ASBTRACT
Rab GTPases serve as master regulators of membrane traffic, each typically controlling several different aspects of a specific stage of membrane traffic by recruiting diverse effector proteins such as cytoskeletal motors, vesicle tethering proteins and regulators of SNARE complex assembly. Rabs, in turn, are regulated by specific guanine nucleotide exchange factors (GEFs), which catalyze the displacement of GDP and binding of GTP, as well as GTPase activating proteins (GAPs) that stimulate the slow intrinsic rate of GTP hydrolysis. Here I review our studies on the final stages of the yeast secretory pathway that have led us to propose that adjacent Rabs on a pathway are networked to one another through their regulators; specifically we have shown that the Rab, Ypt32, in its GTP-bound form recruits both Sec2, the GEF that activates the downstream Rab, Sec4, as well as Gyp1, the GAP that inactivates the upstream Rab, Ypt1. The postulated effect of these counter-current cascades is a programmed series of abrupt Rab transitions that lead to critical changes in the functional identity of the membrane as it flows along the exocytic pathway. Phosphoinositides also play key roles in the temporal and spatial regulation of membrane traffic. The Golgi pool of phosphatidylinositol 4-phosphate (PI(4)P) works in concert with Ypt32 to initially recruit Sec2, yet a subsequent drop in PI(4)P levels directs a regulatory switch in Sec2 function in which it binds to the Sec4 effector Sec15 generating a positive feedback loop. PI(4)P distribution together with Sec2 phosphorylation by a casein kinase determines when and where each regulatory circuit is used.
KEYWORDS: exchange factor, GAP cascade, GEF cascade, GTPase activating protein, membrane traffic, Rab
Introduction
Rab GTPases regulate various aspects of membrane traffic and have been proposed to play a central role in defining organelle identity as well as the direction of vesicular transport.1 Activation requires a guanine nucleotide exchange factor (GEF), while GTP hydrolysis requires a GTPase activating protein (GAP). The GDP-bound form is subject to extraction from the membrane by GDP dissociation inhibitor (GDI). In principle, by locating its GEF on one compartment and its GAP on another compartment, a gradient of Rab activation could be established that would define the direction of transport.2 Rab effectors recognize a specific GTP-bound Rab and constitute a diverse collection of molecules that includes molecular motors that drive the vectorial delivery of an organelle or vesicular carrier along a cytoskeletal track, tethers that mediate the initial recognition of the target compartment by a vesicular carrier and SNARE regulators that control the assembly of specific SNARE complexes and their function in membrane fusion.1 As membrane flows along the exocytic or endocytic pathways, the Rabs associated with that membrane change and as each new Rab decorates the membrane, it recruits a distinct set of effectors that, in turn, help to redefine the functional identity of the membrane.
Our studies have focused on the yeast secretory pathway. The first Rab of the pathway, Ypt1, is a Rab1 homolog that promotes ER to Golgi transport as well as the early stages of transport within the Golgi. The second Rab, Ypt32, is a Rab11 homolog that promotes the formation of secretory vesicles from the Golgi and the initial stages of secretory vesicle maturation.3 The final Rab, Sec4, is a Rab 8 homolog that drives the vectorial delivery of those vesicles to sites of polarized cell surface expansion4-6 as well as the docking and fusion of the vesicles to the plasma membrane.1 Additional Rabs control the endocytic pathway and the interconnections between the endocytic and exocytic pathways.
A Ypt32-Sec2-Sec4 GEF cascade
Sec4 is activated by a GEF called Sec2, a homolog of the Rab8 GEF, Rabin8.6 The GEF domain consists of a homodimeric coiled-coil region consisting of amino acids 1–160.7 Sec2, like Sec4, is highly concentrated on the surface of secretory vesicles.8 A number of temperature-sensitive sec2 alleles reside in the latter half of the Sec2 sequence and these mutations do not affect GEF activity as measured in solution, but rather cause mislocalization of Sec2 due to a failure in its recruitment to secretory vesicles.8 To probe the mechanism of recruitment, we screened for high copy number suppressors of sec2-78, a missense mutation. This screen yielded Ypt32.9 Overexpression of either Ypt32 or its redundant paralog, Ypt31, not only suppressed the growth and secretion defect of sec2-78 cells, it also restored polarized localization of Sec2-78, reflecting renewed recruitment to vesicles. Two-hybrid and protein binding studies indicated that Sec2 binds directly and specifically to Ypt32-GTP.9 The binding site lies downstream of the GEF domain. Mutations that block binding to Ypt32-GTP in vitro result in Sec2 mislocalization as well as temperature-sensitive defects in growth and secretion, when expressed in vivo.10 These results point to an interesting and potentially general mechanism in which one Rab, in its GTP-bound form, recruits the GEF that activates the next Rab on a pathway. We termed this a Rab GEF-cascade, and there are now several additional examples of this regulatory mechanism in both yeast and mammalian cells (reviewed in ref. 2).
