A case report presents prenatal and postnatal imaging findings along with an early clinical course of an infant diagnosed with congenital Zika infection. The patient's phenotypical features fall within the fetal brain disruption sequence.
Keywords: Zika virus, microcephaly, MRI, fetal brain disruption sequence, ultrasound
Abstract
Recent Zika virus (ZIKV) outbreaks have been associated with an increased incidence of neonatal microcephaly. Subsequently, tropism for the brain was established in human fetal brain tissue. We present the first congenital ZIKV infection in the United States, confirmed by high ZIKV immunoglobulin M antibody titers in serum and cerebrospinal fluid. The phenotypic characteristics of the patient fall within fetal brain disruption sequence, suggesting impaired brain development in the second half of gestation. Brain imaging revealed an almost agyric brain with diffuse parenchymal calcifications, hydrocephalus ex vacuo, and cerebellar hypoplasia. Ophthalmologic examination revealed macular pigment stippling and optic nerve atrophy. Liver, lungs, heart, and bone marrow were not affected. The patient had progressive neurologic deterioration in the first month of life. The discovery of ZIKV infection in human fetal brain tissue along with serologic confirmation proves the vertical transmission of ZIKV. Therefore, ZIKV has joined the group of congenital infections.
Zika virus (ZIKV) gained more attention when a 2015 outbreak in Brazil was followed by a sharp increase in the number of cases of severe microcephaly [1, 2]. First isolated by Dick et al in 1947 from the serum of a rhesus monkey in Uganda's Zika Forest, this flavivirus was found to be highly neurotropic in infected mice [3]. Since then, the virus has been observed sporadically in several African, Asian, and Micronesian nations, but until recently was only noted to cause a mild, self-limited disease in humans [4]. The first well-documented ZIKV outbreak occurred on the Micronesian island of Yap in 2007 [5], after which it spread through the central and south Pacific Islands [6]. The islands of French Polynesia had a sharply defined outbreak of ZIKV infection in 2013–2014. Retrospective analysis of this outbreak revealed an increased incidence of neonatal microcephaly that aligned temporally with first- and second-trimester infections [7]. The French Polynesian ZIKV strain apparently spread to Brazil during the 2013–2014 period, as this strain is most closely related to the Brazilian strain by phylogenetic analysis [8]. The newborn we present here was conceived during the ZIKV outbreak in Brazil and is the first confirmed case of congenital ZIKV infection in the United States.
CASE REPORT
The mother is a healthy 32-year-old white woman, gravida one, para one, who was living in Pernambuco State, Brazil at the time of conception. Her last menstrual period was 21 March 2015. Pregnancy was confirmed at 4 weeks’ gestation. In early May 2015, at 7 weeks’ gestation, the mother developed a diffuse maculopapular rash, bilateral conjunctivitis, arthralgias in her wrists and elbows, and generalized malaise. No fevers were documented. She was examined by her general practitioner, who was aware that the mother was pregnant, and she was diagnosed clinically with ZIKV infection, but no serologic or virologic workup was done. Symptoms resolved completely after 7 days. The timing of the illness coincided with the ZIKV outbreak in Pernambuco state in May 2015. The parents subsequently moved to Hawai'i at 12 weeks’ gestation.
At 20 weeks’ gestation, fetal ultrasound revealed an asymmetric left lateral ventricular enlargement (left ventricle 11.4 mm, right ventricle 6.8 mm). Cerebral wall thickness was 5.6 mm on the same sections. Periventricular and parenchymal echogenic foci suggestive of calcifications were noted but did not demonstrate posterior acoustic shadowing. The extra-axial spaces were within normal limits (Figure 1). Fetal head circumference and biparietal diameter measurements were 16.5 cm (3.9th percentile, expected 19 weeks 1 day) and 4.3 cm (7.3rd percentile, expected 19 weeks 1 day), respectively. Follow-up fetal ultrasound at 22 weeks’ gestation demonstrated persistence of ventricular anomalies. The significance of these abnormalities was not appreciated in August 2015 in the context of a congenital ZIKV infection, as reports of ZIKV microcephaly in Brazil had not yet been made. The mother was referred for further workup of asymmetric ventriculomegaly, but due to financial constraints and lack of health insurance at that time, no additional imaging was done. Fetal movements were noted on monthly prenatal visits, and the rest of the pregnancy was unremarkable.
Figure 1.
