Abstract
Cholinergic neurons in the medial habenula (MHb) modulate anxiety during nicotine withdrawal although the molecular neuroadaptation(s) within the MHb that induce affective behaviors during nicotine cessation is largely unknown. MHb cholinergic neurons are unique in that they robustly express neuronal nicotinic acetylcholine receptors (nAChRs), although their behavioral role as autoreceptors in these neurons has not been described. To test the hypothesis that nAChR signaling in MHb cholinergic neurons could modulate anxiety, we expressed novel "gain of function" nAChR subunits selectively in MHb cholinergic neurons of adult mice. Mice expressing these mutant nAChRs exhibited increased anxiety-like behavior that was alleviated by blockade with a nAChR antagonist. To test the hypothesis that anxiety induced by nicotine withdrawal may be mediated by increased MHb nicotinic receptor signaling, we infused nAChR subtype selective antagonists into the MHb of nicotine naïve and withdrawn mice. While antagonists had little effect on nicotine naïve mice, blocking α4β2 or α6β2, but not α3β4 nAChRs in the MHb alleviated anxiety in mice undergoing nicotine withdrawal. Consistent with behavioral results, there was increased functional expression of nAChRs containing the α6 subunit in MHb neurons that also expressed the α4 subunit. Together, these data indicate that MHb cholinergic neurons regulate nicotine withdrawal-induced anxiety via increased signaling through nicotinic receptors containing the α6 subunit and point toward nAChRs in MHb cholinergic neurons as molecular targets for smoking cessation therapeutics.
Keywords: Acetylcholine, habenula, nicotine, withdrawal, anxiety
1. Introduction
Adverse symptoms of nicotine withdrawal significantly contribute to the low quit rate of smokers attempting to stop tobacco use (Benowitz, 2008, 2009). Withdrawal symptoms can be divided into at least three separate classes: 1) somatic (physical) symptoms (such as sweating and tingling in the hands and feet), 2) affective symptoms (such as anxiety and depression), and 3) cognitive deficits (such as trouble concentrating)(Jacobsen et al., 2007, Evans and Drobes, 2009). As in humans, rodents chronically exposed to nicotine exhibit somatic (Malin et al., 1994, Kenny and Markou, 2001, Damaj et al., 2003), affective (Suzuki et al., 1996, Damaj et al., 2003, Jackson et al., 2008a, Portugal et al., 2008), and cognitive withdrawal behaviors upon cessation (Portugal et al., 2008, Wilkinson et al., 2013). Of these symptoms, current theories of drug dependence such as the “opponent process” theory posit that affective symptoms such as anxiety significantly contribute to compulsive drug intake and also facilitate relapse in abstinent drug users, highlighting the importance of understanding the neurocircuitry and neuroadaptations elicited by chronic nicotine exposure that leads to affective withdrawal symptoms (Koob and Le Moal, 2008b, a).
Recently, the habenulo-interpeduncular pathway has been hypothesized to modulate both the affective, as well as somatic symptoms of nicotine withdrawal in rodent models (Gorlich et al., 2013, Zhao-Shea et al., 2013, Zhao-Shea et al., 2015). This circuit consists of a small, epithalamic structure, the habenula (Hb), which can be divided into medial (MHb) and lateral (LHb) sub-regions (Hikosaka, 2010). The Hb projects to its target brain regions through a conspicuous bundle of axons that form the fasciculus retroflexus. The LHb projects to the rostromedial tegmental nucleus that is involved in the modulation of dopamine (DA) release from the substantia nigra pars compacta and ventral tegmental area (VTA) (Kaufling et al., 2009, Bromberg-Martin et al., 2010a, Bromberg-Martin et al., 2010b, Balcita-Pedicino et al., 2011, Hong et al., 2011, Lecca et al., 2011). Activation of the LHb reduces DAergic neuron activity and signals aversion (Hong et al., 2011). The MHb, on the other hand, innervates the interpeduncular nucleus (IPN) which, in turn, projects to the median and dorsal raphe nuclei, as well as other brain regions (Morley, 1986), consistent with a role in modulating affective behavior. Selective ablation of septal glutamatergic MHb afferents reduces anxiety-like behavior in mice, suggesting a role for the MHb in controlling anxiety (Yamaguchi et al., 2013). While dorsal MHb neurons are involved in motor behavior, aversion, and reinforcement, the function of cholinergic neurons in the ventral MHb in behavior is largely unknown (Fowler et al., 2011, Frahm et al., 2011, Hsu et al., 2014). We have recently shown that silencing MHb cholinergic neurons alleviates anxiety during nicotine withdrawal in nicotine dependent mice (Zhao-Shea et al., 2015). Of particular interest, MHb cholinergic neurons express nicotinic acetylcholine receptors (nAChRs) at unusually high levels, suggesting that they may act as autoreceptors to regulate affective behavior during nicotine withdrawal (Grady et al., 2009). Thus, we focused on testing the hypothesis that MHb cholinergic neurons regulate anxiety-like behavior during nicotine withdrawal through nAChR signaling.
2. Materials and Methods
2.1 Mice
C57BL/6J, L9′A α4, α4 KO, and ChAT-Cre mice were used in experiments as indicated. ChAT-Cre knock-in mice, were purchased from Jackson Laboratories (stock number: 006410) and bred in the UMMS animal facility. Breeding was conducted by mating heterozygous pairs. L9′A α4 and α4 KO mice on a C57BL/6J background have been described previously (Ross et al., 2000, Tapper et al., 2004). Mice were group-housed four mice/cage on a 12-h light-dark cycle with lights on at 7:00 a.m. and off at 7:00 p.m, and given food and water ad libitum. Male mice were used for all experiments. Mice were at least 8 weeks old at the start of each experiment. Behavioral experiments were performed during the light cycle as nicotine withdrawal symptoms, including increased anxiety, occur in both light and dark phases (Jackson et al., 2008b, Stoker et al., 2008). Independent groups of animals were used for each behavioral experiment unless otherwise noted. All experiments were conducted in accordance with the guidelines for care and use of laboratory animals provided by the National Research Council, as well as with an approved animal protocol from the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
2.2 Drugs and drinking solutions
Nicotine and control drinking solutions were prepared from nicotine hydrogen tartrate or L-tartaric acid (Sigma-Aldrich), which were dissolved in tap water with concentrations of 200 µg/ml (nicotine base) and 300 µg/ml, respectively. Saccharin Sodium (Fisher Scientific) was added to each solution to sweeten the taste with a concentration of 3 mg/ml. For brain infusions, mecamylamine (2 µg/µl), dihydro-β-erythoidine (DHβE) (3 µg/µl), SR16583 (1 µg/µl), and α-conotoxin MII[E11A] (5 µM, 1 µl) were dissolved in artificial cerebrospinal fluid (ACSF) prior to delivery. For i.p. injections, mecamylamine was dissolved in saline.