A Ypt32-Gyp1-Ypt1 GAP cascade
While the GEF cascade mechanism explains how a new Rab can be recruited to a membrane domain that initially carries the upstream Rab, it doesn't explain how the first Rab is removed from the membrane once the downstream Rab has been recruited. Analysis of a GAP called Gyp1, that inactivates Ypt1, the first Rab on the yeast secretory pathway,11 offers a potentially general mechanism. Gyp1 binds to Ypt32-GTP, even though this Rab is not a substrate for its GAP activity, and binding involves a region of Gyp1 distinct from the GAP catalytic domain.12 Gyp1 localizes to the Golgi and this localization requires both the Ypt32 binding region of Gyp1 and Ypt31/32 function. Individual Golgi cisternae in yeast are observed to initially label with Ypt1, but then subsequently acquire Ypt32 and abruptly lose Ypt1. In a strain deleted for gyp1 the Golgi cisternae initially label with Ypt1, however even after they acquire Ypt32, the Ypt1 remains associated, leading to an extended period of overlap.12 These results suggest a Rab GAP cascade mechanism in which a GAP that inactivates the first Rab is recruited to the membrane only after the next Rab on the pathway has been activated, thus limiting the temporal and spatial overlap of the 2 Rab domains. Several examples of GAP cascades have now been documented in the literature (reviewed in ref. 2). GEF and GAP cascades, working in a counter-current fashion, could trigger an abrupt Rab transition that would in turn lead to a transition in Rab effectors and hence a change in the functional identity of the membrane domain.
Regulation by phosphoinositides
Our studies of the yeast exocytic pathway have revealed additional levels of regulation. The starting mutation in our screen for Sec2 suppressors, sec2-78 lies well downstream of the Ypt32 binding site and does not significantly affect Ypt32 binding in vitro.9 If Ypt32 was the only signal for Sec2 recruitment, Sec2-78 should not mislocalize. We therefore surmised that there must be an additional element that works in concert with Ypt32 in Sec2 membrane recruitment. This we showed to be phosphatidylinositol (4) phosphate, PI(4)P.10 This phosphoinositide is produced in the Golgi by the PI(4) kinase, Pik113 and binds directly to Sec2.10 Mutations in either the catalytic domain of pik1 or within a polybasic domain of Sec2 needed for PI(4)P binding, block Sec2 membrane recruitment to secretory vesicles. Overexpression of Ypt32 restores Sec2 membrane recruitment in pik1 mutant cells.10 These results indicate that Sec2 is normally recruited by a combination of Ypt32 and PI(4)P and can be thought of as a coincidence detector, recognizing only those membrane domains marked by both ligands. This is directly analogous to certain endosomal proteins that are recruited by the combination of Rab5-GTP and PI(3)P .
A GEF-effector positive feedback loop regulated by PI(4)P
Sec2 is also involved in another type of regulatory circuit; it binds to one of the effectors of Sec4-GTP, Sec15.14 Sec15 is a subunit of the octomeric exocyst complex that tethers secretory vesicles to exocytic sites on the plasma membrane. The interaction of a GEF with an effector of the Rab that it activates could, in principle, generate a positive feedback loop and thereby promote the creation of a domain marked by high concentrations of the activated Rab, the GEF that activates it and the effector that responds to it. Binding studies indicated that the region of Sec2 required for binding to Sec15 overlaps with the region required for binding Ypt32-GTP and that the 2 ligands compete against each other for binding to Sec2.14 These observations led us to probe the mechanisms by which Sec2 would choose one ligand over the other.