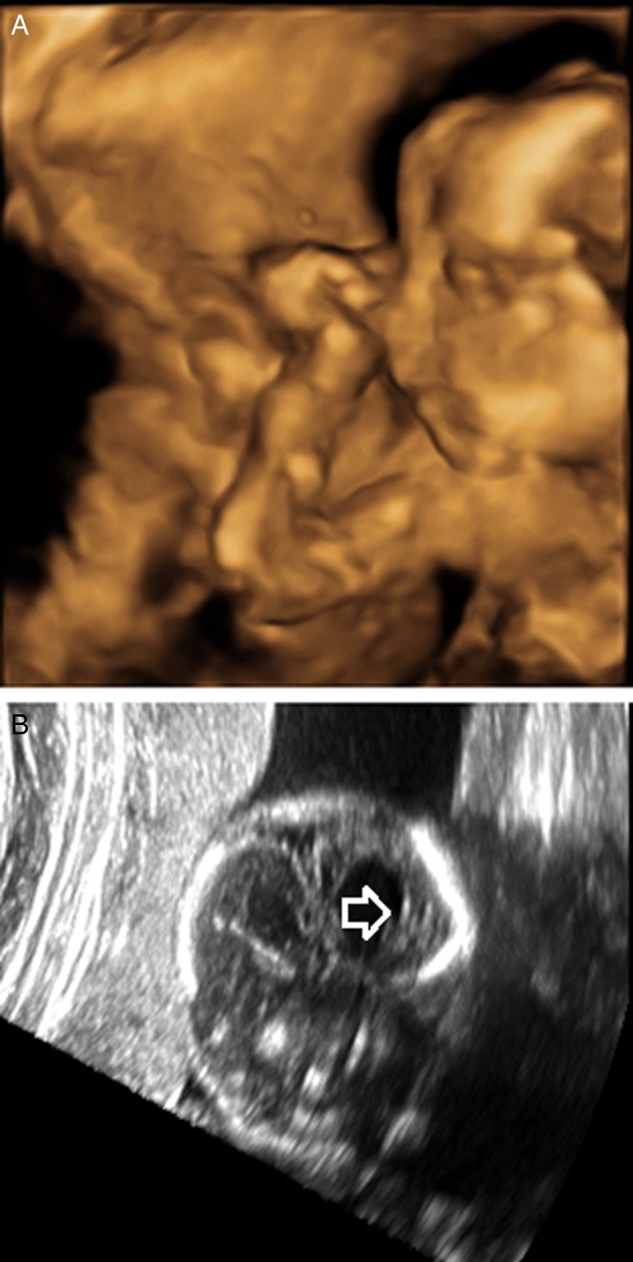
Fetal ultrasounds at 20 weeks’ gestation. A, 3D reconstruction. Note the borderline microcephalic appearance, with normal-appearing facies and normal-appearing forehead. B, Note the asymmetric lateral ventriculomegaly, scattered parenchymal echogenic foci with no acoustic shadowing, and normal extra-axial spaces.
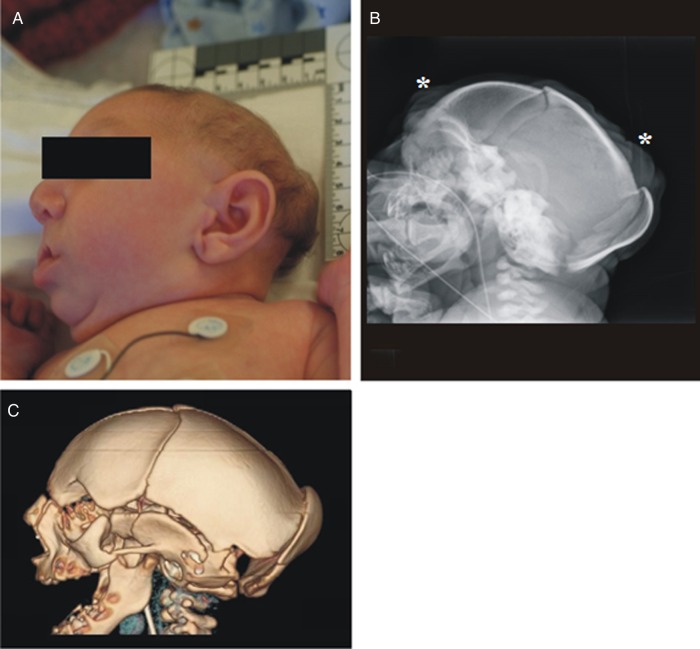
The male newborn was delivered at 39 weeks’ gestation via normal spontaneous vaginal delivery. Apgar scores were 7 at 1 minute and 8 at 5 minutes. His birth length was 45.7 cm (<5th percentile, z = −2.2), weight was 2063 g (<5th percentile, z = −3.2), and head circumference was 27.3 cm (<5th percentile, z = −5.6). On initial exam, the infant was noted to be severely microcephalic, with overriding sutures, occipital plate prominence, and scalp skin rugae (Figure 2). He had a normal scalp hair pattern, and no facial dysmorphic features except for a sloping forehead. Examination of his body was normal except for coronal hypospadias and bilateral cryptorchidism. The rest of the body was proportionally developed (Figure 3). No hepatosplenomegaly or rashes were noted.
