Significance
During vertebrate evolution, Hox gene function was coopted through the emergence of global enhancers outside the Hox gene clusters. Here, we analyze the regulatory modalities underlying Hoxd gene transcription into the developing mammary glands where Hox proteins are necessary. We report the existence of a long-distance acting mammary bud enhancer located near sequences involved in controlling Hox genes in the limbs. We argue that the particular constitutive chromatin structure found at this locus facilitated the emergence of this enhancer element in mammals by hijacking a regulatory context at work in other cell types, supporting a model wherein enhancer sequences tend to cluster into large regulatory landscapes due to an increased probability to evolve within a preexisting regulatory structure.
Keywords: enhancers, TAD, mammalian development, mammary gland
Abstract
Vertebrate Hox genes encode transcription factors operating during the development of multiple organs and structures. However, the evolutionary mechanism underlying this remarkable pleiotropy remains to be fully understood. Here, we show that Hoxd8 and Hoxd9, two genes of the HoxD complex, are transcribed during mammary bud (MB) development. However, unlike in other developmental contexts, their coexpression does not rely on the same regulatory mechanism. Hoxd8 is regulated by the combined activity of closely located sequences and the most distant telomeric gene desert. On the other hand, Hoxd9 is controlled by an enhancer-rich region that is also located within the telomeric gene desert but has no impact on Hoxd8 transcription, thus constituting an exception to the global regulatory logic systematically observed at this locus. The latter DNA region is also involved in Hoxd gene regulation in other contexts and strongly interacts with Hoxd9 in all tissues analyzed thus far, indicating that its regulatory activity was already operational before the appearance of mammary glands. Within this DNA region and neighboring a strong limb enhancer, we identified a short sequence conserved in therian mammals and capable of enhancer activity in the MBs. We propose that Hoxd gene regulation in embryonic MBs evolved by hijacking a preexisting regulatory landscape that was already at work before the emergence of mammals in structures such as the limbs or the intestinal tract.
Animal development is orchestrated by a limited number of signaling molecules and transcription factors that cooperate in complex gene regulatory networks (1). The various elements of these networks are repeatedly used in time and space and were often coopted in the course of evolution to support the appearance of anatomical novelties (2–6). Hox genes are a good example of genes that underwent several rounds of neofunctionalization to accompany the emergence of morphological and functional novelties of many kinds, such as tetrapod digits (7) or pregnancy in mammals (8). They encode transcription factors with critical roles in the development of various embryonic structures, including the main body and appendicular axes, as well as virtually all major systems (9–11).
In amniotes, 39 Hox genes are generally found located in four different genomic clusters that arose following two rounds of whole-genome duplications (12–14). Although this clustered organization facilitates their coordinated transcriptional activation during the elongation of the major body axis (10, 15), it also favors the evolution of global regulations outside the gene clusters themselves (16). Consequently, specific subgroups of Hox genes, at least within the HoxA and HoxD clusters, are controlled by the same series of global enhancers; hence, they are coregulated in different contexts, which provide the system with both quantitative and qualitative modulations. This regulation has been well documented for the HoxD cluster, which contains a range of long-acting enhancers of different specificities within its two large flanking gene deserts (17–20).
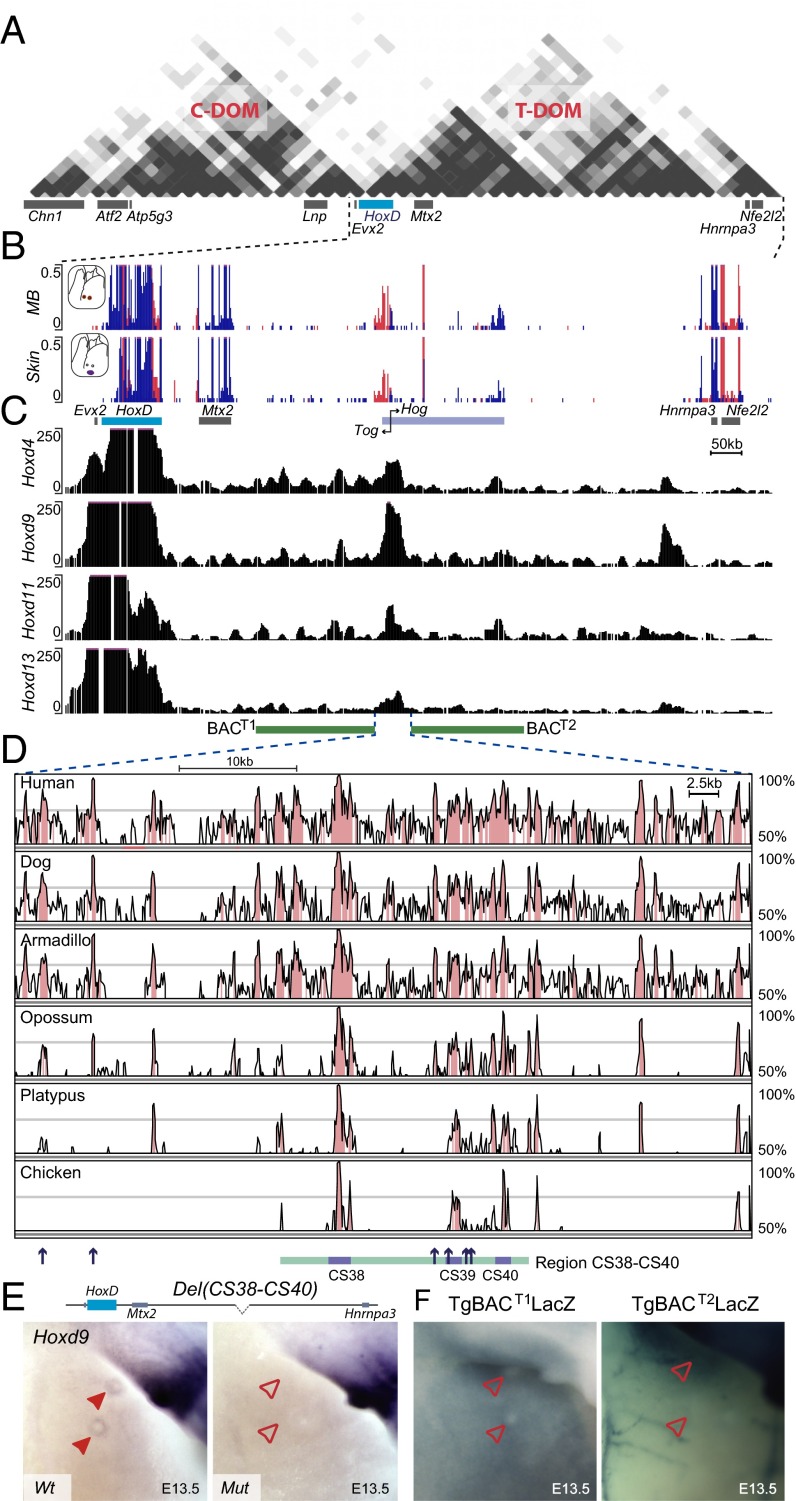
The application of chromosome conformation capture approaches (21, 22) on this locus using in vivo material has revealed that these two large regulatory landscapes match in their extent two topologically associating domains (TADs) [i.e., chromosome domains where specific and constitutive physical interactions are privileged (23, 24)]. These two TADs are separated by a tight boundary localized within the HoxD cluster itself, isolating those genes controlled by the telomeric TAD (T-DOM) from those genes responding to the centromeric regulation domain (C-DOM) (17) (see Fig. 5A). Within the T-DOM, series of enhancers are found, which regulate groups of genes lying in the central part of the cluster, as if the regulatory sequences would contact a chromatin pocket encompassing Hoxd8 to Hoxd11 (18). These large and apparently constitutive chromosome domains (TADs) may facilitate the required regulatory switches at important developmental loci, and were also proposed to have triggered the evolution of pleiotropy by providing the regulatory context for evolving novel enhancers (16, 19).
Fig. 5.
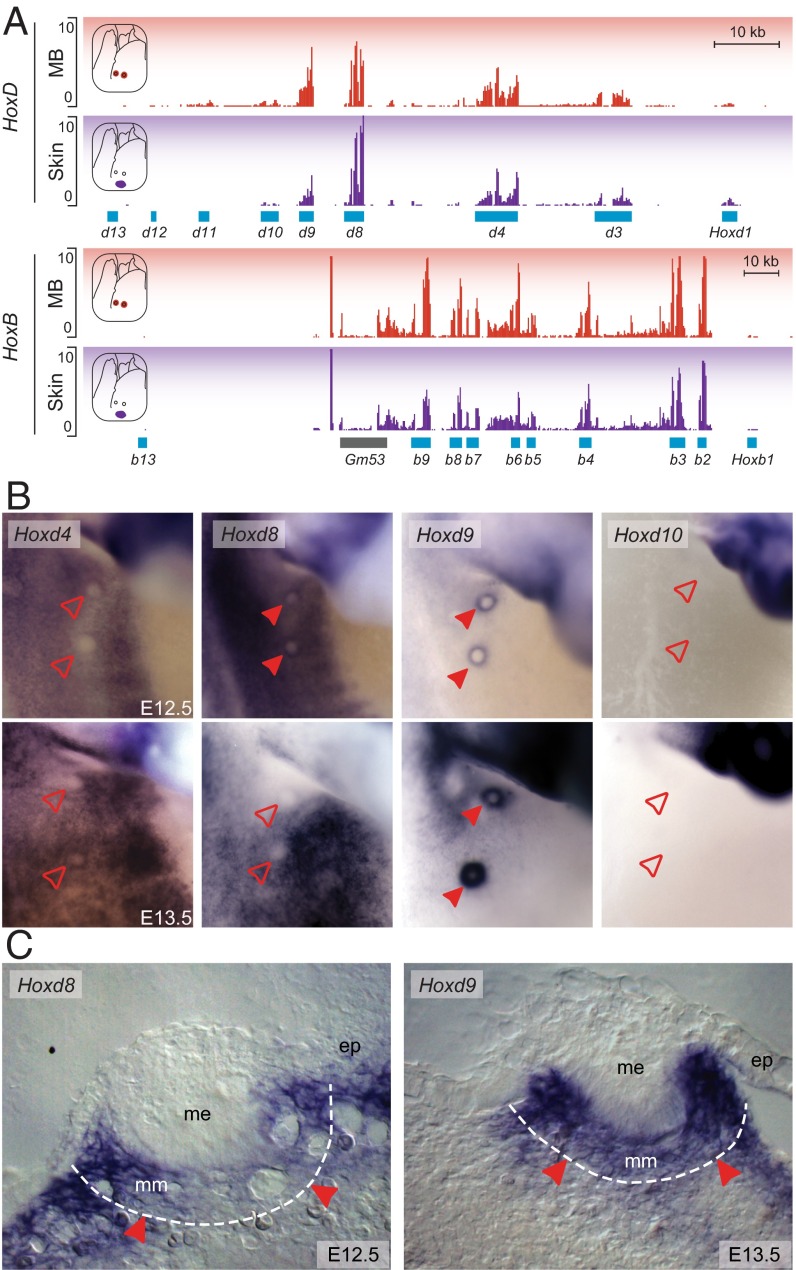
A 20-kb-large region of the HoxD telomeric gene desert specifically interacts with Hoxd9 and is required for its expression in MBs. (A) Hi-C data adapted from Dixon et al. (23) showing the C-DOM and T-DOM flanking the HoxD cluster. The light blue and gray boxes, respectively, represent the HoxD cluster and the other gene loci contained within the region analyzed. (B) RNA-seq analysis comparing the mouse E13.5 MBs with the adjacent epidermal tissue (skin). The HoxD cluster is depicted by a blue rectangle, whereas other, non–Hox-coding genes are represented by gray boxes. Light blue rectangles point to the Hog and Tog lncRNAs, and black arrows represent the transcription start site of these two genes. (C) Chromosome conformation capture analysis of different Hox genes in E11.5 mouse embryos aligned with the RNA-seq from B. All 4C samples were normalized for the respective number of mapped reads for each sample. Hoxd9 displays increased interactions with a short region, including the CS38-CS39 regions and containing the transcription start site of the Hog and Tog lncRNAs. Data are from Guerreiro et al. (75). Green rectangles represent the region of the HoxD telomeric gene desert contained within the BACT1 and BACT2 transgenic lines tested in this study. (D) Vista alignment showing the noncoding conserved elements across different vertebrate species within a 60-kb window around the CS38–CS40 region. Regions of conservation (defined by at least 70% sequence identity over 100 bp) are shown in pink. Several eutherian-specific sequences shown by blue arrows at the bottom were identified in this area. The turquoise rectangle represents the region spanned by the Del(CS38–CS40), which removes most of these eutherian-specific sequences. (E) WISH analysis comparing Hoxd9 expression in E13.5 MBs from either Wt or embryos homozygous for the Del(CS38–CS40) deletion (Mut). A schematic representation of the Del(CS38–CS40) allele is shown above the panels. The dashed line represents the deleted region. (F) LacZ staining of embryos carrying random integrations of BACs coding for the HoxD telomeric regions on both sides of the CS38–CS40 elements. In E and F, filled red arrowheads represent MBs where Hoxd9/LacZ expression was detected. Empty red arrowheads represent MBs lacking expression of these genes. (Magnification: 18×.)
