Abstract
Human papillomavirus (HPV) infections are deemed to play a role in the pathogenesis of oral cavity cancer (OCC). However, their exact prevalence and clinical significance remain unclear. Herein, we investigated the prevalence and prognostic value of HPV infections in a large sample of Taiwanese OCC patients.
This study was designed as a retrospective cohort study. Between 2004 and 2011, we identified 1002 consecutive patients with newly diagnosed OCC who were scheduled for standard treatment. HPV genotyping was performed in tumor specimens using polymerase chain reaction-based HPV blots. To investigate the temporal trends of HPV infections and their impact on 5-year overall survival (OS), patients were divided into 2 cohorts according to calendar periods: “2004 cohort” (2004–2007; n = 466) and “2008 cohort” (2008–2011; n = 536). Univariate and multivariate Cox regression models were also used to identify the independent predictors of OS in the 2 cohorts. A weighted risk score was assigned to each factor based on the range of their corresponding hazard ratios and validated in both cohorts using the c-statistic.
The overall prevalence of HPV infections was 19%, with a trend toward decreasing rates from 2004 to 2011. In patients without risky oral habits, the 5-year OS rate of HPV-positive patients was significantly lower than that of HPV-negative cases (49% vs 80%; P = 0.021). In the 2004 cohort, multivariate analysis identified HPV16, pathological T3/T4, pathological N1/N2, and extracapsular spread as independent adverse prognostic factors for OS. In the 2008 cohort, pathological N1/N2, pathological stage III/IV, and histological tumor depth >8 mm were identified as independent adverse prognostic factors. Using a weighted grading system incorporating HPV16 infection, we devised a prognostic index that identified 4 distinct risk categories with 5-year OS rates ranging from 25% to 89% (c-statistic = 0.76) in the 2004 cohort. The validity of the index was internally confirmed in the 2008 cohort (c-statistic = 0.71).
We conclude that HPV infections are common in Taiwanese OCC patients and predict 5-year OS. If independently validated, our composite prognostic score comprising HPV16 infection may be useful for allocating OCC patients to risk-adapted therapies.
INTRODUCTION
Oral cavity cancer (OCC) and oropharyngeal cancer (OPC) are among the most common cancers worldwide, with an estimated 440,000 new cases occurring in 2012.1 Despite being an established cause of OPC (>50%), molecular epidemiology data demonstrated that the risk of OCC attributable to human papillomavirus (HPV) infections is relatively low (∼3%).2,3 Notably, the incidence of OPC has overall increased slightly over time, although not significantly so. Such an increase was largely driven by HPV-related OPC from 1983 to 2002, whereas OCC concomitantly showed a decreasing trend.4,5 In general, the role of HPV infections in the pathogenesis of OCC seems to be less prominent in industrialized countries.6
Despite the reduction in risky oral habits (ie, betel chewing, tobacco smoking, and alcohol drinking) observed between 1980 and 2010, the incidence of OCC in Taiwan is still increasing.7–9 Notably, HPV infections seem to be common in Asian OCC patients, being detectable in 20% to 50% of all cases.6,10–16 Moreover, there is evidence to suggest that HPV infections may predict distant metastases,12,16 second primary cancers,13 disease-free survival,14,16 disease-specific survival,12,14–16 and overall survival (OS).12,15,16 Unfortunately, previous studies on the role of HPV in OCC were limited by small sample sizes and did not provide sufficient evidence to modify current clinical guidelines and management protocols. Importantly, a better understanding of the role played by HPV infections in OCC is paramount to assess whether or not an HPV vaccine may be useful for these patients.
The aims of this retrospective study were to investigate the prevalence of intratumor HPV infections in formalin-fixed paraffin-embedded primary tumor samples from a large cohort of OCC patients, and to assess the independent prognostic significance of HPV infections for 5-year OS after treatment with radical surgery(either with or without adjuvant therapy).
MATERIALS AND METHODS
Ethics Statement
The local Institutional Review Board at Chang Gung Memorial Hospital approved the study (No. 99-0112B), which followed the tenets of the Helsinki Declaration. Written informed consent was obtained from each patient.
Participants
This study was designed as a retrospective research. In total, 1002consecutive Taiwanese OCC patients were enrolled between 2004 and 2011. The inclusion criteria were as follows: newly histology-proven OCC; previously untreated tumor scheduled for radical surgery (either with or without neck dissection); absence of suspected distant metastases detected by imaging; and willingness to undergo imaging-guided biopsy or exploratory surgery if necessary. The exclusion criteria included the refusal or inability to undergo radical surgery of OCC.
Procedures
All participants underwent an extensive presurgical evaluation.12,15 Cancer staging was performed according to the 2002 American Joint Committee on Cancer (AJCC) 6th edition staging criteria.17 All patients underwent radical excision of the primary tumor with ≥1 cm gross safety margins. Patients with advanced-stage cancer and/or close margins ≤4 mm underwent adjuvant radiotherapy or concomitant chemoradiotherapy, as reported elsewhere.12 The following variables were collected in all participants: sex, age at disease onset, alcohol drinking (ever/never), betel quid chewing (ever/never), cigarette smoking (ever/never), tumor subsite, differentiation, pathological T-status, pathological N-status, pathological stage, extracapsular spread (ECS), level IV/V metastases, treatment modality, and patient status at the last follow-up.
HPV Typing
Formalin-fixed, paraffin-embedded tumor samples collected during radical surgery were prepared for DNA extraction as described elsewhere.12 DNA was extracted using a Lab Turbo 48 automatic nucleic acid extraction system and a Lab Turbo Virus Mini Kit LVN500 (Taigen, Taipei, Taiwan). We utilized a commercially available HPV L1 gene polymerase chain reaction assay (EasyChip HPV Blot genotyping assay, King Car Ltd, Ilan, Taiwan) to screen for HPV infections. The assay underwent strict quality-check procedures,18,19 and it has been successfully utilized in previous OCC studies.11–13,15,16 A 192-bp DNA fragment was produced after amplification of HPV DNA using the MY11/biotinylated GP6+ L1 consensus primer system. Samples that were β-globin-negative were repeatedly extracted. Briefly, the resultant amplimers (15 μL) were hybridized to a nylon membrane that was coated with 39 HPV type-specific oligonucleotide probes.
Definitions of HPV Infections
The prevalence of genotype-specific HPV infections was reported both individually and in distinct groups (all types, oncogenic types, and nononcogenic types). The category “any HPV infection” was defined as a positive test result for 1 (single) or more than one (multiple) of the 39 distinct HPV genotypes included in the assay. Patients with both oncogenic and nononcogenic HPV types were classified as having an “oncogenic HPV infection”. Because more than half of oncogenic HPV infections are caused by HPV16,6,10–14 oncogenic HPV infections were further divided into 2 subgroups: “HPV16 infections” and “other oncogenic HPV infections”.