Figure 2.
Photograph of the side head (A) shows severe microcephaly with occipital keel-like prominence, and normally developed face. Craniogram (B) and 3D computed tomography volumetric reformat of the calvarium (C) also show collapse of the calvarium with extensive overlap of the calvarial bones. Note the skin rugae (*) on the craniogram.
Figure 3.
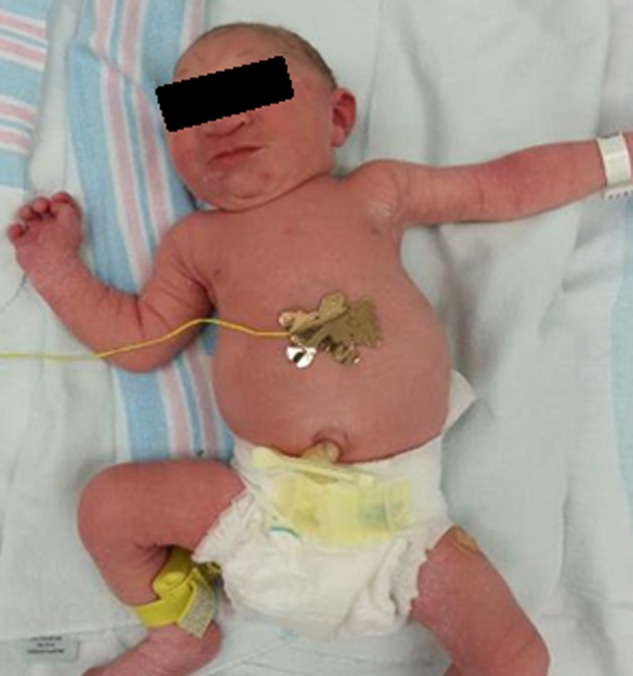
Whole-body photograph of the patient immediately after birth, showing severe microcephaly, but otherwise proportionally developed body.
Placenta weighed 390 g (10th–25th percentile), and pathology revealed acute chorioamnionitis, deciduitis, and amniotic membranes with mildly pigmented macrophages suggestive of meconium soilage. The placental villi showed diffuse chorangiosis and scattered intervillous thrombi, which may be suggestive of anoxic or ischemic stress. One of 4 placental tissue blocks was positive for ZIKV NS5 and envelope genes by reverse transcription polymerase chain reaction (RT-PCR) performed at the Centers for Disease Control and Prevention (CDC) [9]. Viral cultures were not performed, as the rest of the placental tissue was formalin fixed.
At 5 hours of life, the infant developed respiratory distress and hypoglycemia, both of which resolved after a formula feed. At 8 hours of life a self-resolving 30-second seizure was noted, not associated with hypoglycemia. The seizure was characterized by opisthotonus, globally increased muscle tone, and rhythmic jerking of the upper extremities, accompanied by desaturation and bradycardia. Cerebral function monitor was concerning for sharp wave forms. Patient was treated with phenobarbital, and continued on maintenance dosing. By day 4 of life no further seizures were noted, electroencephalography showed no abnormal tracings, and phenobarbital was discontinued. No further clinically apparent seizures were observed. On initial detailed neurologic examination at day 4 of life, the newborn had symmetric facies, and pupils were equal and reactive to light, although he did not track light. Suck reflex was present. He withdrew to cold touch in all 4 extremities. Muscle tone was decreased centrally and in upper extremities, but appropriate in lower extremities. There were no focal deficits in strength. Moro reflex was symmetric. Tendon reflexes in upper and lower extremities were appropriate and symmetric. By 6 weeks of life there was no increase in head circumference. Pertinent new findings included conjugate roving eye movements with preserved pupillary reaction to light, suggestive of cortical blindness. Moro reflex was now exaggerated and patient exhibited frequent arching of the back. Central and peripheral muscle tone was increased, with mild hyperreflexia of upper and lower extremities.