Mammary glands (MGs) are a defining characteristic of mammals. They are highly specialized skin appendages that allow for extensive postnatal care of the progeny by providing a primary source of nutrition and immune protection (25, 26). Their early development depends on complex epithelial–mesenchyme interactions (27, 28), starting with the formation of the mammary lines, a thickening of the ectoderm on the lateral sides of the embryo stretching between the forelimbs and hind limbs. At embryonic day (E) 11.5, the murine milk lines split into five symmetrical pairs of mammary placodes (MPs). Each placode is associated with a specialized underlying mammary mesenchyme, which drives mammary ectoderm invagination and formation of the duct system. By E12.5, the placodes invaginate, and around E13.5, they form the mammary buds (MBs), which will then elongate and sprout to create a ductular structure deeper in the dermal mesenchyme. The mammary mesenchyme remains associated with the surface ectoderm, driving the formation of the mammary papilla or nipple, which plays a critical role in lactation (29). Nipples are found in eutherian mammals and marsupials but not in monotremes. The molecular bases of these complex intertissue interactions have recently started to be investigated in some detail (30).
The function of Hox genes during the development of skin-derived appendages has been documented in several instances, including hair follicles and feathers (31–35). Likewise, these genes have been implicated in MG development (36–38), with Hoxc8 being one of the first markers of MP specification, where it contributes to define the number and positions where these structures will form (37). The expression of Hoxc8 is nevertheless completely abrogated by E12.5. In contrast, Hox gene members of the group 9 are transcribed during both embryonic and postnatal development of the MGs, and their combined loss of functions resulted in severe MG hypoplasia and the death of offspring due to milk starvation (36).
We analyzed the transcriptomes of microdissected MBs and observed that Hoxd8 and Hoxd9 are the only members of the HoxD cluster expressed during MB development. By using a panel of mutant alleles, we show that expression of these two genes in the MB depends on two different and largely independent regulatory mechanisms. Although DNA sequences located near Hoxd8 are required to drive its expression in the MBs, Hoxd9 transcription seem to depend mainly on a regulatory input located 450 kb downstream of the cluster, within the T-DOM. We further isolated a 200-bp long DNA segment, which displayed enhancer activity in the MBs. This DNA sequence is conserved among therian mammals and is located near a previously described limb enhancer. Finally, the regulatory potential of this sequence, when isolated from either the platypus or the opossum, was tested to address the evolutionary origin of this element across the mammalian lineage.
Results
Characterization of MB-Enriched Transcripts by RNA-Sequencing.
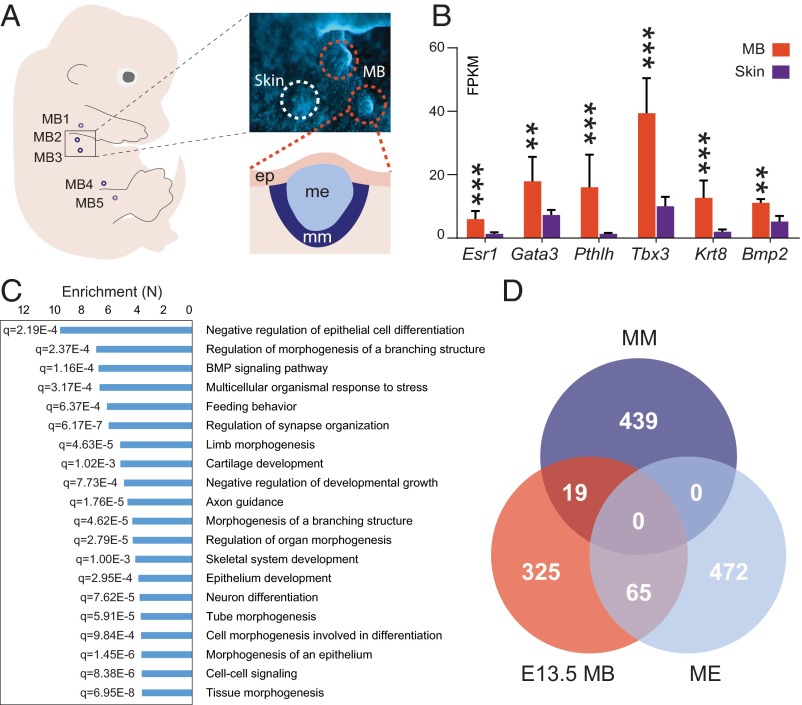
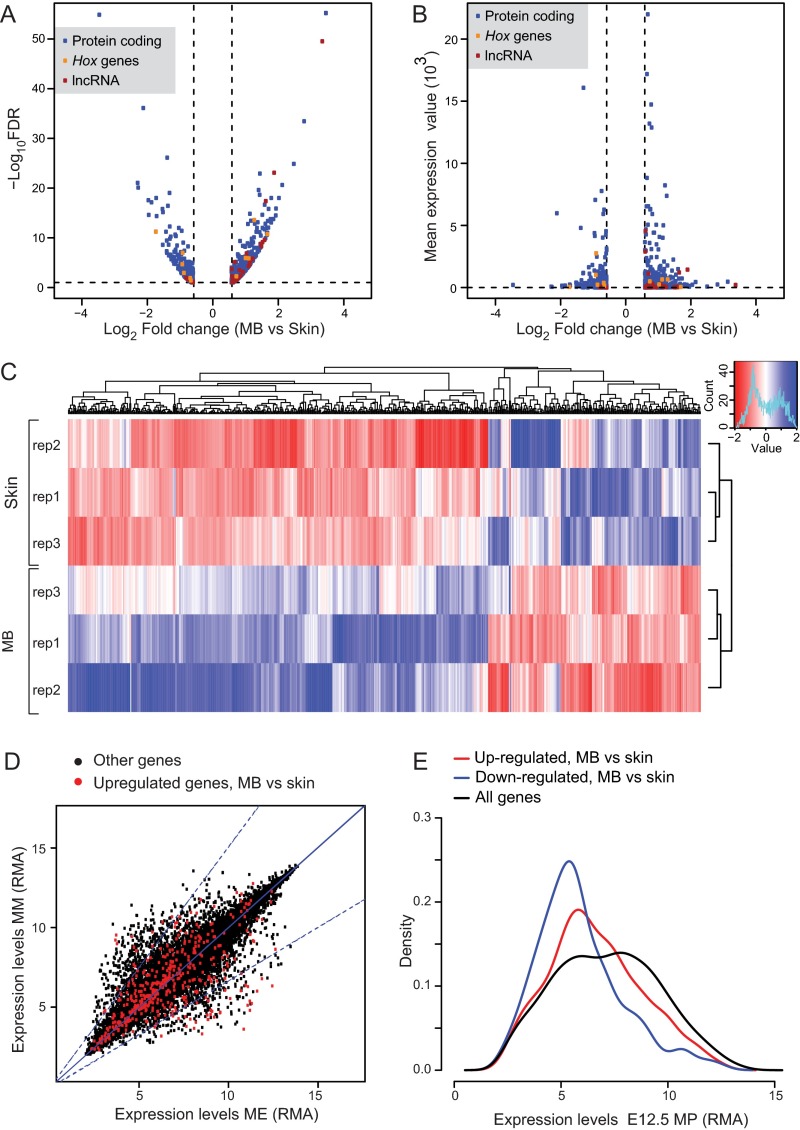
We complemented previous transcriptome studies of the adult MG (39–42) by a transcriptional analysis of microdissected E13.5 embryonic MBs. MB pairs 2 and 3 were thus obtained, and their expression profiles were established by RNA-sequencing (RNA-seq). To distinguish MB-specific expression from the background transcription found in the presumptive dermis and epidermis, we dissected from the same animals an equivalent area of tissue immediately adjacent to the MB (hereafter referred to as “skin”), including the nonmammary epithelium and its underlying mesenchyme (Fig. 1A). We identified 409 genes specifically up-regulated (SI Materials and Methods) and 204 down-regulated genes in the MBs compared with control skin (false discovery rate < 10%, minimum expression fold change = 1.5; Fig. S1 A–C and Datasets S1 and S2). Up-regulated genes included the mammary epithelium markers Krt8, Bmp2, Fgf17, Gata3, Pthlh, and Tbx3 (30, 43–47), as well as the mammary mesenchyme-specific genes Tbx18, Esr1, and Meox2 (30), thus validating our experimental approach (Fig. 1B and Datasets S1 and S2). Among the differentially expressed genes, 28 long noncoding RNAs (lncRNAs) were up-regulated and four were down-regulated in the E13.5 MBs, compared with embryonic skin (Fig. S1 A and B and Datasets S1 and S2).
Fig. 1.
Transcriptome analysis of MG. (A) Schematic view of an E13.5 embryo showing the location of the different MB pairs (MB1–MB5) along the anteroposterior axis (Left) and zoomed-in lateral view of a whole-mount DAPI-stained embryo (Right) showing the MB2 and MB3 dissected in this study (red dashed circles). A portion of the adjacent skin tissue (including nonmammary ectoderm and mesenchyme) was used as a control (white dashed circle) (Magnification: 30×.). A schematic view of a transversal section of an E13.5 MB is depicted below. ep, epithelium; me, mammary ectoderm; mm, mammary mesenchyme. (B) Bar graph plot showing the expression levels of mammary ectodermal (Gata3, Tbx3, Pthlh, Bmp2) and mesodermal (Esr1, Lhx9, Tbx18, Ptx2) markers measured in fragments per kilobase of exon per million mapped reads (FPKM). **P < 0.001, ***P < 0.0001. (C) Bar graph displaying the 20 most enriched GO term categories and their respective false discovery rate (q) values. GO terms significantly enriched among MG up-regulated genes were identified using Gorilla (P < 10−4). To reduce the number of redundant GO term categories, we used the Revigo algorithm (similarity threshold = 0.7). (D) Venn diagram showing the overlap of genes specifically expressed in the E13.5 MBs with mammary ectodermal (ME)-specific or mesenchymal (MM)-specific genes identified by Wansbury et al. (30). Among the 409 E13.5 MB-specific genes, 19 corresponded to mesenchyme-specific genes, 65 were specifically enriched in the mammary ectoderm, and 19 were up-regulated in the mammary mesenchyme.
Fig. S1.
(Related to Fig. 1). (A) Volcano-plot representation showing genes significantly up- or down-regulated in the MB, as identified in this study [Wald test fold change (FC) >1.5, FDR < 0.1]. (B) Dot-plot representation comparing the FC versus mean expression values of the different genes either up-regulated or down-regulated in the MB. (C) Heat map representation of the 409 genes up-regulated and 204 genes down-regulated in the E13.5 MBs, compared with their adjacent skin control region (Wald test FC > 1.5, FDR < 0.1). The graph shows the three replicates (rep1–rep3) of each experimental condition. (D) Dot-plot graph showing expression level values [expressed in robust microarray average (RMA) units] of genes expressed in the E12.5 mammary ectoderm and/or mesoderm (30). Red dots represent expression values of the E13.5 MB specific genes identified in our study. (E) Density distribution plot showing the expression value distribution (RMA) of genes expressed in the E12.5 MBs (black line) (30). Red and blue lines represent the density distribution of E13.5 genes either up-regulated or down-regulated, respectively, in the latter study. According to the dataset of Wansbury et al. (30), most of the MB-specific genes identified in our study are expressed at similar levels in the mammary ectoderm and mesoderm. Nevertheless, they display higher expression values than genes down-regulated in the mammary mesenchyme. They are generally transcribed at low to moderate levels.
To classify the MB-enriched genes functionally, we performed gene ontology (GO) term enrichment analysis (SI Materials and Methods) using Gorilla (48) (P < 10−4). Enriched GO terms were further clustered using Revigo (49) (similarity threshold = 0.7). Among the 20 most enriched GO term categories, we found several terms related to epithelial structures and MG development, as well as BMP signaling, previously described to be essential for MG development (e.g., refs. 43, 50) (Fig. 1C and Dataset S3).
To evaluate spatial expression patterns within the MB further, we analyzed the overlap between our list of MB up-regulated genes and an existing estimate of both ectoderm- and mesenchyme-specific genes derived from a microarray-based analysis of the posterior MB at E12.5 (30). We found that of our 409 identified MB up-regulated genes, 65 had been reported as ectoderm-specific in this previous work, whereas 19 of them were found to be enriched in mammary mesenchyme (Fig. 1D). However, the majority of the MB up-regulated genes in our dataset were expressed at similar levels in the E12.5 mammary mesenchyme and ectoderm (Fig. S1D). We think that differences in the embryonic stage and tissue [E12.5 posterior MB analyzed by Wansbury et al. (30) versus E13.5 anterior MB analyzed here], as well as the increased sensitivity of RNA-seq compared with microarrays (51), may account for the moderate overlap between the two datasets. In support of the latter, most of our MB-enriched genes were expressed at moderate to low levels in E12.5 mammary primordia (Fig. S1E), and were thus likely not detectable as being differentially expressed with microarrays. The distribution of these genes was nevertheless clearly shifted compared with the distribution of genes classified as down-regulated in our analysis, which tended to be expressed at lower levels in the E12.5 mammary primordium (Fig. S1E).
The Embryonic MG Hoxome.