2.3 Laser Capture Microdissection
Six weeks after nicotine or control solution exposure, mice were decapitated and the brains were removed, snap-frozen in dry ice-cooled 2-methylbutane (−60°C) and stored at −80°C. Coronal serial sections (10 µm) of MHb were cut using a cryostat (Leica Microsystems Inc.) and mounted on pre-cleaned glass slides (Fisher Scientific). The sections were immediately placed in a slide box on dry ice until completion of sectioning, followed by storage at −80°C. A quick immunofluorescence staining protocol was used to identify cholinergic neurons. First, frozen sections were allowed to thaw for 30 s. Slides were immediately fixed in ice-cold acetone for 4 min and then washed in PBS, incubated with mouse anti-ChAT primary antibody (Millipore, 1: 50 dilution for 10 min), washed in PBS once, followed by incubation in secondary fluorescently-labeled antibody (Molecular Probes; donkey anti-goat Alex Fluor 594, 1: 100) for 10 min. The slides were washed in PBS once, and then subsequently dehydrated in graded ethanol solution (30 s each in 70% ethanol, 95% ethanol, 100% ethanol, and once for 5 min in xylene). Slides were allowed to dry for 5 min. All antibodies were diluted in PBS, including 2% BSA and 0.2% Triton X-100. PBS and dH2O were treated with diethylpyrocarbonate (DEPC) for RNA preservation. All ethanol solutions and xylene were prepared fresh to preserve RNA integrity. A Veritas Microdissection System Model 704 (Arcturus Bioscience) was used for laser capture microdissection (LCM). ChAT immunopositive neurons were captured on CapSure Macro LCM caps (Arcturus Bioscience) for total RNA isolation.
2.4 Reverse Transcription-PCR
For quantitative reverse transcription-PCR (qRT-PCR), Total RNA was extracted from individual replicate samples using a Micro Scale RNA Isolation Kit (Ambion). RNA samples extracted from cholinergic neurons were reverse-transcribed into cDNA using a TaqMan Reverse Transcription Kit (Applied Biosystems). qRT-PCR was performed using an Applied Biosystems 7500 Real-Time System and TaqMan assays (Applied Biosystems). Samples containing no reverse transcriptase were used as negative controls. Expression values are relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Samples were analyzed in triplicate. Student’s t-test was used for statistical analysis. For single-neuron RT-PCR, MHb slice neuron cytoplasm was aspirated into a recording patch pipette and the contents were expelled into a microcentrifuge tube containing 75% ice-cold ethanol and stored at −20°C for at least 2 h before single-cell RT-PCR experiments were done as previously described (Zhao-Shea et al., 2011). Primer sequences for single-neuron RT-PCR experiments are indicated in Table S2.
2.5 Immunostaining
All mice were deeply anesthetized with sodium pentobarbital (200 mg/kg, i.p.) and perfused transcardially with 10 ml of ice-cold 0.1 M phosphate-buffered saline (PBS) followed by 10 ml ice-cold 4% (W/V) paraformaldehyde dissolved in 0.1 M PBS (pH 7.4). Brains were removed and immunolabeled as previously described with either rabbit anti-c-Fos (Santa Cruz, 1:800) or goat anti-ChAT (Millipore, 1:100) primary antibody and secondary antibodies. The number of c-Fos-immunopositive neurons was counted via fluorescence microscopy (Zeiss, Carl Zeiss MicroImaging Inc., NY, USA). Positive signals were counted only if they were at least two-fold above background.
2.6 Plasmid engineering, virus packaging, and virus microinjection
The engineering of AAV-Ef1A-DIO-Leu9′Ser-YFP viral plasmid has been described previously (Ngolab et al., 2015). Briefly, the L9′S-YFP α4 nAChR subunit cDNA was sub-cloned into the double-inverted open (DIO) reading frame of the pAAV-EF1a-DIO viral plasmid in the antisense orientation. Thus, the cDNA insert is flanked by two pairs of distinct Lox sites that regulate L9′S-YFP α4 subunit cDNA expression by rearranging the cDNA into the sense orientation wherever Cre recombinase is expressed (Fig. 2A) (Tsai et al., 2009, Ngolab et al., 2015). Packaging of AAV-Ef1A-DIO-Leu9′Ser-YFP and AAV-eGFP plasmids into AAV2 viral particles was done by the University of Massachusetts Medical School Viral Vector Core. For viral particle injection, ChAT-Cre mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) (VEDCO). The surgical area was shaved and disinfected. Mice were placed in a stereotaxic frame (Stoelting Co.) with mouse adaptor and a small incision was cut in the scalp to expose the skull. Using bregma and lambda as landmarks, the skull was leveled in the coronal and sagittal planes. The AAV2-Ef1A-DIO-Leu9′Ser-YFP or AAV-eGFP viral particles (1 × 1012 viral particles/µl) were injected into the MHb according to the coordinates: AP, −1.94, ML, 0.2, DV, −2.25. The viral particles (1.5 µl/side to maximize cholinergic neuron infection) were injected bilaterally at a flow rate of 0.3 µl/min. The injection needle was kept in place for 5 min post-injection. The incision was sutured and mice were returned to their home cages. Mice were allowed to recover 4–6 weeks post-infection to maximize plasmid expression.
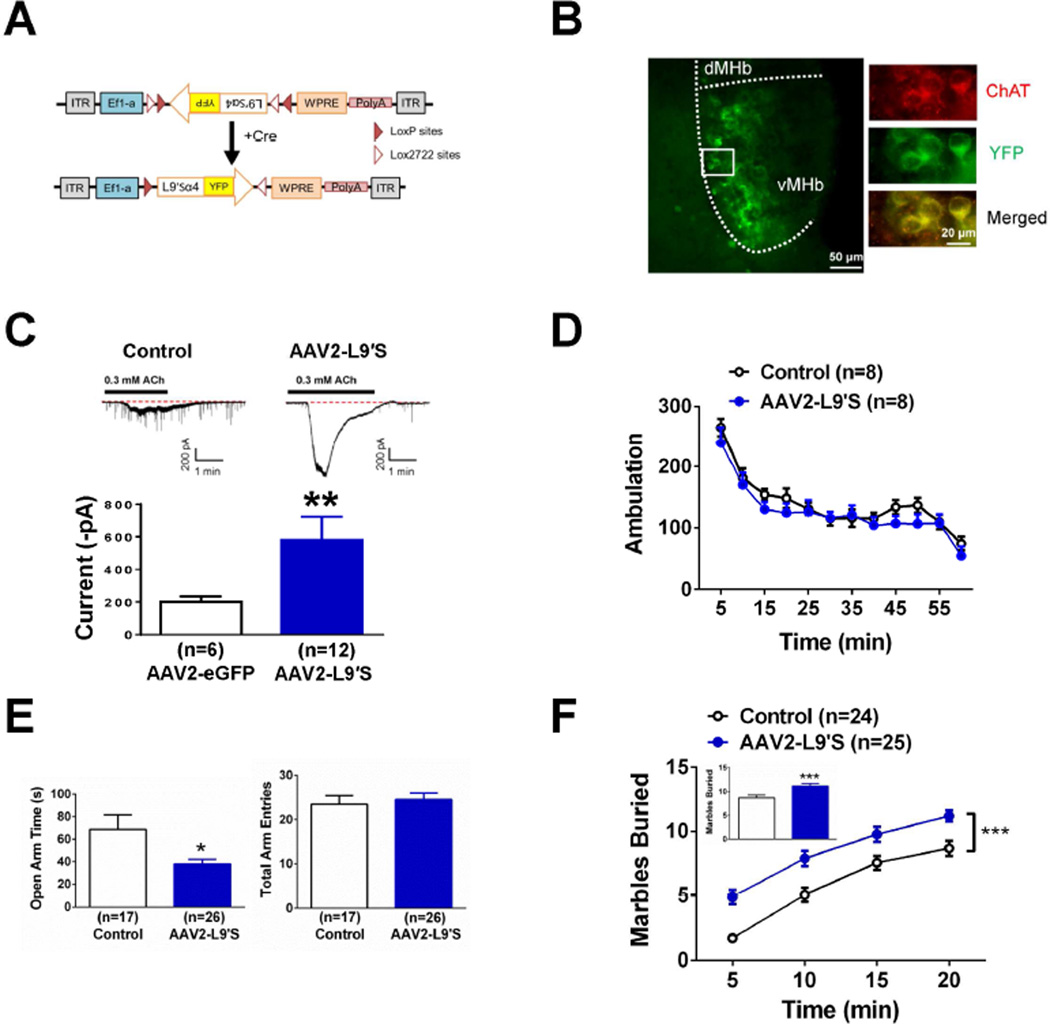
Figure 2.
