonuclease. Interactions with single-stranded DNA. J Biol Chem. 1975 Jul 25;250(14):5438–5445. [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA. V. Terminally repetitive sequences. J Virol. 1976 Feb;17(2):503–512. doi: 10.1128/jvi.17.2.503-512.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S., Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975 Jun;15(6):1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B., Savage T., Spear P. G., Mizell M., Durr F. E., Sypowicz D. Characterization of the DNA of herpesviruses associated with Lucké adenocarcinoma of the frog and Burkitt lymphoma of man. Virology. 1970 Sep;42(1):257–261. doi: 10.1016/0042-6822(70)90265-5. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Becker Y. Studies on EB virus of Burkitt's lymphoblasts. Virology. 1969 Oct;39(2):312–321. doi: 10.1016/0042-6822(69)90051-8. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Hayward G. S., Bujard H. Physical mapping of the HindIII, EcoRI, Sal and Sma restriction endonuclease cleavage fragments from bacteriophage T5 DNA. Mol Gen Genet. 1976 Feb 2;143(3):279–290. doi: 10.1007/BF00269404. [DOI] [PubMed] [Google Scholar]