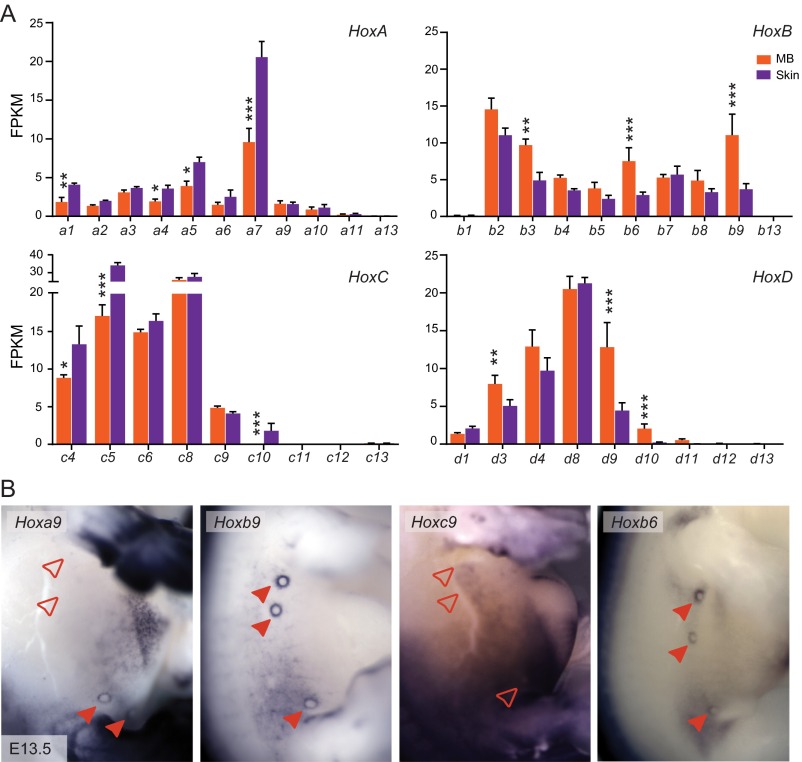
Due to their potential role in MG development (discussed above), we particularly looked at the Hox gene family (Fig. S2A) and noticed that members of the HoxB and HoxD clusters were expressed at high levels in the MB compared with the nearby skin control, whereas HoxA and HoxC paralogs were either not differentially expressed or even down-regulated in these structures (Fig. 2A and Fig. S2A). Among HoxB and HoxD genes, Hoxb3, Hoxb6, Hoxb9, and Hoxd9 were significantly up-regulated in the MBs compared with the skin tissue (Fig. 2A and Fig. S2A). Hoxd3 was also up-regulated in the MBs, although to a lower level than the former genes. Although the absolute mRNA levels of Hoxd10 were also significantly up-regulated in the MB, the levels of Hoxd10 remained very low (Fig. 2A and Fig. S1F). We performed whole-mount in situ hybridization (WISH) at different stages of MB development (Fig. 2B and Fig. S2B) and detected strong Hoxd9 expression in all five MB pairs at E12.5 and E13.5, whereas neither Hoxd10 nor Hoxd11 transcripts were scored. Hoxd8 expression was also observed, but only in the first to third pairs of MBs at E12.5. Its expression was strongly down-regulated to become virtually nondetectable 1 day later (Fig. 2B).
Fig. S2.
(Related to Fig. 2). (A) Bar plot showing the fragments per kilobase of exon per million mapped reads (FPKM) analysis of the different Hox genes in the E13.5 MBs and in control skin tissue (*FDR < 0.05, **FDR < 0.01, ***FDR < 0.0001). (B) WISH showing Hox gene expression in the developing MBs at E13.5. Filled red arrowheads indicate the MBs where the different Hox genes are expressed. Empty red arrowheads point to Hox-negative MBs. (Magnification: 16×.)
Fig. 2.
Hoxd8 and Hoxd9 are expressed during MB development. (A) RNA-seq profiles of E13.5 MG and neighboring skin showing the expression of either Hoxd (Top) or Hoxb (Bottom) genes. (B) WISH showing Hoxd expression in MB2 to MB3. Filled red arrowheads represent those MBs where Hoxd gene expression was detected. Empty red arrowheads represent MBs lacking Hoxd expression. (Magnification: 23×.) (C) Coronal cross-section of MB2 showing expression of Hoxd8 (E12.5) and Hoxd9 (E13.5) in the MBs. The dashed line delimits the mammary mesenchyme from the surrounding nonmammary mesoderm. Red arrowheads point to the expression of Hoxd8 and Hoxd9 in the mammary mesenchyme. ep, nonmammary epithelium; me, mammary epithelium. (Magnification: 125×.)
In support of our RNA-seq data, WISH also revealed expression of Hoxb6 and Hoxb9 in all MBs, whereas Hoxa9 transcripts were only scored in the fourth and fifth MB pairs (Fig. S2B). We confirmed that Hoxc9 was not transcribed in the MBs at any of the developmental times analyzed (36), and we could not detect any Hoxd3 transcripts by WISH, despite a slight, yet significant increase, compared with skin (Fig. S2A), likely due to the different sensitivities of the two approaches. To determine the precise localization of the Hoxd8 and Hoxd9 transcripts, we cryostat sectioned WISHed embryos, and both Hoxd8 and Hoxd9 were detected in the mammary mesenchyme below the mammary epithelium (Fig. 2C). Although Hoxd9 was detected at a high level in all mammary mesenchymal cells, Hoxd8 transcripts were found in the most lateral portion of this mesenchyme and were barely detectable in its most central part at E12.5. This expression pattern likely paralleled the rapid down-regulation of Hoxd8 transcription occurring in the MB between E12.5 and E13.5. This dataset, along with previous contributions (36–38), indicates that Hox genes are differentially expressed both among the various pairs of MBs and during the development of these structures, suggesting that temporal and spatial Hox combinations may contribute to MG development and specification.
Hoxd8 and Hoxd9 Transcription Depends on Distinct Regulations.
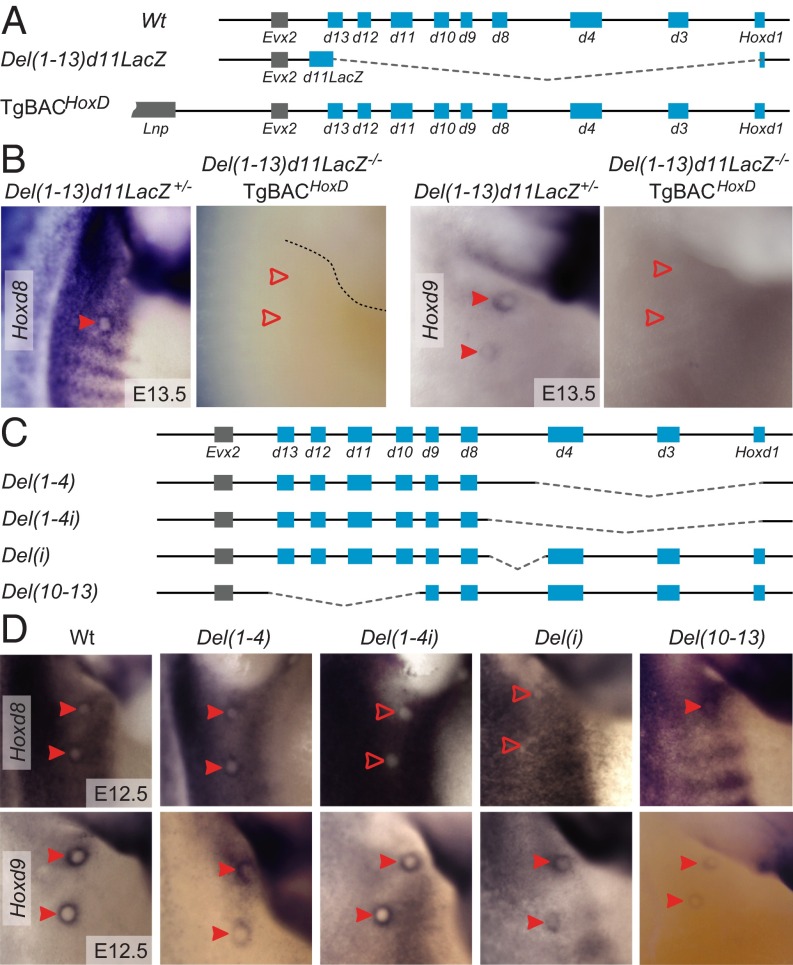
To search and identify regulatory elements controlling Hoxd8 and Hoxd9 expression in MBs, we initially analyzed a BAC transgenic line containing the entire HoxD cluster (TgBACHoxD; Fig. 3A). To discriminate transgenic Hox genes from their endogenous counterparts, TgBACHoxD mice were crossed with the HoxDDel(1–13)d11Lac line [also known as Del(1–13)d11lac] where all Hoxd genes are deleted (Fig. 3A). Backcrosses generated Del(1–13)d11lac−/−;TgBACHoxD embryos where only transgenic Hoxd genes were present. Neither Hoxd8 nor Hoxd9 was expressed in the MB in these embryos (Fig. 3B), suggesting that DNA elements located outside the region covered by the BACHoxD were required.
Fig. 3.
Hoxd8 and Hoxd9 expression in embryonic MBs depends on different regulatory mechanisms. (A and B) WISH analysis showing expression of Hoxd8 and Hoxd9 in a mouse carrying a transgenic BAC coding for the HoxD cluster randomly integrated into the genome. The BAC insertion was analyzed in Del(1–13)d11LacZ−/− animals, which lack the endogenous HoxD cluster. Del(1–13)d11LacZ+/− animals were used as controls. The different alleles used in this study are schematized in A. Hoxd genes are represented by light blue boxes, whereas gray rectangles represent other, non–Hox-coding genes. The deleted region is depicted with dashed lines. (C and D) Mice carrying deletions of different portions of the HoxD cluster were analyzed to identify potential regulatory elements controlling Hoxd8 and/or Hoxd9 expression in the MBs. (C) Different Hoxd genes and Evx2 are represented with light blue and gray boxes, respectively. Dashed lines represent the deleted DNA segments. (D) WISH analysis showing Hoxd8 and Hoxd9 expression in E12.5 MBs in the homozygous embryos of the different lines shown in C, compared with wild-type (Wt) embryos. Expression of Hoxd9 remained unchanged in all of the deletions analyzed. Hoxd8 expression in the MB, however, was lost in all lines carrying the deletion of region i. In B and D, filled red arrowheads represent MBs where expression of Hoxd8 and/or Hoxd9 was detected. Empty red arrowheads represent MBs lacking expression of these genes. (Magnification: B, 20×; D, 18×.)
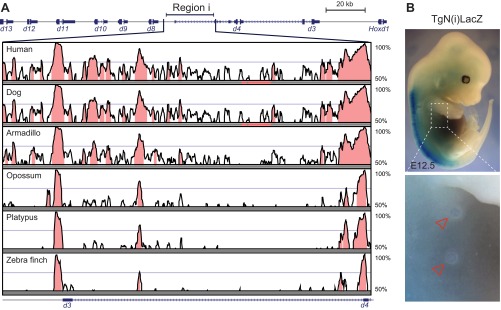
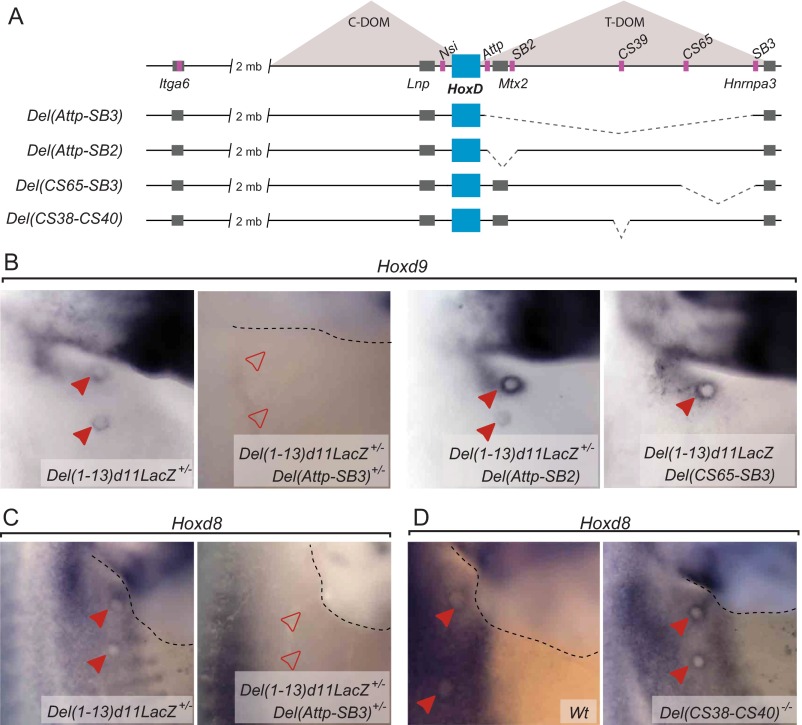
We further analyzed a series of targeted deletions within the HoxD cluster spanning different intervals around the Hoxd8-to-Hoxd9 locus (Fig. 3C) and obtained contrasting results. Although Hoxd9 expression was not affected in any of these deletions (Fig. 3D, Upper), Hoxd8 transcripts were strongly down-regulated in all homozygous mutant embryos where the Hoxd4-to-Hoxd8 intergenic region was absent [HoxDDel(1–4i) or HoxDDel(i); Fig. 3D, Bottom]. Hoxd8 transcription was scored in other deletions maintaining this DNA region, however, such as the HoxDDel(1–4) or HoxDDel(10–13). Although phylogenetic foot-printing analysis of the Hoxd4-Hoxd8 intergenic region revealed the presence of sequences conserved in mammals (Fig. S3A), a transgenic construct carrying this region upstream of a LacZ reporter cassette did not display any β-gal activity in the E12.5 MBs (Fig. S3B), thus corroborating the results obtained with the BACHoxD transgenic fetuses. Altogether, these results indicated that although enhancers outside of the HoxD cluster drive Hoxd9 expression in the MBs, Hoxd8 transcription depends on the combined activity of regulatory elements located in its immediate 3′ vicinity and in the flanking gene deserts.