Statistical Analysis
The main endpoint was OS (defined as the time period between primary surgery and death by any cause). Follow-up visits were continued until April 30, 2014. Patients without a documented event were censored at time of last follow-up. To investigate the temporal trends of HPV infections and their impact on 5-year OS, patients were divided into 2 cohorts according to calendar periods: “2004 cohort” (2004–2007; n = 466) and “2008 cohort” (2008–2011; n = 536). Continuous variables were categorized using published thresholds, laboratory norms, and quartiles. Data were compared using the Mann–Whitney U test and the chi-square test.
A prognostic scoring system was devised based on clinicopathological factors identified as significant for OS by multivariate analysis in the 2004 cohort. Internal validity was examined using the 2008 cohort. Survival curves were plotted using the Kaplan–Meier method and compared with the log-rank test. We used Cox proportional-hazard regression analysis with a bootstrap approach (200 runs) to identify the variables associated with survival and to estimate unadjusted and adjusted hazard ratios (HRs) and their corresponding 95% confidence intervals (95% CIs). Dichotomized variables were considered for further analysis only when they were of prognostic significance in univariate analysis. Correlations between variables were assessed with the Spearman correlation. All variables that showed significant association with OS on univariate analysis and variables of interest were consequently included in multivariate stepwise Cox proportional-hazard regression models were fitted using a backward selection procedure, with a significance level for retention of any given variable into the model of 0.05. Independent factors associated with OS in the final model were included in the prognostic index.
We assigned a weighted risk score to each factor based on the range of their corresponding HRs. The total risk score was then calculated by the sum of the ratings of individual factors. The discrimination ability of the developed models was evaluated using the Harrell c-statistic. Models are typically considered acceptable when c-values are between 0.7 and 0.8, excellent when between0.8 and 0.9, and outstanding when ≥0.9.20 All calculations were performed using the SPSS 23.0 statistical package for Windows (SPSS Inc., Chicago, IL), the R software (Version 3.2.2; R Foundation for Statistical Computing, http://www.r-project.org/), and GraphPad Prism 6.0 for Windows (GraphPad Software, Inc., San Diego, CA). Two-tailed P values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics and Clinical Outcomes
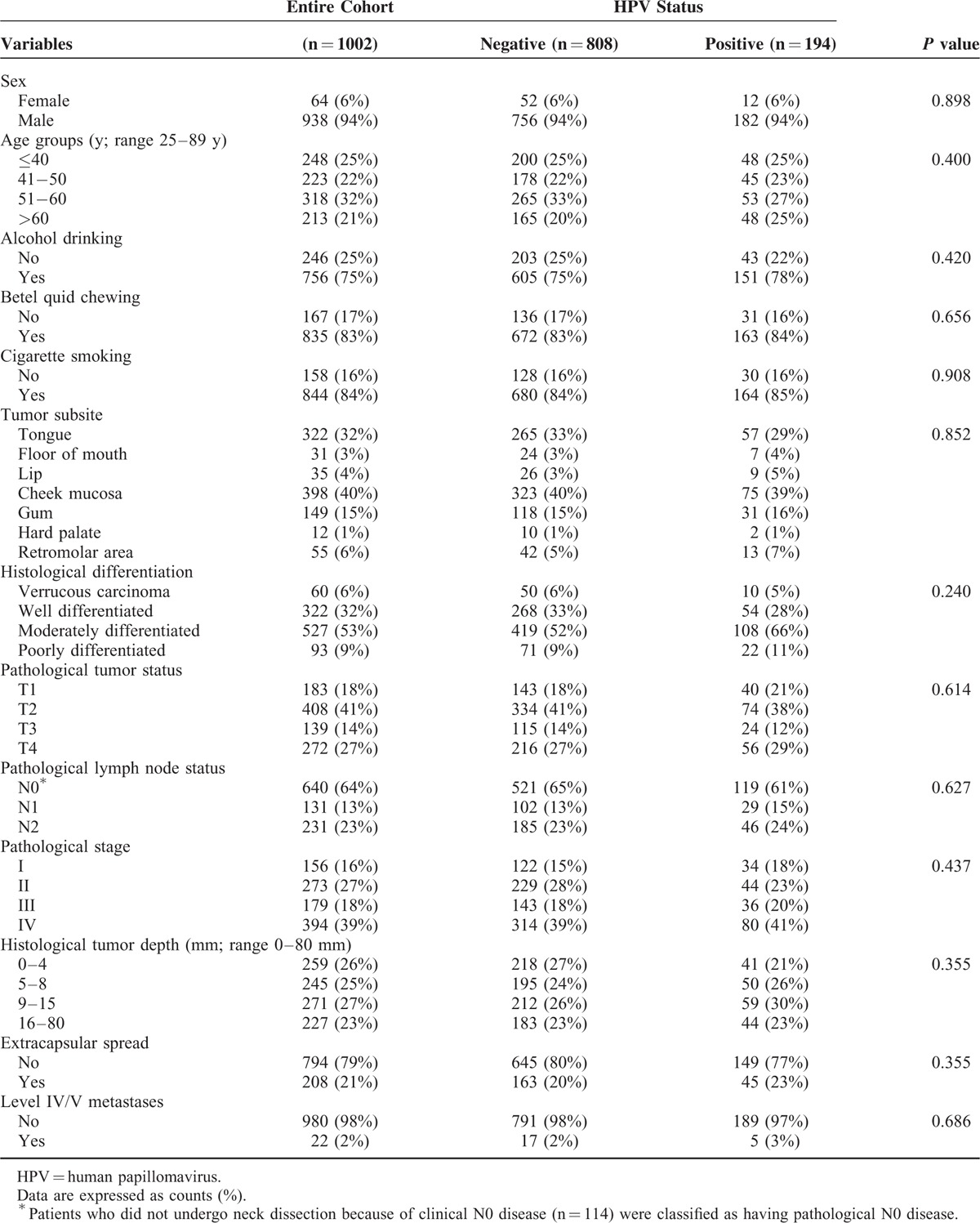
Sixty-four females and 938 males who were scheduled to receive primary surgery at our hospital were enrolled. In Taiwan, the incidence of OCC has been found to be markedly lower in females than in males (2.23/106 vs 23.67/106, respectively [2004]; 2.71/106 vs 25.52/106, respectively [2011]).8,9 The discrepancy of tobacco and betel quid use may account for the different incidence of OCC between male Taiwanese and female Taiwanese.21 On the basis of the observed sex-related differences in the incidence of OCC in our country, it is not surprising that males outnumbered females in the current study. Table 1 depicts the general characteristics of the study participants. There were 723 (72%) alive patients who completed a minimum follow-up of 5 months (median: 59 mos, interquartile range [IQR]: 37–83 mos). A total of 279 (28%) patients died during the study period (median time to death: 15 mos, IQR: 9–30 mos). The 5-year OS rate in the entire study cohort was 72% (95% CI 70%–75%).