Selective expression of agonist-hypersensitive α4* nAChRs in MHb cholinergic neurons. A. Schematic diagram of the double-floxed Cre-dependent AAV vector expressing L9′S α4-YFP nAChR subunit cDNA under the control of the Ef-1α promoter. B. Left, Representative images of coronal sections illustrating the expression of L9′S-YFP (green signal) in the MHb (20x). Right, 60x photomicrograph from the section indicated in the square outline of the 20x image. YFP signal (green, top panel), cholinergic signal (red, middle panel) and merged signals (yellow, bottom panel) are shown. C. Representative whole-cell voltage-clamp recording from eGFP-positive (left, control) or L9′S-YFP-positive neurons (middle) in response to 0.3 mM ACh in acute MHb slices from ChAT-Cre mice. Average currents recorded from eGFP- and L9′S-YFP-positive neurons are shown on the right. D. Locomotor activity (ambulation) did not differ between Control-infected and AAV2 L9′S-infected ChAT-Cre mice. Each data point represents summed 5 min total ambulation. E. Time spent in the open arms of the EPM in Control- and AAV2 L9′S-infected ChAT-Cre mice (left). Total EPM arm entries of each group (right). F. Cumulative number of buried marbles at different time points in the MBT from Control-infected and AAV2 L9′S-infected ChAT-Cre mice. Inset, averaged marbles buried at t=20 min. Data are expressed as average values ± S.E.M. *p < 0.05, ** p < 0.01, *** p < 0.001.
2.7 Marble burying test (MBT)
As described previously (Zhao-Shea et al., 2013), mice were habituated in a standard mouse cage with 5 – 6 cm layer of bedding for 2 days (45 min/day) before the test. On the test day, 15 clean 1.5-cm glass marbles were evenly spaced in five rows of three, each 4 cm apart on the thick bedding. A mouse was placed in its test cage and left for 30 min and the number of marbles buried (to at least 2/3 their depth) with bedding was counted every 5 min or after 30 min (as indicated in the figures), then mice were returned to their home cages.
2.8 Elevated plus maze (EPM)
The EPM apparatus consisted of a central axis; four arms elevated 45 cm above the floor, with each arm positioned at 90° relative to the adjacent arms. One 60W red fluorescent light placed 100 cm above the maze was used as the source of illumination. The mice were individually placed in the center of the maze with their heads facing one of the open arms and allowed 5 min of free exploration. The number of entries into the open and closed arms and the total time spent in the open and closed arms were measured by MED-PC IV software (MED associates, Inc.). The time spent in open and closed arms was calculated as standard anxiety indices. The total number of arm entries was considered as an index of locomotor activity. The apparatus was cleaned thoroughly between trials. Mice that froze at the initial placement point without moving were not included in the analysis.
2.9 Locomotor activity
Locomotor activity was recorded using an automated system (San Diego Instruments, La Jolla, CA, USA) with photobeams, which recorded ambulation (consecutive beam breaks). Four weeks after the virus microinjection, mice were placed individually in a novel cage and recorded for 60 min for their baseline locomotor activity.
2.10 Guide cannula placement
Guide cannulas were surgically implanted at least 3 days prior to the experiment. Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) (VEDCO) and placed on a stereotaxic frame (Stoelting Co.). The surgical area was shaved and disinfected and the scull bone was exposed. Using bregma and lambda as landmarks, the skull was leveled in the coronal and sagittal planes. A hole was drilled and a stainless steel guide cannula was inserted into the brain and secured to the skull with instant adhesive. Guide cannulas were implanted into the MHb or LHb according to the coordinates (in mm): AP −1.94 ML 0.2 DV −2.25 and AP −1.43 ML 0.5 mm DV −2.5, respectively. After surgery, the mice were kept on a heating pad to prevent hypothermia. Mice were allowed to recover in individual cages for at least 3 days prior to drug infusion and behavioral testing.
2.11 Intra-cerebral infusions
Mice were lightly anesthetized with 2% isoflurane via a nose cone adaptor at a flow rate of 800 ml/L. Once anesthetized, an infusion cannula designed to reach 0.5 mm below the guide cannula was inserted into the guide cannula. Vehicle, mecamylamine, DHβE, SR16583, or α-conotoxin MII[E11A] was infused at a rate of 1 µl/min. After infusion, the infusion cannula was left in place for 2 min prior to retraction. Mice were placed back into their home cages until awake and the behavior tests were conducted. After completion of behavioral experiments, mice were culled, brains isolated, and cannula placement verified. To verify the guide cannula placement, the brains were cut in 30-µm sections and the locations of the guide cannulae were determined by neutral red staining using light microscopy. In addition, the injection sites were double verified histologically by microinjection of DiI cell labeling solution (Invitrogen, Fig. S2) prior to brain isolation. Little structural damage to tissue caused by drug infusion was noted. Only mice with cannulae in the correct positions were included in the statistical analysis.
2.12 Electrophysiology
Brains were quickly removed and placed in oxygenated ice-cold high sucrose artificial cerebrospinal fluid (SACSF) containing kynurenic acid (1 mM, Sigma, St. Louis, MO). SACSF solution contained (in mM): 250 sucrose, 2.5 KCl, 1.2 NaH2PO4•H2O, 1.2 MgCl2•6H2O, 2.4 CaCl2•2H2O, 26 NaHCO3, 11 D-Glucose. Coronal brain slices (180 µm) containing MHb brain regions were made using a Leica VT1200 vibratome. Current responses to ACh or nicotine were obtained in the whole-cell configuration and gap-free acquisition mode in Clampex (Axon Instruments). Neurons were held at a resting membrane potential of −70 mV. For whole-cell responses to nicotine and ACh, neurons were held at −70 mV and nicotine (10 µM) or ACh (300 µM) was applied via bath perfusion for 10 min. Whole-cell responses to nicotine were measured in the presence of atropine (1 mM) to block muscarinic receptors, bicuculline (20 µM) to block GABAA receptors, CNQX (10 µM) to block AMPA receptors, and AP5 (50 µM) to block NMDA receptors. All recordings were filtered at 1 kHz using the amplifier's four-pole, low-pass Bessel filter, digitized at 10 kHz with an Axon Digidata 1440A interface and stored on a personal computer. ACSF was used for bath solution and contained (in µM): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4•H2O, 1.2 MgCl2•6H2O, 2.4 CaCl2•2H2O, 26 NaHCO3, 11 D-Glucose.
2.13 Experimental Design
The timelines for behavioral experiments are illustrated in Figure S1. For data described in Results Section 3.3 and depicted in Fig. 3A–D, three week old ChAT-cre mice were infected with AAV2 L9′S-YFP α4 or control virus. Four weeks post-infection mice received single daily intraperitoneal (i.p.) saline pre-injections for three days. On the test day, mice received a vehicle or mecamylamine (i.p.) injection. Anxiety-like behavior was recorded in the EPM and MBT assays 1 hr post injection (Fig. S1A). For data described in Results Section 3.3 and depicted in Fig. 3E–H, three week old ChAT-cre mice were infected with AAV2 L9′S-YFP or control virus. Four weeks post-infection, guide cannulas were implanted targeting the MHb. Four days post-surgery, drug was infused. Fifteen minutes after infusion, anxiety-like behavior was recorded in the EPM and MBT (Fig. S1B). For data described in Results Section 3.4 and depicted in Fig. 4A–C and Fig.S3, three week old C57BL/6J mice received chronic nicotine or control drinking solutions. After 45 d chronic treatment, guide cannulas were implanted targeting either MHb or LHb. Four days post-surgery, nicotine solution was replaced with control solution to induce withdrawal. After 24 h withdrawal from nicotine, mice were infused with nAChR antagonists. Fifteen min post-infusion, anxiety-like behavior was measured in the EPM and MBT assays. Oral nicotine consumption was used to induce nicotine dependence because it more closely mimics nicotine exposure experienced by smoking as compared to chronic treatment in osmotic minipumps or injections. In addition, previous studies indicate that blood cotinine concentrations achieved in this model are equivalent to that found in “heavy smokers” and results in little variability of cotinine levels between mice on a C57BL/6J background (Zhao-Shea et al., 2015).
