Abstract
TRPV4 ion channels are osmo-mechano-TRP channels with pleiotropic function and expression in many different types of tissues and cells. They have also been found involved in pain and inflammation. Studies have focused on the role of TRPV4 in peripheral sensory neurons, but its expression and function in central nervous glial cells and neurons has also been documented. In this overview, based on the senior author’s lecture at the recent physiology meeting in Dublin, we concisely review evidence of TRPV4 expression and function in the CNS, and how TRPV4 function can be modulated for therapeutic benefit of neuro-psychiatric disorders. Novel TRPV4-inhibitory compounds developed recently in the authors’ lab will also be discussed
Introduction
Transient receptor potential, vanilloid type 4 (TRPV4) ion channels (Liedtke et al., 2000) are also expressed in cells of the central nervous system (CNS) including neurons, astrocytes and microglia (White et al., 2016). Cell-physiologic experiments indicate that TRPV4 channels can function as Ca2+-permeable cation channels that are gated by various stimuli such as cell swelling, low pH, mechanical stress and temperature (Liedtke & Kim, 2005; Liedtke, 2008; Guilak et al., 2010; McNulty et al., 2015; White et al., 2016). Whether this currently established functional profile is particularly relevant for the role that TRPV4 plays in the CNS remains to be determined. As of today, 16 years after its initial description (Liedtke et al., 2000; Strotmann et al., 2000), studies on specific functions of TRPV4 in various regions of the CNS are not further advanced than the early stages of exploration. Slow progress could be due to low levels of TRPV4 expression in certain cell types within the CNS, yet still contributing to important function. TRPV4 could function both developmentally as well as post-developmentally as a sensor-signaling molecule. Despite this lack of a clear picture, TRPV4 appears to function in glial cells and neurons in basic physiology as well as under specific pathologic conditions, which will be concisely reviewed here. One particular focus of this article will be to relate known findings on TRPV4 in the CNS to what this possibly means for medicinal-translational purposes.
Role of TRPV4 in astrocytes
TRPV4 channels have been found to be expressed in astrocytes. These channels were localized in the plasma membrane of astrocytes and exhibited activation in response to selective agonist showing a typical outwardly rectifying cation current (Benfenati et al., 2007). As a Ca2+ permeable channel, TRPV4 was shown to influence neurovascular coupling through Ca2+-induced Ca2+ release from inositol trisphosphate receptors (IP3Rs) in astrocytic endfeet (Fig. 1A) (Dunn et al., 2013). Studies indicate that astroglial cells can modulate neuronal excitability in hippocampus, cortex and hypothalamus and that TRPV4 might function as a key player in this excitation (Simard & Nedergaard, 2004; Shibasaki et al., 2014). In hippocampus, for example, TRPV4 is highly expressed in astrocytes of the CA1 region and its enhanced expression in this region coincides with the development of astrogliosis (Shirakawa et al., 2010; Butenko et al., 2012).
Fig. 1. TRPV4 in astrocytes in physiology and up-regulated in response to injury.
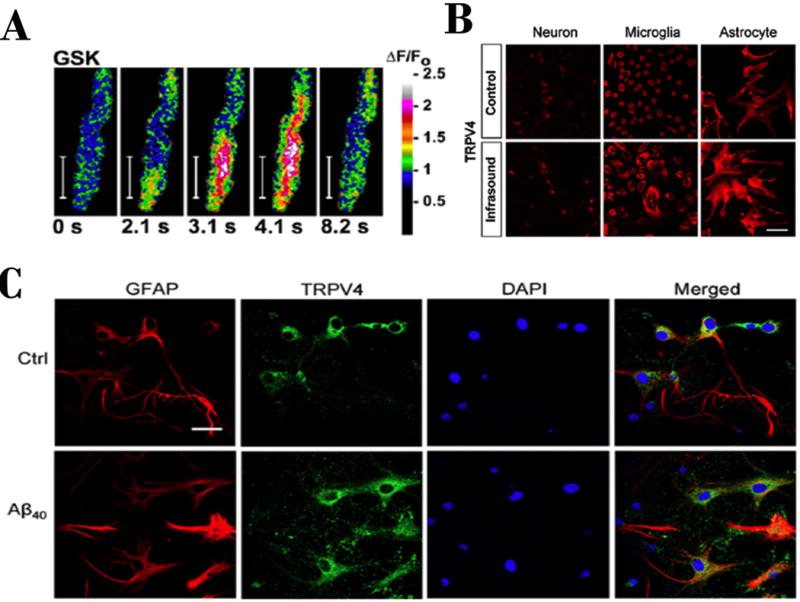
(A) Time course of an astrocytic endfoot Ca2+-transient evoked by selective stimulation of TRPV4, using selective activator compound GSK101; from [9]. (B) Infrasound induced mechanical stress-response of cultured CNS lineages, note up-regulation of TRPV4 in response to infrasound in microglia and astrocytes; from [17]. (C) Alzheimer’s Disease-associated amyloid peptide Aß40 exposure of cultured astrocytes induces increased expression of astrocytic intermediate filament protein, GFAP, and TRPV4 ion channels; from [16].