Fig. S3.
(Related to Fig. 3). (A) Vista alignment showing the evolutionary conserved elements within a 13-kb-large intergenic region located between the Hoxd4 and Hoxd8 genes (region i). Gene exons are illustrated by blue boxes, whereas introns are depicted with blue arrows. Pink peaks represent noncoding evolutionary conserved elements (70% identity over a 100-bp window). (B) β-Gal staining of a stable transgenic line carrying a construct coding for a LacZ reporter cassette under the control of “region i” displayed in A. Empty arrowheads indicate the lack of expression of the TgN(i)LacZ construct in the MBs. (Magnification: Top, 7×; Bottom, 30×.)
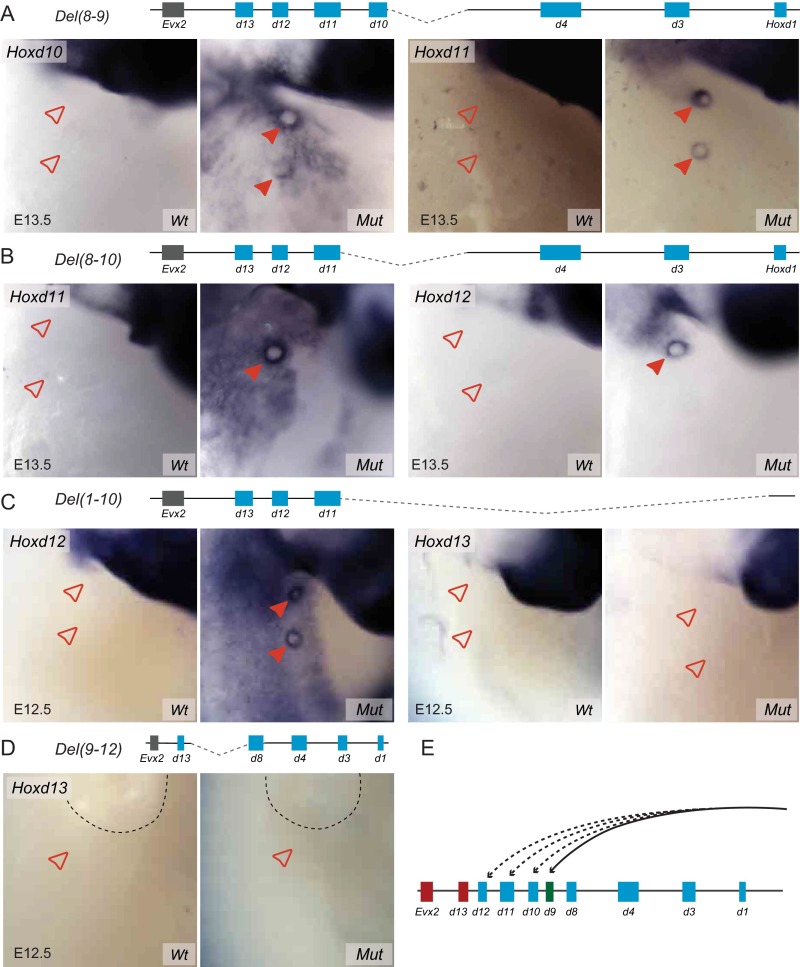
The selective expression of Hoxd8 and Hoxd9 in the MBs was not due to the incapacity of other Hoxd genes to be transcribed there, because mutant lines carrying deletions encompassing the Hoxd8 and/or Hoxd9 locus ectopically transcribed Hoxd10, Hoxd11, or Hoxd12 (Fig. S4). This ectopic expression was at least partially driven by regulatory elements located outside the cluster because it was also scored in embryos homozygous for the HoxDDel(1–10) deletion, which removes the putative local Hoxd8 enhancer(s) [Fig. S4C; Del(1–10)]. In all of these cases of ectopic expression, the concerned Hoxd genes were positioned closer to the T-DOM as a result of the deletion, suggesting that the T-DOM may contain embryonic MB enhancers specifically interacting with this central part of the gene cluster (Fig. S4E). Ectopic expression of Hoxd13 was nevertheless never scored in any of the mutant lines analyzed in this study, suggesting that not all promoters are responsive to the MB enhancers (Fig. S4).
Fig. S4.
(Related to Fig. 3). (A–D) WISH analysis showing Hoxd gene expression in the MBs of either control (Wt) embryos or various mouse homozygous mutant (Mut) embryos. A schematic representation of the HoxD cluster with the region deleted in each allele is shown above the various panels. Light blue boxes represent the genomic sequence spanning the Hoxd coding regions. The deleted DNA segments are shown as dashed lines. Filled red arrowheads represent MBs where Hoxd gene expression was detected. Empty red arrowheads represent MBs lacking expression of these genes. (E) Schematic representation showing Hoxd gene regulation in E13.5 MBs. Hoxd9 (green) is the only Hoxd gene specifically expressed in E13.5 MBs, driven by regulatory elements located in the HoxD T-DOM (black arrow). In the different deletions removing Hoxd9, ectopic expression of Hoxd10, Hoxd11, or Hoxd12 was observed (dashed black lines). Hoxd13 and Evx2 (red), however, were never ectopically expressed in any of these deletions. (Magnification: 28×.)
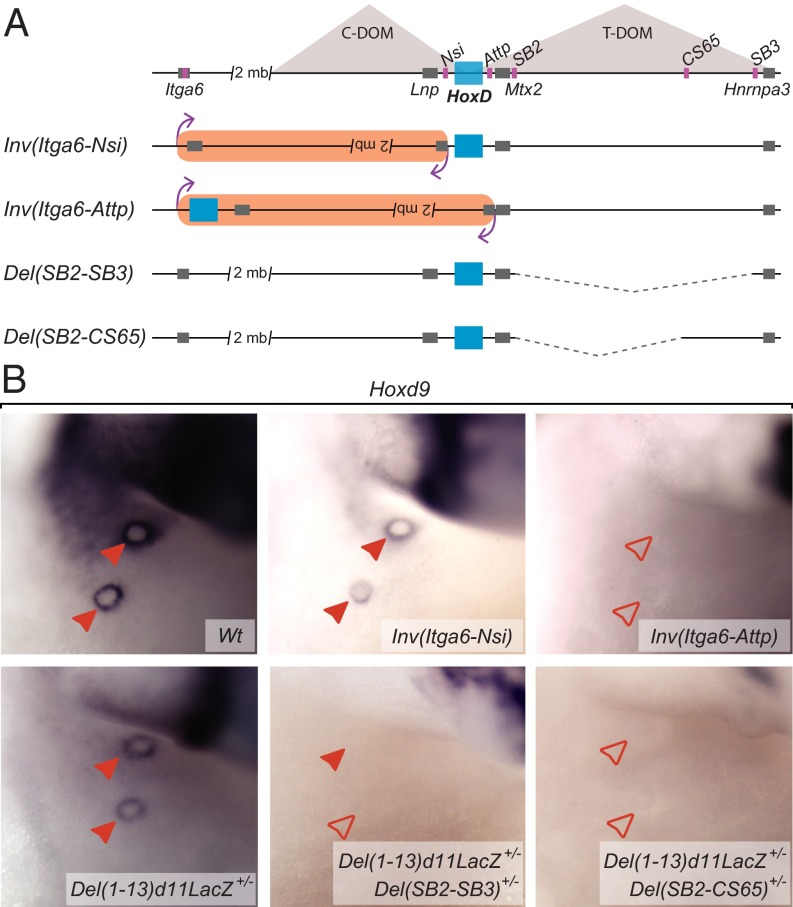
An MB Enhancer Region Is Located in the T-DOM.
To verify that distal MB enhancer(s) were located in the T-DOM, we tested two large inversions that separate the HoxD cluster from either one or the other gene desert (Fig. 4A). In the HoxDInv(Itga6-TgHd11LacNsi) inversion, a 3-Mb region containing the entire C-DOM was inverted, repositioning potential enhancers far away from the gene cluster [Fig. 4A; Inv(Itga6-Nsi)]. On the other hand, the HoxDInv(Itga6-Attp) inversion disconnected the HoxD cluster from the T-DOM, still keeping its relative distance with potential C-DOM enhancers [Fig. 4A; Inv(Itga6-Attp) and SI Materials and Methods]. Although Hoxd9 expression in the MBs remained unaffected in homozygous Inv(Itga6-Nsi) embryos, it was fully abrogated in the MBs of Inv(Attp-Itga6) homozygous mice (Fig. 4B, Upper). To rule out a potential negative effect of the novel neighboring DNA sequence associated with the HoxD cluster following the latter inversion, we also analyzed a large deletion removing almost the entire T-DOM [Fig. S5A; Del(Attp-SB3)]. Embryonic lethality was prevented by balancing this allele with a deletion of the HoxD cluster [Del(1–13)d11Lac; SI Materials and Methods], and Hoxd8 and Hoxd9 expression in the mammary mesenchyme was expectedly lost in Del(Attp-TpSB3)+/−/Del(1–13)d11Lac+/− embryos, compared with the Del(1–13)d11Lac+/− control littermates (Fig. S5 B and C).
Fig. 4.
HoxD telomeric gene desert is required for Hoxd9 expression in the MBs. (A) Schemes representing the alleles used in this study and carrying different inversions or deletions spanning the HoxD centromeric or telomeric deserts. The HoxD cluster is represented by a light blue rectangle. The T-DOM and C-DOM are represented by light gray triangles. Gray boxes illustrate other, non-Hox genes. Breakpoints for the different inversions/deletions are shown with pink boxes. The inverted DNA regions are highlighted in pale orange, whereas dashed lines represent the deleted DNA segments. (B) WISH analysis showing Hoxd9 expression in E13.5 MBs in the different alleles depicted in A, compared with control embryos. Embryos homozygous for the Inv(Itga6-Attp) and Inv(Itga6-Nsi) alleles were compared with Wt littermates. Deletions spanning the HoxD telomeric gene desert were crossed with mice carrying the balancer mutation Del(1–13)d11lacZ, as in Fig. 3, to avoid the embryonic lethality observed in homozygous embryos. In this case, Del(1–13)d11LacZ+/− littermates were used as controls. Filled red arrowheads represent MBs where Hoxd9 expression was detected. Empty red arrowheads represent MBs lacking Hoxd9 expression. (Magnification: 38×.)
Fig. S5.
Regulatory elements controlling Hoxd8 and Hoxd9 expression in the MB are located in the telomeric gene desert. (A) Scheme representing the alleles used in this study and carrying different inversion or deletions spanning the HoxD centromeric or telomeric desert. The T-DOM and C-DOM are represented by light gray triangles. The HoxD cluster is represented by a light blue rectangle. Light gray boxes illustrate other non-Hox genes. Breakpoints for the different inversions/deletions are shown with pink boxes. The dashed lines represent the deleted DNA segments. The inverted DNA region is highlighted in pale orange. WISH analysis showing Hoxd8 and Hoxd9 expression in E13.5 (B) and E12.5 (C and D) MBs from embryos carrying different mutant alleles, compared with their respective control [Wt or Del(1–13)d11lac+/−] littermates. Filled red arrowheads represent MBs where Hoxd8 and Hoxd9 expression was detected. Empty red arrowheads represent MBs lacking expression of these genes. (Magnification: 26×.)
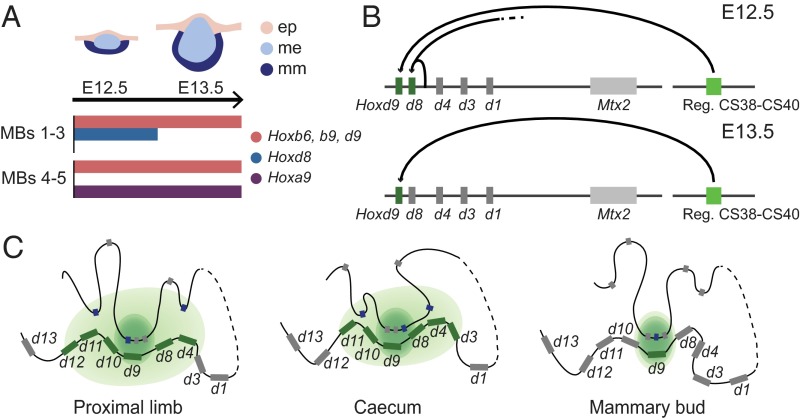
To locate the MB regulatory region more precisely within the T-DOM, we used targeted deletions covering this area, including the HoxDDel(Attp-TpSB2), HoxDDel(TpSB2-TpSB3), HoxDDel(65-TpSB3), and HoxDDel(TpSB2-65) alleles (Fig. 4A and Fig. S5A), which were all analyzed over the Del(1–13)d11Lac balancing allele. The expression of Hoxd8 and Hoxd9 in the MB was not significantly affected in either Del(Attp-SB2) or Del(CS65-SB3) embryos (Fig. 4B and Fig. S5B). In contrast, expression was no longer scored in Del(SB2-SB3) or Del(SB2-CS65) embryos (Fig. 4B, Bottom). It is noteworthy that this region was previously shown to contain regulatory elements controlling Hoxd gene expression both in the proximal limb buds and the intestinal cecum (17, 18). This DNA segment also contains the transcriptional start sites of Hog and Tog, two lncRNAs coregulated with selected Hoxd genes in the developing cecum (18), and whose transcription was also slightly enriched in E13.5 MBs compared with the surrounding control tissue (Fig. 5B).