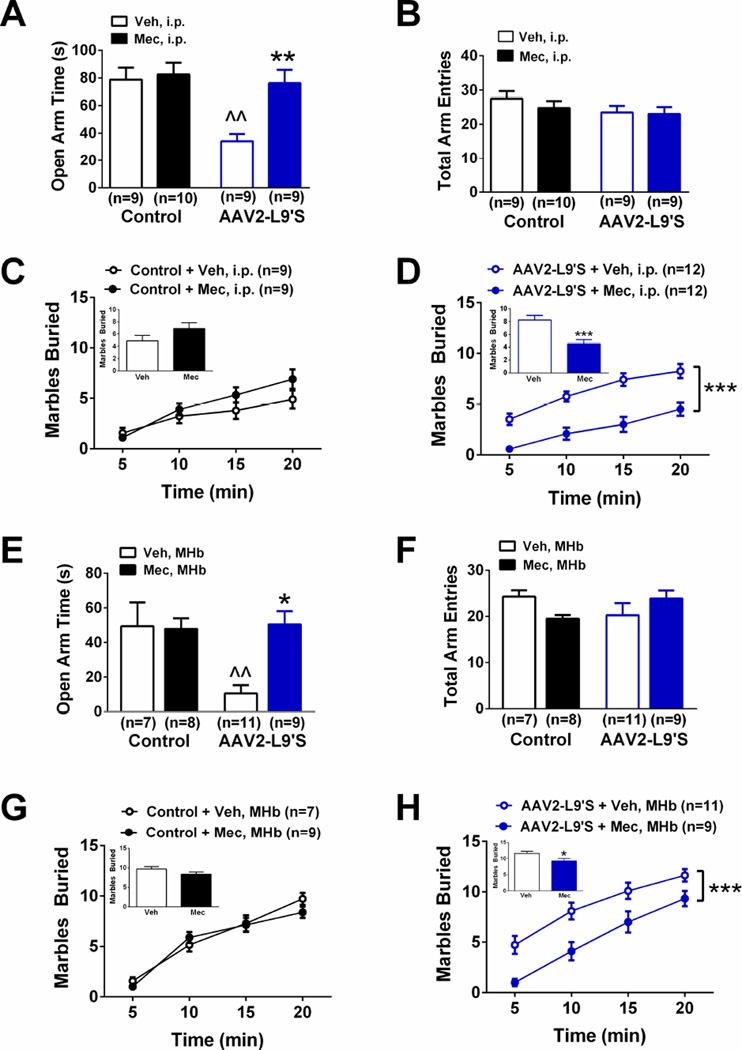
Figure 3.
Increased anxiety-like behavior is mediated by increased nAChR activity in mice expressing Leu9′Ser α4-YFP nAChRs in MHb cholinergic neurons. A. Time spent in the open arms of the EPM in Control- and AAV2 L9′S-infected ChAT-Cre mice after challenge with saline (Sal) or low dose mecamylamine (Mec, 0.3 mg/kg, i.p.). B. Total EPM arm entries of each group from panel A. C. Cumulative number of buried marbles at different time points in the MBT from control-infected ChAT-Cre mice challenged with Sal or Mec. Inset, averaged marbles buried at t=20 min. D. Cumulative number of buried marbles at different time points in the MBT from AAV2-L9′S-infected ChAT-Cre mice challenged with Sal or Mec. Inset, averaged marbles buried at t=20 min. E. Time spent in the open arms of the EPM in Control- and AAV2 L9′S-infected ChAT-cre mice after infusion of vehicle (Veh) or low dose mecamylamine (Mec, 0.5 µg). F. Total EPM arm entries of each group from panel E. G. Cumulative number of buried marbles at different time points in the MBT from control-infected ChAT-cre mice infused with Veh or Mec. H. Cumulative number of buried marbles at different time points in the MBT from AAV2-L9′S-infected ChAT-cre mice infused with Veh or Mec. ^^p<0.01 compared to between group control. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to within group vehicle.Data are expressed as average values ± S.E.M.
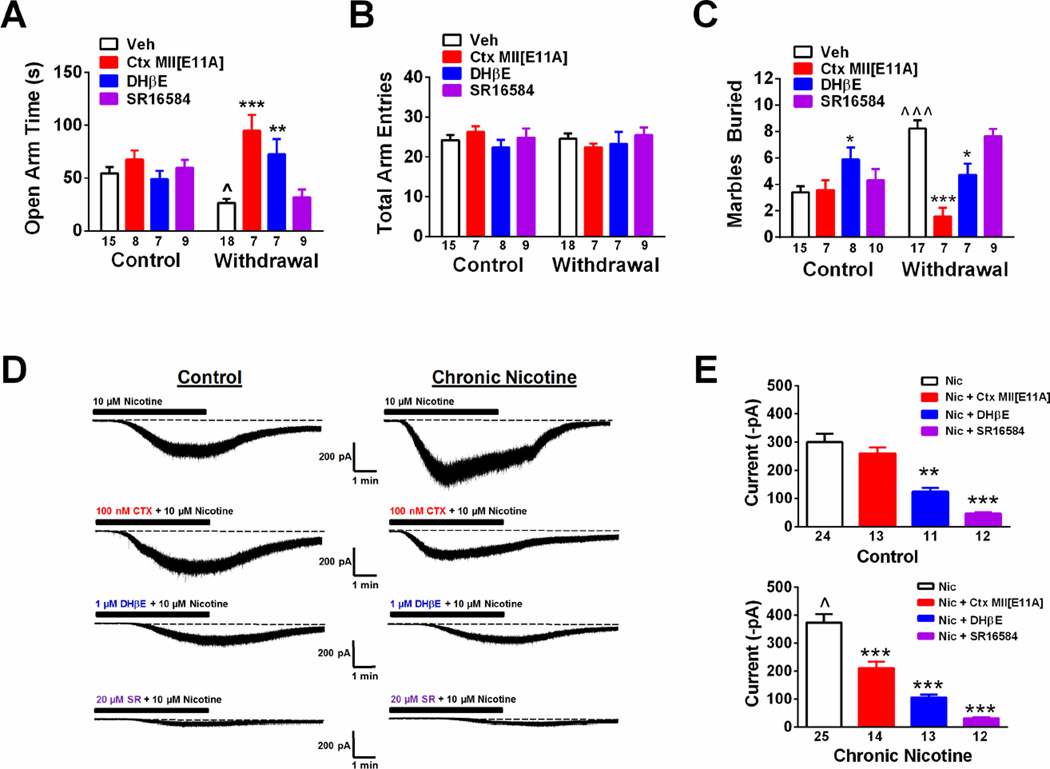
Figure 4.
