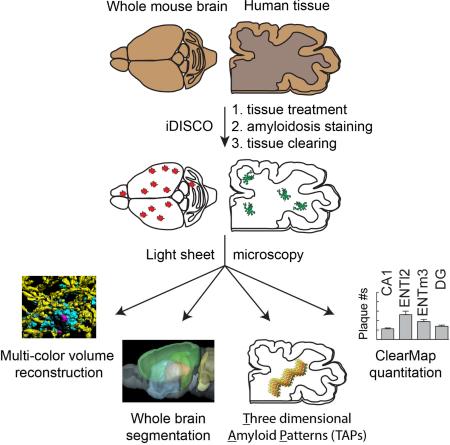
Summary
Amyloidosis is a major problem in over one hundred diseases including Alzheimer's disease (AD). Using the iDISCO visualization method involving targeted molecular labeling, tissue clearing and light-sheet microscopy, we studied plaque formation in the intact AD mouse brain and up to twenty-seven months of age. We visualized amyloid plaques in 3D together with tau, microglia and vasculature. Volume imaging coupled to automated detection and mapping enables precise and fast quantification of plaques within the entire intact mouse brain. The present methodology is also applicable to analysis of frozen human brain samples without specialized preservation. Remarkably, amyloid plaques in human tissues showed greater three-dimensional complexity and surprisingly large three-dimensional amyloid patterns or TAPs. The ability to visualize amyloid in 3D, especially in the context of their micro-environment, while simultaneously analyzing two other markers, and the discovery of large TAPs, may have potentially important scientific and medical implications.
Keywords: amyloid plaques, tau, iDISCO, brain clearing, Alzheimer's disease, amyloidosis, three-dimensional amyloid patterns (TAPs), Congo red, 3D rendering, microglia, ClearMap
Graphical Abstract
INTRODUCTION
Alzheimer's Disease (AD), the most common neurodegenerative disorder, is characterized by initial short-term memory loss followed by subsequent severe deficits attributed to neuronal and neuritic loss (Probst et al., 1991; Holtzman et al., 2011). The two main pathological hallmarks of the disease are 1) the abnormal accumulation of the β amyloid peptide (Aβ) and its aggregation leading to amyloid plaques localized in the brain parenchyma, and 2) the presence of intracellular hyperphosphorylated tau protein inclusions (neurofibrillary tangles, or NFTs) (Haass et al., 2007; Castellani et al., 2010). There are still many unknowns about the origin and the role of these hallmarks, including the debate about the importance of oligomers versus amyloid plaques and NFTs (Berger et al., 2007; Lesne et al., 2006 ;Walsh et al., 2002), but we believe that a better understanding of the three-dimensionality of disease hallmarks and their complex association with the immediate environment (e.g. vasculature, microglia) will help clarify the underlying mechanisms of neurodegeneration.
Until recently, volume imaging of a human brain sample or an entire mouse brain hemisphere has been restricted by microscopy techniques with limited light penetration and/or insufficient resolution; the workhorse techniques of confocal and 2-photon microscopy are not compatible with large volume imaging, and positron emission tomography has a relatively poor resolution, especially when studying rodents (Pan W.J., et al., 2016). Due to a renewed interest in light-sheet fluorescence microscopy, also referred to as selective plane illumination microscopy (Huisken et al., 2004, Dodt et al., 2007), coupled with the development and optimization of tissue clearing technologies, it has become possible to investigate large intact tissue volumes, including the mouse brain, with cellular resolution (Hama et al., 2011; Ertürk et al., 2012; Renier et al., 2014; Kuwajima et al., 2013; Ke et al., 2013; Chung et al., 2013a, Tomer et al., 2014; Yang et al., 2014; Susaki et al., 2014). Recent attempts at comprehensive 3D imaging of AD mouse models have given momentum to volume imaging strategies for the field of AD, but have failed to reveal the entirety of the plaque content in the brain due to volume limitations, incomplete illumination/detection or restricted quantitation (Jährling et al., 2015; Ando et al., 2014; Hama et al., 2015).
The combination of multiple technologies (volume immunolabeling, tissue clearing, light-sheet microscopy and computational analysis) described previously (iDISCO, Renier et al., 2014 and Renier et al., 2016) allowed us to study AD hallmarks in the intact mouse brain hemisphere. As immunodetection was crucial for this study, iDISCO was the ideal method for labeling and volume imaging of beta amyloid, tau protein, cell nuclei, microglia and vasculature, allowing visualization of up to three parameters simultaneously. We compared 3D imaging using optical slices to the conventional immuno-histochemical (IHC) method performed on physical sections. As a faster and more economical alternative for beta amyloid plaque labeling, we showed that antibodies can be replaced by the small molecule Congo red [PubChem CID: 11313]. We studied the progression of amyloid plaque accumulation in the 2xTg AD mouse model (APPswe/PSEN1dE9) in animals ranging from 4.4 to 27 months of age. iDISCO facilitated more comprehensive detection of beta amyloid plaques at earlier stages than allowed by the classical IHC method, which typically does not effectively sample plaques until later stages due to the scarcity of plaques in thin sections. Five major brain regions were optically dissected and analyzed for plaque density. For more comprehensive and non-biased quantification of the entire brain, we applied ClearMap, a recently developed method for automated detection and alignment to an anatomical atlas. The combination of volume imaging and ClearMap analysis allowed us to automate detection of amyloid plaques and alignment to the Allen Brain Atlas.
Remarkably, volume analysis of archived human brain tissue led to the discovery of large three-dimensional amyloid patterns (TAPs) measuring up to 27 mm3. In these human samples, contrary to the mouse brains, we observed a larger diversity of amyloid plaques in terms of size and three-dimensional shape. These intriguing differences between animal models and human samples might highlight a trait of, and specific to, human AD pathogenesis.
The combination of various parameters (3D co-labeling with up to three antibodies and/or small molecules, light-sheet/confocal/two-photon microscopy) provides access to an increased set of possibilities for facilitated quantitative pre-clinical studies and for pathological exploration through correlative examination using retrospective studies of archival tissues, which may even lead to disease classification.
RESULTS
Detecting three-dimensional beta amyloid plaques in a full mouse brain hemisphere, with anti-Abeta peptide antibodies or Congo red
Here we explored the possibilities of studying amyloidosis in 3D. We propose that studies of beta amyloid plaques and their surroundings could gain substantially by resolving beta amyloid plaques in their three-dimensional context. We applied iDISCO (immunolabeling-enabled three-dimensional imaging of solvent cleared organs), a method developed (Renier at al., 2014) to combine whole-mount immunolabeling with the high transparency offered by the solvent-based clearing technique 3DISCO (Erturk et al, 2012). We first tested eleven anti-Abeta antibodies for compatibility with the iDISCO methanol-based immunostaining protocol for their capacity to reveal beta amyloid plaques in large mouse tissue blocks (see Table S1). Based on label specificity and effective tissue penetration (see Figure S1), one antibody was selected (referred to as PAb #10) for Abeta labeling throughout the study. Full mouse hemispheres were then prepared and treated with PAb #10 and imaged utilizing high-speed light-sheet microscopy, resulting in image data sets that enable optical isolation of arbitrary image planes (optical slice) or volumes throughout the brain. A representative sagittal optical slice obtained from a 27-month-old 2xTg mouse brain reveals the presence of beta amyloid plaques in the cortex and hippocampus (Figure S2A, top panel). A magnified view of this optical slice shows a large concentration of amyloid plaques throughout the cortex and in the hippocampus (Figure 1A, top image). A direct comparison (two hemispheres from the same mouse brain) between the most promising antibodies (PAb #10 and PAb #9) showed a similar distribution even though many fewer amyloid plaques are visible in the center of the sample labeled with PAb #9, presumably due to reduced penetration depth for PAb #9 as a result of antibody depletion (Figure S2B, S3).