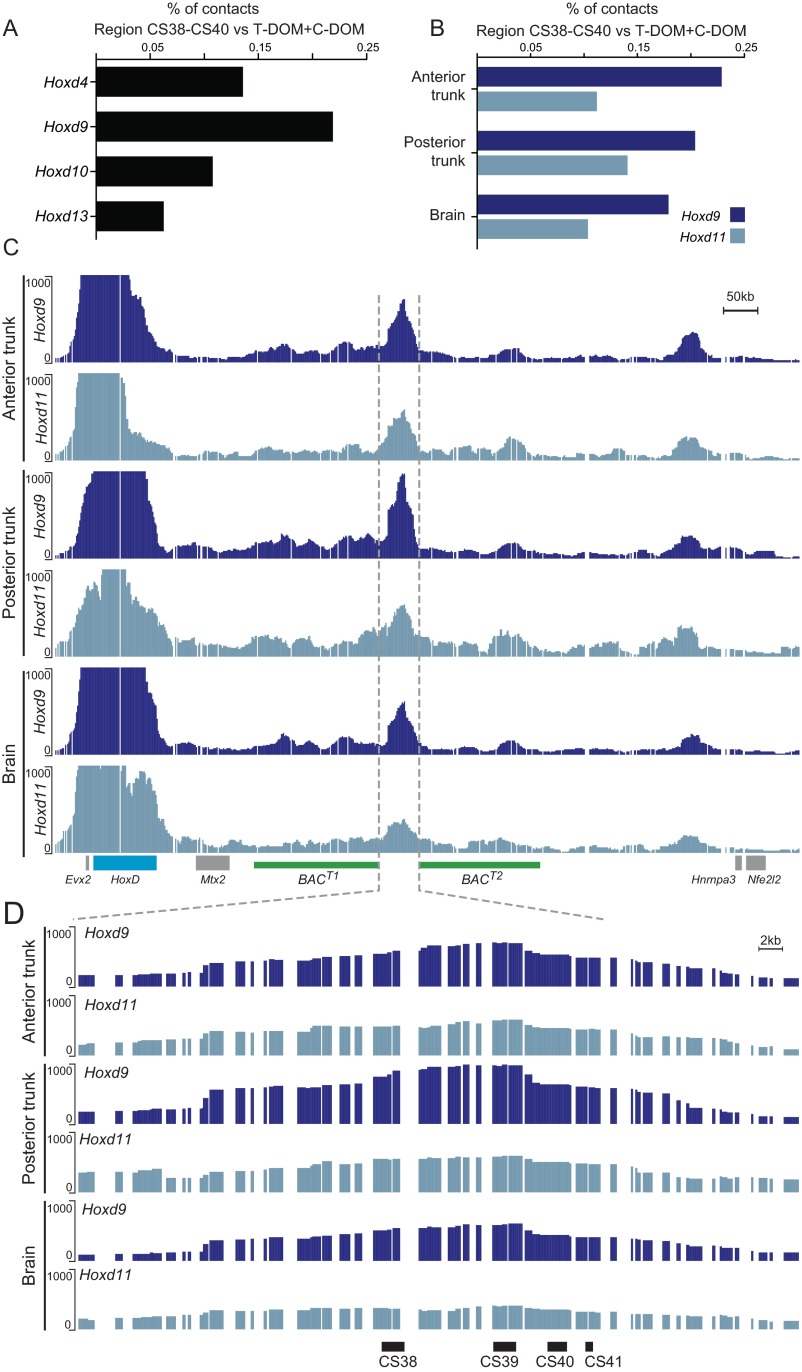
This particular DNA region maps at the boundary between two sub-TADs within the T-DOM (17) (Fig. 5A), and chromosome conformation capture approaches have revealed its strong contacts with the HoxD gene cluster in either transcriptionally active or inactive cellular contexts (17, 18, 23, 52). Of note, the interactions observed between this DNA region and Hoxd9 are usually stronger than with the other Hoxd genes (Fig. 5C and Fig. S6 A and B). This privileged interaction involving Hoxd9 was not scored with any other sequence within the T-DOM, except for the other limb-specific enhancer CS65 (17), ruling out a technical bias associated with the Hoxd9 probes used for chromosome conformation capture (4C) studies. The same preferential interaction was observed when Hoxd9 and Hoxd11 4C profiles were compared from either E10.5 brain cells (where Hoxd genes are not expressed) or anterior and posterior trunk cells (expressing different combinations of Hoxd genes) (53) (Fig. S6 B and C), supporting the idea that Hoxd9 specifically contacts this region, regardless of its transcriptional activity.
Fig. S6.
Hoxd9 locus preferentially interacts with the CS38–CS40 regulatory region. (A and B) Bar graphs representing the relative proportion of contacts between different Hox genes and the CS38–CS40 region, normalized by the total of their interactions within the two flanking gene deserts, using either whole-mouse embryos or different embryonic tissues [no. 4581 in the study by Noordermeer et al (53)]. The 4C-seq analysis of the interactions established between Hoxd9 or Hoxd11, used as viewpoints, and either the HoxD telomeric gene desert (C) or the region encompassed by the Del(CS38–CS40) deletion (D) are shown. The HoxD cluster is depicted by a blue rectangle, whereas other genes are shown in gray. Green rectangles represent the region of the HoxD telomeric gene desert included in the BACT1 and BACT2 clone transgenic lines tested in this study. Black rectangles represent the previously identified vertebrate-conserved elements CS38, CS39, CS40, and CS41. Hoxd9-specific interactions are maximal over the CS38–CS39 regions.
A high density of DNA sequences conserved among mammals were found in the 60-kb surrounding this Hoxd9 interacting region, with six of them specifically conserved in all eutherian mammals and marsupials but not in monotremes (Fig. 5D, blue arrows). A 20-kb-large deletion was engineered, which removed four of the six eutherian-specific elements, as well as the previously reported conserved sequences CS38 to CS40 (17). Embryos homozygous for this deletion [HoxDDel(CS38–CS40), also known as Del(CS38–CS40)] were viable and displayed no major alteration in Hoxd gene expression in either the proximal limb or the cecum at E13.5. Likewise, the expression of Hoxd8 in the E12.5 MBs remained unchanged (Fig. S5D). In contrast, Hoxd9 transcripts were no longer observed in these structures (Fig. 5E), confirming that expression of Hoxd8 and Hoxd9 in the MBs depends on different regulatory mechanisms. Despite the loss of Hoxd9 expression in the MB of Del(CS38–CS40)−/− embryos, no major alterations were observed either in the milking behavior or in the survival of the offspring of knockout females, in agreement with the apparent lack of phenotype observed in the Hoxd9−/− mice (36).
To assess whether additional regulatory elements controlling Hoxd8/Hoxd9 in the developing MB were located outside of this deleted region, we tested two stable transgenic lines carrying random insertions of BAC clones flanking the CS38–CS40 region (Fig. 5B; BACT1 and BACT2) carrying an integration of a LacZ reporter under the control of the β-globin minimal promoter. Neither of the lines displayed any β-gal activity in the MB at any stage analyzed (Fig. 5E). Therefore, although the CS38–CS40 region is required for Hoxd9 transcription, the control of Hoxd8 transcription depends on the combined activities of local and distal regulatory elements.
A Eutherian-Specific MB Element.
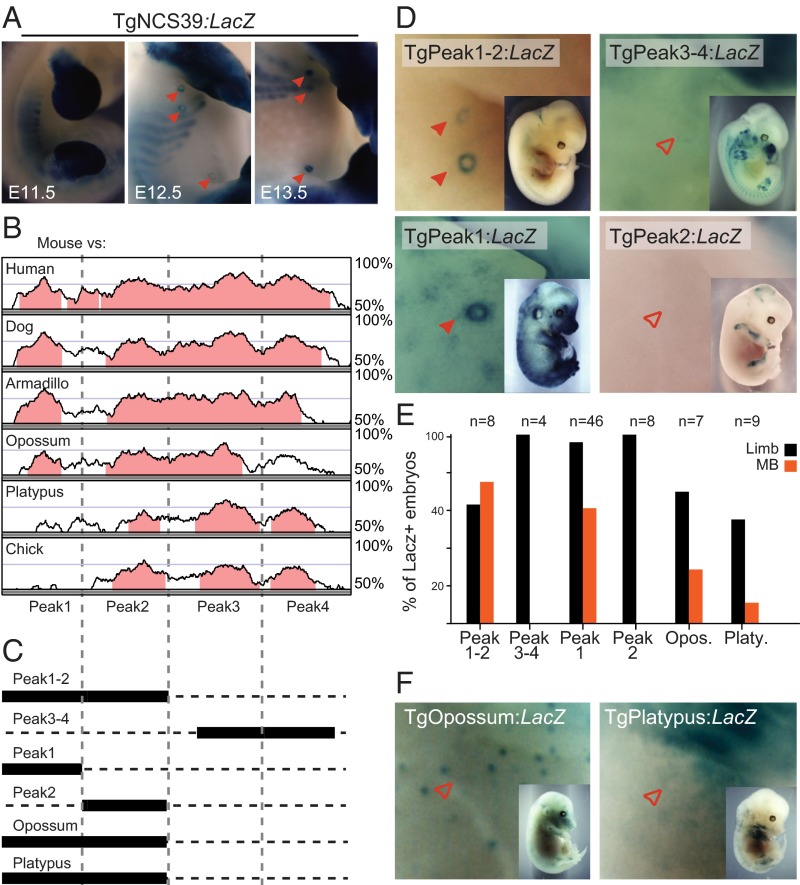
This 20-kb-large DNA region necessary to drive Hoxd9 expression in the developing MB interacts strongly with the HoxD cluster in all tissues analyzed thus far (17, 18, 53). A close examination of 4C interaction profiles revealed that the strongest contacts coincide with the CS38 and CS39 conserved elements (Fig. S6D). The latter had been previously described as an enhancer driving Hoxd expression in developing limb buds (17, 52). Immediately 5′ of CS39, we identified a ca. 200-bp sequence conserved among eutherian mammals. A stable transgenic line carrying this eutherian-specific element, the CS39 sequence, and a LacZ reporter cassette revealed β-gal activity in the developing MBs at E12.5 and E13.5, but not in the E11.5 MPs (Fig. 6A), thus matching the expression of endogenous Hoxd9. Instead, the CS38 region did not display any enhancer activity when tested in a classical transgenic assay.
Fig. 6.
Eutherian-specific sequence displays MB enhancer activity. (A) LacZ staining of mouse transgenic embryos carrying a 1.2-kb construct, including the CS39 regulatory element and its 5′ adjacent therian-specific conserved element. Robust expression of the LacZ reporter is scored in the developing MBs (red arrowheads). (B) Vista alignment of the sequence encoded by the TgNCS39 transgene, showing four peaks of conservation (defined by at least 70% sequence identity over 100 bp). The most 5′-located sequence (Peak1) is conserved in therian mammals, yet not in the monotreme platypus. (C) Scheme showing the different transgenic constructs used in this study, including either different portions of the TgNCS39 transgene or the opossum and platypus regions paralogous to the Peak1-Peak2 sequence (black rectangles). (D and F) LacZ staining of E12.5 mouse embryos transgenic for the different lentivirus constructs described in C. Filled red arrowheads represent MBs where LacZ expression was detected. Empty red arrowheads represent MBs lacking expression of the reporter. (Magnification: 18×.) (E) Bar graphs representing the percentage of LacZ-stained embryos showing specific LacZ expression either in the MB or the limb for each of the analyzed lentiviral constructs over the total number of LacZ-stained embryos.
Phylogenetic footprinting of the sequence contained in the TgNCS39 transgenic construct (Fig. 6B) revealed four regions of high conservation (Peak1–Peak4). Whereas Peak1 is specific to therian mammals, Peak2–Peak4 matched the vertebrate conserved CS39 region (17). We generated transgenic constructs carrying different combinations of these conserved regions (Fig. 6C) to define which sequence displayed the MB enhancer capacity. All constructs carrying the Peak1 sequence activated LacZ transcription in the MBs (Fig. 6 D and E), in agreement with the conservation of Peak1 in therian mammals. In contrast, the TgPeak3-Peak4/LacZ reporter displayed β-gal staining in the limb buds but not in the E12.5 MB (Fig. 6 D and E). We next generated lentiviral reporter constructs carrying either Peak1 or Peak2 individually, and only the TgPeak1/LacZ construct was able to drive LacZ reporter expression in the developing MBs, in addition to a high background activity in the rest of the embryo (Fig. 6 D and E). Both constructs were consistently active in the limb bud. However, the TgPeak1/LacZ construct showed significantly less expression intensity and robustness than observed with the TgPeak1-Peak2/LacZ reporter. In addition, β-gal activity was detected in the vibrissae and in the incipient hair follicles, as well as in the presumptive skin dermis, although at low levels. These results suggest that, albeit Peak1 is the minimal region required for MB enhancer activity, other sequences might contribute to drive robust and specific transcription into these structures.
The Peak1 sequence [hereafter referred to as mammary bud regulatory element (MBRE)] was not detected in the monotreme platypus or in other nonmammalian vertebrates (Fig. 6B). To evaluate the regulatory potential of this region across eutherian and noneutherian mammals, we cloned a DNA segment orthologous to the murine Peak1-Peak2 sequence from both the platypus and opossum, and tested it in lentiviral transgenic assays. In agreement with its lack of conservation, the platypus transgene failed to drive LacZ activity in the developing MBs (one of nine), although it had similar expression in the developing limb (five of nine) compared with the murine sequence. The opossum sequence also displayed reproducible LacZ staining in the proximal limb (five of seven) but showed only sporadic expression in the developing MB (two of seven). Nevertheless, it could control expression in the hair follicle placodes, thus partially mimicking the reporter activity of the murine TgPeak1/LacZ construct.