TABLE 1.
General Characteristics of Patients With Oral Cavity Cancer According to the Presence of Human Papillomavirus Infections
Prevalence and Temporal Trends of HPV Infections
The overall prevalence of any HPV infection in the study cohort was 19%. Notably, 2% of the participants had multiple HPV infections. No statistically significant differences in terms of baseline variables were found between HPV-positive (any HPV infection) and HPV-negative patients (Table 1). More individuals were infected with oncogenic HPV types (16%) than nononcogenic types (4%). The 3 most common genotypes (including single and multiple infections) were HPV16 (8%), HPV18 (5%), and HPV52 (2%) (Table 2). The prevalence of HPV infections in OCC patients showed a decreasing trend across calendar-years for all assays (Fig. 1). We observed a significantly lower prevalence of HPV infections in the 2008 cohort compared with the 2004 cohort (16% vs 23%; P = 0.011). Notably, oncogenic HPV infections were significantly less frequent (12% vs 21%; P < 0.001), whereas nononcogenic HPV types were significantly more common (5% vs 2%; P = 0.022). Similar decreases were observed for the prevalence of both HPV16 (6% vs 10%; P = 0.022) and HPV18 (3% vs 7%; P = 0.007) infections.
TABLE 2.
Prevalence of Human Papillomavirus Infections in Oral Cavity Cancer
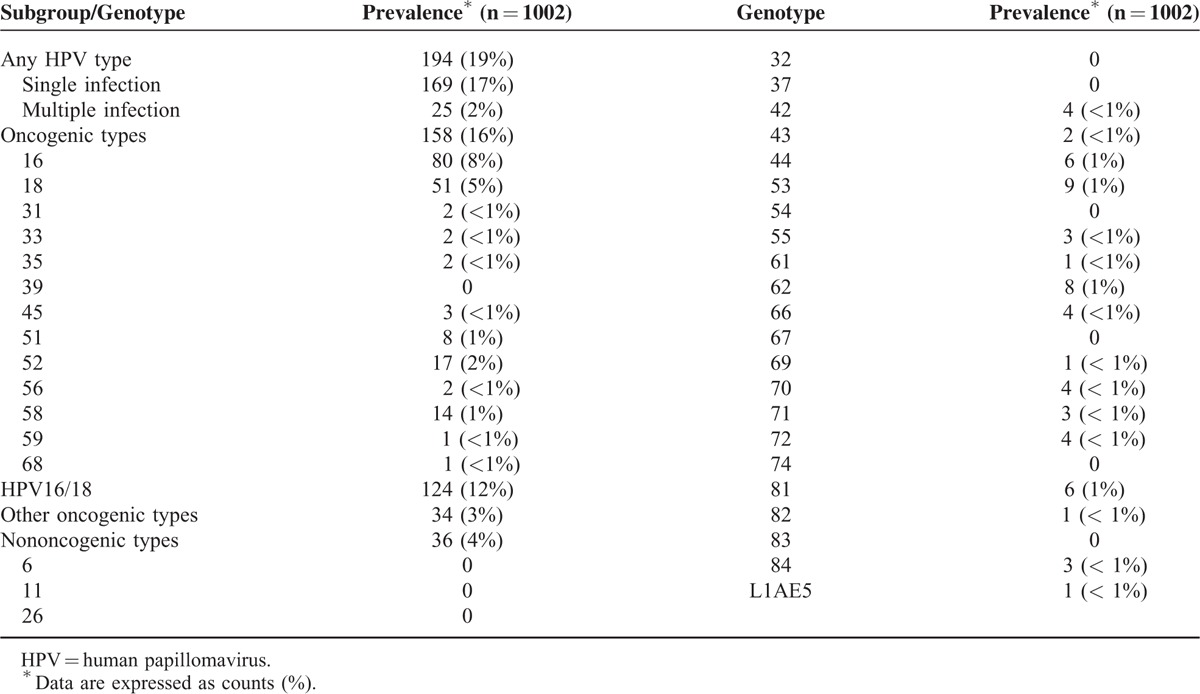
FIGURE 1.
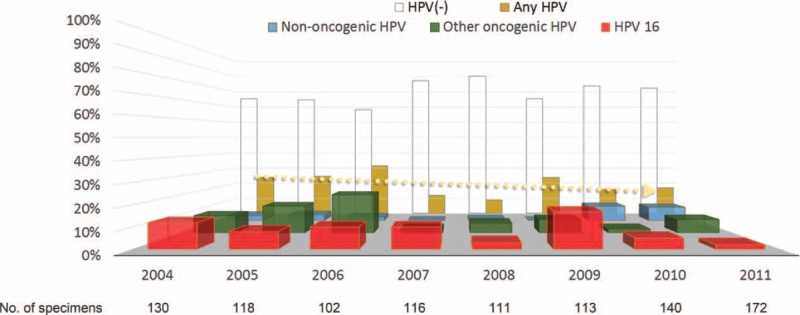
Prevalence of human papillomavirus (HPV) infections in patients with oral cavity cancer between 2004 and 2011. The dotted line indicates the prevalence trend for any HPV infection across the examined calendar-years. The number of specimens assessed for each assay is shown below the X-axis.
Impact of HPV Infections on Survival Rates
The 5-year OS rate was 68% in HPV-positive patients (n = 194) and 73% in HPV-negative patients (n = 808; P = 0.243). Moreover, OCC patients with HPV16 infections (n = 80; 5-year OS, 67%), other oncogenic HPV infections (n = 78; 5-year OS, 78%), or nononcogenic HPV infections (n = 36; 5-year OS, 81%) did not significantly differ from those without HPV infections in terms of OS (P = 0.341).
Prognostic Impact of HPV Infections According to Risky Oral Habits
We then analyzed the prognostic impact of HPV infections according to the presence of risky oral habits (alcohol drinking, betel quid chewing, or cigarette smoking). The study cohort was divided according to the presence or absence of risky oral habits. Interestingly, patients without risky oral habits and HPV infections (n = 12) showed a worse OS (49% vs 80%; P = 0.021) than those with no evidence of HPV infections (n = 56). In contrast, HPV infections were not associated with OS in patients with risky oral habits (HPV-positivity [n = 182] vs HPV-negativity [n = 752]: 70% vs 73%; P = 0.187).