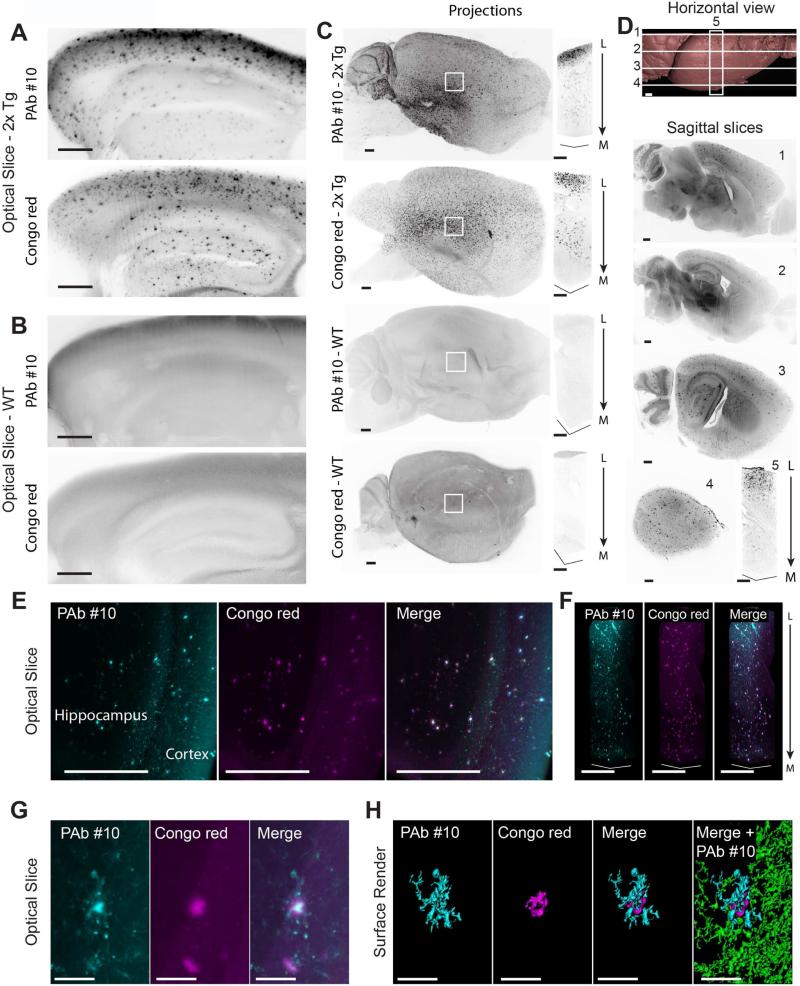
Figure 1. Ultramicroscopic evaluation of beta amyloid staining in 27-month-old cleared mouse brains.
(A) Magnified views of cortices and hippocampi from cleared brain hemispheres from a 2xTg AD mouse. See also Figure S2.
(B) Control images for A on a 37-month-old WT mouse.
(C) Maximum intensity projections from light-sheet images of entire hemispheres and associated central column (1 mm2 cross-section) through the indicated regions (white squares).
(D) Staining of a 2xTg AD mouse hemisphere (17 months old) with 2 hours Congo red incubation. Horizontal view of surface render from light-sheet images indicating positions of subsequent sagittal optical planes and central column projection (1 mm2 cross-section).
(E) Light-sheet sagittal optical slice through hippocampus of a 2xTg AD mouse (10 months old) co-stained for beta amyloid plaques with PAb #10 and Congo red.
(F) Maximum intensity projections of columns (0.25 mm2 cross-section) through volume of the respective double labeled hemispheres shown in E.
(G) High magnification optical section (100 μm thick) with double labeled plaque.
(H) Surface render of the plaque shown in G. Beta amyloid staining associated with plaque (cyan) and surrounding deposits (green).
Scale bars represent 500 μm, except G and H: 50 μm.
Columns are oriented from lateral (L) to medial (M).
2xTG AD: double transgenic Alzheimer's disease mouse model (see also methods); WT: wild-type.
Next, in an attempt to circumvent some of the limitations inherent to antibodies (cost, slow diffusion and occasional unspecific surface labeling), we tested the possibility that a small molecule known to bind beta amyloid plaques, Congo red, could be effectively used in combination with iDISCO, as demonstrated with other small molecules and clearing protocols (Jahrling et al, 2015). We performed a comparison between Congo red and PAb #10 on the two hemispheres of a 27-month-old 2xTg brain. Single optical slices and orthogonal planes reveal that both antibody and Congo red stainings of amyloid plaques are visible throughout the volume of the hemispheres (Figure S2A). Magnified views of cortices and hippocampi clearly demonstrate the specificity of both antibody and dye staining of plaques (Figure 1A) when compared with control wild type tissue (Figure 1B). Maximum projection images highlight the distribution of staining in the intact large tissues. Staining of full hemispheres with PAb #10 and Congo red is shown in an aged 2xTg AD mouse and an aged wild type control mouse (Figure 1C). Highlighting the time efficiency of using Congo red for plaque visualization, we found effective staining throughout an intact hemisphere after only two hours of dye incubation (Figure 1D).
To illustrate the feasibility of increased lateral resolution imaging, whole hemispheres were also imaged using confocal microscopy. Optical slices further confirm the specificity of PAb#10 labeling in the 2xTg AD mouse brain compared to wild type brain staining (Figure S3D). Maximum intensity projections and animated volume rendering of intact brain hemispheres reveal plaques with exceptional detail, combining the resolving capabilities of confocal microscopy with the volume capacity enabled by clearing (Figure S3E-F and Movie S1), though axial resolution is reduced and acquisition times are longer compared to light sheet microscopy.
To directly compare the efficacy of the antibody versus small molecule dye staining of beta amyloid plaques, we simultaneously applied both labels to a single brain hemisphere of a 2xTg AD mouse. Optical slices and volume projections within the hippocampus and cortex demonstrate a comparable efficacy of the two labeling strategies for targeting large amyloid plaques deep in the brain (Figure 1E-F and Movie S2). Based on these results, the two staining methods are generally comparable. Interestingly, detailed inspection of enlarged images of a cortical region reveals differences in labeling patterns between the two methods. Magnification of thin optical slices shows prominent labeling of the plaque core in both cases, whereas subtle outer structures surrounding the core are seen only with antibody staining, suggesting a greater sensitivity of the antibody detection method (Figure 1G). Surface rendering further emphasizes the 3D differences between the two stains and clarifies the plaque ramifications (Figure 1H; cyan) visible around the core of the plaque (Figure 1H; red) and surrounding beta amyloid deposits labeled by antibodies (Figure 1H; green).
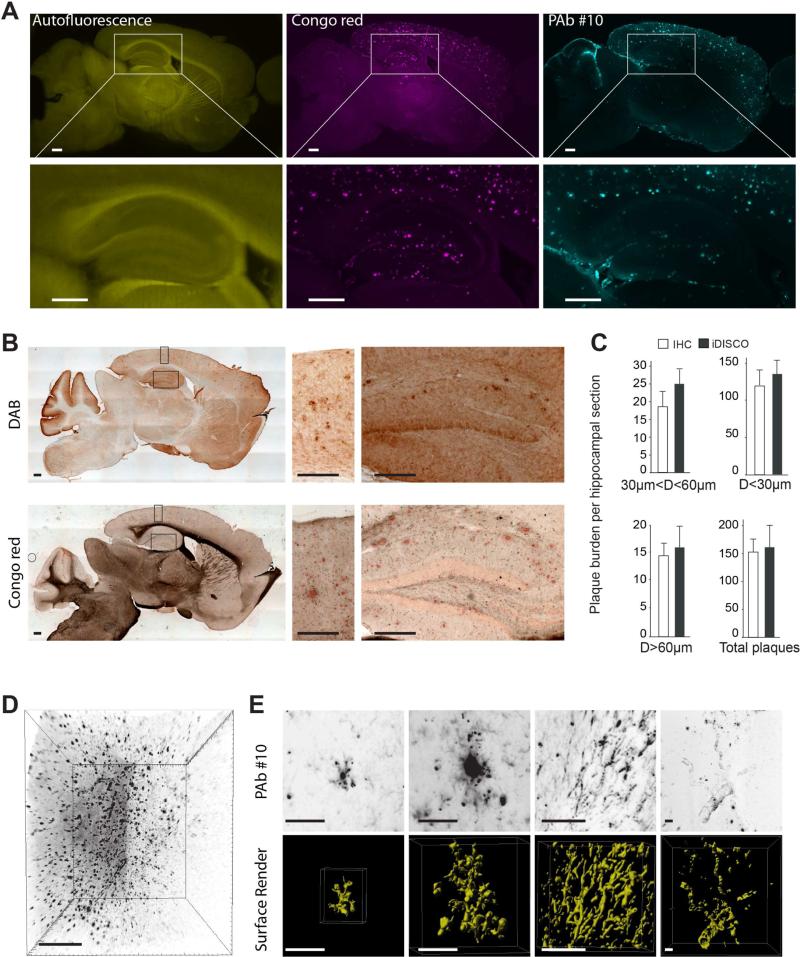
Next, as immunohistochemistry (IHC) is the prevailing method of choice to detect beta amyloid plaques in brain tissue, we compared quantitation of plaques in physical sectioning and DAB staining versus optical sectioning in age-matched animals. In one 17-month-old 2xTg AD mouse, we used iDISCO to stain plaques with both Congo red and PAb #10 and acquire serial sagittal sections (Figure 2A). An additional animal was treated according to standard IHC sectioning and staining for plaques (Figure 2B). A comparative quantification of antibody-labeled plaques in tissue sections versus matched optical planes from iDISCO labeling validates that there is statistically equivalent plaque detection between strategies (Figure 2C). When plaque staining is combined with the autofluorescence in the green spectrum, iDISCO provides optical sectioning with detailed plaque morphology as well as local topography. Volume imaging of plaques with iDISCO also accommodates additional labels, such as nuclear staining to reveal neighboring cell density (Figure S4A).
