Abstract
The tolerogenic hepatic microenvironment impedes clearance of viral infections but is an advantage in viral vector gene transfer, which often results in immune tolerance induction to transgene products. Although the underlying tolerance mechanism has been extensively studied, our understanding of antigen presentation to transgene product-specific CD4+ T cells remains limited. To address this, we administered hepatotropic adeno-associated virus (AAV8) vector expressing cytoplasmic ovalbumin (OVA) into wt mice followed by adoptive transfer of transgenic OVA-specific T cells. We find that that the liver-draining lymph nodes (celiac and portal) are the major sites of MHC II presentation of the virally encoded antigen, as judged by in vivo proliferation of DO11.10 CD4+ T cells (requiring professional antigen-presenting cells, e.g., macrophages) and CD4+CD25+FoxP3+ Treg induction. Antigen presentation in the liver itself contributes to activation of CD4+ T cells egressing from the liver. Hepatic-induced Treg rapidly disseminate through the systemic circulation. By contrast, a secreted OVA transgene product is presented in multiple organs, and OVA-specific Treg emerge in both the thymus and periphery. In summary, liver draining lymph nodes play an integral role in hepatic antigen presentation and peripheral Treg induction, which results in systemic regulation of the response to viral gene products.
Introduction
The microenvironment of the liver has a strong immune regulatory capacity, thereby controlling responses to antigens derived from the blood or intestinal uptake.1 When compared to other solid organ transplants, this ability to exert immune tolerance is a major advantage in liver transplantation, requiring much lesser use of immune suppressive drugs. However, the partially immune suppressive environment of the liver attenuates immune responses against liver tumors and can also be exploited by viral pathogens, resulting in chronic infections such in hepatitis B or C.2 In gene therapy, one can take advantage of these unique immunological features to promote sustained therapeutic gene expression. The capacity of liver-directed gene transfer to induce immune tolerance to transgene products has been demonstrated for several diverse viral vectors such as lentivirus or adeno-associated virus (AAV).1,3–6 Expression of an antigen in hepatocytes can induce peripheral tolerance through induction of CD4+CD25+FoxP3+ regulatory T cells (Treg).7 In case of a secreted transgene product that may reach the thymus through the circulation, central tolerance mechanisms may also apply.8
High levels of hepatic transgene expression increase Treg induction and favor immune tolerance, resulting in suppression of antibody and T cell responses at extrahepatic sites as well.9–12 Therefore, induced liver tolerance may become dominant over activation of immune responses in other tissues or to systemically delivered antigens. Hence, hepatic gene transfer is in development for immune tolerance induction for inherited protein deficiencies and is also considered as an immune modulatory therapy for autoimmune disease and allergies.4,13–15 For example, antibody (“inhibitor”) formation against coagulation factors in the treatment of hemophilia could be prevented and even reversed in murine and canine models.10,16–18 In addition to Treg induction, direct inhibition of memory B cells by high antigen levels likely contributed to these successes.10 Another example for a potential application of this approach is gene therapy for Pompe disease. Here, gene transfer to muscle in order to replace the lysosomal storage enzyme GAA (acid α-glucosidase) bears a risk of immune responses to GAA that can be suppressed by simultaneous hepatic gene transfer.19–21
The mechanism of liver gene transfer-induced immune tolerance has been extensively studied over the past years.4 Both induction and maintenance of tolerance are dependent on Treg.10 Induction of transgene product-specific Treg requires TGF-β and is in part dependent on the GITR – GITR-L costimulatory pathway.22–24 Expression of the cytokine IL-10 by Treg or Kupffer cells helps to suppress CD8+ T cell responses in the liver but may not be required for suppression of antibody formation.22,25 In addition to Treg induction, other mechanisms contribute to shifting the balance from an effector to a regulated response. These include both Fas-FasL-dependent and -independent programmed cell death and T cell anergy.8,26 All these events require presentation of transgene product-derived antigen by MHC II to CD4+ T cells. However, much less is known about this aspect. Previously, we found a requirement for both dendritic cells (DC) and macrophages/Kupffer cells (KCs) for in vivo presentation to CD4+ T cells specific to the transgene product expressed from an AAV vector upon hepatic gene transfer.10 Others found that liver sinusoidal endothelial cells (LSEC) are capable of acquiring hepatocyte-derived antigens and inducing Treg.27 There is an ongoing debate in the field about the role of antigen presentation in the liver itself as opposed to in lymphoid organs. In order to be able to identify sites of antigen presentation and track activation of CD4+ T cells and induction of Treg, we designed experiments using T cell receptor-transgenic CD4+ T cells with specificity to a cytoplasmic protein expressed from AAV vector upon hepatic gene transfer. We identify the celiac and portal lymph nodes as a major site of transgene product presentation to CD4+ T cells induction but also found evidence for CD4+ T cell activation in the liver itself. Activated CD4+ T cell egress and then re-enter liver and lymphoid organs. Further, CD4+CD25+FoxP3+ Treg were peripherally induced, initially in the liver draining lymph nodes, and rapidly disseminated through the blood, representing one mechanism by which hepatic gene transfer establishes systemic tolerance even to nonsecreted proteins. These data have implications for development of hepatic gene transfer and for immune responses to viral infections of the liver.