Although Sec2 can simultaneously bind to Ypt32 and PI(4)P, the interaction of Sec2 with Sec15 is strongly inhibited by the addition of liposomes carrying PI(4)P.10 Furthermore, in pik1 mutants deficient in PI(4)P synthesis, the association of Sec2 with Sec15 is increased several fold, supporting the in vivo significance of this inhibitory mechanism.10 Although secretory vesicles are formed from a region of the Golgi enriched in PI(4)P, 2 different fluorescent probes for PI(4)P fail to show a concentration at sites of polarized cell surface growth, suggesting that the PI(4)P concentration is reduced by the time the vesicles arrive at these sites.10,15 Together these results suggest that when secretory vesicles are initially formed and the PI(4)P concentration is high, Sec2 is predominantly bound to Ypt32 and the interaction with Sec15 is blocked. As secretory vesicles mature, the PI(4)P concentration is reduced leading to a shift toward a Sec2-Sec15 interaction that prepares the vesicles for docking and fusion with the plasma membrane.10
An oxysterol binding protein reduces PI(4)P levels as vesicles mature
To address the mechanism by which PI(4)P is depleted during vesicle maturation, we examined a number of candidate strains for mutants that fail to reduce the level of PI(4)P on secretory vesicles, including mutants in the oxysterol binding proteins (osh). Oxysterol binding proteins not only bind sterols, they also bind PI(4)P at an overlapping site and are thought to exchange these 2 lipids between membranes.16 A strain deleted for 6 of the 7 osh genes and carrying a temperature sensitive allele of the remaining osh4 gene exhibited prominent concentrations of PI(4)P at sites of polarized cell surface expansion.15 This pattern of localization, revealed with 2 different fluorescent probes, indicates an aberrantly high concentration of PI(4)P on mature secretory vesicles. Of the 7 Osh proteins, only Osh4 associates with secretory vesicles and a strain deleted for osh4 alone also exhibits elevated levels of PI(4)P at polarized exocytic sites.15 A reduction in the interaction of Sec2 with Sec15 is observed in osh4Δ mutant cells, consistent with the inhibition of the Sec2-Sec15 interaction by elevated PI(4)P observed in vitro. We also observed a shift of Ypt32 toward exocytic sites and a shift of Sec15 away from exocytic sites in osh4Δ cells.15 Together these data establish an important role for an Osh4-dependent reduction of PI(4)P levels in the regulatory switch of Sec2 from a GEF cascade involving Ypt32-GTP to a GEF-effector positive feedback loop involving Sec15 during vesicle maturation.
Sec2 phosphorylation directs a switch in regulatory binding partners
Phosphorylation also plays an important role in the regulatory switch of Sec2 from its interaction with Ypt32, in a GEF cascade, to binding Sec15, in a GEF-effector positive feedback loop. Under normal growth conditions, Sec2p is predominantly phosphorylated.8 We found that the phosphorylated form of Sec2 purified from yeast, binds to Sec15 several-fold more efficiently than does bacterially expressed, unphosphorylated Sec2.17 Phosphatase treatment of yeast Sec2 reduces Sec15 binding to the level of the bacterially expressed protein, confirming a role for phosphorylation in the regulation of binding. We identified a patch of 3 phosphosites (S181, S186 and S188) within the region of Sec2 that binds to both Ypt32 and Sec15 and demonstrated that phosphorylation regulates the interaction of Sec2p with its binding partners Ypt32p, Sec15p and PI(4)P.17 Using phosphomimetic (S181-8D/E) or non-phosphorylatable (S181-8A) SEC2 alleles, we established that the unphosphorylated form binds preferentially to Ypt32p-GTP and PI(4)P, while the phosphorylated or phosphomimetic form of Sec2p binds preferentially to Sec15p. Live cell imaging and synthetic lethality studies confirmed the in vivo relevance of these binding studies.17 Thus phosphorylation of Sec2p, in conjunction with the reduction of PI(4)P, drives a switch in its regulatory binding partners that facilitates secretory vesicle maturation and helps to promote the directionality of vesicular transport (Fig. 1).
Figure 1.