Figure 2. Comparison of optical light-sheet sectioning of a cleared brain and conventional mechanical tissue sectioning for imaging of beta amyloid plaques in 2xTg AD mouse brains.
(A) Sagittal optical slice of a hemisphere from an adult mouse brain labeled for beta amyloid plaques with an anti-beta amyloid antibody and Congo red. Lower images are magnifications of the hippocampus regions indicated.
(B) Wide field images of 50 μm cryosections from the same mouse as above (A) after staining with anti-beta amyloid antibody and DAB or Congo red. Right images are magnifications of the hippocampus and cortex regions indicated.
(C) Quantification of beta amyloid plaques from age matched samples labeled with iDISCO and IHC. Data are represented as mean ± SD.
(D) Maximum intensity projection of anti-beta amyloid staining from cleared 2xTg AD mouse brain.
(E) Examples of amyloid deposition variability (from D). Top: projection of optically dissected amyloid formation. Bottom: surface render of amyloid deposits showing 3D morphology of single plaques, elongated structures and vasculature deposits (from left to right).
Scale bars represent 500 μm, except E: 50 μm.
As cleared samples are imaged intact (without sectioning), a more complete and accurate morphological characterization of beta amyloid deposits is possible. iDISCO permits staining and imaging of large intact tissue volumes (Figure 2D), upwards of one cm3, while simultaneously maintaining resolved details of individual amyloid deposits. Furthermore, representative maximum intensity projections and surface renderings of individual stained structures from small image volumes reveal the diversity of amyloid deposits found in the brain, ranging from plaques of varying sizes and shapes, integrated or not in larger networks (e.g. vasculature deposits) (Figure 2E and Movie S2). iDISCO is compatible with a multitude of accessible microscopy techniques, as demonstrated with laser scanning multiphoton (Figure S4B) and confocal (Figure S4C) microscopies. With each imaging system, we can access volume data, while resolving a variety of plaque sizes, morphologies and aggregates, as well as other relevant parameters such as vascular deposits.
Evaluating dynamics and regionality of plaque formation and distribution in the whole mouse brain
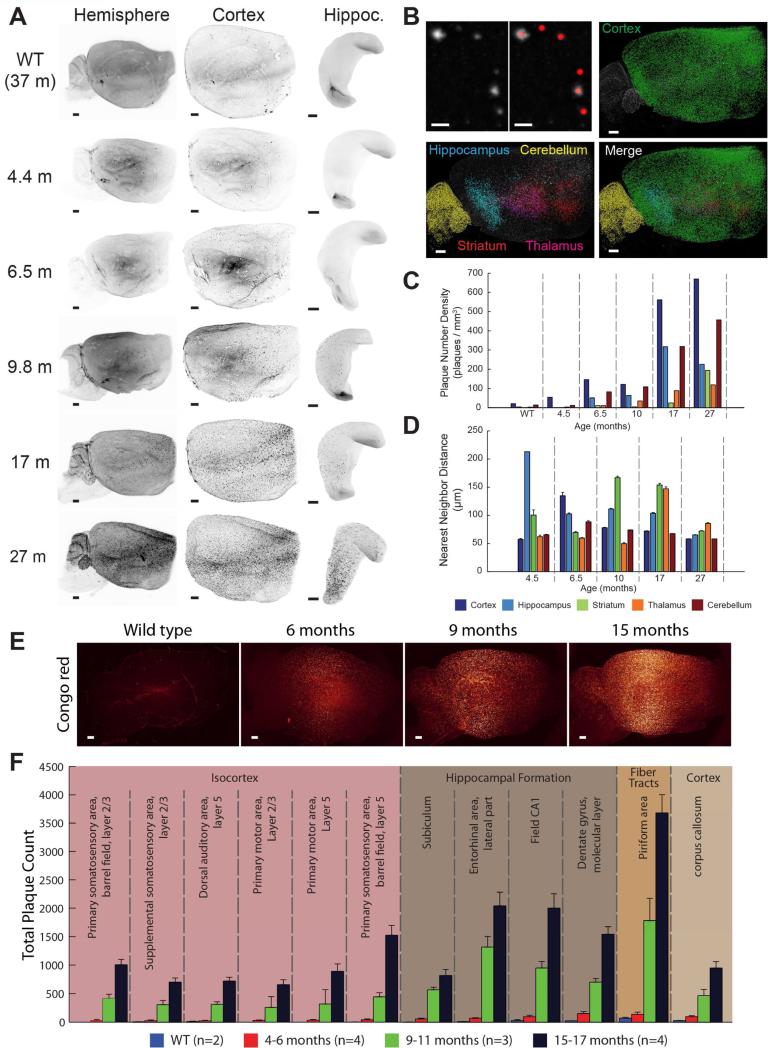
We evaluated the development of beta amyloid plaques in the 2xTg AD mouse line starting with a time at which mice are usually considered beta amyloid plaque-free and ending with old mice with high amyloid plaque burden. Maximum intensity projections of the entire hemispheres show a gradual progression of amyloid plaque staining at each time point (Figure 3A, left panel). With whole hemisphere image data, smaller volumes of interest can be digitally segmented to compare the plaque development profile in any of the prominent structures of the brain (Figure 3A). With images of the complete population of beta amyloid plaques in the intact brain hemisphere, we can directly quantify plaque abundances. The object detection algorithm from the volume image analysis software (Imaris spot detection) was applied to each of the digitally dissected regions of our AD time profile image dataset (Figure 3B). Quantification of each region and age shows the time course of amyloid plaques appearance in the 2xTg mouse model, starting in the cortex and, surprisingly, increasing in the cerebellum earlier than in the hippocampus (Figure 3C). Furthermore, with volume analysis we have the ability to evaluate distances, in 3D space, between plaques in a given region over time (Figure 3D).
Figure 3. Time course of beta amyloid plaque development and automated quantification with ClearMap analysis.
(A) Maximum intensity projections of anti-beta amyloid staining imaged on a light-sheet microscope from cleared WT and 2xTg AD mouse brains at indicated ages. Subregions of hemispheres are digitally isolated.
(B) Spot detection with Imaris. Upper left: sample beta amyloid plaque image and overlay with sphere at each detected beta amyloid plaque. Remaining images: beta amyloid plaque detection in specified brain regions of a 27-month-old 2xTg AD mouse (green: cortex; blue: hippocampus; yellow: cerebellum; magenta: striatum; red: thalamus).
(C) Quantification of beta amyloid plaque density (plaques per mm3) and comparison over time for each analyzed region at the specified age for 2xTg AD and WT mouse brains.
(D) Quantification of beta amyloid plaque nearest neighbor distance and comparison of over time for each digitally isolated brain region. Data are represented as mean ± SEM.
(E) Sample maximum projections of intact hemispheres from 2xTg AD and WT mice of indicated ages stained with Congo red.
(F) ClearMap automated detection and quantification of plaques from the indicated regions. Data are represented as mean ± SEM.
Scale bars represent 500 μm.
Automated whole brain quantitation using ClearMap
Whole brain image data sets require manual input for region-specific quantitation. To circumvent this, we applied a recent tool to streamline region segmentation and allow automated mapping of segmented data, such as amyloid deposits, to annotated references such as the mouse Allen Brain Atlas (Renier et al., 2016). This available toolbox for python, called ClearMap, uses open source modules to rapidly align the autofluorence reference channel from the aligns light-sheet stacks to a reference brain (here, a STP reference aligned to the Allen Brain Standard Annotation). To demonstrate the applicability of ClearMap for comparative studies on plaque distribution, we performed a time course evaluation of 2xTg AD mouse brains using rapid Congo red staining. Intact hemispheres from mice of increasing age were incubated overnight for convenience (shorter incubation times are sufficient) in Congo red before clearing and light-sheet imaging (Figure 3E). In addition to atlas alignment, ClearMap also provides object detection, which works especially well for regular-shaped objects like spherical cell bodies or beta amyloid plaques. Quantitation can then be reported for any region of interest annotated in the Allen Brain Atlas. In this case we present the twelve regions containing the largest plaque burden in the oldest mouse tissue (Figure 3F). The combination of Congo red staining, volume imaging and ClearMap analysis should help to streamline the analysis of the plaque burden in mouse models of AD, as a brain can be prepared overnight, scanned in under two hours on the light sheet microscope, and analyzed in less than one hour.