Skeletal survey was significant for marked microcephaly with a collapsed calvarium (Figure 2), mild metaphyseal lucent bands involving bilateral lower extremities, and bilateral acetabular dysplasia, which was confirmed by hip ultrasound. Echocardiography was normal for age. Patient passed the newborn hearing screen at 10 days of life. Scrotal ultrasound revealed testes with normal echogenicity, located in the lower pelvis above the inguinal rings. The patient's newborn genetic metabolic screen was normal.
The infant underwent an initial, full ophthalmologic examination at 5 days of life. There was no behavioral reaction to light. Anterior segment exam was normal. Dilated funduscopic examination revealed pallor of both optic nerves. Pigment stippling of the macula and hypopigmentation of the fundus were noted in each eye. No other abnormalities were noted at the retinal periphery. Vascular size and caliber were normal, and there was no sign of ocular inflammation. Follow-up ophthalmologic examination at 5 weeks of life revealed no interim changes.
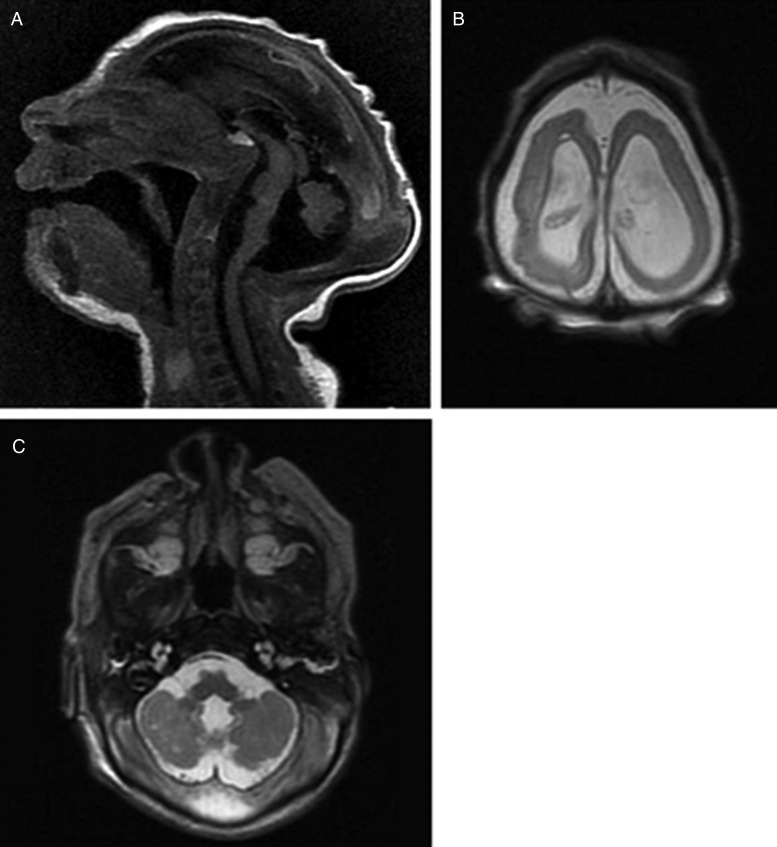
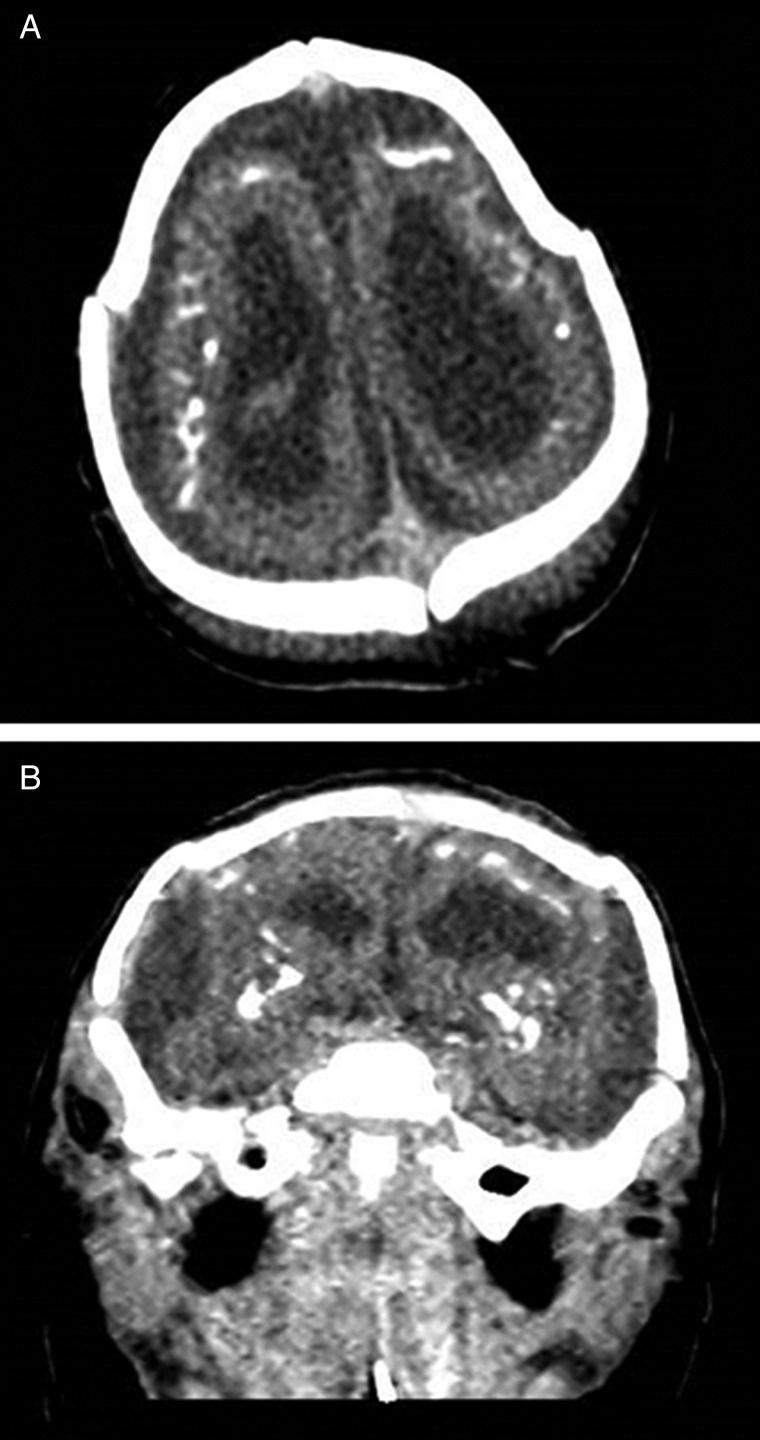
Neuroimaging findings were as follows. Brain magnetic resonance imaging (MRI) was performed on first day of life. T2-weighted sequences showed a small nearly agyric brain with an abnormally smoothed cortical surface (Figure 4). There were <5 gyri in each cerebral cortex, with the cerebral sulci less than one-third the depth of normal. The width of the cerebral wall in the frontoparietal regions ranged between 4.8 mm and 6.6 mm. There was diffuse white matter volume loss with enlargement of extra-axial spaces and asymmetric enlargement