Results
Secreted ovalbumin antigen is presented to specific CD4+ T cells in multiple organs following hepatic AAV8 gene transfer
AAV8 expressing a secreted Ovalbumin (Ova) transgene was administered intravenously to wt BALBc mice (Figure 1a). This vector has strong liver tropism and is highly effective in transduction of murine hepatocytes in vivo.28,29 After 2 weeks, half of the mice were given gadolinium chloride, which specifically inactivates Kupffer cells and M1 macrophages.30 Gadolinium chloride was injected i.p. at 10 mg/kg on day 13 and 14 after AAV8-Ova administration. On day 14, Ova-specific CD4+ T cells labeled with CellTrace Violet dye were adoptively transferred into all mice. These CD4+ T cells had been isolated from BALB/c mice that are transgenic for the Ova-specific T cell receptor (TCR) DO11.10.8,31 Five days after adoptive transfer, livers and spleens were collected, and single cell suspensions were analyzed by flow cytometry. Substantial proliferation of DO11.10-tg CD4+ T cells was observed in both tissues. This was significantly decreased in mice receiving gadolinium chloride (Figure 1b). This experiment was repeated but this time numerous additional lymphoid tissues were analyzed by flow cytometry. Substantial proliferation of adoptively transferred DO11.10 CD4+ T cells is readily detected in all tissues surveyed. Proliferation was highest in liver and spleen, as would be expected for a protein expressed in systemic circulation. After inactivation of Kupffer cells and M1 macrophages, proliferation was most dramatically reduced in liver and spleen, while all other organs (except for the mesenteric lymph node) also showed a reduction in the average rate of proliferation (Figure 1c). Taken together, the data indicate that professional APCs, and in particular Kuppfer cells/macrophages, are required for presentation of transgene-derived antigen to CD4+ T cells.
Figure 1.
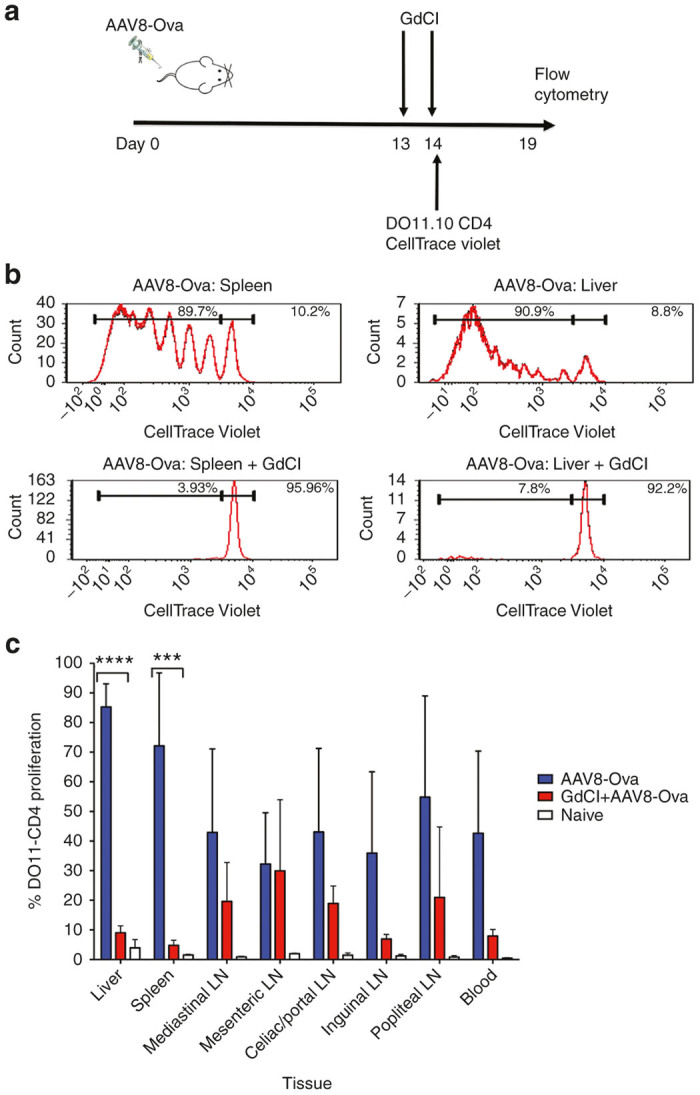
Proliferation of adoptively transferred Ova-specific DO11.10-tg CD4+ T cells in wt BALBc mice transduced with AAV-Ova (expressing secreted Ova) with or without inactivation of Kupffer cells and M1 macrophages via gadolinium chloride (GdCl). (a) Experimental plan. BALBc mice were transduced with 1 × 1011 vg of AAV8-Ova vector. After 2 weeks, half of the mice are given GdCl, followed by adoptive transfer of DO11.10-tg CD4+ T cells labeled with CellTrace Violet dye to all mice. Proliferation of transferred cells was measured by flow cytometry 5 days later. (b) Representative examples of proliferation of transferred cells in the spleen and the liver with or without GdCl treatment (n = 3/experimental group). (c) Proliferation in liver, spleen, and different lymph nodes in a repeat experiment (n = 5–7/experimental group). Naive: Mice that received neither vector nor GdCl. Data are average ± SD. **P < 0.01, ***P < 0.0001.
Gene delivery of cytoplasmic Ova induces proliferation primarily in specific liver-draining lymph nodes
In spite of the strong liver tropism of AAV8, using a secreted transgene product did not allow us to pinpoint the location of presentation of hepatic expressed antigen, because of dissemination to multiple organs via the systemic circulation. In order to determine where hepatic (rather than systemically) expressed antigen is MHC II presented, we employed an AAV8 vector that expresses nonsecreted, cytoplasmic Ova (Cyto-Ova). DO11.1 CD4+ T cells labeled with CellTrace Violet were adoptively transferred into wt BALBc mice that had previously been transduced with AAV8-Cyto-Ova. We then assessed proliferation of the donor DO11.10 CD4+ T cells in liver, spleen and various lymph nodes, including celiac and portal lymph nodes (which are both located just outside the liver where the hepatic portal vein enter) (Figure 2b).32,33 To allow for antibody staining and flow cytometry, we combined cells from celiac and portal lymph nodes,34 i.e., the liver-draining lymph nodes. As shown in Figure 2a, antigen presentation occurred chiefly in the draining lymph nodes of the liver and not in other lymph nodes or other organs such as spleen or liver. Administering of gadolinium chloride caused a substantial decrease in proliferation (Figure 2a). Therefore, a similar requirement for professional APCs exists for presentation of cytoplasmic or secreted transgene product. We conclude that the transgene product, whether cytoplasmic or secreted, has to be taken up by professional APCs for MHC II presentation that is adequate to induce CD4+ T cell proliferation.