Combined volume imaging of beta amyloid and microglia detection
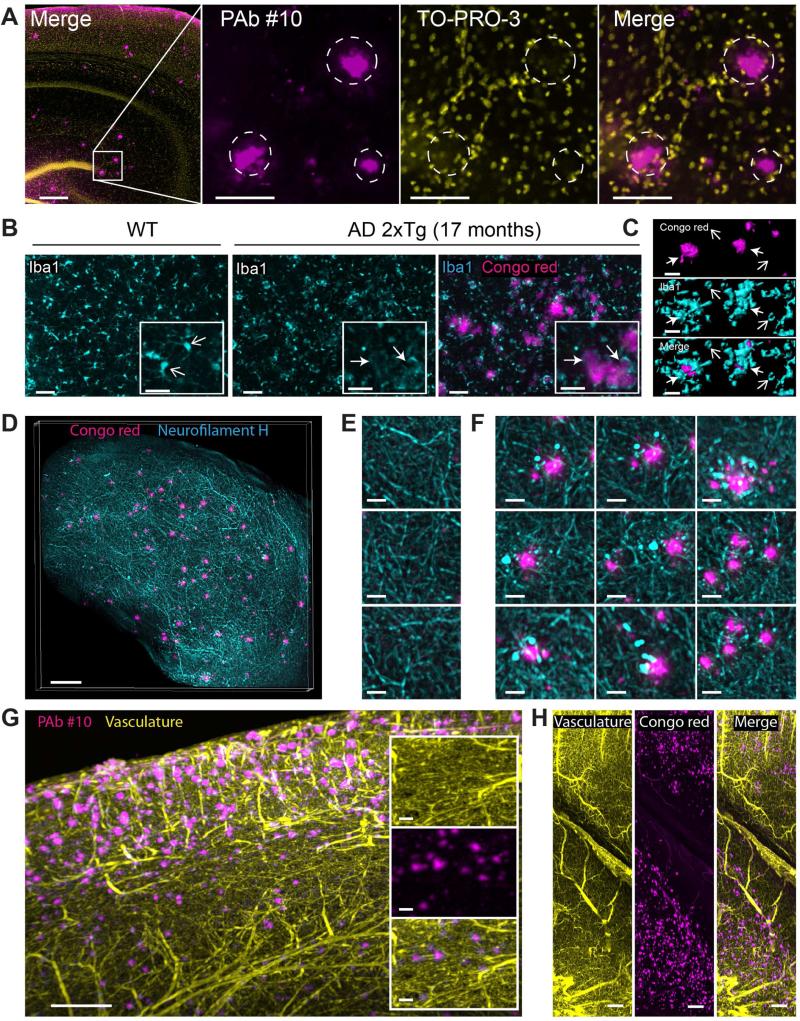
High resolution confocal imaging of combined beta amyloid and To-Pro-3 staining revealed that each plaque is surrounded by a perimeter of nuclei forming a nest-like ring (Figure 4A, circles), suggesting recruitment or displacement of cells, possibly microglia, by the plaques upon amyloid buildup. Though activation of microglia has been associated with AD since the mid 80s (Vostrikov 1985; Rozemuller et al., 1986), the role of reactive gliosis in beneficial plaque clearance or adverse neurotoxicity is yet to be fully clarified. Staining for glia in wild type tissue revealed a homogeneous dispersion of densely labeled cells with discrete arborization (Figure 4B, left image). In comparison, microglia the 2xTg model form clustered patterns of more diffusely labeled cells with enlarged bodies and less discrete branching (Figure 4B, right panel). Upon comparison with the distribution of beta amyloid, it is evident that the clustered microglia are principally associated with amyloid plaques. High resolution volume imaging and surface rendering emphasize that microglia surrounding the plaques enlarge and envelop the plaques, in contrast to the smaller, isolated microglia distant from amyloid plaques (Figure 4C and Movie S3).
Figure 4. Double-labeling of beta amyloid plaques and reactive microgliosis, axonal dystrophy or vasculature in cleared AD brains with volume imaging.
(A) Left: confocal image of beta amyloid plaques and cell nuclei double-staining in a 17-month-old 2xTg AD cleared mouse brain with an anti-beta amyloid antibody (PAb #10) and To-Pro-3, respectively. Magnified images are from the region adjacent to the dentate gyrus blade (white square in left image). White rings highlight nuclei rings surrounding beta amyloid plaques.
(B) Confocal optical slices of microglia stained with anti-Iba1 antibody. Left: WT cleared mouse brain. Open arrows highlight densely labeled microglia with discrete branches (non-activated state). Right panel: images with and without beta amyloid plaque labeling with Congo red. Closed arrows highlight aggregation and hypertrophied microglia cells surrounding beta amyloid plaques. Inserts are magnified regions from the respective images.
(C) 3D surface rendering of 4 isolated beta amyloid plaques and neighboring microglia cells from the AD brain described in B. Open and closed arrows highlight dense microglia away from beta amyloid plaques and clustering reactive microglia surrounding beta amyloid plaques, respectively.
(D) Maximum intensity projection of beta amyloid plaques and neurofilament H staining in a 10-month-old 2xTg AD cleared mouse cortex (500 μm thick).
(E) Magnified region from D showing labeled axons in absence of beta amyloid plaques.
(F) Magnified region from D showing dystrophic axons surrounding labeled beta amyloid plaques.
(G) Maximum intensity projection of optical section (1 mm) from the cortex of an 11-month-old 2xTg AD mouse brain labeled for plaques (anti-beta amyloid antibodies) and vasculature. Inserts are magnified region showing individual and merged channels.
(H) Maximum intensity projection of optical section (1 mm) from the cortex and hippocampus of an 8-month-old 2xTg AD mouse brain labeled for plaques (Congo red) and vasculature. See Figure S5 for additional triple staining. Scale bars represent 30 μm, except first image in A, D and G: 200 μm.
Combined volume imaging of beta amyloid and axonal dystrophy
As mentioned previously, it is crucial to re-consider the way we analyze beta amyloid plaques while emphasizing their surroundings. Among the numerous mechanisms contributing to the dementia associated with Alzheimer's disease is the deterioration of axon projections in proximity of plaques described as dystrophic neurites (For a review see Reitz, et al., 2014). Visual access to axons in intact tissue will aid studies looking to characterize the toxicity of plaque buildup or evaluate strategies for reversing axonal dystrophy (Brendza et al., 2005). Simultaneous imaging of beta amyloid plaques and axon filaments was demonstrated with Congo red and neurofilament H antibodies, respectively (Figure 4D). Owing to the high density of axons and abundance of neurofilament antibody binding sites, labeling is dramatically reduced beyond 1 mm from the tissue surface. Completeness of filament labeling is enhanced by sectioning tissue into blocks. Filaments detected in regions void of beta amyloid plaques were regular in diameter and largely undisrupted (Figure 4E), whereas filaments surrounding prominent plaques were markedly dystrophic (Figure 4F).
Evaluation of amyloid plaques and the complete brain vascular network
Because brain vasculature and associated abnormalities remain an important theme in the deposition and clearance of beta amyloid, we included a strategy for labeling the full network of brain vasculature. Intact adult 2xTg brains were labeled for amyloid plaques and blood vessels, providing complete access to the entire brain vasculature network and total plaque deposition. Combined plaque and vasculature imaging was demonstrated with beta amyloid antibodies and Congo red (Figure 4G and Figure 4H, respectively).