Other studies suggest that TRPV4 may form a complex with aquaporin-4 (AQP4/TRPV4) and function in control of cell-volume in astrocytes and be involved in regulating interstitial tonicity in the brain, also in formation of brain edema (Benfenati et al., 2011). Furthermore TRPV4 in hippocampal astrocytes were considered to function in oxidative stress-induced cell damage (Bai & Lipski, 2010). Other evidence suggests that astrocytic TRPV4 may be involved in neuronal toxicity evoked by the Alzheimer’s Disease associated peptide, Aß40 (Fig. 1C) (Bai & Lipski, 2014). Astrocytes may therefore play crucial roles in the homeostatic regulation of the CNS not only in physiological but also pathological conditions. TRPV4 channels expressed in astro-glial cells were also shown to mediate infrasound-induced neuronal injury (Fig 1B), a model for blast-induced traumatic brain injury, impairing learning and memory in rats (Shi et al., 2013). This latter study also indicated that injury was more pronounced in the hippocampal CA1 region where astrocytic and microglial activation were observed prior to neuronal apoptosis. Inhibition of TRPV4 protected neurons from infrasound injury by decreasing the expression levels of glial cell-released pro-inflammatory cytokines Il-1β and TNF-α.
Role of TRPV4 in microglia
Interestingly, microglial activation after an injection of lipopolysaccharide (LPS) into the mouse cerebral ventricle was inhibited by concurrent administration of a TRPV4 agonist, 4α-phorbol 12, 13-didecanoate (4α-PDD) (Shirakawa et al., 2010; Konno et al., 2012). Refs (Shirakawa et al., 2010; Konno et al., 2012; Shi et al., 2013) describe seemingly opposing roles of TRPV4 in microglial activation in response to mechanical stimulation vs. chemical stimulation via LPS, indicating the important but complex role of TRPV4 in microglia which is currently under-explored, thus in need of future study.
Role of TRPV4 in CNS neurons
In hippocampal neurons the influx of cations through TRPV4 at physiological temperature may control neuronal excitability by regulating the resting membrane potential (Shibasaki et al., 2007; Shibasaki et al., 2015a) (see also (Shibasaki et al., 2015b)), possibly also in other neurons with wide implications on brain functioning, manifested in several abnormal behavioral parameters in Trpv4−/− mice, as recently documented (Shibasaki et al., 2015a). Possibly, TRPV4 expression and function in neurons may play a significant role in seizures. For example in larval zebrafish, febrile seizure-related neural activity, which was triggered by a rise in brain temperature was shown to be blocked by TRPV4 antagonist and not GABA re-uptake inhibitors (Hunt et al., 2012). Furthermore, increased TRPV4 expression in cortical lesions of patients with Focal Cortical Dysplasia (FCD), a known form of therapy-refractory epilepsy, indicate that TRPV4 may contribute to cortical malformation and maldevelopment which may facilitate epileptogenesis (Chen et al., 2016). As an important qualifier, bespeaking of the superficial level of inquiry in this field, changed expression levels do not conclusively substantiate any association with disease pathogenesis. In other studies, mutations in TRPV4, hereditary TRPV4 channelopathies, were shown to cause excessive Ca2+ influx related to motor nerve axonopathy and spinal muscular atrophy (Fecto et al., 2011; Jang et al., 2012). Although the human genetics of these disorders are clear, it is unclear why some TRPV4 channelopathy mutations cause skeletal malformations, whereas other mutations detrimentally affect spinal motoneurons. For the former, mechanistic evidence as to a feasible patho-mechanism has been provided, at least an initial inroad (Leddy et al., 2014a; Leddy et al., 2014b). In contrast, for the sequence of events between excess Ca2+ influx and subsequent moto-neuron dysfunction, this has hitherto remained elusive.