of lateral ventricles (Figure 4). The corpus callosum was diffusely thin and markedly hypoplastic. The cerebellum was hypoplastic with abnormal signal within the cerebellar white matter and cerebellar nuclei. Brain stem structures including the pons and medulla appeared small for patient age. T1-weighted sequences demonstrated scattered areas of T1 hyperintensity within the brain parenchyma and bilateral thalami consistent with intracranial calcifications (Figure 4). MRI volumetry showed decreased brain volume (98.9 mL; median for term-equivalent age: 372.9 mL [10]) and increased ratio of total cerebrospinal fluid (CSF) to intracranial volume (0.59; normal: 0.08–0.1 [10]). To better characterize the calcifications, head computed tomography (CT) was performed on day 5 of life. Noncontrast head CT demonstrated extensive calcifications located primarily at the junction between the cortex and subcortical white matter, with additional coarse calcifications within the basal ganglia (Figure 5).
Figure 4.
A, Midline sagittal T1-weighted sequence demonstrates microcephaly and abnormal head shape with a thinned and dysplastic-appearing corpus callosum. The cerebellum appears hypoplastic with enlargement of the extra-axial spaces within the posterior fossa. Brain stem structures, including the pons and medulla, appear relatively small. B, Axial T2-weighted sequence demonstrates a simplified gyral pattern with diffuse cortical thinning as well as white matter loss, and enlargement of the extra-axial spaces and lateral ventricles. C, Axial T2-weighted sequence demonstrates a hypoplastic cerebellum with abnormal signal within the cerebellar white matter and nuclei.