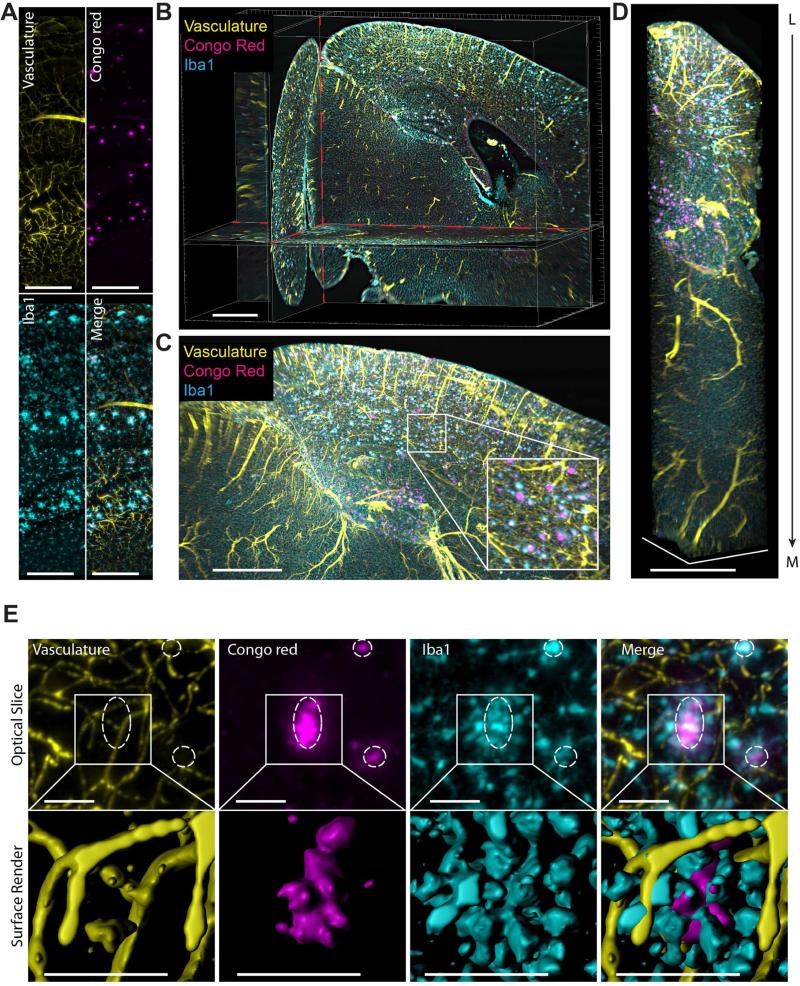
Optimal tissue evaluation would maximize the information extracted from single brain volumes. A few studies have suggested the removal of antibodies for serial labeling of multiple targets (Chung et al., 2013a; Murray et al., 2015), though this approach could potentially lead to incomplete stain removal and difficult image interpretation, especially in intact mouse brains. Previously used as a background image for topographical reference, the green spectrum channel can also be utilized for a third label. Here we present simultaneous labeling of beta amyloid plaques, microglia and vasculature for effective triple-labeling of the entire brain hemisphere. Maximum intensity projections from sagittal optical sections, rendered columns, and animations within the mouse brain hemisphere show efficient labeling of all three stains throughout the tissue (Figure 5A-D and Movie S4). We observed a large occurrence of co-segregation between the three parameters. Samples of magnified optical sections from the hippocampus show clustered reactive microglia and blood vessels coincident with amyloid plaques (Figure 5A, 5E upper panel). Surface rendering of a magnified optical volume captures the near complete encompassing of plaques by the recruited microglia and the immediate proximity of nearby vessels (Figure 5E lower panel and Movie S4). The flexibility of iDISCO for evaluating AD mouse models allows for comparison of a large list of potential targets. As a demonstration of an alternate combination for full brain analysis, we stained an intact 2xTg AD mouse hemisphere for vasculature, cell nuclei and beta amyloid (antibody PAb #10). From a single block of imaged tissue, we can evaluate amyloid plaque correlation with the intricate vascular network and regional cell density (Figure S5A-B). Importantly, this can be achieved from the scale of the whole brain down to the resolution of a single cell (Figure S5A, S5C).
Figure 5. Triple labeling imaged with light-sheet microscopy enables simultaneous whole brain investigation of beta amyloid plaques with relevant molecular or macroscale markers.
(A) Optical slice acquired on a light-sheet microscope from an 11-month-old 2xTg AD mouse brain labeled for vasculature, beta amyloid plaques and microglia.
(B) Orthogonal optical planes from the cleared mouse brain hemisphere shown in A.
(C) Maximum intensity projection of magnified optical section (1 mm thick) from the AD brain in A. Insert is magnification of indicated region (white rectangle).
(D) Maximum intensity projection of column (0.25 mm2 cross-section) through cortex and hippocampus of triple labeled hemisphere shown in A. Column is oriented from lateral (L) to medial (M).
(E) Upper row: optical section (150 μm thick) of each label from the brain in A. Circles highlight regions where plaques, reactive microglia aggregates and vessels converge. Lower row: magnified surface render of each label in upper row.
Scale bars represent 300 μm, except E: 50 μm.
Variation of three-dimensional amyloid patterns (TAPs) in archived brain tissues from human AD patients
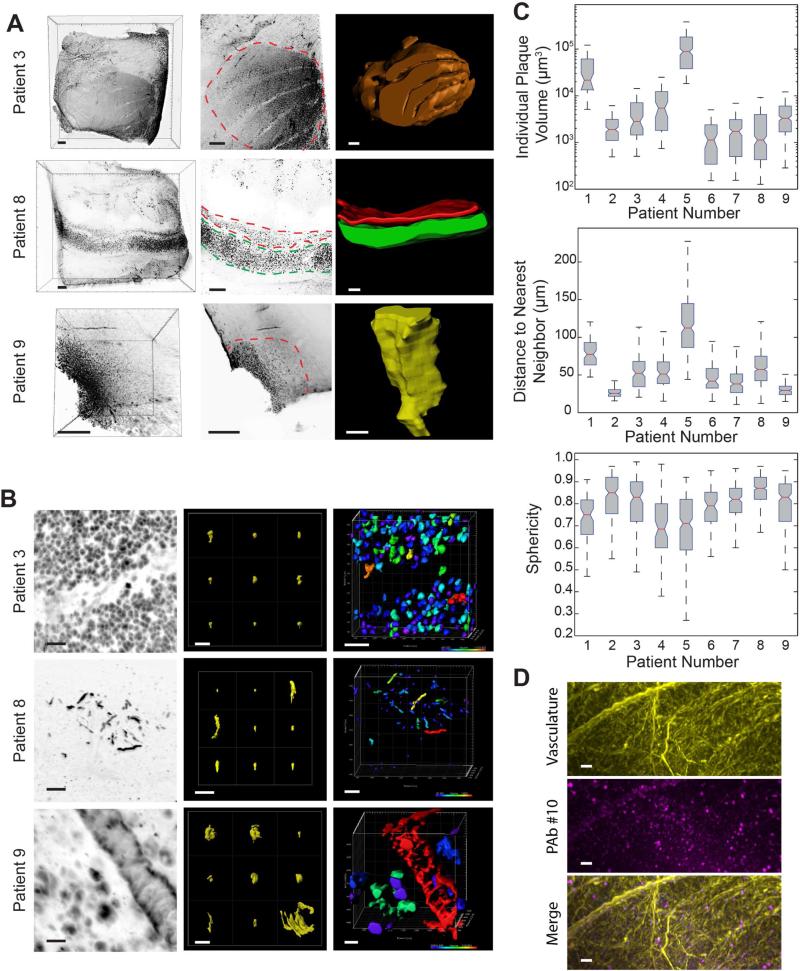
Clearing methods have been previously applied to mouse embryos and various adult mouse tissues including brains. Gaining insight into amyloidosis in AD pathology also necessitates the evaluation of human tissues. With a collection of frozen post-mortem tissue blocks from nine patients, we applied iDISCO for 3D fluorescence analysis of amyloid plaques in large volumes of human brain tissue. All archived AD human samples at our disposal were of hippocampal origin. It is critical to note that these were fresh-frozen samples, none of which had been handled or prepared in any specific way prior to iDISCO application. Most samples were effectively labeled, cleared and imaged with the iDISCO method. Notably, interesting features of amyloid staining with PAb #10 were revealed; perhaps most striking was the presence of complex tertiary structures forming three-dimensional amyloid patterns (TAPs) of up to 27 mm3 (>Figure 6A). Volume imaging also revealed a remarkable 3D diversity of individual amyloid plaque morphology and 3D formations ranging from dense, strongly labeled patches to less intensely labeled and more isolated plaques. 3D images reveal TAPs that appear as dense volumes, thin sheets, parallel ribbons, and density gradients (Figure 6A, see patient 3, 8 and 9, respectively). High magnification volume projection images again reveal pronounced differences in beta amyloid plaque morphology and size. As with the AD mouse model, staining appeared as large and small spheres, thin filament-like structures and vasculature deposits (Figure 6B). Surface rendering highlights the dramatic differences in density, shape and size between samples (Figure 6B and Movie S5). In addition to visualization of beta amyloid plaques in large volumes of human tissue, volume imaging enables direct measurement of amyloidosis parameters such as individual plaque volume, inter-plaque distance and morphological characteristics like sphericity (Figure 6C). Inclusion of additional markers can increase the informative value of amyloid volume imaging. As with the mouse tissue, simultaneous imaging of multiple stains in human tissue can provide anatomical reference or enable correlative evaluation. As proof of principle, we present successful double staining of a human tissue block with PAb #10 and vasculature (Figure 6D). Volume imaging of amyloid plaques in human brain tissue can feasibly be combined with any of the previously demonstrated markers or other iDISCO-compatible stains of interest.