Figure 2.
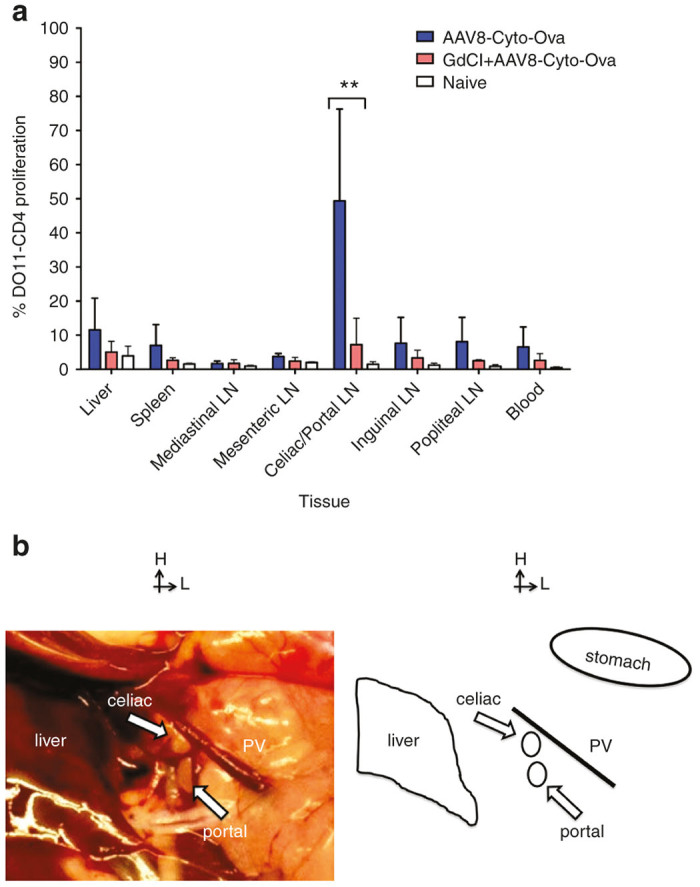
Proliferation of adoptively transferred DO11.10-tg CD4+ T cells in wt BALB/c mice transduced with AAV-Cyto-Ova (expressing cytoplasmic Ova) with or without inactivation of Kupffer cells and M1 macrophages via gadolinium chloride. The experimental timeline was identical to that in Figure 1. (a) Proliferation in liver, spleen, and different lymph nodes (n = 5–6/experimental group). Naive: Mice that received neither vector nor GdCl. Data are average ± SD. **P < 0.01. (b) Location of the liver draining celiac and portal lymph nodes in a BALB/c mouse. Anatomical structures are indicated to the right.
Whereas initial activation of Ova-transgene-specific CD4+ T cells occurs in the liver and liver-draining lymph nodes, antigen-induced Treg distribute through the systemic circulation
An alternative method to detect antigen presentation is to assay for expression of early activation markers in the T cells. AAV8-Cyto-Ova was injected into DO11-10-tg Rag-2-/- BALB/c mice. Due to targeted deletion of recombinase activating gene 2 (rag-2), these mice lack endogenous rearranged TCRs. However, transgenic expression of the DO11.10 TCR assures that CD4+ T cells develop, which are all Ova-specific. Furthermore, these mice lack naturally occurring nTreg.28,35–37 Upon hepatic gene transfer, upregulation of the early activation marker CD69+ in CD4+ T cells was shown first and predominately in the liver and liver-draining celiac/portal lymph nodes (Figure 3a). This CD4+ T cell activation remained highest over time in these locations, implicating these tissues as the primary sites of antigen presentation leading to CD4+ T cell activation. On the other hand, induced regulatory T cells (iTreg) were seen early on in all tissues surveyed at a similar level, except for the blood, which showed higher Treg frequencies early on (Figure 3b). At later time points, Treg were found in all tissues, with the celiac/portal lymph nodes and liver showing the highest frequencies. We conclude that the liver and its draining lymph nodes are major sites of early activation of transgene product-specific CD4+ T cells, as well as sites for accumulation of activated CD4+ T cells and iTreg. Furthermore, the data suggests that iTreg rapidly disseminate through the blood.
Figure 3.
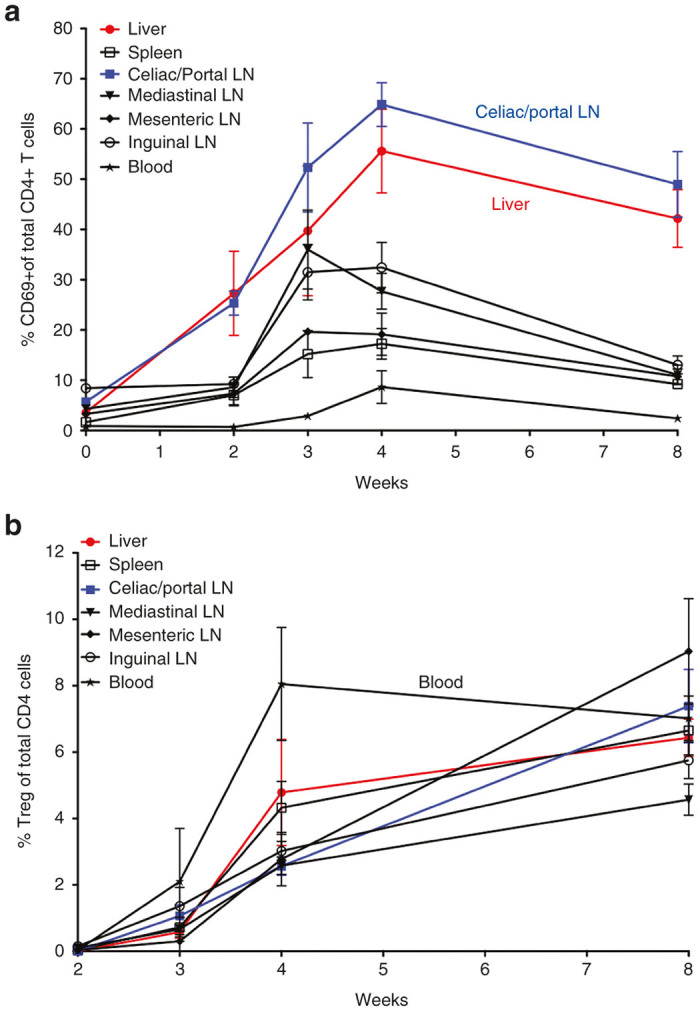
Frequencies of activated CD69+CD4+ T cells (a) and of CD4+CD25+FoxP3+ iTreg (b) in different tissues after as a function of time after AAV8-Cyto-Ova administration to DO11.10-tg Rag-2-/- BALB/c mice (1 × 1012 vg/mouse, n = 3–6/time point), as determined by flow cytometry. Data are average ± SD.