Interestingly in aging, age related expression changes of TRPV4 have been reported in pyramidal cortical neurons, thalamus, basal nuclei of the cerebellum and in the spinal cord (Lee & Choe, 2014). The expression of TRPV4 in neurons in these brain regions could be the basis for (mal-) function of TRPV4 in pathologic conditions. For example, findings by Lee et al [21] on age-dependent expression of TRPV4 channels may be indicative of TRPV4’s role in pathogenesis of age-related neurodegenerative diseases. Neuronal TRPV4 channels may therefore be an important therapeutic target for cognitive, motor and aging-related disorders. TRPV4 inhibitors have been described and were recently described as orally-available compounds (Jia et al., 2004; Krause et al., 2005; Phan et al., 2009; Vincent et al., 2009; Morty & Kuebler, 2014; Feetham et al., 2015; Qi et al., 2015), and also as “dual-inhibitors” of TRPV4/TRPA1 (Kanju et al., 2016), which might be an attractive possibility given the postulated function of astrocytic TRPA1 (Lee et al., 2012; Shigetomi et al., 2012; Shigetomi et al., 2013; Wei et al., 2016). Such inhibitors, in case they are intended for oral use will have to pass through the blood-brain-barrier (although for example the blood-brain-barrier is more penetrable in certain CNS disorders such as multiple sclerosis). As an alternative, in case TRPV4 or TRPV4/TRPA1 (dual-)blockers can potently treat more severe neuropsychiatric illnesses safely and effectively, intra-thecal administration remains a plausible alternative (e.g. therapy-refractory high-intensity chronic pain, note its treatment with intra-thecal ziconotide (McDowell & Pope, 2016)).
Although an interesting topic, the role of TRPV4 in the CNS in systemic osmo- and hydro-mineral regulation is not reviewed here (but see e.g. ref. (Liedtke & Friedman, 2003; Janas et al., 2016)), mainly because it was covered in a separate presentation at the recent joint meeting of APS/The Physiological Society in Dublin, Ireland, and therefore not covered in the overview-talk by the senior author. This qualifier also applies to the important topic of the role of TRPV4 in the CNS in the choroid plexus (Takayama et al., 2014), first described in (Liedtke et al., 2000).
Conclusion
In the current concise review we highlight the important, yet currently under-explored role of TRPV4 in the CNS in glial cells and neurons which has so far not been met with appropriate attention and matching investigative scrutiny.
New Findings.
In this concise review we highlight insights into the role of TRPV4 ion channels in the central nervous system, results that have been contributed over the last 16 years since the channel’s initial discovery. TRPV4 has been found to function in neurons, astroglia and microglia, both in physiology (e.g. astrocytic neurovascular coupling, neuronal membrane potential at physiologic temperature), and also under pathologic conditions (e.g. mechanical trauma), so far recorded as exciting findings in need of more in-depth mechanistic clarifications.
Acknowledgments
This work was supported in part by the US National Institutes of Health (grants DE018549, AR48182, AR48182-S1), US Department of Defense (W81XWH-13-1-0299) and the Harrington Discovery Institute (Cleveland OH).
Footnotes
Author contributions
PK and WL conceived the idea to write this paper and wrote it together.
References
- Bai JZ, Lipski J. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology. 2010;31:204–214. doi: 10.1016/j.neuro.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Bai JZ, Lipski J. Involvement of TRPV4 channels in Abeta(40)-induced hippocampal cell death and astrocytic Ca(2+) signalling. Neurotoxicology. 2014;41:64–72. doi: 10.1016/j.neuro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A. 2011;108:2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One. 2012;7:e39959. doi: 10.1371/journal.pone.0039959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sun FJ, Wei YJ, Wang LK, Zang ZL, Chen B, Li S, Liu SY, Yang H. Increased Expression of Transient Receptor Potential Vanilloid 4 in Cortical Lesions of Patients with Focal Cortical Dysplasia. CNS Neurosci Ther. 2016;22:280–290. doi: 10.1111/cns.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci U S A. 2013;110:6157–6162. doi: 10.1073/pnas.1216514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecto F, Shi Y, Huda R, Martina M, Siddique T, Deng HX. Mutant TRPV4-mediated toxicity is linked to increased constitutive function in axonal neuropathies. J Biol Chem. 2011;286:17281–17291. doi: 10.1074/jbc.M111.237685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feetham CH, Nunn N, Barrett-Jolley R. The depressor response to intracerebroventricular hypotonic saline is sensitive to TRPV4 antagonist RN1734. Front Pharmacol. 2015;6:83. doi: 10.3389/fphar.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010;1192:404–409. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Hortopan GA, Gillespie A, Baraban SC. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp Neurol. 2012;237:199–206. doi: 10.1016/j.expneurol.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas S, Seghers F, Schakman O, Alsady M, Deen P, Vriens J, Tissir F, Nilius B, Loffing J, Gailly P, Devuyst O. TRPV4 is associated with central rather than nephrogenic osmoregulation. Pflugers Arch. 2016;468:1595–1607. doi: 10.1007/s00424-016-1850-5. [DOI] [PubMed] [Google Scholar]