Figure 5.
Axial image (A) and coronal reformat (B) from noncontrast computed tomography demonstrates extensive parenchymal calcifications with additional coarse calcifications involving the bilateral basal ganglia. There is simplification of the sulcation pattern as well as enlargement of the extra-axial spaces and asymmetric enlargement of the lateral ventricles (left greater than right).
Patient's initial complete blood count showed mild lymphocytopenia (absolute lymphocyte count: 5150), but was otherwise normal. CSF had elevated total protein at 184 mg/dL with normal glucose level, and normal white blood cell count. Basic metabolic panel, liver function tests, and bacterial blood and CSF cultures were normal or negative. Rapid plasma reagin, Toxoplasma immunoglobulin G (IgG) and immunoglobulin M (IgM), rubella IgG, surface herpes simplex virus (HSV) cultures, HSV-1 and HSV-2 CSF PCR, and blood and urine cytomegalovirus PCR were all negative. Total IgM was 14 mg/dL (reference values: 13–54 mg/dL).
IgM capture enzyme-linked immunosorbent assay conducted at the CDC was positive for ZIKV and negative for dengue virus serotypes on both mother and infant. Similarly, infant serum IgM on cord blood and blood on the seventh day of life was positive for ZIKV by double antigen enzyme immunoassay (My Biosource, catalog number MBS109003). The mother's postpartum blood was also positive for anti-ZIKV IgM antibody. False-positive ZIKV results due to the presence of dengue antibodies were ruled out by serum dilution–plaque reduction neutralization tests (PRNT) conducted at CDC on both maternal and infant sera. The mother's ZIKV titer, based on PRNT, was 2560; the infant's titer was >20 480. PRNT ZIKV titer on infant CSF was >4096. By CDC guidelines [11], the antibody tests confirmed fetal ZIKV infection. Placental tissue was positive for ZIKV by RT-PCR, but stained negative by histochemistry as tested at CDC. Mother and infant blood and infant CSF were negative by RT-PCR using probes specific for ZIKV envelope region (env) [9].
DISCUSSION
With ZIKV, as with classic congenital infections of the toxoplasmosis-cytomegalovirus-rubella-herpes complex, a mild or even an inapparent maternal infection can cause severe brain, ocular, and auditory damage in the developing fetus. Intrauterine growth retardation and fetal death are also known outcomes of all these infections [12–16]. Beyond these similarities, there are many differences and still unanswered questions about ZIKV effects on the developing fetus. In the case of congenital ZIKV infection, there appears to be no evidence of liver, lung, heart, bone marrow, or skin involvement in the infant we studied or those reported from Brazil [12–16]. The deleterious effects of congenital ZIKV infection appear to be primarily neurologic, with reported associated features such as clubfoot and arthrogryposis more likely resulting from neurologic impairment than direct musculoskeletal damage [12]. It is not yet certain that there is substantial intrauterine growth retardation or placental insufficiency in ZIKV embryopathy. In our patient, the low birth weight for gestational age was the result of the very small head size and weight rather than decreased muscle, organ, or subcutaneous tissue mass. The relevance of timing of maternal illness in relation to neurologic defect remains uncertain.
In a Brazilian cohort of ZIKV-infected infants with microcephaly, maternal illness occurred in 57% in the first trimester and 14% in the second trimester, with the rest unknown [12]. The risk of fetal/infant abnormality with maternal ZIKV infection is another unanswered question with conflicting preliminary evidence. A retrospective population-based study that focused on the outbreak in French Polynesia identified 8 microcephalic infants and estimated the maternofetal transmission risk at 1% with first-trimester infection [17]. In contrast, the prospective study of ZIKV infection in pregnancy showed a 29% risk of adverse fetal effect regardless of the timing of maternal infection [15].
Another important conundrum in maternofetal virus transmission involves the mechanism by which maternal virus reaches the fetus early in gestation before the establishment of maternal blood flow to the placenta, which is known to occur at 10 weeks’ gestation. By the third postconceptional week there are abundant chorionic villi surrounding the embryo in the myometrium. It has been hypothesized that the routes of entry of ZIKV to the embryo/fetus before establishment of maternal circulation in the placenta could include spread through uterine gland secretions, leakage through trophoblastic plugs, or diffusion through intervillous spaces [18, 19].
Finally, information to date on congenital ZIKV has stressed the most severe cases like ours with very abnormal fetal ultrasound and neonatal imaging. The full spectrum of neonatal outcome from severe to mild to subclinical has not been defined.