Nicotine regulation of MHb α4/α6β2* nAChRs mediates increased anxiety during nicotine withdrawal. A. Time spent in the open arms of the EPM in control and nicotine withdrawn mice after vehicle (Veh), α-conotoxin MII[E11A] (α-Ctx[E11A]), DHβE, or SR16584 infusion into the MHb. B. Total arm entries in the EPM from the groups in panel A. C. Total number of marbles buried in the MBT (t = 20 min) in control and nicotine withdrawn mice after Veh, a-Ctx[E11A], DHβE, or SR16584 infusion into the MHb. Data are expressed as average values ± S.E.M. * p < 0.05, ** p < 0.01, *** p < 0.001, Two-way ANOVA, Bonferroni post-hoc. D. Representative whole-cell response to 10 µM nicotine under control conditions and in the presence of 100 nM α-Ctx[E11A], 1 µM DHβE, or 20 mM SR16584 in MHb slices from control-treated mice (left recordings) and chronic nicotine-treated mice (right recordings). B. Averaged peak whole-cell current responses to 10 mM nicotine from the indicated groups as recorded in panel D from control mice (top) or chronic nicotine-treated mice (bottom). Data are expressed as average values ± S.E.M. **p < 0.01, ***p < 0.001 compared to Veh within groups.
2.14 Data analysis
Data were analyzed using One- or Two-way ANOVA followed by Bonferroni post-hoc tests or two-tailed t-tests as indicated. Results were considered significant at p < 0.05. All data are expressed as means ± SEM.
3. Results
3.1 Nicotinic receptor subunit expression in MHb cholinergic neurons
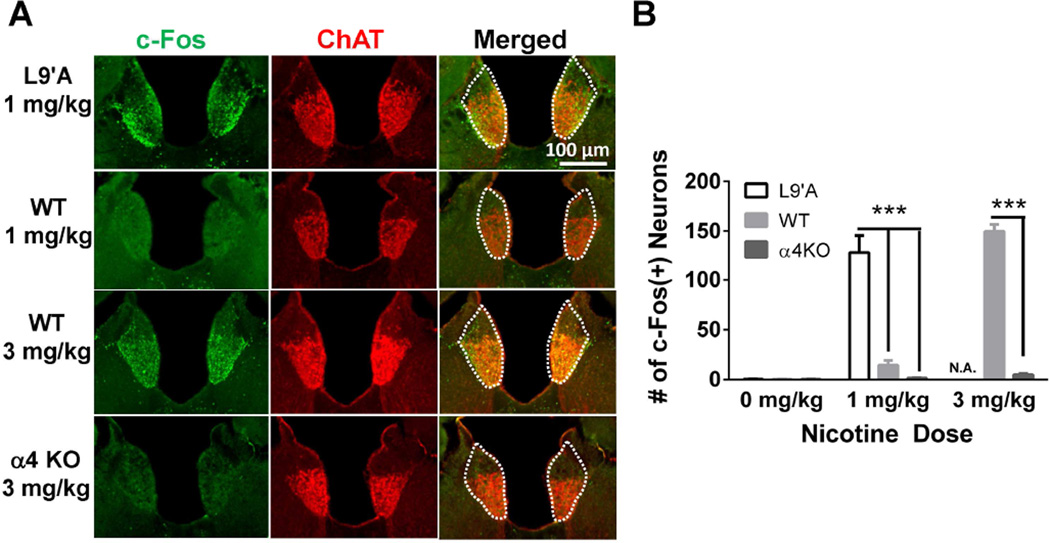
Cholinergic neurons of the MHb exhibit strong expression of genes encoding distinct nAChR subunits (Sheffield et al., 2000, Grady et al., 2009) suggesting that they could serve as a point of regulation for anxiety-like behavior. To determine the precise repertoire of nAChR subunits specifically expressed in MHb cholinergic neurons, cholinergic neurons were laser-microdissected and neuronal nAChR subunit gene expression (α2-α7 and β2-β4 nAChR subunit genes) was measured using qRT-PCR (Table S1). All nAChR subunit genes except α2 were detected in cholinergic MHb neurons. Expression of the nAChR α3 and α4 subunit genes was relatively strong compared to the house-keeping gene GAPDH; whereas, expression of the α5, α6, and α7 genes was less robust. In addition, expression of the nAChR β3 and β4 subunit genes was high relative to GAPDH. As α4 nAChR subunit gene expression was high compared to other nAChR alpha subunit genes, we verified α4 subunit functional expression by measuring nicotine activation of MHb cholinergic neurons in two complementary mouse lines using the immediate early gene c-Fos as a molecular marker of neuronal activation (Cole et al., 1989). For this analysis we used the α4 knockout (KO) mouse line which does not express chrna4 and the L9′A α4 mouse line, a knock-in mouse line that expresses a single point mutation in chrna4 that renders nAChRs hypersensitive to agonist (Ross et al., 2000, Tapper et al., 2004). When challenged with 1 mg/kg nicotine, L9′A α4 animals exhibited prominent c-Fos expression in MHb cholinergic neurons compared to WT or α4 KO mice which, in contrast, had little c-Fos expression (Fig. 1A, B). Conversely, when challenged with 3 mg/kg nicotine, WT mice exhibited significant c-Fos expression in MHb cholinergic neurons; whereas c-Fos expression was dramatically reduced in cholinergic MHb neurons of α4 KO mice. Together, these data indicate that functional nAChRs containing the α4 subunit are critical for nicotine-induced c-Fos activation of MHb cholinergic neurons.
Figure 1.
α4* nAChRs are necessary and sufficient for nicotine activation of MHb cholinergic neurons. A. Representative images of coronal sections from the MHb of C57BL/6J wild type mice, α4 knockout mice or L9′A mutant mice injected with saline or nicotine. Immunolabeling was performed to detect c-Fos expression (green, left columns) and ChAT expression (red, middle columns). Merged images are represented in the right column. The MHb is highlighted by the white-dotted line. B. Quantification of the number of c-Fos immunopositive neurons within the MHb after each drug treatment (10–15 slices analyzed per mice, n = 3 mice/treatment). Note that L9′A mice were not tested at the 3 mg/kg dose (N.A. = Not Analyzed). *** p < 0.001, Student’s t test.
3.2 Expression of L9′S α4* nAChRs subunits in MHb cholinergic neurons increases anxiety
To test the hypothesis that activation of nAChRs specifically in MHb cholinergic neurons could regulate anxiety-like behavior, we selectively expressed novel mutant α4 nAChR subunits that render nAChRs hypersensitive to ACh in MHb cholinergic neurons of adult mice. This was done by using a viral plasmid for expression of mutated α4 nAChR subunits selectively in Cre-expressing neuronal populations (Ngolab et al., 2015). This plasmid includes the α4 nAChR subunit cDNA containing an agonist-hypersensitive L9′S mutation in the M2 transmembrane sequence in addition to a yellow fluorescent protein (YFP) tag, engineered in the M3-M4 intracellular loop, where it does not interfere with expression or function of nAChRs (Labarca et al., 1995, Labarca et al., 2001, Nashmi et al., 2003). To selectively infect cholinergic MHb neurons, L9′S-YFP α4 and eGFP control AAV2 viral particles were intracranially injected into the MHb of ChAT-Cre mice. To verify expression, brains from infected animals were sliced and immunolabeled for ChAT and YFP (Fig. 2B). YFP-expression was primarily restricted to the ventral MHb (i.e., where cholinergic neurons are clustered) and in ChAT-immunopositive neurons from L9′S-YFP α4-infected animals. To confirm functional expression, acute coronal sections containing the MHb were isolated and whole-cell currents in response to ACh were measured from YFP- or GFP (control)-positive neurons using patch-clamp electrophysiology. Bath application of 300 µM ACh elicited inward currents in both control and L9′S-YFP α4-infected neurons (Fig. 2C). However, currents in L9′S-YFP α4-infected neurons were significantly larger compared to controls, indicating increased functional expression of nAChRs in these animals (Fig. 2C, p < 0.01, Mann-Whitney test). Together, these data indicate that L9′S α4 nAChR subunits were properly expressed in MHb cholinergic neurons and formed “gain-of-function” nAChRs to boost nAChR signaling in MHb cholinergic neurons.