SI Materials and Methods
Mouse Mutant Lines.
The Inv(Itga6-Attp) mutant allele was generated using the STRING method (76). The parental alleles contained a LoxP site at the Itga6 locus located 3 Mb 5′ of the cluster (77) and at an Attp located 3.6 kb 3′ of Hoxd1 (17). This allele was maintained on a B6/CBA F1 hybrid background. The deletion Del(SB2-SB3) was obtained by targeted meiotic recombination (TAMERE) (78) by recombining the LoxP sites at the SB2 and SB3 loci insertion sites (17) located 151 kb and 1,000 kb downstream of the HoxD cluster, respectively. The Del(CS38–CS40) line was produced via homologous recombination of a loxP-PGKneo-loxP cassette. The cells containing the insertion were selected by neomycin resistance. Mice were crossed with a Hprt-Cre strain to obtain Del(CS38–CS40)+/− animals. A cassette containing the LacZ coding sequence under the control of the SV40 promoter was introduced at the HindIII site of the Hoxd1 second exon of the BAC RP23-400H17. A stable transgenic line carrying a random integration of the modified BAC was generated by pronuclear injection and maintained on a B6/CBA F1 hybrid background.
RNA-Seq Data Analysis.
We aligned raw RNA-seq reads on the mouse mm10 genome assembly using TopHat 2.0.9 (79), implemented in Galaxy (80). We computed gene expression levels based on genomic annotations from Ensembl release 82 (81). We extracted uniquely mapping reads from TopHat alignments using Galaxy tools, and we counted the number of reads attributable to each gene using HTSeq (82). We further computed reads per kilobase of exon per million mapped reads gene expression levels using Cufflinks (83).
We performed differential expression analyses using DESeq2 (84). Specifically, we contrasted a generalized linear model that explains the variation in read counts for each gene, as a function of the tissue (MB or adjacent skin), to a null model that assumes no effect of the tissue. We ran the Wald test and the likelihood ratio test (LRT) implemented in DESeq2 independently. The P values were corrected for multiple testing with the Benjamini–Hochberg approach. We posed that genes are differentially expressed if the false discovery rate (FDR) was below 0.1, if the absolute fold expression change estimated with the Wald test was higher than 1.5, and if the absolute fold change estimated with the LRT test was higher than 2.
A GO term search was performed with Gorilla (48) by comparing MB enriched gene (Wald test fold change > 1.5, FDR < 0.1) versus all expressed genes (mean base levels > 5), using a P value threshold <10−4. The identified GO terms were further analyzed using the Revigo algorithm to extract relevant GO categories by clustering closely related GO terms using a similarity threshold of 0.7 (49).
Phylogenetic Footprinting Analysis.
Sequences corresponding to the genomic intervals containing the MBRE-CS39 enhancers and the intergenic region i from different species were downloaded from the University of California Santa Cruz (UCSC) genome browser (Table S4). The sequences were aligned using the Vista Shuffle Lagan algorithm (85). Sequences were considered as evolutionary conserved when showing at least 70% base pair identity over a 100-bp window.
Table S4.
Genomic coordinates of the regions used in the Vista sequence alignments
| Region analyzed | Species | Chromosome | Start coordinate | End coordinate | Genome assembly |
| Region i | Mouse | 2 | 74,709,498 | 74,722,577 | GRCm38/mm10 |
| Human | 2 | 176,126,079 | 176,127,978 | hg38 | |
| Dog | 36 | 19,934,480 | 19,963,329 | canFam3 | |
| Opossum | 4 | 187,467,979 | 187,500,797 | 188168441 | |
| Platypus | Ultra514 | 14,676,323 | 14,707,466 | ornAna1 | |
| Zebra finch | 7 | 17,059,909 | 17,084,527 | taeGut2 | |
| Telomeric gene desert region (60 kb) | Mouse | 2 | 75,112,340 | 75,171,806 | GRCm38/mm10 |
| Human | 2 | 176,612,667 | 176,676,149 | hg38 | |
| Dog | 36 | 20,401,543 | 20,460,595 | canFam3 | |
| Opossum | 4 | 188,122,933 | 188,209,388 | 188168441 | |
| Platypus | Ultra514 | 15,068,184 | 15,120,101 | ornAna1 | |
| Chicken | 7 | 15,592,073 | 15,563,383 | galGal4 | |
| MBRE-CS39 | Mouse | 2 | 75,147,221 | 75,148,669 | GRCm38/mm10 |
| Human | 2 | 176,648,611 | 176,650,069 | hg38 | |
| Dog | 36 | 20,434,198 | 20,435,663 | canFam3 | |
| Opossum | 4 | 188,166,939 | 188,168,441 | 188168441 | |
| Platypus | Ultra514 | 15,095,573 | 15,096,979 | ornAna1 | |
| Chicken | 7 | 15,579,562 | 15,581,244 | galGal4 |
Chromatin Conformation Capture (4C) Sequencing Dataset Analysis.
The global quantifications of Hoxd gene interactions with the telomeric and centromeric gene deserts, as well as with the CS38–CS40 region, were calculated as in the study by Andrey et al. (17). The coordinates [in Mus musculus 9 (mm9)] used for these quantifications correspond to the following intervals: chr2:74,619,573–75,443,573, chr2:73,752,211–74,474,511, and chr2:74,958,108–75,003,828, respectively. The counts over the CS38–CS40 region were normalized to the sum of those counts scored in both the C-DOM and T-DOM.
Discussion
Hox Genes in the MGs.
Our comparative transcriptome analysis identified 409 genes enriched in the E13.5 MBs, among which were several Hox genes. The mammary mesenchyme is instrumental in the development of the MGs and can induce ectopic expression of mammary ectoderm markers in the nonmammary skin epithelium of different tetrapod species (54, 55). Also, the mesenchyme underlying the ectodermal precursor of different skin appendages determines the type of structure to be formed (56, 57). Therefore, the molecular identities of these different mesenchymes must be distinctly identified. In a study by Wansbury et al. (30), the authors dissected E12.5 posterior MBs and separated the ectoderm from the underlying mammary mesenchyme, making it difficult to identify which genes are specifically expressed in this structure. Indeed, an important number of the genes identified in that study are also expressed in the adjacent epithelium and mesenchyme (30). Our data, thus, represent a substantial complement in the description of the gene network operating during MB development. We confirmed the strong expression of the Hoxa9, Hoxb9, and Hoxd9 paralogous genes (36) in the MBs and report the detectable transcription of additional Hox genes, such as Hoxd8, Hoxb3, and Hoxb6.
Hoxd8 transcription is rapidly down-regulated, however, whereas other Hox gene mRNAs remain at high steady-state levels. Also, Hoxa9 and Hoxd8 expression is restricted to a subset of MBs, with the latter expressed only in MB1 to MB3 and the former in the posteriorly located MB4 and MB5. These observations, along with the reported role of Hoxc8 in the early formation of the MPs from the milk line (36), suggest that spatial and temporal Hox combinations contribute to the patterning and development of the different pairs of MGs (Fig. 7A). Hairs and feathers are other skin-derived appendages whose early development shares similarities with MP induction and invagination (58–61). Of note, they also express different Hox gene combinations (32–34, 62). Furthermore, within the same skin structure, a considerable level of interspecies heterogeneity in the expression of these genes can be observed (31), suggesting that various Hox codes were differentially coopted during evolution for the specification of epidermal appendages. In this context of multiple Hox gene expression, it is not surprising that our deletion of the entire CS38–CS40 region, including the Hoxd9 MBRE element, did not elicit a strong abnormal phenotype. Functional redundancy between group 9 HOX proteins in these and other developing structures was indeed previously reported (9, 36, 63).
Fig. 7.
Model for Hoxd gene regulation in the MB. (A) Schematic representation of Hox gene expression in E12.5 and E13.5 MBs. The different Hox genes analyzed in this study are represented with bars of different colors spanning the two developmental stages. ep, epithelium; me, mammary epithelium; mm, mammary mesenchyme. (B) Scheme representing the regulation of Hoxd gene expression in the MBs. Hoxd genes specifically expressed in the MBs are represented with green rectangles, whereas others are shown in gray. The regulatory (Reg.) region CS38–CS40 identified in this study is shown in light green. The activation controlled by either proximal or distal regulatory elements is represented by black arrows. The activity of the telomeric gene desert is required for the expression of both genes in these structures. However, Hoxd9 expression depends strictly on the regulatory activity of the CS38–CS40 region containing the therian-specific enhancer MBRE, whereas Hoxd8 transcription requires the combined activity of the HoxD telomeric gene desert and a local regulatory element located within the cluster, at the 3′ end of the Hoxd8 locus. (C) Schematic of the CS38–CS40 regulatory hub. Strong constitutive contacts (depicted here as a dark green sphere) are fixed between the CS38–CS40 region and the central part of the HoxD cluster, particularly with Hoxd9. These contacts drag specific enhancers toward this part of the gene cluster (76–85). The number of genes responding to these enhancers, as well as the number of additional active enhancers located nearby, varies from tissue to tissue (shown by the light green cloud). As a result of this preformed chromatin architecture, novel enhancers can evolve by hijacking this potent regulatory module, as may have been the case for the MB-specific regulatory sequence. Transcriptionally active genes are represented by green boxes, whereas inactive Hox genes are depicted by gray rectangles.
Distinct Regulations in the MB.
We show that the telomeric gene desert is required for the expression of both Hoxd8 and Hoxd9 in the embryonic MB. These two genes are nevertheless regulated by different mechanisms. Hoxd9 transcription depends on a 20-kb-large DNA segment (region CS38–CS40) containing the eutherian conserved element MBRE, whereas sequences inside the HoxD cluster have little impact upon its regulation. On the other hand, the MBRE-containing region only weakly, if at all, contributes to Hoxd8 expression in the MBs, which instead requires a 13-kb-large region located 3′ of the Hoxd8 locus within the cluster (Fig. 7B). Surprisingly, although the Del(SB2-CS65) deficiency abrogated Hoxd8 expression in the MBs, none of the BAC clones covering this region displayed enhancer activity in the MBs. One explanation is that regulatory elements driving Hoxd8 expression may be located within the SB2–SB3 region, yet outside of the transgenic BAC tested in this study. Also, the concomitant activity of various Hoxd8 enhancers located both in the 3′ vicinity of the gene and in the telomeric gene desert may be required such that neither one of these sequences alone would be sufficient. Therefore, although the precise mechanisms behind these regulations remain to be elucidated, our results reveal that Hoxd8 and Hoxd9 are differentially controlled in the developing MBs. This observation is intriguing, because long-range regulations involving the HoxD flanking gene deserts were previously reported to involve series of contiguous genes systematically, thus allowing for a coordinated function.
A Regulatory Hub.
The particular topology of chromatin domains at Hox loci could have triggered the emergence of novel enhancers, through preexisting structural and regulatory contacts (16). Noteworthy, region CS38–CS40 is located at the boundary between the two sub-TADs of the T-DOM and was reported to establish robust contacts with the HoxD cluster in all tissues analyzed thus far, regardless of the transcriptional state of the gene cluster (17, 53, 64). These constitutive contacts may be associated with the presence of several CTCF binding sites clustered in this region. Here, we show that among Hoxd genes, the strongest interaction with this region, and particularly with the CS39 enhancer, is established by Hoxd9, suggesting that this locus may help secure a robust constitutive interaction with this region, which might be used by various regulatory regions located around the CS3–CS40 region to interact with their sets of target genes in the vicinity of Hoxd9 (Fig. 7C). In this view, these constitutive contacts may serve as a regulatory hub by dragging various tissue-specific enhancers always at the same position within the HoxD cluster, centered on Hoxd9, as previously observed for the proximal limbs and the cecum (17, 18) (Fig. 7C). Also, this strong constitutive interaction with Hoxd9 could have been instrumental in the emergence of MB enhancers in mammals by providing the necessary chromatin architecture, and thus optimal conditions, for a novel regulatory sequence to evolve, a mechanism proposed for the de novo appearance of enhancers located in the centromeric gene desert (19).
Evolution of an MB Enhancer.
MGs likely originated from an apocrine gland already present before the divergence of the sauropsid and synapsid lineages, which further evolved in the synapsid lineage (the precursors of mammals). It is believed that the ancestral MG was a unit composed of a hair follicle, a sebaceous gland, and an apocrine gland, thus termed the apo-pilo-sebaceus unit (APSU) (65, 66). Monotremes have ∼200 repetitions of APSUs per MG, which secrete directly to the body surface; hence, they lack mammary papilla (or nipples). In contrast, marsupial and eutherian MGs release their secretion into mammary ducts that converge toward a larger duct opening into nipple-like structures (65). Therian MGs differ in their number and positions, as well as in the shape and function of the nipples, which have adapted according to the nursing behavior and number of the progenies (29, 67). It is therefore possible that changes in the transcription profiles of the mammary mesenchyme, which is required for the development of both structures, accompanied the evolution and diversification of MG morphology and functions across the mammalian lineages.
Our investigations on the evolutionary origin of the enhancer activity driving Hoxd9 expression in the mouse embryonic mammary mesenchyme identified a sequence (MBRE) capable of directing reporter gene expression in the mesenchyme of the developing MBs. The MBRE, however, requires the presence of sequences located nearby (within Peak2 conserved throughout vertebrates) to ensure specific activity into the MBs. We were not able to identify the monotreme MBRE, and the orthologous sequence from platypus did not display enhancer activity in the mammary mesenchyme. Of note, the marsupial orthologous sequence (from opossum) did trigger LacZ reporter expression in the mesenchyme underlying the forming hair follicle placodes, whereas expression in the developing MBs was only sporadic.