Univariate and Multivariate Analyses
In univariate analysis, we identified the following clinicopathological factors as significantly associated with lower 5-year OS: histological differentiation, pathological tumor status, pathological lymph node status, pathological stage, histological tumor depth, ECS, and level IV/V metastases (2004 cohort; Table 3). No significant associations were found between OS and any HPV infection, HPV16 and other oncogenic HPV infections, HPV16 infection, and HPV18 infection. However, additional univariate analysis with stratified data revealed that, among patients with advanced OCC (pathological stage III/IV), the presence of HPV16 infection (n = 23) was associated with a significantly worse 5-year OS (n = 234; 34% vs 56% in those without HPV16 infection; P = 0.038). These results suggest that HPV16 infection was not a significant prognostic factor in univariate analysis because of confounding effects; therefore, HPV16 infection was entered into the multivariate regression model.
TABLE 3.
Variables Associated With Risk of Death in the 2004 Cohort (n = 466) and 2008 Cohort (n = 536)
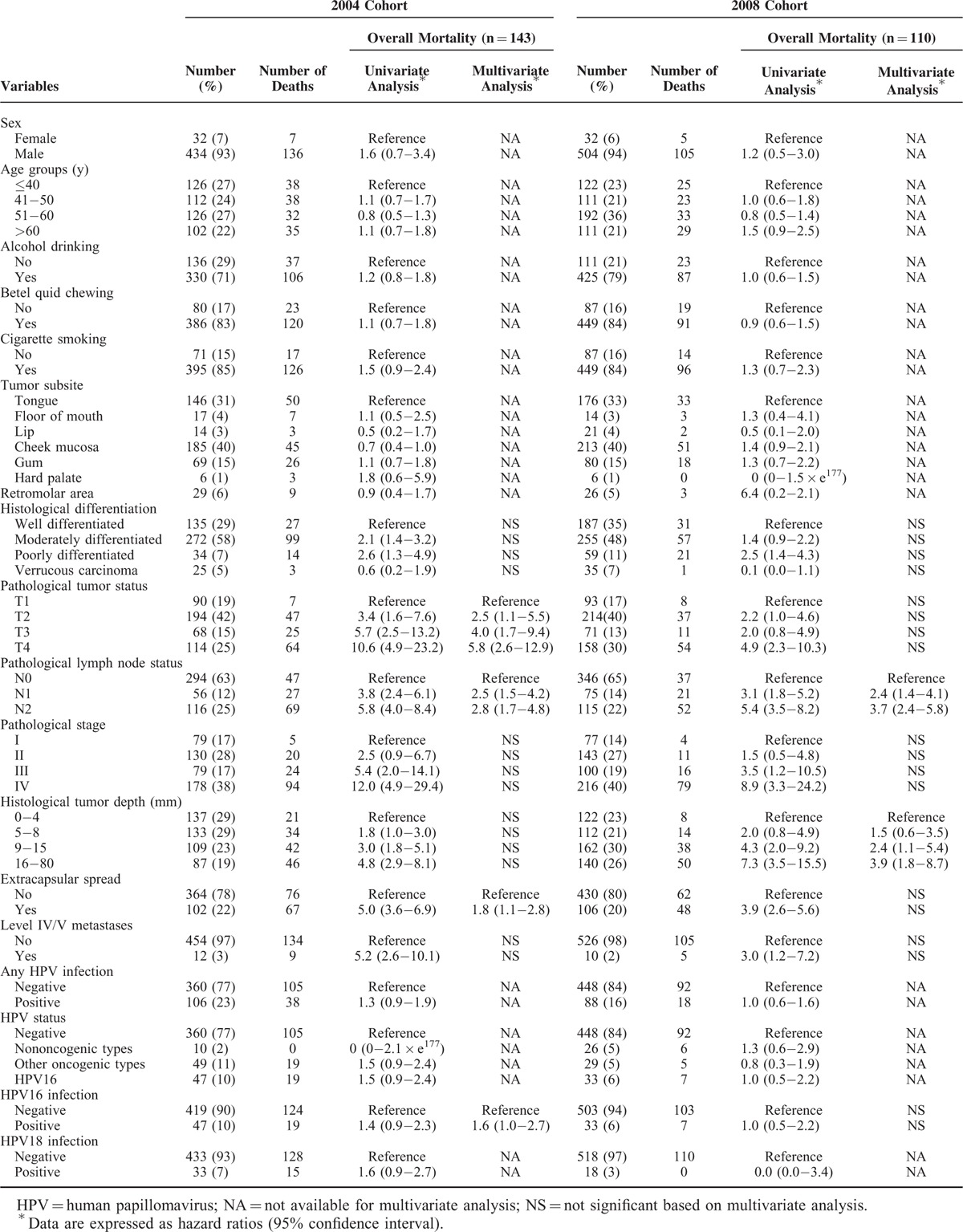
Correlation analyses between clinicopathological variables showed that all of the pathological factors were significantly correlated with each other, whereas HPV16 infection was not associated with any pathological factor. The results of multivariate analyses identified 4 adverse independent prognostic factors for OS (T2, T3, and T4 status; pathological N1 and N2 status; ECS; and HPV16 infection; Table 3).
In the 2008 cohort (Table 3), the same clinicopathological factors (histological differentiation, pathological tumor status, pathological lymph node status, pathological stage, histological tumor depth, ECS, and level IV/V metastases) were identified as being significantly associated with lower 5-year OS in univariate analyses. When these variables and HPV infections were entered as covariates into a multivariate regression model, pathological lymph node status (N1 and N2) and histological tumor depth (9–15 mm and 16–80 mm) were found to independently predict 5-year OS.
Prognostic Scoring System
For translational purposes, we further dichotomized continuous variables according to their optimal cut-off values(age >50 y; tumor depth >8 mm) in the 2004 cohort (model 1; Table 4). Multivariate analysis demonstrated that 4 factors were independent adverse prognostic predictors of 5-year OS: pathological T3/T4 status (HR 2.4 [95% CI 1.7–3.4]); pathological N1/N2 status (HR 2.9 [95% CI 1.8–4.5]); ECS (HR 1.9 [95% CI 1.2–3.0]); and HPV16 infection (HR 1.7 [95% CI 1.0–2.8]). In the 2008 cohort (model 2), 3 factors were independent adverse prognostic predictors for 5-year OS: pathological N1/N2 status (HR 2.4 [95% CI 1.5–3.9]); pathological stage III/IV (HR 2.0 [95% CI 1.0–4.1]); and histological tumor depth >8 mm (HR 2.1 [95% CI 1.3–3.5]). The weighting was based on a simple algorithm assigning the integer value of the corresponding HR to each factor (ie, 2 point for HR 1.5–2.4; 3 points for HR 2.5–3.4).