Blocking lymphocyte mobilization reveals sites of initial CD4+ activation, egression, and re-entry to immune organs
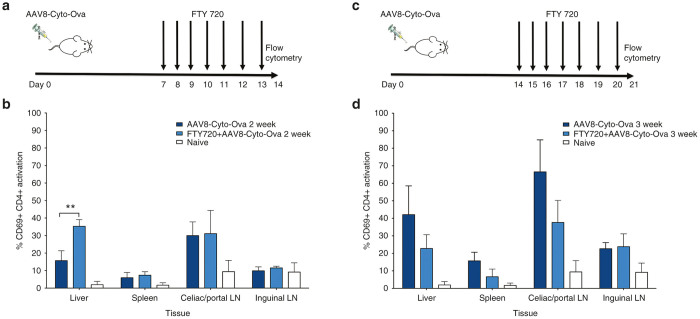
The experiment with AAV8-Cyto-Ova in DO11-10-tg Rag-2-/- BALB/c mice was repeated with the following modification. Starting 1 week after gene transfer, half of the mice were additionally given daily doses of FTY720 for 1 week, followed by tissue harvest (Figure 4a). FTY720 prevents the upregulation of Sphingosine 1-phosphate (SIP) adhesion molecules, inhibiting lymphocyte migration, though still allowing T cell activation.38–40 Two weeks after gene transfer, elevated frequencies of CD69+CD4+ T cells were again found predominately in the liver and celiac/portal lymph nodes in control mice not receiving FTY720 (Figure 4b). FTY720 treatment, to “lock” activated CD4+ T cells in place, again resulted elevated frequencies of CD69+CD4+ T cells in the liver. Frequencies remained unchanged and high in liver-draining lymph nodes (Figure 4b). This result suggests that activated CD4+ T cells more rapidly egress from the liver than from the draining nodes. However, these do not accumulate in peripheral circulation, as no activated T cells were found in circulation (data not shown).
Figure 4.
Frequencies of activated CD69+CD4+ T cells in different tissues of DO11.10-tg Rag-2-/- BALB/c mice after AAV8-Cyto-Ova administration with or without FTY720 treatment (to block T cell migration). Vector was given to all mice on day 0. (a, b) Experiment 1: Half of the mice were given daily injections of FTY720 from d7 to d13, followed by flow cytometric analysis on day 14 to determine frequencies of CD69+CD4+ T cells. (c, d) Experiment 2: Half of the mice were given daily injections of FTY720 from d14 to d20, followed by flow cytometric analysis on day 21 to determine frequencies of CD69+CD4+ T cells. Data are average ± SD for n = 3–5/experimental group. **P < 0.01.
By 3 weeks after gene transfer (Figure 4c), frequencies of CD69+CD4+ T cells had further increased in liver, celiac/portal nodes, and spleens of control mice (Figure 4d). When the 1-week regimen with FTY was performed starting 2 weeks after gene transfer, followed by flow cytometric analysis, the frequencies of activated CD4+ T cells in liver, liver-draining lymph nodes, and spleens were significantly reduced compared to control mice. Therefore, at this later time point, activated Ova-specific CD4+ T cells in these tissues in part represented cells that had by now re-entered lymphoid organs and the liver.
Induction of Cyto-ova-specific Treg occurs exclusively extra-thymic and at least in part in liver-draining lymph nodes
Consistent with our previous studies, we find Treg emerging at low frequency in the thymus after hepatic gene transfer of secreted Ova (Figure 5a).7,8 This was in addition to the more pronounced induction of iTreg in liver and secondary lymphoid organs (data not shown). In contrast, Treg were largely undetectable in the thymus when analyzed 5 weeks after delivery of Cyto-Ova (Figure 5a). At this time, the Treg frequency in spleen and blood in response to Cyto-Ova was about half of that achieved with secreted Ova. Since peripherally induced iTreg already appear by ~3 weeks after AAV-Cyto-Ova gene transfer, we sought to determine their origin using the same experimental protocol outlined in Figure 4c, which is to block T cell migration during the third week after gene transfer with FTY720. Drug treatment significantly increased the frequency of iTreg in the liver-draining lymph nodes (but not other organs, Figure 5b), suggesting that these nodes are also major sites of Treg induction to hepatic expressed transgene product.
Figure 5.