ZIKV neurotropism and glial tropism has been proven in mouse tissues [2, 10], human fetal brain tissue [20, 21], and neurospheres and brain organoids [22]. The mechanism of cell infection and established viral production involves impaired cellular autophagy process and the formation of “viral factories” in the endoplasmic reticulum [23–25]. Vertical transmission of the virus in humans has been confirmed by detection of viral RNA in amniotic fluid [16, 26], and by detecting ZIKV RNA and viral particles in the brain tissue [20, 21].
The findings outlined in the Brazilian cohorts and our report fall within the fetal brain disruption sequence. First described in 1984 [27], this rare entity is characterized by severe microcephaly, overlapping bones of the neurocranium, prominence of the occipital bone plate, and scalp skin rugae. Several underlying pathologies have been described, primarily impaired vascular perfusion, genetic disorders, and congenital infection [27–29]. The collapse of the calvarium was postulated to be the result of diffuse brain necrosis, unbalanced CSF production and resorption, or a combination of both mechanisms. In addition to sharing the phenotype, the majority of these patients reached a similar developmental maximum, not exceeding the milestones of a 2-month-old infant [28]. It is important to emphasize that this pattern of microcephaly is far more profound than the spectrum of small head size usually encountered in other congenital infections.
Our patient presumably had normal brain development until the time of maternal infection. A transient period of maternal viremia and fetal infection must have occurred during pregnancy, accompanied at some point by infection of the placenta. The negative ZIKV PCR tests on postnatal maternal and neonatal blood support the presumed transient nature of the viremia. Between 7 and 20 weeks of gestation, ZIKV must have established itself within the fetal central nervous system (CNS), causing tissue damage, as evidenced by asymmetric ventriculomegaly on fetal ultrasound at 20 weeks’ gestation. The patient's normal scalp hair pattern suggests that the majority of brain parenchymal injury occurred close to 20 weeks’ gestation and later in the second half of gestation. Early studies on scalp hair patterning indicate that its normal appearance reflects normal brain development up to 16–18 weeks’ gestation [30]. Normal head circumference and extra-axial spaces at 20 weeks’ gestation indicate that there was no overwhelming death of cerebral parenchyma at that time.
High ZIKV IgM titer levels in neonatal CSF indicate a recent or ongoing CNS infection. One previous report confirmed an active ZIKV infection in the neurons of the fetal forebrain at 32 weeks’ gestation, about 20 weeks after initial maternal infection [20]. Imaging of our patient at term showed that there was no significant increase in cerebral thickness between 20 and 40 weeks of gestation, indicating that a large part of brain destruction or failure of neuronal maturation occurred in the second half of gestation. This is consistent with previous findings [13, 14, 20]. As in other cases, gyration was extremely impaired, and has been associated with migrational disorders such as pachygyria and polymicrogyria [31]. We speculate that ZIKV infection during the formation of transient fetal zones of the telencephalic wall [32] affects neuronal progenitor cells and impairs radial glial scaffolding required for appropriate neuronal migration. The former would lead to decreased brain volume, whereas the latter would cause migrational disorders. Cystic lesions seen in the white matter indicate that leukomalacia was present earlier in the course, which is aligned with murine studies [33].
The CDC has developed interim guidelines for management of pregnant women during a ZIKV outbreak and interim guidelines for evaluation for suspected congenital ZIKV infection [11, 34]. Most notably, if found positive for ZIKV, pregnant women should undergo fetal ultrasound every 3–4 weeks.
The rapid spread of ZIKV in the Pacific, South and Central America, and the Caribbean has been alarming. The potential for severe brain damage in infants makes it imperative to find means to bring it under control and ultimately eliminate it. The outbreak in Brazil, the Caribbean, and the Pacific Islands is still active, and it is reasonable to expect the virus to keep spreading north into Central and North America, including the southern United States, following the distribution of its vector, the Aedes mosquito.
Notes
Financial support. V. R. N. and M. K. are partly supported by the Centers of Biomedical Research Excellence, National Institute of General Medical Sciences, National Institutes of Health (grant number P30GM114737).