The lack of evidence about Hox gene expression in the MBs of noneutherian mammals makes it difficult to interpret such changes in the regulatory activity of the MBRE in these lineages. Also, interspecies divergence within enhancer sequences does not necessarily mean that their regulatory output would be fundamentally modified (68), and enhancers associated with genes under positive selection in mammals were shown to evolve rapidly (69). As a possible scenario however, this sequence may have acquired a nonspecific enhancer activity in APSU-associated mesenchyme in the common ancestor of metatherians and eutherians, thus preluding the actual murine MB regulatory element. Alternatively, this sequence exerted its MB activity in the therian common ancestor and further diversified toward a hair follicle-specific activity in the marsupial lineage. Our observation that a transgenic construct carrying only MBRE retains its broad APSU enhancer activity, thus resembling the opossum sequence, nevertheless suggests that this activity was an ancestral property. In this view, the MB specificity of this sequence evolved through the acquisition of responsiveness to upstream mammary mesenchyme determinants. The nature of these upstream factors is currently under study.
It was recently shown that transposable elements can acquire a regulatory potential during evolution, and thus have an impact on gene expression (e.g., refs. 70–72). On the other hand, vertebrate Hox clusters are largely devoid of transposable elements, unlike their flanking regulatory regions. Interestingly, the CS38–CS40 region is also relatively poor in transposable element insertions compared with the surrounding HoxD telomeric gene desert. This low number of transposons could be due either to the high density of regulatory elements found within this region or to the importance of this DNA segment in the establishment of a regulatory conformation at the HoxD locus.
Materials and Methods
Mouse Strains.
Mice were handled according to the Swiss law on animal protection (LPA), with the requested authorization (GE/81/14 to D.D.). Mice were raised and killed according to good laboratory practice standards. Genetically modified mice were maintained and crossed in heterozygosis. The mouse mutant lines used in this work and the primers used to genotype them are described in Table S1. The description of the Inv(Attp-Itga6) and Del(CS38–CS40) alleles can be found in SI Materials and Methods.
Table S1.
Primers used to genotype the different mouse lines analyzed in this study
| Mouse line | Allele | Forward | Reverse |
| Del(1–13)D11Lac | WT | GAGCCCGACGCATCGAGATAGC | CAAGGTCCTCAGCCTTAAGAGTGG |
| Mutant | GAGTTTCTCTTTGCTGTAATGAAGAGCTG | CAAGGTCCTCAGCCTTAAGAGTGG | |
| Del(TpSB2-AttP) | Mutant | ATCCCGGGGGATCCACTAGAG | GCTATATAGTAGTTTCAAGGCCAGCCC |
| Del(TpSB3-AttP) | Mutant | ATCCCGGGGGATCCACTAGAG | GCTTCCCTAGTTTATGGGGGAGG |
| Del(TpSB2-TpSB3) | Mutant | ATCCCGGGGGATCCACTAGAG | GCTTCCCTAGTTTATGGGGGAGG |
| Inv(TgHd11lacNsi-Itga6) | WT | CCGTCCAATGTGCGTGTTTTCC | GCAAGCCACTTGGAAACAACTGTTAATGG |
| Mutant | CCGTCCAATGTGCGTGTTTTCC | GAGTTTCTCTTTGCTGTAATGAAGAGCTG | |
| Inv(attP-Itga6) | WT | GACAGACTCTGTTAAATGCTGGCACAG | GCAAGCCACTTGGAAACAACTGTTAATGG |
| Mutant | CACTTGAAGTTCCCGTCCAATGTGC | GGGAGGGGGATGTAAACATTA | |
| Del(8–13)d11lac | WT | TGGAACAGAGAGCAAGGACG | AGTGTGGCCCTAAACGAAGG |
| Mutant | GAGTTTCTCTTTGCTGTAATGAAGAGCTG | AGTGTGGCCCTAAACGAAGG | |
| Del(65-TpsB2) | WT | CAGCCAGCTCCTGAAAGGAGG | CGTTTCAAAACATGCTCTCTAACCACC |
| Mutant | GTGCATCTCTTCTTACTCAGAACCCAC | GTCACGTGACATCCAGAAGGCG | |
| Del(65-TpsB3) | WT | CAACCCCCTGTAACTCCAGAGG | GCTTCCCTAGTTTATGGGGGAGG |
| Mutant | TGACAGCTCGTTTTGTGCTC | GTTTTCCCAGTCACGACGTTG | |
| Del(1–4) | WT | TATGGTTCTGCAGAGCCGGGAC | CTGCCCCACAACTCTAGAGTTATTGTG |
| Mutant | TATGGTTCTGCAGAGCCGGGAC | CAAGGTCCTCAGCCTTAAGAGTGG | |
| Del(1–4i) | WT | TGAGAGCCCAGCTGGAAAACAGC | GGGGAGCATTCTTGAGTTGGAGATAC |
| Mutant | TGAGAGCCCAGCTGGAAAACAGC | CTCTCAAGTCTATTCAAAGGTGGGGAG | |
| Del(i) | WT | TGGAACAGAGAGCAAGGACG | CTGCCCCACAACTCTAGAGTTATTGTG |
| Mutant | TGGAACAGAGAGCAAGGACG | AGTGTGGCCCTAAACGAAGG | |
| Del(1–10) | WT | AAACGCATTTCTAAATCTTTGGT | CAAACAAACAGTATGATCCCAGA |
| Mutant | AAACGCATTTCTAAATCTTTGGT | CCTGAGAAGGGCTTCCTAGATTCC | |
| Del(8, 9) | WT | GTGGTTTGGTAGAAGTACAAGCGCAAC | CCTGCAGTGGGTTCTTACTTACCAG |
| Mutant | GTGGTTTGGTAGAAGTACAAGCGCAAC | AGTGTGGCCCTAAACGAAGG | |
| Del(8–10) | WT | CTAGAGCTAGGGTGTACTGAGAATTTGG | CAAACAAACAGTATGATCCCAGA |
| Mutant | TTGTTTCGTAAACGCATTTCT | AGTGTGGCCCTAAACGAAGG | |
| Del(8–13)d11lac | WT | TGGAACAGAGAGCAAGGACG | AGTGTGGCCCTAAACGAAGG |
| Mutant | GAGTTTCTCTTTGCTGTAATGAAGAGCTG | GGGGAGCATTCTTGAGTTGGAGATAC | |
| Del(8–13)RXII | WT | TGGAACAGAGAGCAAGGACG | AGTGTGGCCCTAAACGAAGG |
| Mutant | GAGTTTCTCTTTGCTGTAATGAAGAGCTG | GGGGAGCATTCTTGAGTTGGAGATAC | |
| Del(9, 10) | WT | CTAGAGCTAGGGTGTACTGAGAATTTGG | CAAACAAACAGTATGATCCCAGA |
| Mutant | TTGTTTCGTAAACGCATTTCT | TCCCCCATTCTTTAACCTCCCAGC | |
| Del(9–12) | WT | GACAAATAGACCGCTTATCCAATTCGGTTC | TCCCCCATTCTTTAACCTCCCAGC |
| Mutant | CTTCTGCCTCCTCCCACCTAC | TCCCCCATTCTTTAACCTCCCAGC | |
| Del(10–13) | WT | GTGGTTTGGTAGAAGTACAAGCGCAAC | CCTGCAGTGGGTTCTTACTTACCAG |
| Mutant | GTGGTTTGGTAGAAGTACAAGCGCAAC | CCAAGAACTTGCAGGGACATCTAGC | |
| Del(CS38–CS40) | WT | GGAGCTTACTACTTGAGGGTGAGC | GAGCTGTGCACAGGTGTGATGC |
| Mutant | CTCAGGGACCACAGTGTTCGG | GCTATCCGAGTTGCTATATAGCTCTGG | |
| BACHoxD | GGTAAACTGGCTCGGATTAGGG | CTATTCAAAGGTGGGGAGCAGTC | |
| TgNCS39 | AAGCGGGGTTCCAACAGAAACCC | GGGGGATGTGCTGCAAGGC | |
| Lentiviral constructs (LacZ) | CCTGCTGATGAAGCAGAACA | CAGCGACCAGATGATCACAC | |
WT, wild type.
RNA Extraction and RNA-Seq.
Pairs of embryonic MBs 2 and 3 from E13.5 embryos were dissected in cold PBS and immediately processed for RNA extraction. To evaluate MB-specific gene expression, we dissected an equivalent portion of ectoderm and its underlying mesoderm tissue immediately adjacent to the MB. RNA extraction was performed using the RNAeasy Micro Kit (Qiagen) following the manufacturer’s instructions. The RNA-seq libraries were prepared from 100 ng of pure total RNA using the TruSeq Stranded mRNA protocol from Illumina with polyA selection. Libraries were sequenced on a HiSeq 2500 machine as single-end, 100-bp reads. The RNA-seq datasets produced in this study are publically available in the Gene Expression Omnibus (GEO) database (accession no. GSE84943).
Cloning.
The probe sequences of Hoxa9, Hoxb6, Hoxb9, and Hoxc9 were amplified using Toptaq (Qiagen) and specific primers (Table S2), and cloned into pGEMT-easy vector following the manufacturer’s instructions. For the cloning of lentiviral constructs, the target DNA was amplified by PCR using the Expand High Fidelity PCR system (Sigma) and specific primers (Table S3), and ligated in the PCR8/GW/TOPO (Thermo Fisher Scientific). Evolutionary conserved sequences were identified using the Vista alignment algorithm (see SI Materials and Methods). Coordinates used for the alignments are listed in Table S4. The opossum and platypus Peak1 and Peak2 regions were identified based on sequence conservation corresponding to the mouse orthologous CS39 Peak2. The cloned sequences correspond to the coordinates chr4:188,167,036–188,167,486 (monDom5) and Ultra514:15,095,67–15,096,077 (ornAna1) of the opossum and platypus genomes, respectively. These sequences were synthesized in vitro and inserted into the pENTR vector (GeneArt; Thermo Fisher Scientific). All of the PCR8/GW/TOPO or pENTR clones were verified by standard Sanger sequencing using the T7 primer located in the vector backbone, and the clones carrying the correct insert were recombined into the pRRLbLacGW (18) by LR reaction (Thermo Fisher Scientific). The final recombined constructs were verified by Sanger sequencing.
Table S2.
Primers used for the cloning of Hoxa9, Hoxb9, Hoxc9, and Hoxb6 probes and enzymes used to digest the plasmid and transcribe the digoxigenin-labeled probe
| Cloning | Probe synthesis | ||||
| Gene | Forward | Reverse | Vector | Digest | Polymerase |
| Hoxa9 | GGTTGTATCATCGACGGTG | ATGTAACAACTTGGTGGCAC | pGEM-T | SpeI | T7 |
| Hoxb9 | GCTCCACTGTACTTTGTTGG | CTTTGTCCTCGCTTCCTTCG | pGEM-T | SpeI | T6 |
| Hoxc9 | TCCCTGATGAGATATGATTCG | AGCTACAGGACGGAAAATCG | pGEM-T | SpeI | T7 |
| Hoxb6 | TCCCGATGAGTTCCTATTTC | GAAGTCTCACTCACTGTTGCAC | pGEM-T | NcoI | Sp6 |
Table S3.
Primers used for the cloning of the different CS39 transgenic constructs
| Genomic region | Cloning | ||
| Forward | Reverse | ||
| Mouse CS39 | Peak 1 (MBRE) | ACAGTGCAGGTAGTAGAGAG | TGGACTGATAAAGACTTCAG |
| Peak 2 | CTGAAGTCTTTATCAGTCCA | CTGGCAACAACTTTGGGAG | |
| Peak 1 + 2 | ACAGTGCAGGTAGTAGAGAG | CTGGCAACAACTTTGGGAG | |
| Peak 3 + 4 | CCCTTTGAACACGAAGAGAAAG | CCAGTCAGACACCACAAGAAG | |
Lentiviral Transgenesis and β-Gal Staining.
Lentiviral transgenesis was performed as in the study by Friedli et al. (73), and E12.5 embryos were dissected and stained for β-gal activity following a standard protocol.
WISH.
Probes used in WISH were synthesized using T3, T7, or SP6 RNA polymerase (Table S2). They were subsequently purified using the RNA Easy Mini Kit (Qiagen). The WISH protocol was the protocol used by Woltering et al. (74).
Chromosome Conformation Capture (4C) Sequencing Dataset Analysis.
The 4C-sequencing data from E11.5 whole embryo (75) were downloaded from the GEO database (accession no. GSE79048), whereas the 4C-sequencing data from E10.5 brain and anterior and posterior trunk were obtained from Noordermeer et al. (53). The pipeline used to analyze the 4C-seq data is described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. B. Howard from the Breakthrough Breast Cancer Research Centre (London) for sharing information about the MB microarray data reported by Wansbury et al. (30), as well as members of the Duboule laboratories for sharing ideas and discussions. We also thank Dr. B. Mascrez, S. Gitto, and J. Couderey for help with mutant stocks. We thank the Geneva genomics platform (University of Geneva), the transgenesis unit of the Geneva Medical Centre, and the transgenic core facility at the Ecole Polytechnique Fédérale de Lausanne (EPFL) for their assistance. This work was supported by funds from the EPFL, the University of Geneva, Swiss National Research Fund Grant 310030B_138662, European Research Council SystemHox Grant 232790 and RegulHox Grant 588029, and the Claraz Foundation (D.D.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The RNA sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE84943).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617141113/-/DCSupplemental.