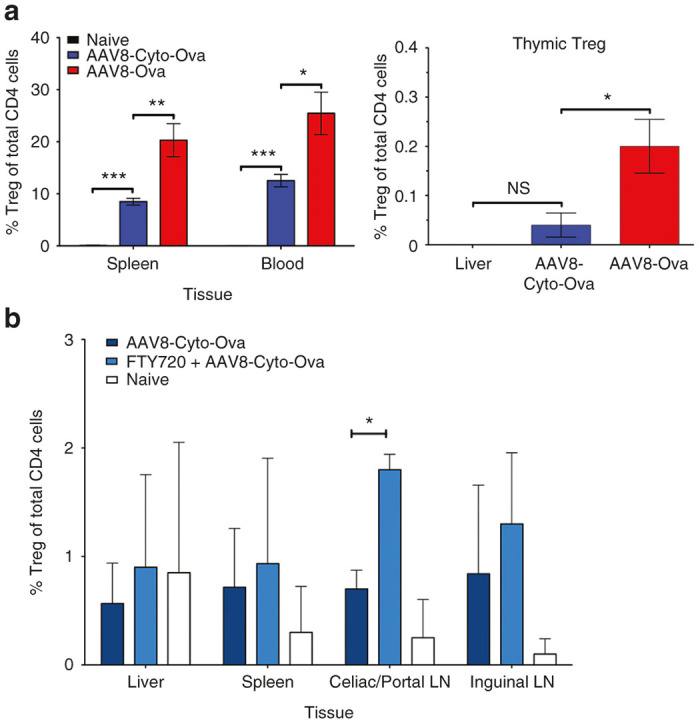
Peripheral induction of Treg in liver draining lymph nodes after AAV8-Cyto-Ova gene transfer. (a) Flow cytometric analysis of CD4+CD25+Foxp3+ Treg in the thymus 5 weeks after AAV8-Ova (1 × 1011 vg/mouse) or AAV8-Cyto-Ova (1 × 1012 vg/mouse) administration. In contrast to AAV8-Ova administration, which results in systemic delivery of secreted Ova and emergence of Treg in the thymus, AAV8-Cyto-Ova induced Treg are not found in the thymus. Data are average ± SD for n = 5–6/experimental group. (b) Frequencies of peripherally induced CD4+CD25+Foxp3+ T cells 3 weeks after AAV-Cyto-Ova administration. Half of the mice were given daily injections of FTY720 from d14 to d20, followed by flow cytometric analysis on day 21. Data are average ± SD for n = 3/experimental group. *P < 0.05, **P < 0.001, ***P < 0.0001.
Discussion
Antigen presentation is a critical part of an adaptive immune response and also of tolerance induction to a specific antigen. The architecture and composition of liver provide an environment that often promotes immune tolerance.41 For example, APC residing in sinusoids such as KCs and LSEC allow for antigen presentation to T cells present in the blood. The liver also contains a larger number of plasmacytoid DCs than other organs. These have important innate immune functions but also facilitate Treg induction, possibly via expression of IDO (Indoleamine-pyrrole 2,3-dioxygenase).35,42 Expression of immune-suppressive cytokines such as IL-10 and negative regulators of T cell activation such as PD-L1 (Programmed cell Death-Ligand 1) further contribute to a tolerogenic environment.2 Hence, gene therapy based on expression in the liver is an excellent approach to induce immune tolerance to a transgene product. AAV vectors, characterized by eliciting only low and transient innate immunity and by inefficient transduction of professional APCs, are ideal for this purpose.43–45 However, other vector systems, such as microRNA-regulated integrating and integration-deficient lentiviral vectors, have also been highly effective in tolerance induction.5,6 Although critical to liver tolerance induction, surprisingly little is known about presentation of the expressed antigen to CD4+ T cells. The study presented here fills some of these gaps in knowledge. Our findings are also viral infections of the liver such as with HBV or HCV.
Liver-draining lymph nodes as a major site of antigen presentation
Because of the unique composition and location of APCs in hepatic environment, it has been thought that antigen presentation in the liver itself leads to T cell activation and to Treg induction. This model is in contrast to the classical view of T cell activation in draining lymph nodes that provide immune surveillance for the organ. Consistent with this second model, we find that liver-draining lymph nodes are the major site of presentation of an antigen expressed in hepatocytes after AAV gene transfer, leading to CD4+ T cell proliferation. Nonetheless, in the DO11-10-tg Rag-2-/- model, we find a substantial increase in activated CD4+ T cells in the liver when T cell migration is blocked. Thus, the liver itself is likely also a site of MHC II presentation of hepatocyte-expressed antigen, leading to activation of CD4+ T cells that rapidly egress from the liver. We conclude that CD4+ T cells are activated the liver and in celiac/portal lymph nodes within the first 2 weeks after gene transfer. They subsequently egress and re-enter lymphoid tissues, with many returning to the liver and liver-draining nodes. Our data suggest that the liver is a site where CD4+ T activation but only limited proliferation takes place. Others found that AAV8 gene transfer upregulated FasL on transduced hepatocytes, thereby increasing T cell apoptosis.26 Hence, the liver environment may favor activation-induced cell death over CD4+ T cell proliferation.
MHC II presentation of cytoplasmic or secreted antigen expressed from an AAV upon hepatic gene transfer, leading to CD4+ T cell proliferation, depended on macrophages. Performing hepatic AAV8-Ova gene transfer for expression of secreted Ova, we previously found that CD11chi DCs were also required, in addition to macrophages.10 Together, these findings show that professional APCs, likely both macrophages and DCs, are required for antigen presentation. Thus, it is likely that nonsecreted antigens are also passed on from hepatocytes to professional APCs. Resident macrophages (e.g., KCs) may help capture antigen, which is transported by DCs to draining lymph nodes for presentation to induce T cell proliferation. Indeed, celiac and portal lymph nodes have been identified as preferential sites of migration for DCs injected under the liver capsule, resulting in T cell activation in these nodes.32
Induced Treg disseminate systemically
In response to hepatic delivery of a cytoplasmic antigen, iTreg were extrathymically induced. Therefore, extrathymic presentation of hepatocyte-derived antigen leads to conversion of conventional CD4+ T cells to FoxP3+ Treg. Literature data show that ectopic expression of an antigen in the liver of a transgenic mouse can lead to immune tolerance induction the absence of thymic T cell education through Treg induction.46,47 Although induced Treg may accumulate in the liver, as shown after hepatic lentiviral gene transfer, they also reside in the lymphoid organs such as the spleen, as shown by adoptive transfer of suppression.5–7,10,12,25,48 A number of studies have documented that liver gene transfer induces systemic immune tolerance, even to nonsecreted antigens.5,6,9,10,16,19,47–49 This feature makes liver gene transfer attractive as a tolerogenic immune modulatory therapy for multiple diseases. Our data show that liver-draining lymph nodes are a major site of Treg induction. The celiac/portal lymph nodes are not only sites for Treg induction to hepatic but also to orally administered antigen.50 This may reflect transport of antigen from the small intestine to the liver via the portal circulation, followed by transport to the celiac/portal nodes by migrating DC. We cannot entirely rule out that Treg induction in the liver itself also occurred in our studies. Others have for example shown that LSEC, a liver-resident cell type with the ability to present antigen, are more potent inducers of Treg than liver DCs or KCs.27 The advantage of Treg induction in sinusoids would be direct and rapid dissemination into the blood to establish systemic tolerance.