TABLE 4.
Multivariate Analysis of Variables Predicting 5-Year Overall Survival
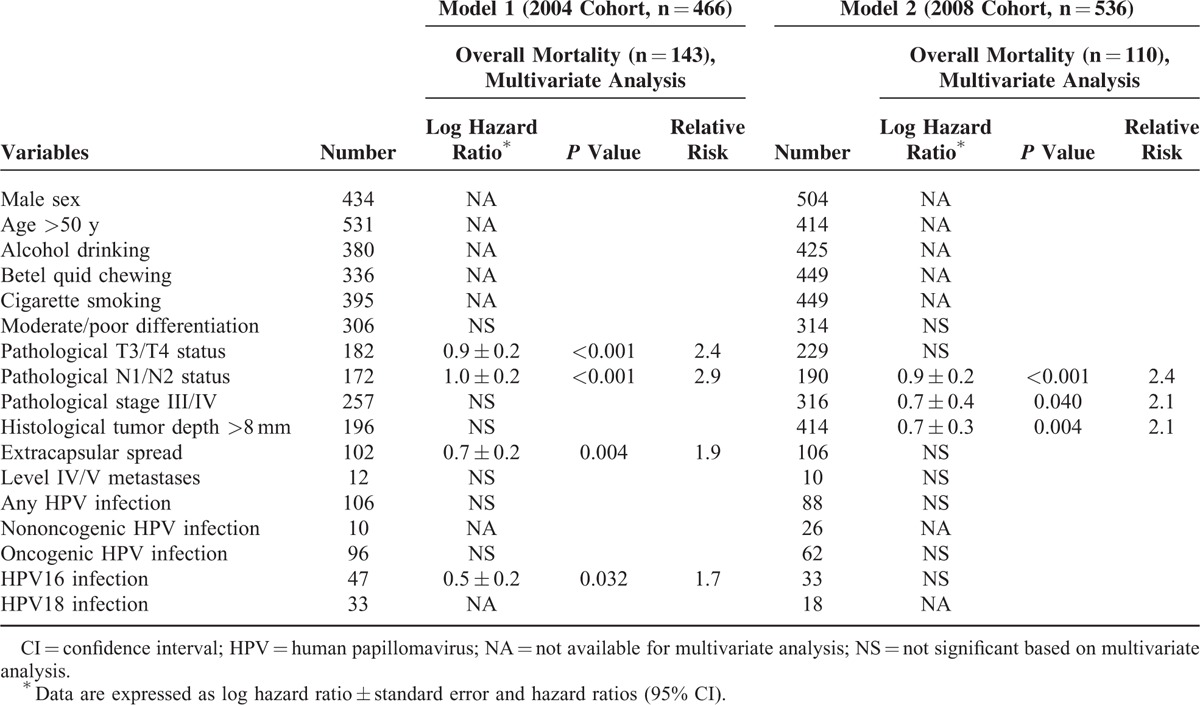
Specifically, a score of 2 was assigned in presence of pathological T3/T4 status, ECS, or HPV16 infection, and a score of 3 was given for pathological N1/N2 status (model 1). The resulting prognostic score (ranging from 0 to 9) was then applied to stratify the study cohort into 4 distinct prognostic groups, as follows: low (score 0 [40%], intermediate (score 1–3 [31%]), high (score 4–6 [14%]), and very high (score 7–9 [16%]; Table 5) risk. Patients with a low risk had an excellent 5-year OS (89%). However, the 5-year OS rates of patients with intermediate, high, and very high risk were 76%, 50%, and 25%, respectively (P < 0.005 for all comparisons). The c-index was 0.76, suggesting a satisfactory prediction performance.
TABLE 5.
Prognostic Scoring System in Relation to 5-Year Overall Survival
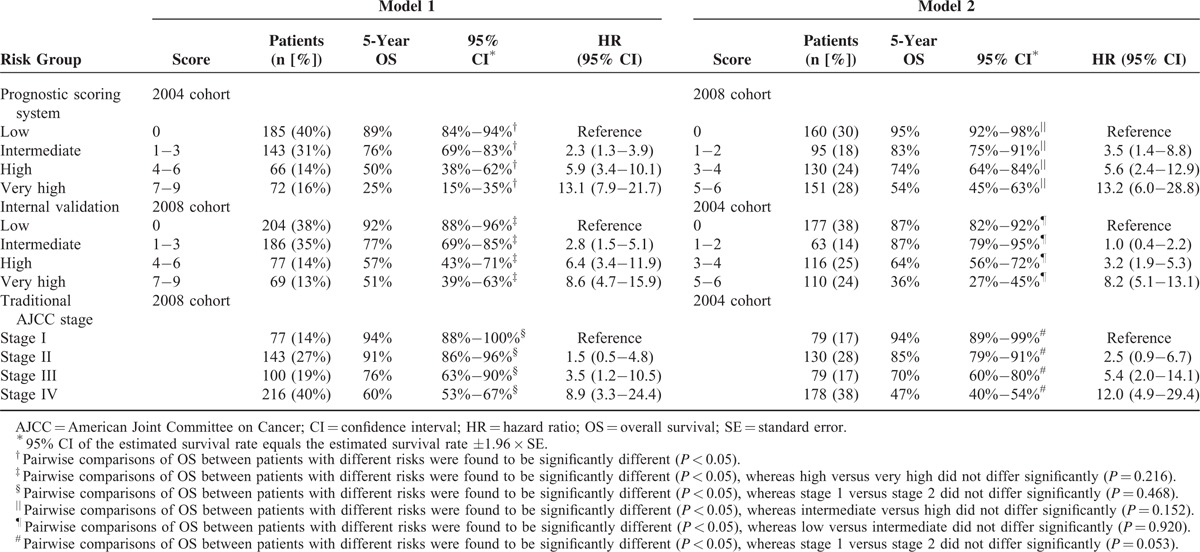
A score of 2 was assigned in presence of each of the following risk factors: pathological N1/N2 status; pathological stage III/IV; and histological tumor depth >8 mm. The resulting prognostic score (ranging between 0 and 6) was applied to stratify the 2008 study cohort into 4 distinct prognostic groups, as follows: low risk (score 0 [30%]), intermediate risk (score 1–2 [18%]), high risk (score 3–4 [24%]), and very high risk (score 5–6 [28%]) (Table 5). Patients with a low risk had an excellent 5-year OS (95%). The 5-year OS rates of patients with intermediate, high, and very high risk were 83%, 74%, and 54%, respectively (P < 0.005 for all comparisons, the only exception being intermediate vs high [P = 0.152]). The c-index was 0.73 (indicating a satisfactory prediction performance of model 2).