Figure 6. Volume analysis of beta amyloid plaque characteristics in human archival tissues, beta amyloid plaques sampling and examples of three-dimensional amyloid patterns (TAPs).
(A) First column: overview maximum intensity projections from postmortem AD human tissue samples labeled with PAb #10. Second column: magnified maximum intensity projections from within the respective sample. Dashed lines highlight TAP-forming beta amyloid plaque staining. Third column: surface render of TAPs from the respective highlighted regions.
(B) First column: magnified maximum projection of human AD samples shown in A. Second column: table of random individual beta amyloid plaque surface renders from the corresponding sample. Third column: Volume image with surface rendering of the corresponding sample shown with surface color coded according to individual amyloid deposit volume (heat map from blue to red scales from smallest to largest volume).
(C) Quantification of beta amyloid deposits in human AD tissue. Data are presented in boxplots with the box representing the interquartile range (IQR), whiskers showing the spread beyond the IQR, and a red line for the median value. Top to bottom: volume of individual amyloid plaques (note logarithmic axis), distance to nearest neighbor and sphericity of amyloid plaques, respectively.
(D) Light-sheet images from block of human tissue labeled for vasculature and beta amyloid plaques with anti-human secondary antibodies (yellow) and PAb #10, respectively.
Scale bars represent 500 μm in A and 50 μm for B and D.
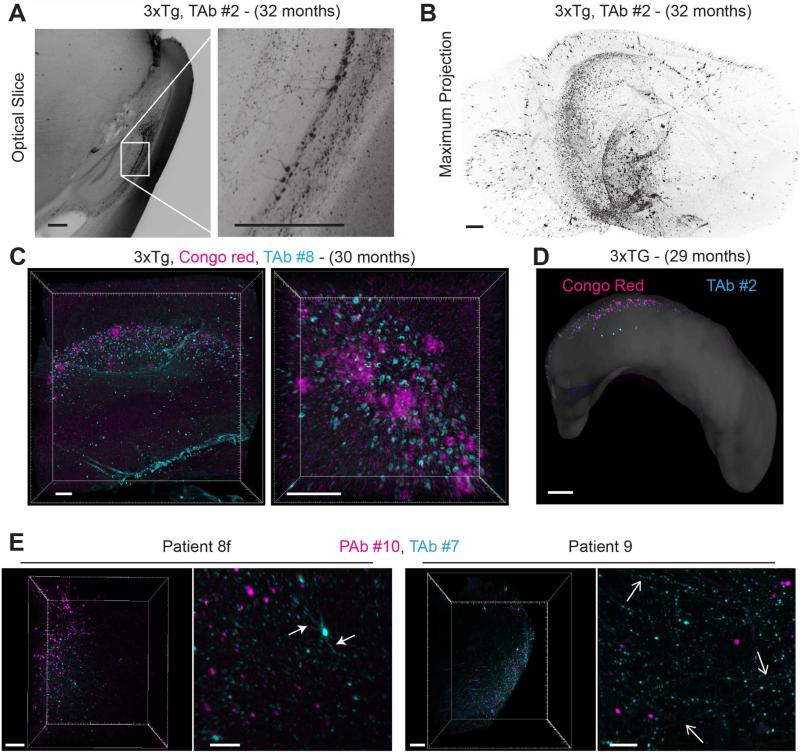
The origin of AD is still largely debated and scientists remain divided between two main hypotheses (Aβ oligomers and amyloid plaques versus Tau-dependent neurofibrillary tangles), while other important parameters such as inflammation and hormonal regulation among others are also important. Accordingly, we also applied iDISCO for volume evaluation of tau in AD tissue, starting with validation of anti-tau antibody compatibility in 3xTg AD mice (see Table S1). Optical slices from cleared brain hemispheres (29 months old mice) obtained after labeling with anti-tau antibodies show prominent staining in pyramidal neurons in CA1 of the hippocampus as well as positive staining in the neighboring subiculum and the stratum oriens (Figure 7A and Movie S6). Full hemisphere imaging shows the expected distribution throughout the hippocampus and around the entorhinal cortex (Figure 7B). Double labeling of 3xTg mouse brains (29 months old) with Congo red and Tau antibodies shows a highly restrictive parallel distribution of beta amyloid plaques and tau positive cells in the subiculum (Figure 7C, D and Movie S6). Volume images reveal that the pattern of plaque distribution is dramatically influenced by the selected animal model, as seen when comparing 1xTg, 2xTg and 3xTg AD mouse models (Figure S6). Interestingly, co-labeling for the two AD hallmarks in human AD brain samples reveals a degree of regional co-localization. Tau staining is clearly prominent in dendritic branching of individual cells as well as thin axons projecting through the cleared tissue blocks, possibly highlighting the presence of neuropil threads. (Figure 7E).
Figure 7. Ultramicroscopic evaluation of tau accumulation in 3xTg AD cleared mouse brains and double-labeling with beta amyloid plaques, and comparison to human cleared brain samples.
(A) Left image: maximum intensity projection of 100 μm optical section stained with an anti-tau antibody, TAb #2 (see Table S1). Image is a sagittal view containing the hippocampus and cortex from a cleared, 32-month-old 3xTg AD mouse brain. Right image: magnification of indicated region (white rectangle).
(B) Maximum intensity projection of full hemisphere shown in A.
(C) Confocal maximum projection of hippocampus from a 30-month-old 3xTg AD cleared mouse brain labeled with Congo red and TAb #8.
(D) Digitally dissected full hippocampus from a 29-month-old 3xTg AD cleared mouse brain stained for beta amyloid plaques and tau with Congo red and TAb #2, respectively.
(E) Human tissue, cleared and labeled for beta amyloid plaques and neurofibrillary tangles. Left image of each pair: maximum intensity projection of cleared human tissue from the hippocampus, stained with PAb #10 (magenta) and TAb #7 (cyan). Right image of each pair: magnifications of corresponding images. Closed and open arrows highlight dendritic and axonal staining, respectively.
Scale bars represent 500 μm, except A and C: 100 μm.
DISCUSSION
Three-dimensional perspective on neurodegeneration
We have combined the iDISCO clearing method, light-sheet microscopy, and biochemical and chemical tools relevant to AD (e.g. beta amyloid antibodies, tau antibodies, Congo red, and axon, vasculature and microglia detection) to examine the diseased brains of several AD mouse models and patients in three-dimensions. This revealed clear differences between mouse brains from AD models and material from patient brains. Our volume image analysis unveiled strikingly large patterns of amyloid staining in human brain samples, which we refer to as Three-dimensional Amyloid Patterns or TAPs. We did not observe comparable large amyloid patterns in any of the mouse AD models. This inconsistency highlights a possible difference in the aggregation process between human and mouse species. However, it is not clear if the time required for the TAPs to form is compatible with studies carried out on mice due to their short life expectancy.
AD mouse models present clear differences compared to human samples
AD mouse models are in constant need of refinement to address the biochemical, temporal and phenotypic discrepancies that exist between those mouse models and AD pathogenesis (for a review see Ashe et al., 2010). Beta amyloid plaque distribution can also dramatically differ between the animal models (Fig S6). The present work reinforces the notion that better mouse models are needed, and it offers the possibility of efficiently and globally evaluating the anatomical and temporal distributions of AD hallmarks in promising mouse models (Liu et al., 2015) and future genetic models aiming to better recapitulate the pathology presented in humans.
Amyloid plaques, microglia and vasculature
The immune surveillance provided by microglia is an important topic for AD pathology (for a review see Aguzzi et al., 2013). Here we present detailed 3D renderings and videos that unequivocally demonstrate strong association between microglia and beta amyloid plaques. It is remarkable to observe the targeted activation of microglia in contact with plaques. The precise role(s) of microglia segregating around plaques remains unclear, but their visualization in 3D opens avenues to better understand where, and perhaps, how they are recruited.
Cerebral amyloid angiopathy (CAA) is an integral component of a series of important disorders strongly associated with dementia as well as cerebral hemorrhage. It is known that, in the case of AD, some arteries are more targeted by deposition of beta amyloid plaques than others (Viswanathan et al., 2011; Yarchoan et al., 2012). We demonstrated that we could visualize such associations and that this is facilitated by 3D volume rendering. As such, the present study provides a relevant approach to examine the relationship between amyloidosis and CAA. Additionally, further 3D characterization will help clarify why the amyloid plaques congregate around blood vessels. Do they have a specific affinity for blood vessels or, on the contrary, do the plaques appear as a consequence of other phenomena such as local inflammation, micro-hemorrhage, etc?