To test the hypothesis that increased nAChR signaling in MHb cholinergic neurons modulates anxiety, we evaluated anxiety-like behavior in the EPM and MBT assays. In the EPM, mice infected with the L9′S-YFP α4 virus spent significantly less time in the open arms compared to control-infected mice (t19.4 = 2.24, p < 0.05, Fig. 2E, left). Total arm entries between groups did not differ (Fig. 2E, right). In the MBT, two-way ANOVA with repeated measures indicated a significant main effect of time (F5, 282 = 86.3, p < 0.001) and virus (F1, 282 = 66.8, p < 0.001) on cumulative number of marbles buried. L9′S-YFP α4-infected animals buried significantly more marbles at each time point in the assay, but most importantly, there was an overall increase in the number of marbles buried at the end of the assay (Fig. 2F, inset). To determine if increased burying in L9′S α4-expressing mice compared to control mice was a non-specific phenotype elicited by increased locomotor activity, we measured ambulation during one hour in both groups (Fig. 2D). Locomotor activity did not significantly differ between groups.
3.3 L9′S α4* nAChRs expression in MHb cholinergic neurons increases anxiety through nAChR signaling
To test the hypothesis that increased anxiety-like behavior in L9′S α4-infected mice was due to ACh-mediated activation of the agonist-hypersensitive nAChRs in cholinergic MHb neurons, control and L9′S α4-infected mice were challenged with either saline or a low dose of the nAChR antagonist mecamylamine (0.3 mg/kg, i.p.) prior to testing in the EPM and MBT assays (Fig. S1A, Fig. 3). In the EPM, two-way ANOVA indicated an overall main effect of virus (F1, 33 =9.64, p < 0.01) and drug injection (F1, 33 = 7.83, p < 0.01) and significant virus × drug interaction (F1,33 = 5.41, p<0.05). Post-hoc analysis revealed that mice expressing L9′S α4 nAChRs in MHb cholinergic neurons spent significantly less time in the open arms of the EPM after saline treatment compared to control mice receiving saline (Fig. 3A, p<0.01). Mecamylamine injection of L9′S α4-expressing animals significantly increased time spent in the open arm compared to a saline injection (Fig. 3A, p<0.01); whereas the low dose of antagonist did not significantly alter time spent in the open arms in control mice. Neither virus infection nor drug injection significantly altered total arm entries in the EPM (Fig. 3B). In the MBT assay, mecamylamine did not significantly alter the number of marbles buried in control animals compared to a saline injection (Fig. 3C). In contrast, two-way ANOVA with repeated measures indicated a significant effect of mecamylamine injection (F1, 11 = 32.1, p < 0.001) and time on marble burying (F5, 55 = 41.2, p < 0.001). Mecamylamine significantly reduced the number of marbles buried in mice expressing L9′S nAChRs in MHb cholinergic neurons compared to a saline injection at each time point and such that, at the end of the assay, the cumulative number of marbles buried was significantly reduced (p < 0.001, Fig. 3D, inset). To verify that systemic antagonist injections were actually acting at mutant nAChRs in the MHb, we implanted cannulas targeting the MHb and infused vehicle or mecamylamine (0.5 µg) in the MHb of L9′S α4-expressing and control ChAT-cre mice prior to testing in the EPM and MBT assays (Fig. S1B, Fig. 3E–H). In the EPM, two-way ANOVA indicated a significant effect of virus (F1, 28 = 8.74, p < 0.01) and drug infusion (F1, 28 = 4.52, p < 0.05), and a virus × infusion interaction (F1, 28 = 6.23, p < 0.05). Post-hoc analysis revealed that in control-virus expressing mice, 0.5 µg mecamylamine had little effect compared to vehicle infusion; whereas the antagonist significantly increased time spent in the open arms compared to a vehicle infusion in L9′S α4-expressing ChAT-cre mice (Fig. 3E). Infusion of mecamylamine did not alter total arm entries (Fig. 3F). Similarly, infusion of 0.5 µg mecamylamine did not alter marble burying in control mice compared to vehicle infusion (Fig. 3G) but significantly reduced the number of marbles buried in the MBT of L9′S α4-expressing ChAT-cre mice compared to vehicle (Main effect of drug infusion: F1,72 = 32.2, p < 0.0001, Fig. 3H). Proper placement of cannulas and drug infusions were verified after behavioral experiments as described in Methods (and shown in Fig. S2). Together, these data indicate that L9′S α4 expression in MHb cholinergic neurons increased anxiety-like behavior through increased endogenous activation of L9′S α4* nAChRs (“α4*” indicates that in addition to the α4 subunit, other subunits may be present in the mature receptor).
3.4 Anxiety during nicotine withdrawal is alleviated by blocking upregulated α6* nAChR in the MHb
To test the hypothesis that the anxiogenic effects of nicotine withdrawal are mediated, in part, through increased MHb nAChR signaling, we measured anxiety in the EPM and MBT assay during spontaneous withdrawal from nicotine or control solution in C57Bl/6J mice after MHb infusion of vehicle or subtype selective nAChR antagonists including the α4β2* nAChR-selective antagonist DHβE, the α6* nAChR-selective antagonist α-conotoxin MII[E11A] (McIntosh et al., 2004), and the α3β4 nAChR selective antagonist SR16584 (Zaveri et al., 2010) (Fig. S1C, Fig. 4A–C). In the EPM, two-way ANOVA revealed a significant main effect of drug infusion (F3,72 = 9.68, p < 0.0001) and significant drug infusion × chronic treatment interaction (F3,72 = 7.01, p < 0.001). Post-hoc analysis indicated that infusion of the antagonists into the MHb did not significantly modulate time spent in the open arms of the EPM in nicotine-naïve mice compared to vehicle infusion (Fig. 4A). However, mice undergoing nicotine withdrawal and infused with vehicle spent less time in the open arms compared to nicotine-naïve animals (p < 0.05) and time spent in the open arms was significantly increased by infusion of DHβE (p < 0.01) and α-conotoxin MII[E11A] (p < 0.001), but not SR16584. Total arm entries in the EPM did not significantly differ between nicotine-naïve and –dependent mice regardless of antagonist infusion into the MHb (Fig. 4B). In the MBT, two-way ANOVA indicated a significant main effect of drug infusion (F3,72 = 8.26, p < 0.0001) and chronic treatment (F1,72 = 5.87, p < 0.05) and a drug infusion × chronic treatment interaction (F3,72 = 11.34, p < 0.0001). Marble burying was not significantly affected by antagonist infusion in nicotine-naïve mice; whereas mice undergoing nicotine withdrawal had significantly increased marble burying compared to control mice (p < 0.001, Fig 4C). This increase was significantly decreased by MHb infusion of the antagonist DHβE (p < 0.05) and α-conotoxin MII[E11A] (p < 0.01), but not SR16584. Proper placement of cannulas and drug infusions were verified after behavioral experiments (Fig. S2). In addition, to determine if the effects of DHβE and α-conotoxin MII[E11A] in alleviating anxiety were specific to the MHb and not due to blockade of nAChRs in the adjacent LHb, we implanted cannulas and infused the antagonists directly into the LHb of nicotine naïve and withdrawn animals immediately prior to anxiety-like behavior measurements. Interestingly, both antagonists decreased time spent in the open arms of the EPM in control animals and also increased the number of marbles buried indicating an anxiogenic effect (Fig. S3). However, during withdrawal neither antagonist alleviated anxiety-like behavior. These data suggest that anxiety induced by nicotine withdrawal is mediated by increased signaling through α6/α4β2*, but not α3β4* nAChRs specifically in the MHb.