The analysis of OS in the 2008 cohort also demonstrated the internal validity of the model 1 system for the main endpoint. The 5-year OS rates of the 4 risk groups were 92%, 77%, 57%, and 51%, respectively (P < 0.001; Table 5), with a c-statistic of 0.71. Subgroup analyses corroborated the discriminative strength of the prognostic scoring system for all comparisons, the only exception being the very-high–risk group compared with the high-risk group (P = 0.216). As the prevalence of HPV16 infection significantly decreased from 10% (2004 cohort) to 6% (2008 cohort), the proportion of HPV16 infections was similarly reduced from 19% to 10% in the very-high–risk group. The proportion of the very-high–risk group was also reduced from 16% to 13% in model 1, albeit not significantly so (P = 0.121 and 0.461, respectively). As long as risk scores were analogous, similar survival rates were observed. This phenomenon caused a decreased discriminative strength for the comparison between the very high and high-risk groups in the 2008 cohort. The internal validity of model 2 for the main outcome measure was investigated in the 2004 cohort. The 5-year OS rates of the 4 risk groups were 87%, 87%, 64%, and 36%, respectively (P < 0.001; c-statistic = 0.74; Table 5). Only the low and intermediate-risk groups did not differ from each other (P = 0.920). All other comparisons were statistically significant.
Comparison Between Prognostic Scoring System and Sixth Edition AJCC Stage System
The 5-year OS differed significantly according to the traditional AJCC pathological stage in the 2004 (Table 3) and 2008 (Table 5) cohorts, the only exception being a marginal difference between patients with a pathological stage of 2 (85% and 91%, respectively) and those with a pathological stage of 1 (94% and 94%, respectively; P = 0.053 and 0.68, respectively). The c-statistics for pathological stage were 0.72 for the 2004 cohort and 0.70 for the 2008 cohort, respectively. The Kaplan–Meier survival curves for OS according to the pathological stage and the model 1 and model 2 systems in the entire cohort are shown in Figure 2. In the entire study cohort, the c-statistics for pathological stage, model 1, and model 2 were 0.71, 0.73, and 0.72, respectively.
FIGURE 2.
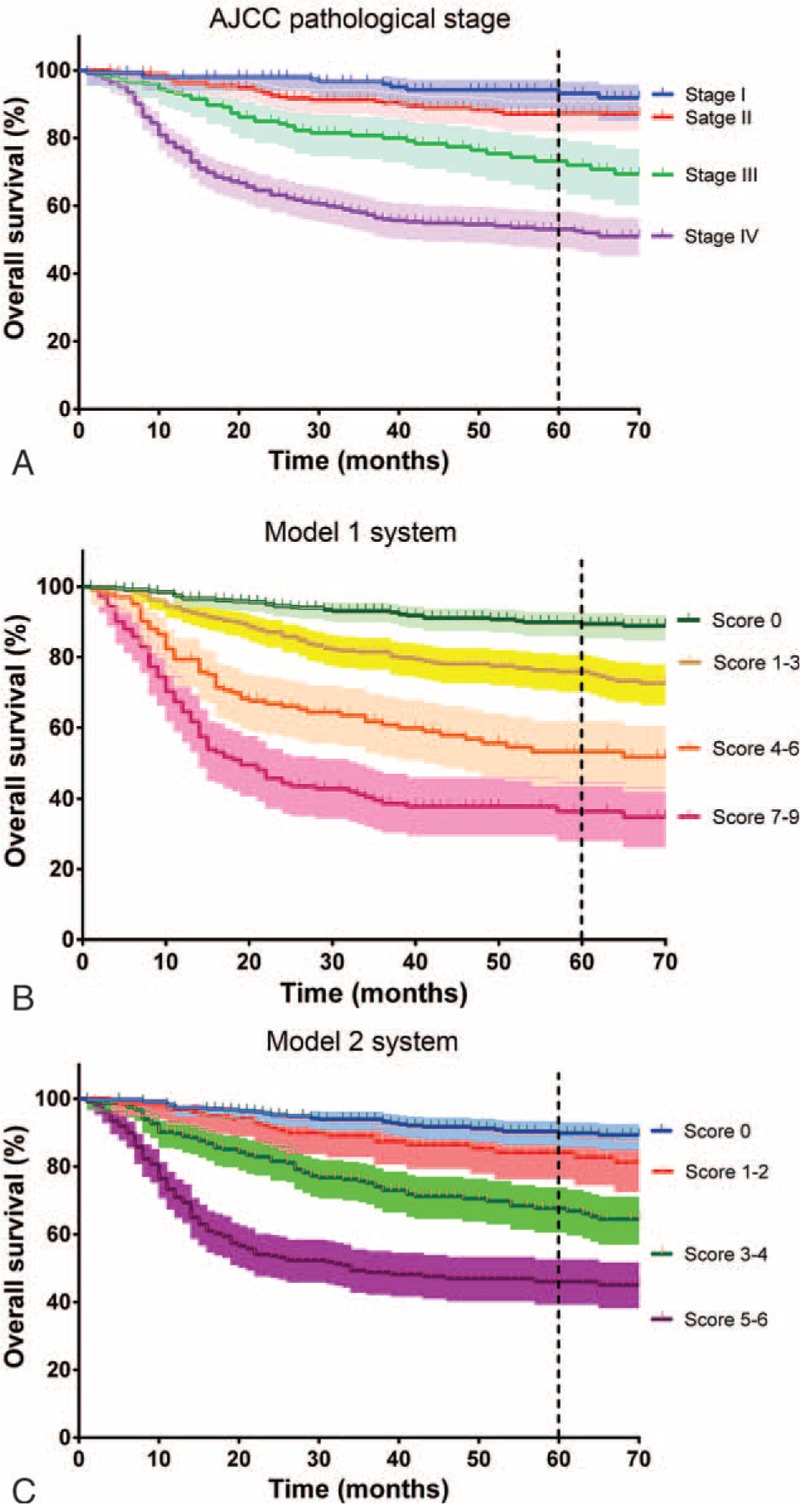
Kaplan–Meier estimates of the 5-year overall survival in patients with oral cavity cancer (n = 1002). A, Risk stratification according to the 2002 American Joint Committee on Cancer (AJCC) pathological stage (log-rank test, P < 0.001). B, Risk stratification according to model 1 (P < 0.001). C, Risk stratification according to model 2 (P < 0.001). Shallow areas indicate the 95% confidence interval (CI).