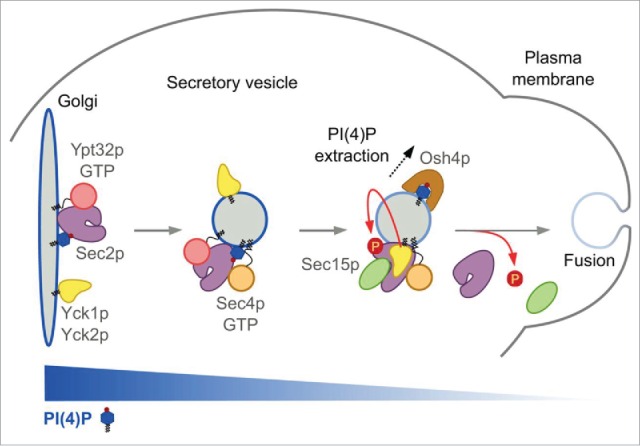
(from left to right) Sec2 is recruited to membranes by the combination of Ypt32-GTP and PI(4)P. Sec2 phosphorylation by Yck1/2 is initially inhibited by the high level of PI(4)P. The secretory vesicle buds off and Sec2 activates Sec4, which then recruits its effector, Sec15. During vesicle maturation, PI(4)P is removed by Osh4 and Ypt32 is released from the vesicle. This permits Sec15 to interact with Sec2 and also allows Sec2 phosphorylation by Yck1/2, further enhancing the Sec2–Sec15 interaction. This process pushes the reaction forward and creates a positive-feedback loop that generates a micro-domain of high Sec4-GTP and high Sec15, facilitating the delivery, tethering, and fusion of the vesicle with the plasma membrane. Sec2 is dephosphorylated by an as yet unknown phosphatase that allows Sec2 to dissociate from Sec15 and thus dissociate from the vesicle. The dephosphorylated Sec2 is then available to associate with a new round of vesicles.
The casein kinases Yck1 and Yck2 regulate Sec2
A screen of all protein kinase deletion mutants for strains that exhibit an increased mobility form of Sec2, led to the identification of 2 redundant members of the casein kinase family, Yck1 and Yck2.18 Deletion of either YCK gene alone causes a shift of about a third of Sec2 to an increased mobility form, while phosphatase treatment leads to co-migration of Sec2 from all strains at the higher mobility. A strain deleted for yck1 and expressing a ts allele of yck219 showed a nearly complete shift of Sec2 to the higher mobility form. Only a minor mobility shift was seen when comparing WT and yck1 yck2 ts strains if they expressed the Sec2 phosphomimetic (S181-8D/E) or non-phosphorylatable (S181-8A) alleles, indicating that the 181–188 region is the principle site of phosphorylation by the Yck kinases.18 We observed a substantial reduction in the amount of Sec15 that co-precipitates with Sec2 from yck1 yck2 ts lysates, consistent with our model of Sec2 phospho-regulation (Fig. 1).
Yck2 binds to GST-Sec2 and the region of Sec2 from residues 450 to 508 proved critical for Yck2 binding, even though the sites of phosphorylation lie between residues 181 and 188.18 Thus, Yck2 recognizes the region of Sec2 between residues 450–508, but then phosphorylates residues between 181 and 188. The Yck1/2 binding region of Sec2 is also important for binding PI(4)P 10 and addition of liposomes containing PI4P inhibits phosphorylation of Sec2 by Yck2 in vitro. Analysis of Sec2 phosphorylation in various secretory mutants suggests that Sec2 phosphorylation occurs only after its association with secretory vesicles and persists through the vesicle transport reaction.18 Dephosphorylation at the end of the reaction may facilitate recycling. In total our studies support the model shown in Figure 1.
Future perspectives
It seems likely that most of the components that play a direct role in the exocytic pathway in yeast have already been identified and their mechanisms resolved at some level, but how all of these components are organized into a robust, stable pathway is only poorly understood. Here, I have reviewed our evidence for several mechanisms involving Rab proteins that have evolved to coordinate sequential steps on the exocytic pathway. Interconnections with other systems are certainly involved as well, such as the role of the COP II coat in recruitment of the Ypt1 GEF, TRAPP I,20 and the roles of Ypt1 and Ypt32 in the regulation of ARF-directed vesicle budding from the Golgi.21 Further study will almost certainly reveal additional layers of regulation. Can these various mechanisms, working in concert fully explain the self-assembly, organization and function of the secretory pathway or are additional, undiscovered and unrelated mechanisms involved as well? Formal proof might require in vitro reconstitution of the entire secretory pathway, certainly a daunting prospect. Mathematical modeling may offer some insight into the likelihood that the known mechanisms can account for the organization of the secretory pathway. In the mean time, artificially “rewiring” the various regulatory circuits offers one means by which specific predictions can be tested in vivo.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the NIH to PN (GM35370 and GM082861).