To test the hypothesis that increased sensitivity to anxiolytic effects of α-conotoxin MII[E11A] and DHβE during nicotine withdrawal were induced by functional upregulation of α6/α4β2* nAChRs in MHb neurons, we measured whole-cell responses to nicotine in MHb neurons from nicotine-naïve and nicotine-dependent animals and tested current sensitivity to α-conotoxin MII[E11A], DHβE, and SR16584 (Fig. 4D, E). As a recent study indicated that α6* nAChRs are predominantly located in the medial ventral portion of the MHb, we focused our recordings on this sub-region (Shih et al., 2014). In control mice, 10 µM nicotine elicited robust whole-cell currents. One-way ANOVA revealed a significant main effect of antagonist on current amplitude (Kruskal-Wallis test, p < 0.001). Dunn’s post-hoc test indicated that currents were relatively insensitive to 100 nM α-conotoxin MII[E11A], but were significantly blocked by DHβE and SR16584. In MHb neurons from chronic nicotine-treated mice, whole-cell responses were significantly larger compared to control neurons (Mann-Whitney test, p < 0.05). In addition, one-way ANOVA indicated a significant main effect of antagonist on nicotine response (F3, 60 = 34.17, p < 0.0001). Unlike nicotine-naïve mice, post-hoc analysis revealed that whole-cell responses from nicotine-treated mice were significantly reduced in the presence of α-conotoxin MII[E11A], as well as DHβE and SR16584. Analysis of nAChR subunit gene expression in MHb cholinergic neurons of nicotine-dependent mice revealed an increase in α6 nAChR subunit transcript compared to MHb cholinergic neurons from control mice (Table S1). Together, these data indicate an increase in functional α6* nAChRs in the MHb after chronic nicotine treatment.
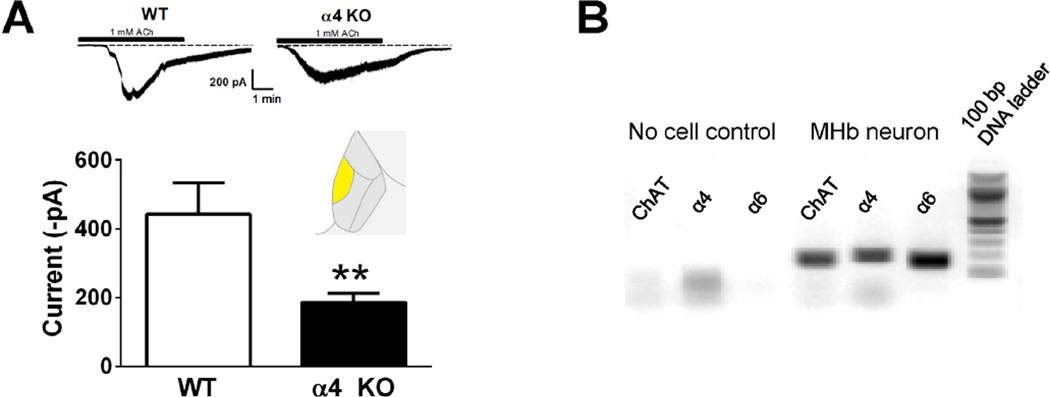
Recently, we identified a novel nAChR subtype containing both α4 and α6 subunits within the VTA that is sensitive to α6* nAChR antagonists as well as DHβE (Zhao-Shea et al., 2011, Liu et al., 2012). To test if α4* nAChRs are expressed within the medial ventral sub-region of the MHb, where α6 nAChRs are predominantly expressed (Shih et al., 2014), we measured whole-cell responses to ACh in WT and α4 KO mice. Peak ACh-induced current amplitudes were significantly reduced in α4 KO mice compared to WT (Fig. 5A). In addition, single neuron RT-PCR revealed a majority of cholinergic neurons in the ventral medial MHb of C57Bl/6J mice expressed both α4 and α6 subunit genes (18 out of 24 neurons, Fig. 5B).
Figure 5.
Expression of α4* nAChRs in ventral medial MHb. A. Representative whole-cell traces from the medial ventral portion of the MHb in WT and α4 KO mice (top, (recorded region is highlighted in inset). Averaged peak current in response to 1 mM nicotine in WT and α4 KO mice (bottom). B. A representative DNA agarose gel is shown illustrating a typical result from a cholinergic neuron from the ventral medial MHb. α4 and α6 nAChR subunit transcript was detected in 18 out of 24 neurons from C57Bl/6J mice (as recorded in Fig. 3). ** p < 0.01, unpaired t-test.
4. Discussion
Within the MHb-IPN circuit, there has been great attention focused on nAChRs as their dense expression within this axis has been linked to acute nicotine intake, reinforcement, withdrawal, and aversion (Grady et al., 2009, Salas et al., 2009, Fowler et al., 2011, Frahm et al., 2011, Zhao-Shea et al., 2013, Slimak et al., 2014, Zhao-Shea et al., 2015). In order to probe the behavioral consequences of these receptors specifically in MHb cholinergic neurons, we expressed “gain-of-function” L9′S α4 nAChR subunits in discreet neuron populations using viral-mediated gene delivery combined with the Cre-Lox system. Our data indicate that cholinergic signaling through nicotinic autoreceptors in MHb cholinergic neurons can regulate anxiety-like behavior in mice. Expression of “gain-of-function” α4 nAChR subunits selectively in MHb cholinergic neurons increased anxiety-like behavior. Anxiety was alleviated by blocking the hypersensitive nAChRs with a low dose of antagonist either systemically or locally within the MHb, indicating that heightened anxiety was mediated through activation of L9′S α4* nAChRs and not due to additional factors such as nAChR desensitization or excitotoxicity.
Recently, the MHb→IPN circuit has been critically implicated in increased anxiety during nicotine withdrawal (Zhao-Shea et al., 2015). Expression of L9′S α4* nAChRs in MHb cholinergic neurons increased anxiety-like behavior, essentially mimicking what may happen if one or more nAChR subtype was functionally upregulated after chronic nicotine exposure. Confirming this idea, heightened anxiety during nicotine withdrawal was alleviated by infusion of an α4β2* and an α6* nAChR-selective antagonist into the MHb. These antagonists had little effect on anxiety in nicotine-naïve mice suggesting that nAChR signaling in MHb cholinergic neurons under baseline conditions may be low. In addition, anxiolysis mediated by blocking MHb α4β2* and α6* nAChRs during nicotine withdrawal was not a byproduct of diffusion into the nearby LHb as direct infusion of the antagonists into the LHb was anxiogenic independent of nicotine treatment. Because the LHb receives DAergic/GABAergic input from the VTA, and DAergic terminals robustly express α4* and α6* nAChRs, the antagonists would be expected to reduce VTA innervation of the LHb resulting in increased anxiety/aversion (Tapper et al., 2004, Drenan et al., 2008, Stamatakis et al., 2013).