DISCUSSION
The results of the present study indicate that HPV infections are common among Taiwanese OCC patients, this finding being in line with a recent meta-analysis (pooled HPV-DNA prevalence 24%).22 However, the prevalence of all HPV infections, oncogenic HPV infections, and both HPV16 and HPV18 infections decreased significantly between 2004 and 2011. In contrast, nononcogenic HPV infections showed an increasing trend in the same calendar period. Importantly, we demonstrate that HPV16 infection is an independent adverse prognostic factor for OS in OCC patients. To our knowledge, this study is the first to internally validate a prognostic index that included HPV16 infection for predicting 5-year OS in OCC patients. The index had a high discriminatory power and was a significant predictor of prognosis at the individual patient level. However, further external validation is required before it could be implemented in clinical practice.
A better understanding of the pathogenic role of HPV infections in OCC patients is needed for assessing the potential utility of HPV vaccination campaigns. The reported prevalence of oncogenic HPV infections in apparently healthy US adults aged between 25 and 69 years is 2% to 7%.23 Most of our knowledge about HPV epidemiology relates to the oropharynx, where HPV infections are becoming more prevalent, tend to affect younger people, and are associated with a better prognosis than HPV-negative tumors.24–26 In this large study conducted in OCC patients, we have shown that HPV infections tended to occur less frequently during the past decade, being fairly stable across different age groups. However, we cannot exclude the occurrence of year-to-year variations, ultimately requiring data at 10 years to clearly establish a temporal trend. Notably, HPV-positive tumors were associated with a worse OS, especially in patients without risky oral habits. Among the HPV types (n = 158), HPV16 was the most commonly observed among our OCC patients (51% [n = 80]), although at a significantly lower frequency than that previously reported for HPV-positive OPC (>90%).27 HPV18 (26% [n = 51]) and HPV58 (7% [n = 14]) were other frequently encountered oncogenic types in this study.
Human papillomavirus molecular oncogenesis is significantly different from that of tobacco and alcohol-related head and neck malignancies in terms of genetic mutations or deletions.28 The superior response to treatment in HPV-positive OPC patients may result from a higher likelihood of restoring a normal cell growth control.25 The observation that HPV infections are related to a worse prognosis in OCC patients without risky oral habits suggests that detection of intratumor HPV DNA may not characterize the tumor as being caused by the virus. The use of p16-based assays can detect oncogenic HPV activity,29 but immunohistochemical analysis of p16 expression lacks prognostic utility in OCC patients, unless its intracellular localization is considered.28 A more recent study suggested that a combined p16/HPV testing is necessary to identify HPV-associated nonoropharyngeal head and neck cancers characterized by favorableoutcomes.29 However, the detection of HPV-DNA using PCR and p16 expression in cancer biopsies lacks specificity, and HPV E6/E7 antibody detection lacks sensitivity.30,31 Therefore, such tests remain suboptimal for the detection of HPV infections.2 Moreover, the genetic diversity of OCC patients (regardless of HPV infections) may play a key role in determining prognosis and treatment outcomes. In our previous studies, several molecular markers, including Gα12,32 crucial upstream drivers,33 miRNA-491-5p and GIT1,34 BST2,35 oncomiR-196,36 and somatic copy numbers,37 have been linked to clinical outcomes in small case series. Studies aimed at investigating the prognostic impact of genetic variants in larger clinical cohorts are currently ongoing.
These caveats notwithstanding, our results suggest that HPV16 infections are independently associated with a lower OS in OCC patients. In previous smaller studies, we have shown that the detection of HPV16 DNA in patients with advanced OCC12 and high HPV16/18 E7 viral load in OCC patients16 are related to distant metastasis and OS. We further developed a prognostic scoring system that incorporates both common prognostic factors and the presence of HPV16 infections for predicting OS in a more accurate manner than traditional pathological stage. The results indicated that the projected 5-year OS rates were89% for low-risk, 76% for intermediate-risk, 50% for high-risk, and 25% for very-high–risk patients, respectively. One potential strategy to improve outcomes in patients at high risk of death after combination therapy (ie, those with a prognostic score ≥7) may rely on a more aggressive initial treatment, based on intensive chemoradiation regimens. Such a stratification system might be useful as part of the prognostic evaluation in OCC patients and as an enrichment strategy for clinical trials.
Recently, the International Consortium for Outcome Research in Head and Neck Cancer has attempted to review the prognostic performance of the AJCC classification of OCC. The results indicated that the inclusion of histological tumor depth (depth of invasion) can improve T-staging system.38 Moreover, the number of metastatic lymph nodes in patients with both N2b and N2c may improve the prognostic performance of the N-staging system.39 Finally, T3N1 and stage IVa disease in OCC have been shown to be similar.40 The results of the current study indicate that ECS and HPV16 can help refining the prognostication of OCC patients in model 1 (distinction between low vs intermediate risk, and high vs very high risk). Moreover, histological tumor depth can help identifying high-risk patients in model 2. On the basis of the similar c-statistics, we suggest that these categories could be grouped together in future revisions of the AJCC staging system with the overall goal of enhancing prognostic accuracy. In contrast, the validation 2008 cohort had a decreased prevalence of HPV16 infection and a favorable OS in the very-high–risk group, suggesting the clinical usefulness of preventing HPV16 infections. Since 2 licensed prophylactic vaccines against HPV16 and HPV18 has been marketed for almost a decade,41,42 it is expected that both the prevalence of HPV infections and their impact on OS of OCC patients will be declining in the upcoming decades.
In summary, our findings suggest that HPV16 infections may increase the risk of death by any cause in high-risk OCC patients. If independently confirmed, the prognostic score may be useful for allocating OCC patients to risk-adapted therapies in countries with a high prevalence of HPV infections. However, patients with HPV-positive OCC should be currently treated using standard-of-care approaches unless otherwise demonstrated in future studies. Because of potential biases (eg, ethnical differences, variations in risky oral habits, retrospective nature of the study, different laboratory assays for detecting HPV infections), our data need to be validated in ethnically diverse populations. This cohort study provides a strong rationale for implementing such collaborative efforts. Larger studies focusing on a longer time period are warranted to confirm the declining trend of HPV infections.
Acknowledgments
This study was financially supported by the Taiwan Ministry of Health and Welfare (100-TD-C-111-006); Taiwan Ministry of Science and Technology (102-2325-B-182A-005, 103-2325-B-182A-002, 102-2325-B-182A-004, and 103-2325-B-182A-001); and the Chang Gung Memorial Hospital, Taoyuan, Taiwan (390301-3, 391431-4, and 396101-3) for the support.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence interval, ECS = extracapsular spread, HPV = human papillomavirus, HR = hazard ratio, IQR = interquartile range, OCC = oral cavity cancer, OPC = oropharyngeal cancer, OS = overall survival.
LAL and CGH contributed equally to this work.