Potential conflicts of interest. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pan American Health Organization/World Health Organization. Epidemiological update: neurological syndrome, congenital anomalies and ZIKV infection. Washington, DC: PAHO/WHO, 2016:1–8. [Google Scholar]
- 2.Kleber de Olivera W, Cortez-Escalante J, De Olivera WT et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:242–7. [DOI] [PubMed] [Google Scholar]
- 3.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 4.Buathong R, Hermann L, Thaisomboonsuk B et al. Detection of Zika virus infection in Thailand, 2012–2014. Am J Trop Med Hyg 2015; 93:380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 6.Tognarelli J, Ulloa S, Villagra E et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol 2016; 161:665–8. [DOI] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Rapid risk assessment: microcephaly in Brazil potentially linked to the Zika virus epidemic—24 November 2015. Stockholm: ECDC, 2015. [Google Scholar]
- 8.Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis 2015; 21:1885–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanciotti RS, Kosoy OL, Laven JJ et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hüppi PS, Warfield S, Kikinis R et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol 1998; 43:224–35. [DOI] [PubMed] [Google Scholar]
- 11.Staples JE, Dziuban EJ, Fischer M et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:63–7. [DOI] [PubMed] [Google Scholar]
- 12.Schuler-Faccini L, Ribeiro EM, Feitosa IM et al. ; Brazilian medical genetics society–Zika embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:59–62. [DOI] [PubMed] [Google Scholar]
- 13.Miranda-Filho Dde B, Martelli CM, Ximenes RA et al. Initial description of the presumed congenital Zika syndrome. Am J Public Health 2016; 106:598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner H, Fazecas T, Guedes B et al. Intrauterine Zika virus infection and microcephaly: correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet Gynecol 2016; 47:657–60. [DOI] [PubMed] [Google Scholar]
- 15.Brasil P, Pereira JP, Gabaglia CR et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. New Engl J Med 2016; doi:10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvet G, Aguiar RS, Melo AS et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet 2016; 16:653–60. [DOI] [PubMed] [Google Scholar]
- 17.Cauchemez S, Besnard M, Bompard P et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 2016; doi:10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adibi JJ, Marques ET Jr, Cartus A, Beigi RH. Teratogenic effects of the Zika virus and the role of the placenta. Lancet 2016; doi:10.1016/S0140-6736(16)00650-4. [DOI] [PubMed] [Google Scholar]
- 19.Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester—a review. Placenta 2001; 22(suppl A):S70–7. [DOI] [PubMed] [Google Scholar]
- 20.Mlakar J, Korva M, Tul N et al. Zika virus associated with microcephaly. N Engl J Med 2016; 374:951–8. [DOI] [PubMed] [Google Scholar]
- 21.Driggers RW, Ho CY, Korhonen EM et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016; doi:10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 22.Garcez PP, Loiola EC, Madeiro da Costa R et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016; 352:816–8. [DOI] [PubMed] [Google Scholar]
- 23.Tang H, Hammack C, Ogden SC, Jin P, Song H, Ming G. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016; 18:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tetro JA. Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect 2016; 18:167–8. [DOI] [PubMed] [Google Scholar]
- 25.Jheng JR, Ho JY, Horng JT. ER stress, autophagy, and RNA viruses. Front Microbiol 2014; 5:388 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besnard M, Lastere S, Teissier A et al. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19. [PubMed] [Google Scholar]
- 27.Russell LJ, Weaver DD, Bull MJ, Weinbaum M. In utero brain destruction resulting in collapse of the fetal skull, microcephaly, scalp rugae, and neurologic impairment: the fetal brain disruption sequence. Am J Med Genet 1984; 17:509–21. [DOI] [PubMed] [Google Scholar]
- 28.Moore CA, Weaver DD, Bull MJ. Fetal brain disruption sequence. J Pediatr 1990; 116:383–6. [DOI] [PubMed] [Google Scholar]
- 29.Corona-Rivera JR, Corona-Rivera E, Romero-Velarde E, Hernández-Rocha J, Bobadilla-Morales L, Corona-Rivera A.. Report and review of the fetal brain disruption sequence. Eur J Pediatr 2001; 160:664–7. [DOI] [PubMed] [Google Scholar]
- 30.Smith DW, Gong BT. Scalp hair patterning as a clue to early fetal brain development. J Pediatr 1973; 83:374–80. [DOI] [PubMed] [Google Scholar]
- 31.de Fatima Vasco AM, van der Linden V, Brainer-Lima AM et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ 2016; 353:i1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 2008; 9:110–22. [DOI] [PubMed] [Google Scholar]
- 33.Dick GW. Zika virus (II). Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–34. [DOI] [PubMed] [Google Scholar]
- 34.Petersen EE, Staples JE, Meaney-Delman D et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:30–3. [DOI] [PubMed] [Google Scholar]