Tau and neurofibrillary tangles
In addition to the effort dedicated to the Abeta peptide amyloidosis in this study, we also examined Tau staining in 3D. While we do not aim here to resolve the long standing Abeta-tau debate, tau and neurofibrillary tangles are important in disease progression, if not generation, and therefore warrant examination. It is obvious that the multiple phosphorylation states of Tau considerably increase the complexity of the problem, especially regarding the antibody testing for our current approach (Jicha et al., 1997; Espinoza et al., 2008). We have, however, demonstrated that iDISCO is an effective method for labeling tau with a number of total and phospho-specific tau antibodies, allowing for more detailed evaluation of the volumetric formation of neurofibrillary tangles in the context of AD mouse models and human tissues, a necessary contribution toward complete understanding of the disease.
Future studies
Future studies analyzing large numbers of samples anatomically identical and from various origins will be necessary to fully characterize the extent of these TAPs in human samples. Such large-scale evaluation might allow correlation studies, such as indexing TAPs and ultimately matching them with specific symptoms or clinical traits documented prior to death. While diagnosis of individual patient TAPs might remain limited mostly to post-mortem samples and brain biopsies, we do foresee that comprehensive retrospective analyses may even help categorize patients based on specific three-dimensional pathological features, will contribute to the understanding of some clinical trial failures by retrospective identification of responsive or non-responsive groups, and, ideally, might aid the categorization of patients for future clinical trials. It will be also informative to compare familial versus sporadic AD TAPs, and determine if specific familial AD mutations correspond to unique TAPs. Furthermore, a similar approach using iDISCO clearing should be easily transposable to any form of amyloidosis or related diseases, including non-AD dementias such as vascular dementia (O'Brien et al., 2015), frontotemporal dementia (Bang et al., 2015), dementia with Lewy bodies (Goldberg et al., 2015) and transmissible dementia (Watts et al., 2014; Sanders et al., 2016). This work also may have the potential to advance the evaluation strategies for therapeutic approaches in pre-clinical stages such as immunotherapies (Wisniewski et al., 2015).
In conclusion, we believe that the discovery of large TAPs and the surprising complexity of amyloid plaques in human (but not mouse) brain samples may help revisit disease classification and lead to a better understanding of the initial causes of AD. In more general terms, the methodology developed here to detect beta amyloid plaques in their three-dimensional context, both in murine and human brains, and allowing for the study of up to three markers at a time, including tau protein, is a valuable approach to tackling various characteristics of Abeta amyloidogenesis, and amyloidogenesis in general.
EXPERIMENTAL PROCEDURES
Mouse line genotypes and related work
All procedures were approved by the Rockefeller University Institutional Animal Care and Use Committee. All mice were housed with a 12-hour light/12-hour dark cycle and fed ad libitum. Transgenic mouse lines (2xTg: APPswe/PSEN1dE9; 1xTg: Tg2576 harboring HuAPP695.KM670/671NL; 3xTg: PS1M146V, APPswe and tauP301L], and C57BL/6J WT mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were anesthetized via Nembutal IP injection (50-100 µl) and intracardiac perfusion was performed first with 20 ml ice cold PBS then 20 ml ice cold PBS with 4% paraformaldehyde. Dissected cerebral tissue was post-fixed overnight in PBS with 4% PFA at 4°C followed by 1 hour at room temperature.
Human tissue origin and related work
Frozen human hippocampal brains blocks were obtained from the New York Brain Bank (Columbia University, Alzheimer Disease Research Center, Taub institute) in agreement with our Institutional Review Board. The brain tissues were collected from autopsied patients with AD clinical diagnosis as well as post-mortem histo-pathological signature for AD. Frozen tissue kept at −80°C was cut into blocks approximately 0.125 cm3 and defrosted on ice for 20 minutes before fixation in PBS with 4% PFA overnight at 4°C and 1 hour at room temperature.
Tissue permeabilization
Tissue was pretreated following the methanol protocol provided in Renier et al. (2014). Briefly, whole brains were dehydrated with gradual addition of methanol in PBS (50% x1, 80% x1, 100% x2, each for 1 hr). Overnight bleaching with a 1:5 ratio of hydrogen peroxide:methanol was done at 4 °C. Tissue was the n gradually rehydrated in PBS by removing methanol at 20% increments (1 hr for each step, followed by 2 additional washes in PBS). Detergent washing was then performed in PBS with 0.2% Triton X-100 (2× 1 hr).
Immunolabeling
To block any residual active aldehyde groups, tissue was incubated overnight at 37°C in PBS with 0.2% Triton X-100 and 0.3 M glycine, followed by blocking in PBS with 0.2% Triton X-100 and 6% normal goat serum. Blocking and staining incubation times were 7 days for whole hemispheres and 2 days/mm (maximum of 7 days) for sectioned blocks. For 7 day incubation steps, 0.02% sodium azide was included to prevent contaminants. Following blocking, the tissue was washed for 1 hr twice in PBS with 0.2% Tween-20 and 10 μg/ml heparin (PTwH). Antibodies were applied for the indicated time in PTwH with 3% NGS at 37°C with rocking. Excess primary antibodies were washed for 1 day in PTwH with periodic solution changes before starting incubation of secondary antibodies. After secondary antibody labeling, tissue was washed in PTwH for 2 days with periodic solution changes.
For vasculature staining, tissue was fixed in 4% PFA without perfusion to preserve the endogenous immunoglobulins. Dye-conjugated secondary antibodies (1:100 dilution) against the tissue species were then used without need of primary antibodies. There were no detectable differences in amyloid plaque and glial staining in the non-perfused tissue.
Primary antibodies against amyloid plaques and neurofibrillary tangles were selected by evaluation on 1-2 mm tissue sections after methanol pretreatment described in the iDISCO protocol (Renier et al., 2014) and are listed in Table S1. Microglia and neuronal axons were labeled with rabbit Iba1 antibodies from Wako Chemicals and chicken neurofilament antibodies from EMD Millipore. Secondary antibodies were selected based on the number of labels in a given sample. Alexa 647, if available as a conjugate against the respective primary antibody, was prioritized for single labels, followed by Alexa 568 as a second label, and Alexa 488 as a third label as demonstrated with vasculature staining. Autofluorescence was imaged using 488 nm excitation, eliminating the option for simultaneous Alexa 488 staining. Secondary antibody concentrations were matched to that of the respective primary antibody, with a maximum limit of 20 μg/ml.
For samples additionally stained with chemical dyes, the respective dye was included in the secondary antibody incubation step. For samples stained solely with a chemical dye, the respective dye was incubated at 37°C in PT wH for 2 days before 2 days of washing and clearing. On samples labeled with dye for volume imaging, Congo red and To-PRO-3 were used at 10 μM and 2 μM, respectively.
Standard immunofluorescence labeling was done on 45 μm cryosections. Sections were washed in PBS for 20 min and PBS with 0.2% Triton X-100 for 20 min then blocked in PBS with 6% normal goat serum and 0.2% Triton X-100 for 1 hr, all at RT with rocking. Beta amyloid was stained overnight at 4°C in PBS with 3% normal goat serum and 0.2% Triton X-100 (anti-beta amyloid, 1:250; Congo red, 3.3 μM). Slices were washed 3 x 10 min in PBS prior to secondary labeling (goat anti-rabbit Alexa 647, 1:250) for 2 hr at RT and washed in PBS 3 × 10 min before imaging.
Immunohistochemistry
Mouse brains were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA), washed with PBS, and incubated in 25% sucrose for 48 hours. Brains were then frozen on dry ice with freezing medium (VWR, Radnor, PA) and sliced in 50 μm sections. Sections were stained using PAb #10 and a DAB peroxidase substrate kit (Vector Laboratories). Sections were imaged using a wide field microscope at 2.5x with a QI Click color digital camera. Images were assembled using the Fiji plugin for ImageJ (Preibisch et al., 2009).
Tissue clearing
After washing away excess secondary antibodies, optical clearing of iDISCO samples was performed as described in Renier et al. 2014. Tissue was gradually dehydrated in resistant glassware with tetrahydrofuran in water (50% x1, 80% x1, 100% x2, 1 hour each). Remaining lipids were extracted with dichloromethane for 1 hour, and dibenzyl ether (DBE) was used for refractive index matching. Samples were kept in a full vial of DBE. Samples stained with Congo red for automated ClearMap analysis were treated with a modified protocol to allow for anatomical alignment described in Renier at al. 2016. The most significant change is removal of tetrahydrofuran to avoid shrinkage of some tissue layers that prevents effective atlas matching.