References
- [1].Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 2006; 103(32):11821-7; PMID:16882731; http://dx.doi.org/ 10.1073/pnas.0601617103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase Networks in Membrane Traffic. Annu Rev Biochem 2012; 81:637-659; PMID:22463690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol 1997; 137(3):563-80; PMID:9151665; http://dx.doi.org/ 10.1083/jcb.137.3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, Munson M, Schekman R, Weisman LS. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell 2011; 21(6):1156-70; PMID:22172676; http://dx.doi.org/ 10.1016/j.devcel.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol 1995; 128(6):1055-68; PMID:7896871; http://dx.doi.org/ 10.1083/jcb.128.6.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol 1997; 137(7):1495-509; PMID:9199166; http://dx.doi.org/ 10.1083/jcb.137.7.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dong G, Medkova M, Novick P, Reinisch KM. A catalytic coiled coil: structural insights into the activation of the Rab GTPase Sec4p by Sec2p. Mol Cell 2007; 25(3):455-62; PMID:17289591; http://dx.doi.org/ 10.1016/j.molcel.2007.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elkind NB, Walch-Solimena C, Novick PJ. The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. J Cell Biol 2000; 149(1):95-110; PMID:10747090; http://dx.doi.org/ 10.1083/jcb.149.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 2002; 157(6):1005-15; PMID:12045183; http://dx.doi.org/ 10.1083/jcb.200201003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell 2010; 18(5):828-40; PMID:20493815; http://dx.doi.org/ 10.1016/j.devcel.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Du LL, Novick P. Yeast rab GTPase-activating protein Gyp1p localizes to the Golgi apparatus and is a negative regulator of Ypt1p. Mol Biol Cell 2001; 12(5):1215-26; PMID:11359917; http://dx.doi.org/ 10.1091/mbc.12.5.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci U S A 2009; 106(34):14408-13; PMID:19666511; http://dx.doi.org/ 10.1073/pnas.0906536106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol 1999; 1(8):523-5; PMID:10587649; http://dx.doi.org/ 10.1038/70319 [DOI] [PubMed] [Google Scholar]
- [14].Medkova M, France YE, Coleman J, Novick P. The rab exchange factor Sec2p reversibly associates with the exocyst. Mol Biol Cell 2006; 17(6):2757-69; PMID:16611746; http://dx.doi.org/ 10.1091/mbc.E05-10-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ling Y, Hayano S, Novick P. Osh4p is needed to reduce the level of phosphatidylinositol-4-phosphate on secretory vesicles as they mature. Mol Biol Cell 2014; 25(21):3389-400; PMID:25165144; http://dx.doi.org/ 10.1091/mbc.E14-06-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 2013; 155(4):830-43; PMID:24209621; http://dx.doi.org/ 10.1016/j.cell.2013.09.056 [DOI] [PubMed] [Google Scholar]
- [17].Stalder D, Mizuno-Yamasaki E, Ghassemian M, Novick PJ. Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners. Proc Natl Acad Sci U S A 2013; 110(50):19995-20002; PMID:24248333; http://dx.doi.org/ 10.1073/pnas.1320029110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stalder D, Novick PJ. The casein kinases Yck1p and Yck2p act in the secretory pathway, in part, by regulating the Rab exchange factor Sec2p. Mol Biol Cell 2015; 27:686-701; PMID:26700316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robinson LC, Menold MM, Garrett S, Culbertson MR. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol Cell Biol 1993; 13(5):2870-81; PMID:8474447; http://dx.doi.org/ 10.1128/MCB.13.5.2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 2011; 473(7346):181-6; PMID:21532587; http://dx.doi.org/ 10.1038/nature09969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McDonold CM, Fromme JC. Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network. Dev Cell 2014; 30(6):759-67; PMID:25220393; http://dx.doi.org/ 10.1016/j.devcel.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