The authors declare no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr Accessed December 20, 2014. [Google Scholar]
- 2.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol 2014; 50:370–379. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356:1944–1956. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013; 31:4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu C, Ling Y, Dong C, et al. The relationship between oral squamous cell carcinoma and human papillomavirus: a meta-analysis of a Chinese population (1994–2011). PLoS One 2012; 7:e36294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishna Rao SV, Mejia G, Roberts-Thomson K, et al. Epidemiology of oral cancer in Asia in the past decade-an update (2000–2012). Asian Pac J Cancer Prev 2013; 14:5567–5577. [DOI] [PubMed] [Google Scholar]
- 8.Health Promotion Administration, Taiwan Ministry of Health and Welfare: Taiwan cancer registry annual report (2004, 2010, 2011). Available at: http://www.hpa.gov.tw/ Accessed October 1, 2015. [Google Scholar]
- 9.Liao CT, Wallace CG, Lee LY, et al. Clinical evidence of field cancerization in patients with oral cavity cancer in a betel quid chewing area. Oral Oncol 2014; 50:721–731. [DOI] [PubMed] [Google Scholar]
- 10.Chang JY, Lin MC, Chiang CP. High-risk human papillomaviruses may have an important role in non-oral habits-associated oral squamous cell carcinomas in Taiwan. Am J Clin Pathol 2003; 120:909–916. [DOI] [PubMed] [Google Scholar]
- 11.Luo CW, Roan CH, Liu CJ. Human papillomaviruses in oral squamous cell carcinoma and pre-cancerous lesions detected by PCR-based gene-chip array. Int J Oral Maxillofac Surg 2007; 36:153–158. [DOI] [PubMed] [Google Scholar]
- 12.Lee LA, Huang CG, Liao CT, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One 2012; 7:e40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SF, Li HF, Liao CT, et al. Association of HPV infections with second primary tumors in early-staged oral cavity cancer. Oral Dis 2012; 18:809–815. [DOI] [PubMed] [Google Scholar]
- 14.Hwang CF, Huang CC, Chien CY, et al. Human papillomavirus infection in oral papillary and verrucous lesions is a prognostic indicator of malignant transformation. Cancer Epidemiol 2012; 36:e122–e127. [DOI] [PubMed] [Google Scholar]
- 15.Lee LA, Huang CG, Tsao KC, et al. Increasing rates of low-risk human papillomavirus infections in patients with oral cavity squamous cell carcinoma: association with clinical outcomes. J Clin Virol 2013; 57:331–337. [DOI] [PubMed] [Google Scholar]
- 16.Huang CG, Lee LA, Tsao KC, et al. Human papillomavirus 16/18 E7 viral loads predict distant metastasis in oral cavity squamous cell carcinoma. J Clin Virol 2014; 61:230–236. [DOI] [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th edNew York, NY: Springer-Verlag; 2002. [Google Scholar]
- 18.Huang SL, Chao A, Hsueh S, et al. Comparison between the Hybrid Capture II test and an SPF1/GP6+ PCR-based assay for detection of human papillomavirus DNA in cervical swab samples. J Clin Microbiol 2006; 44:1733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CY, Chen HC, Lin RW, et al. Quality assurance of genotyping array for detection and typing of human papillomavirus. J Virol Methods 2007; 140:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edNew York NY: John Wiley & Sons; 2000. [Google Scholar]
- 21.Liao CT, Wang HM, Ng SH, et al. Good tumor control and survivals of squamous cell carcinoma of buccal mucosa treated with radical surgery with or without neck dissection in Taiwan. Oral Oncol 2006; 42:800–809. [DOI] [PubMed] [Google Scholar]
- 22.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 2014; 15:1319–1331. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi AK, Graubard BI, Pickard RK, et al. High-risk oral human papillomavirus load in the US population, national health and nutrition examination survey 2009–2010. J Infect Dis 2014; 210:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11:9–22. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24:736–747. [DOI] [PubMed] [Google Scholar]
- 26.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol 2015; 33:836–845. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine 2012; 30:F34–F54. [DOI] [PubMed] [Google Scholar]
- 28.Rampias T, Sasaki C, Psyrri A. Molecular mechanisms of HPV induced carcinogenesis in head and neck. Oral Oncol 2014; 50:356–363. [DOI] [PubMed] [Google Scholar]
- 29.Benson E, Li R, Eisele D, et al. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 2014; 50:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gröbe A, Hanken H, Kluwe L, et al. Immunohistochemical analysis of p16 expression, HPV infection and its prognostic utility in oral squamous cell carcinoma. J Oral Pathol Med 2013; 42:676–681. [DOI] [PubMed] [Google Scholar]
- 31.Salazar CR, Anayannis N, Smith RV, et al. Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer 2014; 135:2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jian SL, Hsieh HY, Liao CT, et al. Gα12 drives invasion of oral squamous cell carcinoma through up-regulation of proinflammatory cytokines. PLoS One 2013; 8:e66133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng CH, Jiang YZ, Tai AS, et al. Causal inference of gene regulation with subnetwork assembly from genetical genomics data. Nucleic Acids Res 2014; 42:2803–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang WC, Chan SH, Jang TH, et al. miRNA-491–5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res 2014; 74:751–764. [DOI] [PubMed] [Google Scholar]
- 35.Fang KH, Kao HK, Chi LM, et al. Overexpression of BST2 is associated with nodal metastasis and poorer prognosis in oral cavity cancer. Laryngoscope 2014; 124:E354–E360. [DOI] [PubMed] [Google Scholar]
- 36.Lu YC, Chang JT, Liao CT, et al. OncomiR-196 promotes an invasive phenotype in oral cancer through the NME4-JNK-TIMP1-MMP signaling pathway. Mol Cancer 2014; 13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng CH, Liao CT, Ng KP, et al. Somatic copy number alterations detected by ultra-deep targeted sequencing predict prognosis in oral cavity squamous cell carcinoma. Oncotarget 2015; 6:19891–19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebrahimi A, Gil Z, et al. International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg 2014; 140:1138–1148. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahimi A, Gil Z, Amit M, et al. The prognosis of N2b and N2c lymph node disease in oral squamous cell carcinoma is determined by the number of metastatic lymph nodes rather than laterality: evidence to support a revision of the American Joint Committee on Cancer staging system. Cancer 2014; 120:1968–1974. [DOI] [PubMed] [Google Scholar]
- 40.Amit M, Yen TC, Liao CT, et al. Prognostic Performance of Current Stage III Oral Cancer Patients After Curative Intent Resection: Evidence to Support a Revision of the American Joint Committee on Cancer Staging System. Ann Surg Oncol 2015; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161–2170. [DOI] [PubMed] [Google Scholar]
- 42.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943. [DOI] [PubMed] [Google Scholar]