Imaging and image processing
Images of large samples were principally acquired on a light-sheet ultramicroscope (Ultramicroscope II, LaVision Biotec). High resolution images were takes on a confocal (Zeiss 710 LSM) or multi-photon (FV1000MPE Fluoview, Olympus). The light-sheet and multi-photon microscopes were provided by the Bio-Imaging Recourse Center of The Rockefeller University. All cleared tissue images were acquired with tissue submerged in DBE. Volume images were acquired with 16-bit depth to allow for a broad range of intensity values and rendered using Imaris software from Bitplane. The ultramicroscope was used with a fixed 2x/0.5 N.A. MVPLAPO objective. Exposure time was 100 ms for each frame, spaced axially with a 2.5 μm interval. Laser intensities were set to prevent saturated pixels (typically 50 to 70% of maximum power). The following laser excitations were used: autofluorescence and Alexa 488, 488 nm; Alexa 568, 561 nm; Alexa 647, 640 nm. Images were taken with an Andor NeosCMOS (2160 × 2560 pixels).
For visualization of volume images, gamma corrections and background subtractions were applied to reveal the range of staining intensities and remove global background signal, respectively, for each data set. Automated amyloid plaque detection was done with the Imaris ‘spots’ feature, using an estimated diameter of 30 μm and minimum threshold values for maximum peak intensity (3,000) and standard deviation (500) that most accurately detected visible plaques in random test regions without including background signal. Digital dissection of volumes was done with Imaris by manual tracing of autofluorescence channels at appropriately spaced planes, typically 250 μm and 50 μm for large and small surfaces, respectively. Surface rendering for small volumes of amyloid plaques, glia and vessels was automated on Imaris, with manual threshold adjustments for each channel to remove background.
Automated quantitation with ClearMap
ClearMap, a light-sheet image analysis toolbox, is available upon request by authors (Renier et al., 2016). The free package operates on a Linux operating system with python programming software. Light-sheet images were collected from autofluorescence (488 nm at 100%, 50 ms exposure) and Congo red (561 nm at 100%, 50 ms exposure, this time with a 20-point horizontal focus and contrast blending). Autofluorescence images were applied for automated alignment to the Allen Brain Atlas, and Congo red images were used for object detection. ClearMap detection parameters for the plaques were: background subtraction (7 pixels), difference of Gaussian filter (4,4,6), extended maxima h-max (20) and size (5 pixels), cell shape threshold (125).
Supplementary Material
Highlights.
- iDISCO clearing is used to detect amyloid plaques in a full mouse brain hemisphere
- Three-dimensional amyloid patterns (TAPs) are detected in human brain archival samples
- Triple-labeling of cleared tissues allows highly contextual analysis of amyloidogenesis
- Automated anatomical mapping empowers accurate and fast quantitation of plaques
ACKNOWLEDGMENTS
We thank Dante Varotsis and Lisa Randolph for technical support, and other members of the laboratory of Molecular and Cellular Neuroscience for support. We thank the veterinarian team and in particular Craig Hunter and Alejandra Gonzalez. We thank the members of the Rockefeller University Bio-Imaging Resource Center, and in particular Pablo Ariel, Tao Tong and Alison North. This work was supported, in part, by grants from the U.S. National Institutes of Health (NIA: AG09464; P.G.), and the Fisher Center for Alzheimer's Research Foundation (M.F., P.G.), and the Cure Alzheimer's Fund (M.T-L., P.G.). Support by the Empire State Stem Cell Fund through NYSDOH Contract #C023046. Opinions expressed here are solely those of the author and do not necessarily reflect those of the Empire State Stem Cell Fund, the NYSDOH, or the State of NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
T.L., N.R., M.T.-L. and M.F. designed the study. T.L performed all the experiments except IHC. NR helped with protocol optimization and technical support. K.B. organized mouse work and performed IHC staining and quantification. T.L. generated movies. T.L, and M.F. wrote the paper. N.R., K.B., P.G. and M.T.-L. help revising the paper.
REFERENCES
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Laborde Q, Lazar A, Godefroy D, Youssef I, Amar M, Pooler A, Potier MC, Delatour B, Duyckaerts C. Inside Alzheimer brain with CLARITY: senile plaques, neurofibrillary tangles and axons in 3-D. Acta Neuropathol. 2014;128(3):457–9. doi: 10.1007/s00401-014-1322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66(5):631–45. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang J, Spina S, Miller B. Frontotemporal dementia. Lancet. 2015;386(10004):1672–82. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Rode r H., Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27(14):3650–62. doi: 10.1523/JNEUROSCI.0587-07.2007. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE, Mathis CA, Bales KR, Paul SM, Hyman BT, Holtzman DM. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest. 2005;115(2):428–33. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis.Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim S-Y, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013a;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Leischner U, Schierloh A, Jährling N, Mauch CP, Deininger K, Deussing JM, Eder M, Zieglgänsberger W, Becker K. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods. 2007;4(4):331–6. doi: 10.1038/nmeth1036. [DOI] [PubMed] [Google Scholar]
- Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 2012a;7:1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- Espinoza M, de Silva R, Dickson DW, Davies P. Differential incorporation of tau isoforms in Alzheimer's disease. Alzheimers Dis. 2008;14:1–16. doi: 10.3233/jad-2008-14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg NR, Caesar J, Park A, Sedgh S, Finogenov G, Masliah E, Davis J, Blurton-Jones M. Neural Stem Cells Rescue Cognitive and Motor Dysfunction in a Transgenic Model of Dementia with Lewy Bodies through a BDNF-Dependent Mechanism. Stem Cell Reports. 2015;5(5):791–804. doi: 10.1016/j.stemcr.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, Fukami K, Sakaue-Sawano A, Miyawaki A. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- Hama H, Hioki H, Namiki K, Hoshida T, Kurokawa H, Ishidate F, Kaneko T, Akagi T, Saito T, Saido T, Miyawaki A. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci. 2015;18(10):1518–29. doi: 10.1038/nn.4107. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305(5686):1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- Jährling N, Becker K, Wegenast-Braun BM, Grathwohl SA, Jucker M, Dodt HU. Cerebral β-Amyloidosis in Mice Investigated by Ultramicroscopy. PLoS One. 2014;10(5) doi: 10.1371/journal.pone.0125418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha GA, Lane E, Vincent I, Otvos L, Jr, Hoffmann R, Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer's disease. J Neurochem. 1997;69:2087–95. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- Ke M-T, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 2013;16:1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]
- Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development. 2013;140:1364–1368. doi: 10.1242/dev.091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Liu P, Paulson JB, Forster CL, Shapiro SL, Ashe KH, Zahs KR. Characterization of a Novel Mouse Model of Alzheimer's Disease--Amyloid Pathology and Unique β-Amyloid Oligomer Profile. PLoS One. 2015;10(5):e0126317. doi: 10.1371/journal.pone.0126317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim SY, Choi H, Park YG, Park JY, Hubbert A, McCue M, Vassallo S, Bakh N, Frosch MP, Wedeen VJ, Seung HS, Chung K. Simple, Scalable Proteomic Imaging for High-Dimensional Profiling of Intact Systems. Cell. 2015;163(6):1500–14. doi: 10.1016/j.cell.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Billings JC, Grooms JK, Shakil S, Keilholz SD. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front Neurosci. 2015;9:269. doi: 10.3389/fnins.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A, Langui D, Ulrich J. Alzheimer's disease: a description of the structural lesions. Brain Pathol. 1991;1(4):229–39. doi: 10.1111/j.1750-3639.1991.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640–51. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159(4):896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, Wang VX, Tang CY, Olsen O, Dulac C, Osten P, Tessier-Lavigne M. Mapping of brain activity by automated volume analysis of immediate early genes. Cell. 20162016 doi: 10.1016/j.cell.2016.05.007. pii: S0092-8674(16)30555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozemuller JM, Eikelenboom P, Stam FC. Role of microglia in plaque formation in senile dementia of the Alzheimer type. An immunohistochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):247–54. doi: 10.1007/BF02899034. [DOI] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, Holmes BB, Diamond MI. Prions and Protein Assemblies that Convey Biological Information in Health and Disease. Neuron. 2016;89(3):433–48. doi: 10.1016/j.neuron.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157(3):726–39. doi: 10.1016/j.cell.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Tomer R, Ye L, Hsueh B, Deisseroth K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 2014;9:1682–1697. doi: 10.1038/nprot.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70(6):871–80. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vostrikov VM. Electron-cytochemical study of microglia in Alzheimer's disease and senile dementia]. Zh Nevropatol Psikhiatr Im S S Korsakova. 1985;85(7):974–6. [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Watts JC, Prusiner SB. Mouse models for studying the formation and propagation of prions. J Biol Chem. 2014;289(29):19841–9. doi: 10.1074/jbc.R114.550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goñi F. Immunotherapeutic approaches for Alzheimer's disease. Neuron. 2015;85(6):1162–76. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen C-K, Lubeck E, Shah S, Cai L, Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 2014;158:945–958. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–56. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.