References
- 1.Nowick K, Stubbs L. Lineage-specific transcription factors and the evolution of gene regulatory networks. Brief Funct Genomics. 2010;9(1):65–78. doi: 10.1093/bfgp/elp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glassford WJ, et al. Co-option of an ancestral Hox-regulated network underlies a recently evolved morphological novelty. Dev Cell. 2015;34(5):520–531. doi: 10.1016/j.devcel.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinman VF, Cheatle Jarvela AM. Developmental gene regulatory network evolution: Insights from comparative studies in echinoderms. Genesis. 2014;52(3):193–207. doi: 10.1002/dvg.22757. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro A. Gene regulatory networks reused to build novel traits: Co-option of an eye-related gene regulatory network in eye-like organs and red wing patches on insect wings is suggested by optix expression. BioEssays. 2012;34(3):181–186. doi: 10.1002/bies.201100160. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner M, Gerhart J. Evolvability. Proc Natl Acad Sci USA. 1998;95(15):8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duboule D, Wilkins AS. The evolution of ‘bricolage’. Trends Genet. 1998;14(2):54–59. doi: 10.1016/s0168-9525(97)01358-9. [DOI] [PubMed] [Google Scholar]
- 7.Woltering JM, Duboule D. The origin of digits: Expression patterns versus regulatory mechanisms. Dev Cell. 2010;18(4):526–532. doi: 10.1016/j.devcel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Lynch VJ, et al. Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci USA. 2008;105(39):14928–14933. doi: 10.1073/pnas.0802355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallo M, Wellik DM, Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev Biol. 2010;344(1):7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deschamps J, van Nes J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development. 2005;132(13):2931–2942. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- 11.Zakany J, Duboule D. The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev. 2007;17(4):359–366. doi: 10.1016/j.gde.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Pascual-Anaya J, D’Aniello S, Kuratani S, Garcia-Fernàndez J. Evolution of Hox gene clusters in deuterostomes. BMC Dev Biol. 2013;13:26. doi: 10.1186/1471-213X-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendivil Ramos O, Barker D, Ferrier DE. Ghost loci imply Hox and ParaHox existence in the last common ancestor of animals. Curr Biol. 2012;22(20):1951–1956. doi: 10.1016/j.cub.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134(14):2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- 15.Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301(5631):331–333. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- 16.Darbellay F, Duboule D. Topological domains, metagenes, and the emergence of pleiotropic regulations at Hox loci. Curr Top Dev Biol. 2016;116:299–314. doi: 10.1016/bs.ctdb.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Andrey G, et al. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science. 2013;340(6137):1234167. doi: 10.1126/science.1234167. [DOI] [PubMed] [Google Scholar]
- 18.Delpretti S, et al. Multiple enhancers regulate Hoxd genes and the Hotdog LncRNA during cecum budding. Cell Reports. 2013;5(1):137–150. doi: 10.1016/j.celrep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Lonfat N, Montavon T, Darbellay F, Gitto S, Duboule D. Convergent evolution of complex regulatory landscapes and pleiotropy at Hox loci. Science. 2014;346(6212):1004–1006. doi: 10.1126/science.1257493. [DOI] [PubMed] [Google Scholar]
- 20.Montavon T, et al. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147(5):1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 21.de Wit E, de Laat W. A decade of 3C technologies: Insights into nuclear organization. Genes Dev. 2012;26(1):11–24. doi: 10.1101/gad.179804.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibcus JH, Dekker J. The hierarchy of the 3D genome. Mol Cell. 2013;49(5):773–782. doi: 10.1016/j.molcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon JR, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nora EP, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485(7398):381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefèvre CM, Sharp JA, Nicholas KR. Evolution of lactation: Ancient origin and extreme adaptations of the lactation system. Annu Rev Genomics Hum Genet. 2010;11:219–238. doi: 10.1146/annurev-genom-082509-141806. [DOI] [PubMed] [Google Scholar]
- 26.Vorbach C, Capecchi MR, Penninger JM. Evolution of the mammary gland from the innate immune system? BioEssays. 2006;28(6):606–616. doi: 10.1002/bies.20423. [DOI] [PubMed] [Google Scholar]
- 27.Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11(3):437–458. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- 28.Ahn Y. Signaling in tooth, hair, and mammary placodes. Curr Top Dev Biol. 2015;111:421–459. doi: 10.1016/bs.ctdb.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Koyama S, Wu HJ, Easwaran T, Thopady S, Foley J. The nipple: A simple intersection of mammary gland and integument, but focal point of organ function. J Mammary Gland Biol Neoplasia. 2013;18(2):121–131. doi: 10.1007/s10911-013-9289-1. [DOI] [PubMed] [Google Scholar]
- 30.Wansbury O, et al. Transcriptome analysis of embryonic mammary cells reveals insights into mammary lineage establishment. Breast Cancer Res. 2011;13(4):R79. doi: 10.1186/bcr2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awgulewitsch A. Hox in hair growth and development. Naturwissenschaften. 2003;90(5):193–211. doi: 10.1007/s00114-003-0417-4. [DOI] [PubMed] [Google Scholar]
- 32.Kanzler B, et al. Differential expression of two different homeobox gene families during mouse tegument morphogenesis. Int J Dev Biol. 1994;38(4):633–640. [PubMed] [Google Scholar]
- 33.Chuong CM, et al. Gradients of homeoproteins in developing feather buds. Development. 1990;110(4):1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- 34.Stelnicki EJ, et al. HOX homeobox genes exhibit spatial and temporal changes in expression during human skin development. J Invest Dermatol. 1998;110(2):110–115. doi: 10.1046/j.1523-1747.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 35.Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12(1):11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci USA. 1999;96(2):541–546. doi: 10.1073/pnas.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll LS, Capecchi MR. Hoxc8 initiates an ectopic mammary program by regulating Fgf10 and Tbx3 expression and Wnt/β-catenin signaling. Development. 2015;142(23):4056–4067. doi: 10.1242/dev.128298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garin E, Lemieux M, Coulombe Y, Robinson GW, Jeannotte L. Stromal Hoxa5 function controls the growth and differentiation of mammary alveolar epithelium. Dev Dyn. 2006;235(7):1858–1871. doi: 10.1002/dvdy.20822. [DOI] [PubMed] [Google Scholar]
- 39.Andrechek ER, Mori S, Rempel RE, Chang JT, Nevins JR. Patterns of cell signaling pathway activation that characterize mammary development. Development. 2008;135(14):2403–2413. doi: 10.1242/dev.019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchard A, et al. Gene expression profiling of early involuting mammary gland reveals novel genes potentially relevant to human breast cancer. Front Biosci. 2007;12:2221–2232. doi: 10.2741/2225. [DOI] [PubMed] [Google Scholar]
- 41.Master SR, et al. Functional microarray analysis of mammary organogenesis reveals a developmental role in adaptive thermogenesis. Mol Endocrinol. 2002;16(6):1185–1203. doi: 10.1210/mend.16.6.0865. [DOI] [PubMed] [Google Scholar]
- 42.Stein T, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6(2):R75–R91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phippard DJ, et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122(9):2729–2737. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- 44.Eblaghie MC, et al. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J Anat. 2004;205(1):1–13. doi: 10.1111/j.0021-8782.2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiremath M, Wysolmerski J. Parathyroid hormone-related protein specifies the mammary mesenchyme and regulates embryonic mammary development. J Mammary Gland Biol Neoplasia. 2013;18(2):171–177. doi: 10.1007/s10911-013-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asselin-Labat ML, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9(2):201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 47.Douglas NC, Papaioannou VE. The T-box transcription factors TBX2 and TBX3 in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(2):143–147. doi: 10.1007/s10911-013-9282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6(7):e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho KW, et al. Retinoic acid signaling and the initiation of mammary gland development. Dev Biol. 2012;365(1):259–266. doi: 10.1016/j.ydbio.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Gerstein M, Snyder M. RNA-Seq: A revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beccari L, et al. A role for HOX13 proteins in the regulatory switch between TADs at the HoxD locus. Genes Dev. 2016;30(10):1172–1186. doi: 10.1101/gad.281055.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noordermeer D, et al. The dynamic architecture of Hox gene clusters. Science. 2011;334(6053):222–225. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- 54.Cunha GR, et al. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anat (Basel) 1995;152(3):195–204. doi: 10.1159/000147698. [DOI] [PubMed] [Google Scholar]
- 55.Propper A, Gomot L. Control of chick epidermis differentiation by rabbit mammary mesenchyme. Experientia. 1973;29(12):1543–1544. doi: 10.1007/BF01943907. [DOI] [PubMed] [Google Scholar]
- 56.Dhouailly D, Rogers GE, Sengel P. The specification of feather and scale protein synthesis in epidermal-dermal recombinations. Dev Biol. 1978;65(1):58–68. doi: 10.1016/0012-1606(78)90179-3. [DOI] [PubMed] [Google Scholar]
- 57.Kollar EJ, Fisher C. Tooth induction in chick epithelium: Expression of quiescent genes for enamel synthesis. Science. 1980;207(4434):993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- 58.Chen CF, et al. Development, regeneration, and evolution of feathers. Annu Rev Anim Biosci. 2015;3:169–195. doi: 10.1146/annurev-animal-022513-114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhouailly D. A new scenario for the evolutionary origin of hair, feather, and avian scales. J Anat. 2009;214(4):587–606. doi: 10.1111/j.1469-7580.2008.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duverger O, Morasso MI. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J Cell Physiol. 2008;216(2):337–346. doi: 10.1002/jcp.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikkola ML, Millar SE. The mammary bud as a skin appendage: Unique and shared aspects of development. J Mammary Gland Biol Neoplasia. 2006;11(3-4):187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 62.Kanzler B, Prin F, Thelu J, Dhouailly D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev Dyn. 1997;210(3):274–287. doi: 10.1002/(SICI)1097-0177(199711)210:3<274::AID-AJA8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 63.Xu B, Wellik DM. Axial Hox9 activity establishes the posterior field in the developing forelimb. Proc Natl Acad Sci USA. 2011;108(12):4888–4891. doi: 10.1073/pnas.1018161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noordermeer D, et al. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife. 2014;3:e02557. doi: 10.7554/eLife.02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7(3):225–252. doi: 10.1023/a:1022896515287. [DOI] [PubMed] [Google Scholar]
- 66.Oftedal OT, Dhouailly D. Evo-devo of the mammary gland. J Mammary Gland Biol Neoplasia. 2013;18(2):105–120. doi: 10.1007/s10911-013-9290-8. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert AN. Mammary number and litter size in Rodentia: The “one-half rule”. Proc Natl Acad Sci USA. 1986;83(13):4828–4830. doi: 10.1073/pnas.83.13.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakabe NJ, Savic D, Nobrega MA. Transcriptional enhancers in development and disease. Genome Biol. 2012;13(1):238. doi: 10.1186/gb-2012-13-1-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villar D, et al. Enhancer evolution across 20 mammalian species. Cell. 2015;160(3):554–566. doi: 10.1016/j.cell.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Notwell JH, Chung T, Heavner W, Bejerano G. A family of transposable elements co-opted into developmental enhancers in the mouse neocortex. Nat Commun. 2015;6:6644. doi: 10.1038/ncomms7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chuong EB, Elde NC, Feschotte C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science. 2016;351(6277):1083–1087. doi: 10.1126/science.aad5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lynch VJ, Leclerc RD, May G, Wagner GP. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet. 2011;43(11):1154–1159. doi: 10.1038/ng.917. [DOI] [PubMed] [Google Scholar]
- 73.Friedli M, et al. A systematic enhancer screen using lentivector transgenesis identifies conserved and non-conserved functional elements at the Olig1 and Olig2 locus. PLoS One. 2010;5(12):e15741. doi: 10.1371/journal.pone.0015741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woltering JM, Noordermeer D, Leleu M, Duboule D. Conservation and divergence of regulatory strategies at Hox Loci and the origin of tetrapod digits. PLoS Biol. 2014;12(1):e1001773. doi: 10.1371/journal.pbio.1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerreiro I, et al. Reorganisation of Hoxd regulatory landscapes during the evolution of a snake-like body plan. eLife. August 1, 2016 doi: 10.7554/eLife.16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spitz F, Herkenne C, Morris MA, Duboule D. Inversion-induced disruption of the Hoxd cluster leads to the partition of regulatory landscapes. Nat Genet. 2005;37(8):889–893. doi: 10.1038/ng1597. [DOI] [PubMed] [Google Scholar]
- 77.Gimond C, et al. Cre-loxP-mediated inactivation of the alpha6A integrin splice variant in vivo: Evidence for a specific functional role of alpha6A in lymphocyte migration but not in heart development. J Cell Biol. 1998;143(1):253–266. doi: 10.1083/jcb.143.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hérault Y, Rassoulzadegan M, Cuzin F, Duboule D. Engineering chromosomes in mice through targeted meiotic recombination (TAMERE) Nat Genet. 1998;20(4):381–384. doi: 10.1038/3861. [DOI] [PubMed] [Google Scholar]
- 79.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giardine B, et al. Galaxy: A platform for interactive large-scale genome analysis. Genome Res. 2005;15(10):1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yates A, et al. Ensembl 2016. Nucleic Acids Res. 2016;44(D1):D710–D716. doi: 10.1093/nar/gkv1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12(3):R22. doi: 10.1186/gb-2011-12-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32(Web Server issue):W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.