Interestingly, the blood contained the initially highest frequency of induced Treg. Rapid systemic distribution of induced Treg explains why immune tolerance can be established when nonhepatic AAV gene transfer is performed concomitant with hepatic gene transfer.19,21,49 Increased levels of transgene expression in hepatocytes correlate with increased Treg responses. Here, we had to use higher vector doses to induce Treg to cytoplasmic compared to secreted Ova antigen. As antigen presentation requires uptake by APCs, it is possible that secretion facilitates presentation. However, in prior studies we and others were able to induce Treg/tolerance with modest expression levels of non-secreted antigens in the liver.6,48,51 While such dose responses likely vary depending on the antigen and the host, TCR transgenic models may also overestimate the amount of antigen required because many cells with identical receptor compete for the antigen.
While a nonsecreted protein expressed in the liver will be subject to local mechanisms of antigen presentation, it should be pointed out that a secreted protein that is distributed via the systemic circulation is be presented in multiple tissues, which may include the thymus. It is therefore likely that peripheral Treg induction in extra-hepatic sites contributes to tolerance induction to a secreted protein, and that central tolerance mechanisms, including thymus-derived Treg, are additionally involved in response to transgene products secreted by hepatocytes into the blood.
Conclusions
In conclusion, liver draining lymph nodes play an integral role in presentation of virally encoded antigens in presentation to CD4+ T cells. This is particularly the case for non-secreted antigens, which are not distributed via the blood. Celiac and portal nodes are the major site of antigen presentation and are also a main site of iTreg induction. These iTreg rapidly disseminate to other organs through the blood, which explains why local immune tolerance induction in the hepatic environment can lead to a dominant systemic tolerance (summarized in Figure 6). Hence, liver gene transfer is being developed not only for phenotypic correction of genetic disease but also as an immune modulatory therapy for antigen-specific tolerance induction.
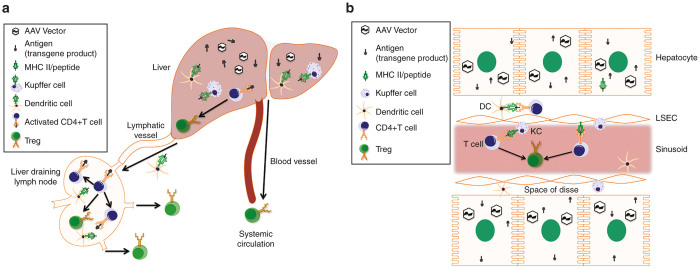
Figure 6.
Model of MHC II restricted antigen presentation and immune cell trafficking in response to transgene product expressed from an AAV in the liver. (a) Model of immune cell trafficking upon antigen expression in hepatocytes from an AAV vector. Antigen-loaded DCs and activated CD4+ T cells migrate from the liver to the liver-draining lymph nodes, where further CD4+ T cell activation/proliferation and Treg induction occurs. (b) Model of antigen presentation by Kupffer cells, dendritic cells, and liver sinusoidal endothelial cells in the liver; CD4+ T cell activation and Treg induction in the liver. Induced Treg migrate systemically via the liver’s extensive circulatory system.
Materials and Methods
Animals
All mice used in this study were 8-week-old male mice. BALBc mice were from Jackson Lab (Bar Harbor, ME). DO11-10-tg Rag-2-/- BALB/c mice were from our in house-breeding colony housed at the University of Florida as previously described.7,28,35 These mice are immune deficient, while their CD4+ T cells exclusively express the DO11.10 TCR specific for the model antigen ovalbumin. Immune-competent DO11.10 mice for the adoptive transfer experiments were purchased from Jackson Lab. All experiments were performed with a minimum of n = 3 animals per group.
Vectors and animal experiments
AAV coding for secreted ovalbumin (AAV-Ova) was as published.7,8,28 In this expression cassette, Ova is under transcriptional control of the human EF1α promoter. AAV-Cyto-Ova vector was constructed by replacing the Ova cDNA with a sequence coding for a nonsecreted, cytoplasmic version of ovalbumin (obtained from Addgene, Cambridge, MA). Vector genomes were packaged into AAV serotype 8 capsids by triple transfection of HEK-293 cells. Vector particles were purified by iodixanol gradient centrifugation, and vector titers were determined by dot blot hybridization and confirmed by Western blot using a reference standard of known titer for comparison. Vectors were administered to mice intravenously via the tail vein using doses that induce Ova-specific Treg in DO11-10-tg Rag-2-/- BALB/c mice (1 × 1011 vg/mouse for AAV8-Ova and 1 × 1012 vg/mouse for AAV8-Cyto-Ova). CellTrace Violet (Invitrogen, Eugene, OR) labeling was performed per the manufacturers recommended protocol. Briefly, cells were labeled at a final cell density of 3 million cells/ml and a final CellTrace Violet concentration of 3 μmol/l in phosphate buffered saline (PBS) containing 1% fetal bovine serum (FBS). These cells were then adoptively transferred into the host mouse via tail vein. Gadolinium chloride (Sigma, St. Louis, MO) was administered intraperitoneally at a dose of 10 mg/kg in PBS. FTY720 (Cayman Chemical, Ann Arbor, MI) was administered intraperitoneally at a dose of 10 mg/kg in sterile tissue culture grade water.