- Jang Y, Jung J, Kim H, Oh J, Jeon JH, Jung S, Kim KT, Cho H, Yang DJ, Kim SM, Kim IB, Song MR, Oh U. Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth. J Biol Chem. 2012;287:6014–6024. doi: 10.1074/jbc.M111.316315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–278. doi: 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, Shahid R, Fan P, Gooden DM, Simon SA, Spasojevic I, Mook RA, Liddle RA, Guilak F, Liedtke WB. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep. 2016;6:26894. doi: 10.1038/srep26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno M, Shirakawa H, Iida S, Sakimoto S, Matsutani I, Miyake T, Kageyama K, Nakagawa T, Shibasaki K, Kaneko S. Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia. 2012;60:761–770. doi: 10.1002/glia.22306. [DOI] [PubMed] [Google Scholar]
- Krause JE, Chenard BL, Cortright DN. Transient receptor potential ion channels as targets for the discovery of pain therapeutics. Curr Opin Investig Drugs. 2005;6:48–57. [PubMed] [Google Scholar]
- Leddy HA, McNulty AL, Guilak F, Liedtke W. Unraveling the mechanism by which TRPV4 mutations cause skeletal dysplasia. Rare Diseases. 2014a:1–5. doi: 10.4161/2167549X.2014.962971. e-publication e962971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy HA, McNulty AL, Lee SH, Rothfusz NE, Gloss B, Kirby ML, Hutson MR, Cohn DH, Guilak F, Liedtke W. Follistatin in chondrocytes: the link between TRPV4 channelopathies and skeletal malformations. Faseb J. 2014b;28:2525–2537. doi: 10.1096/fj.13-245936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Choe SY. Age-related changes in the distribution of transient receptor potential vanilloid 4 channel (TRPV4) in the central nervous system of rats. J Mol Histol. 2014;45:497–505. doi: 10.1007/s10735-014-9578-z. [DOI] [PubMed] [Google Scholar]
- Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat. 2012;45:45–49. doi: 10.1016/j.jchemneu.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Liedtke W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann N Y Acad Sci. 2008;1144:42–52. doi: 10.1196/annals.1418.012. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62:2985–3001. doi: 10.1007/s00018-005-5181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell GC, 2nd, Pope JE. Intrathecal Ziconotide: Dosing and Administration Strategies in Patients With Refractory Chronic Pain. Neuromodulation. 2016;19:522–532. doi: 10.1111/ner.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty AL, Leddy HA, Liedtke W, Guilak F. TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:437–450. doi: 10.1007/s00210-014-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morty RE, Kuebler WM. TRPV4: an exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol. 2014;307:L817–821. doi: 10.1152/ajplung.00254.2014. [DOI] [PubMed] [Google Scholar]
- Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Li Z, Kong CW, Tang NL, Huang Y, Li RA, Yao X. Uniaxial cyclic stretch stimulates TRPV4 to induce realignment of human embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2015;87:65–73. doi: 10.1016/j.yjmcc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Shi M, Du F, Liu Y, Li L, Cai J, Zhang GF, Xu XF, Lin T, Cheng HR, Liu XD, Xiong LZ, Zhao G. Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta Neuropathol. 2013;126:725–739. doi: 10.1007/s00401-013-1166-x. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Ikenaka K, Tamalu F, Tominaga M, Ishizaki Y. A novel subtype of astrocytes expressing TRPV4 (transient receptor potential vanilloid 4) regulates neuronal excitability via release of gliotransmitters. J Biol Chem. 2014;289:14470–14480. doi: 10.1074/jbc.M114.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Sugio S, Takao K, Yamanaka A, Miyakawa T, Tominaga M, Ishizaki Y. TRPV4 activation at the physiological temperature is a critical determinant of neuronal excitability and behavior. Pflugers Arch. 2015a;467:2495–2507. doi: 10.1007/s00424-015-1726-0. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Tominaga M, Ishizaki Y. Hippocampal neuronal maturation triggers post-synaptic clustering of brain temperature-sensor TRPV4. Biochem Biophys Res Commun. 2015b;458:168–173. doi: 10.1016/j.bbrc.2015.01.087. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’;Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Nakagawa T, Kaneko S. Pathophysiological roles of transient receptor potential channels in glial cells. Yakugaku Zasshi. 2010;130:281–287. doi: 10.1248/yakushi.130.281. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Takayama Y, Shibasaki K, Suzuki Y, Yamanaka A, Tominaga M. Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. Faseb J. 2014;28:2238–2248. doi: 10.1096/fj.13-243436. [DOI] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Wei H, Wu HY, Fan H, Li TF, Ma AN, Li XY, Wang YX, Pertovaara A. Potential role of spinal TRPA1 channels in antinociceptive tolerance to spinally administered morphine. Pharmacol Rep. 2016;68:472–475. doi: 10.1016/j.pharep.2015.11.008. [DOI] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]