Tissue harvesting and processing
DO11.10 CD4+ T cells were harvested from the spleens of DO11.10-tg mice and transported in L-15 media on ice. These spleens were pushed through a 70-micron mesh tube top cell strainer and washed with PBS. CD4+ T cells were isolated using a MACS CD4-negative selection column (Miltenyi Biotec, Auburn, CA) per manufactures suggested protocols. Livers, spleens, and lymph nodes were harvested at indicated time points and transported in L-15 media on ice. Tissues were pushed through 70-micron mesh tube top cell strainer, washed with PBS to get single cell suspensions.
Flow cytometry
Antibodies against cell surface markers: CD3, CD4, CD69, CD25, DO11.10, and the intracellular marker Foxp3 were purchased from eBioscience (San Diego, CA). Intracellular Staining kit was also from eBioscience and used per the manufacturers protocol. Data was collected with a BD LSR II cytometer and data generated using FACS Diva software (BD Biosciences, San Jose, CA). Flow cytometry gating schemes are summarized in Supplementary Figure S1.
Statistical analysis
Statistical significance was determined with an unpaired, two-tailed Student’s t-test using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Values at P < 0.05 were deemed significant and indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.0001.
Author Contributions
G.Q.P., I.Z., A.S., and M.B. performed experiments. G.Q.P., M.B., C.T., and R.W.H. designed experiments. G.Q.P., C.T., A.M.D., and R.W.H. analyzed and interpreted data. G.Q.P., C.T., A.M.D., and R.H.W. wrote the manuscript. R.W.H. supervised the study.
Acknowledgments
This work was supported by NIH grants R01 AI51390 and R01 HL097088 (to R.W.H.), R01HL131093 (to R.W.H., Y.dJ., and C.T.), and R01 HL073838 (to A.M.D.).
R.W.H. received royalty payments from Spark Therapeutics for license of AAV-FIX technology. A.M.D. receives patent income from Uniqure for the FIX vector and a patent has been granted for the factor VIII vector. The other authors declared no conflict of interest.
References
- Hoffman, BE and Herzog RW. Immunology of hepatic gene transfer. In: Herzog RW (ed.). Gene Therapy Immunology. Wiley-Blackwell: Hoboken, NJ, 2009. pp. 143–166. [Google Scholar]
- Heymann, F and Tacke, F (2016). Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol 13: 88–110. [DOI] [PubMed] [Google Scholar]
- LoDuca, PA, Hoffman, BE and Herzog, RW (2009). Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther 9: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack, BK, Herzog, RW, Terhorst, C and Markusic, DM (2014). Development of gene transfer for induction of antigen-specific tolerance. Mol Ther Methods Clin Dev 1: 14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni, A, Brown, BD, Cantore, A, Sergi, LS, Naldini, L and Roncarolo, MG (2009). In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood 114: 5152–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátrai, J, Cantore, A, Bartholomae, CC, Annoni, A, Wang, W, Acosta-Sanchez, A et al. (2011). Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 53: 1696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, O, Dobrzynski, E, Wang, L, Nayak, S, Mingle, B, Terhorst, C et al. (2007). Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 110: 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski, E, Mingozzi, F, Liu, YL, Bendo, E, Cao, O, Wang, L et al. (2004). Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 104: 969–977. [DOI] [PubMed] [Google Scholar]
- Hoffman, BE, Dobrzynski, E, Wang, L, Hirao, L, Mingozzi, F, Cao, O et al. (2007). Muscle as a target for supplementary factor IX gene transfer. Hum Gene Ther 18: 603–613. [DOI] [PubMed] [Google Scholar]
- Markusic, DM, Hoffman, BE, Perrin, GQ, Nayak, S, Wang, X, LoDuca, PA et al. (2013). Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med 5: 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi, F, Liu, YL, Dobrzynski, E, Kaufhold, A, Liu, JH, Wang, Y et al. (2003). Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 111: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski, E, Fitzgerald, JC, Cao, O, Mingozzi, F, Wang, L and Herzog, RW (2006). Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA 103: 4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarpour, M, Goudy, KS, Cantore, A, Russo, F, Sanvito, F, Naldini, L et al. (2015). Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci Transl Med 7: 289ra81. [DOI] [PubMed] [Google Scholar]
- Kumar, SR, Markusic, DM, Biswas, M, High, KA and Herzog, RW (2016). Clinical development of gene therapy: results and lessons from recent successes. Mol Ther Methods Clin Dev 3: 16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Lai CW, Wu CJ, Chen LC, Tao MH, Kuo ML (2016). Liver-specific allergen gene transfer by adeno-associated virus suppresses allergic airway inflammation in mice. Hum Gene Ther 27: 631–642. [DOI] [PubMed] [Google Scholar]
- Annoni, A, Cantore, A, Della Valle, P, Goudy, K, Akbarpour, M, Russo, F et al. (2013). Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol Med 5: 1684–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudele, JM, Finn, JD, Siner, JI, Martin, NB, Niemeyer, GP, Zhou, S et al. (2015). AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood 125: 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, JD, Ozelo, MC, Sabatino, DE, Franck, HW, Merricks, EP, Crudele, JM et al. (2010). Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 116: 5842–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler, PA, Todd, AG, Clément, N, Falk, DJ, Nayak, S, Herzog, RW et al. (2016). Copackaged AAV9 vectors promote simultaneous immune tolerance and phenotypic correction of Pompe disease. Hum Gene Ther 27: 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G, Young, SP, Bali, D, Hutt, J, Li, S, Benson, J et al. (2014). Assessment of toxicity and biodistribution of recombinant AAV8 vector-mediated immunomodulatory gene therapy in mice with Pompe disease. Mol Ther Methods Clin Dev 1: 14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P, Sun, B, Osada, T, Rodriguiz, R, Yang, XY, Luo, X et al. (2012). Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum Gene Ther 23: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, BE, Martino, AT, Sack, BK, Cao, O, Liao, G, Terhorst, C et al. (2011). Nonredundant roles of IL-10 and TGF-β in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol Ther 19: 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, G, Nayak, S, Regueiro, JR, Berger, SB, Detre, C, Romero, X et al. (2010). GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int Immunol 22: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, G, O’Keeffe, MS, Wang, G, van Driel, B, de Waal Malefyt, R, Reinecker, HC et al. (2014). Glucocorticoid-induced TNF receptor family-related protein ligand is requisite for optimal functioning of regulatory CD4(+) T cells. Front Immunol 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous, E, Somanathan, S, Vandenberghe, LH and Wilson, JM (2009). Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 50: 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust, SM, Bell, P, Zhu, Y, Sanmiguel, J and Wilson, JM (2013). The role of apoptosis in immune hyporesponsiveness following AAV8 liver gene transfer. Mol Ther 21: 2227–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carambia, A, Freund, B, Schwinge, D, Heine, M, Laschtowitz, A, Huber, S et al. (2014). TGF-β-dependent induction of CD4⁺CD25⁺Foxp3⁺ Tregs by liver sinusoidal endothelial cells. J Hepatol 61: 594–599. [DOI] [PubMed] [Google Scholar]
- Cooper, M, Nayak, S, Hoffman, BE, Terhorst, C, Cao, O and Herzog, RW (2009). Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther 20: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren, K, Hoffman, BE, Zolotukhin, I, Keeler, GD, Xiao, JW, Basner-Tschakarjan, E et al. (2016). Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 24: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin, DL, Sunil, VR, Gardner, CR and Laskin, JD (2011). Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 51: 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L, Dobrzynski, E, Schlachterman, A, Cao, O and Herzog, RW (2005). Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood 105: 4226–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier, L, Tay, SS, McGuffog, C, Triccas, JA, McCaughan, GW, Bowen, DG et al. (2012). Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J Hepatol 57: 352–358. [DOI] [PubMed] [Google Scholar]
- Zheng, M, Yu, J and Tian, Z (2013). Characterization of the liver-draining lymph nodes in mice and their role in mounting regional immunity to HBV. Cell Mol Immunol 10: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell, A, Mongini, C, Gravisaco, MJ, Canellada, A, Tesone, AI, Goin, JC et al. (2016). An oral salmonella-based vaccine inhibits liver metastases by promoting tumor-specific T-cell-mediated immunity in celiac and portal lymph nodes: a preclinical study. Front Immunol 7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, M, Sarkar, D, Kumar, SR, Nayak, S, Rogers, GL, Markusic, DM et al. (2015). Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood 125: 2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, O, Hoffman, BE, Moghimi, B, Nayak, S, Cooper, M, Zhou, S et al. (2009). Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther 17: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, D, Biswas M, Liao G, Seay HR, Perrin GQ, Markusic DM, et al. (2014). Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia. Mol Ther Methods Clin Dev 1: 14030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann, V, Davis, MD, Heise, CE, Albert, R, Cottens, S, Hof, R et al. (2002). The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457. [DOI] [PubMed] [Google Scholar]
- Matloubian, M, Lo, CG, Cinamon, G, Lesneski, MJ, Xu, Y, Brinkmann, V et al. (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360. [DOI] [PubMed] [Google Scholar]
- Brinkmann, V, Pinschewer, DD, Feng, L and Chen, S (2001). FTY720: altered lymphocyte traffic results in allograft protection. Transplantation 72: 764–769. [DOI] [PubMed] [Google Scholar]
- Horst, AK, Neumann, K, Diehl, L and Tiegs, G (2016). Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 13: 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippens, C, Duraes, FV, Dubrot, J, Brighouse, D, Lacroix, M, Irla, M et al. (2016). IDO-orchestrated crosstalk between pDCs and Tregs inhibits autoimmunity. J Autoimmun (epub ahead of print). [DOI] [PMC free article] [PubMed]
- Martino, AT, Herzog, RW, Anegon, I and Adjali, O (2011). Measuring immune responses to recombinant AAV gene transfer. Methods Mol Biol 807: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanathan, S, Breous, E, Bell, P and Wilson, JM (2010). AAV vectors avoid inflammatory signals necessary to render transduced hepatocyte targets for destructive T cells. Mol Ther 18: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, GL, Martino, AT, Aslanidi, GV, Jayandharan, GR, Srivastava, A and Herzog, RW (2011). Innate immune responses to AAV vectors. Front Microbiol 2: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, BE and Herzog, RW (2008). Coaxing the liver into preventing autoimmune disease in the brain. J Clin Invest 118: 3271–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüth, S, Huber, S, Schramm, C, Buch, T, Zander, S, Stadelmann, C et al. (2008). Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest 118: 3403–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, AT, Nayak, S, Hoffman, BE, Cooper, M, Liao, G, Markusic, DM et al. (2009). Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS One 4: e6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini, MA, Bu, J, Fidler, JA, Ziegler, RJ, Foley, JW, Dodge, JC et al. (2007). Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci USA 104: 9505–9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultkrantz, S, Ostman, S and Telemo, E (2005). Induction of antigen-specific regulatory T cells in the liver-draining celiac lymph node following oral antigen administration. Immunology 116: 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietupski, JB, Hurlbut, GD, Ziegler, RJ, Chu, Q, Hodges, BL, Ashe, KM et al. (2011). Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol Ther 19: 1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.