Our biophysical analysis revealed that nicotine-induced currents in the MHb were functionally upregulated in nicotine-dependent animals as compared to nicotine-naïve animals. The upregulated currents became more sensitive to a selective α6* nAChR antagonist compared to currents from nicotine-naïve animals, indicating that α6* nAChRs were increased. Indeed, α-conotoxin MII[E11A] did not significantly block nicotine-induced whole-cell currents in MHb of nicotine-naïve mice at the concentration tested suggesting low functional expression of α6* nAChRs in MHb, but chronic nicotine treatment increased chrna6 gene expression likely resulting in increased incorporation of α6 subunits into nAChR complexes. These data are consistent with a recent study showing that mice harboring α6 subunits with an eGFP tag exhibit an increase in fluorescent signal in the MHb after chronic nicotine treatment compared to control mice, suggesting α6 subunit upregulation after nicotine exposure (Henderson et al., 2014). Previous pharmacological studies have shown that intracerebral injection of an α6 antagonist block expression of affective nicotine withdrawal symptoms (Jackson et al., 2009). Our data indicate that this effect is mediated, at least in part, by upregulated α6* nAChRs in the MHb. Importantly, we previously identified a novel nAChR containing both α4 and α6 subunits in DAergic neurons of the VTA and show here that nicotinic currents in the MHb sub-region that predominantly expresses the α6 subunit also contains the α4 subunit, suggesting that upregulated α6 nAChR subunits may incorporate into α4β2* nAChRs during nicotine exposure and contribute to increased anxiety-like behavior during withdrawal (Zhao-Shea et al., 2011, Liu et al., 2012, Shih et al., 2014). This is also consistent with previous work indicating that blocking β2* nAChRs may promote anxiety relief (Anderson and Brunzell, 2015). Future experiments will need to focus on identifying the precise nAChR subunit composition of upregulated nAChRs in the MHb of nicotine-dependent animals. Interestingly, the α3β4 nAChR-selective antagonist SR16584 did not affect anxiety during nicotine withdrawal. These data are consistent with previous studies indicating a predominant role for β4* nAChRs in somatic, but not affective nicotine withdrawal symptoms (Salas et al., 2004). In addition, a recent study indicates β4* nAChRs in MHb are involved in nicotine reinforcement suggesting that distinct nAChR subtypes within MHb control withdrawal-associated versus reward-associated behaviors (Harrington et al., 2015). It should be noted that SR16584 significantly blocked nicotine-induced whole-cell currents in the medial ventral portion of the MHb even though MHb infusion of the antagonist failed to influence anxiety-like behavior. This discrepancy between physiology and behavior is likely due to the expression pattern of α3β4* nAChR which are expressed throughout the MHb (as compared to α6* and α4* nAChRs which have select expression patterns in discreet sub-nuclei of the MHb (Shih et al., 2014)). Thus, nAChR signaling in MHb sub-nuclei may have opposing roles in modulating affective behaviors such that blockade of α3β4* nAChRs throughout the MHb ultimately may have little effect in modulating withdrawal behavior output compared to α6/α4β2* nAChR antagonists which would be expected to block signaling in distinct MHb sub-regions. Alternatively, the concentration of SR16584 used for our biophysical analysis may have been nonspecific and blocked non-α3β4* nAChRs. Regardless, these data indicate that heightened anxiety during nicotine withdrawal is mediated by increased α6/α4β2* nAChR signaling in MHb cholinergic neurons. Interestingly, the medial ventral MHb where upregulation of nAChRs occurs in nicotine-dependent mice projects to the ventral IPN including the IPN intermediate, which is activated during nicotine withdrawal, triggering anxiety (Grieder et al., 2014, Shih et al., 2014, Shih et al., 2015, Zhao-Shea et al., 2015).
Together, our data indicate that nAChR signaling in MHb cholinergic neurons modulates anxiety-like behavior. In nicotine-dependent animals, upregulation of α6/α4β2* nAChRs in MHb neurons is a critical mediator of increased anxiety induced by nicotine withdrawal. Thus, nicotinic acetylcholine autoreceptors in MHb cholinergic neurons should be considered molecular targets for novel smoking cessation therapeutics.
Supplementary Material
Highlights.
-
-
Nicotinic receptors expressed in habenula cholinergic neurons modulate anxiety
-
-
Chronic nicotine functionally upregulates α6* nicotinic receptors in medial habenula
-
-
Blocking medial habenula α6* nicotinic receptors reduces nicotine withdrawal anxiety
Acknowledgments
We thank Dr. Guangping Gao for AAV-eGFP and for packaging of viral plasmids. This work was supported by award numbers DA035371 (A.R.T. and P.D.G.) and DA034490 (J.N.) from the National Institute on Drug Abuse, as well as award numbers GM103801 and GM48677 (J.M.M.) from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing interest that influences the present work.
References
- Anderson SM, Brunzell DH. Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at beta2*nicotinic acetylcholine receptors. Br J Pharmacol. 2015;172:2864–2877. doi: 10.1111/bph.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:S3–S10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron. 2010a;67:144–155. doi: 10.1016/j.neuron.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Nakahara H, Hikosaka O. Multiple timescales of memory in lateral habenula and dopamine neurons. Neuron. 2010b;67:499–510. doi: 10.1016/j.neuron.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha6* nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Gorlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD, Ibanez-Tallon I. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc Natl Acad Sci U S A. 2013;110:17077–17082. doi: 10.1073/pnas.1313103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, Chwalek M, Maal-Bared G, Freiling J, Schlosburg JE, Clarke L, Crawford E, Koebel P, Repunte-Canonigo V, P PS, Tapper AR, Roberto M, Kieffer BL, Sawchenko PE, Koob GF, van der Kooy D, George O. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci. 2014;17:1751–1758. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, Vinals X, Herrera-Solis A, Flores A, Morel C, Tolu S, Faure P, Maldonado R, Maskos U, Robledo P. Role of beta4* Nicotinic Acetylcholine Receptors in the Habenulo-Interpeduncular Pathway in Nicotine Reinforcement in Mice. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Srinivasan R, Nichols WA, Dilworth CN, Gutierrez DF, Mackey ED, McKinney S, Drenan RM, Richards CI, Lester HA. Nicotine exploits a COPI-mediated process for chaperone-mediated up-regulation of its receptors. The Journal of general physiology. 2014;143:51–66. doi: 10.1085/jgp.201311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YW, Wang SD, Wang S, Morton G, Zariwala HA, de la Iglesia HO, Turner EE. Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. J Neurosci. 2014;34:11366–11384. doi: 10.1523/JNEUROSCI.1861-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008a;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008b doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008b;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao-Shea R, McIntosh JM, Gardner PD, Tapper AR. Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits. Mol Pharmacol. 2012;81:541–548. doi: 10.1124/mol.111.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacol. 1994;115:180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of alphα-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Morley BJ. The interpeduncular nucleus. Int Rev Neurobiol. 1986;28:157–182. doi: 10.1016/s0074-7742(08)60108-7. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. Assembly of alpha4beta2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngolab J, Liu L, Zhao-Shea R, Gao G, Gardner PD, Tapper AR. Functional Upregulation of alpha4* Nicotinic Acetylcholine Receptors in VTA GABAergic Neurons Increases Sensitivity to Nicotine Reward. J Neurosci. 2015;35:8570–8578. doi: 10.1523/JNEUROSCI.4453-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, Drenan RM. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci. 2014;34:9789–9802. doi: 10.1523/JNEUROSCI.0476-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PY, McIntosh JM, Drenan RM. Nicotine Dependence Reveals Distinct Responses from Neurons and Their Resident Nicotinic Receptors in Medial Habenula. Mol Pharmacol. 2015;88:1035–1044. doi: 10.1124/mol.115.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimak MA, Ables JL, Frahm S, Antolin-Fontes B, Santos-Torres J, Moretti M, Gotti C, Ibanez-Tallon I. Habenular expression of rare missense variants of the beta4 nicotinic receptor subunit alters nicotine consumption. Frontiers in human neuroscience. 2014;8:12. doi: 10.3389/fnhum.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol. 1996;314:281–284. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Genetic background influences the effects of withdrawal from chronic nicotine on learning and high-affinity nicotinic acetylcholine receptor binding in the dorsal and ventral hippocampus. Psychopharmacology (Berl) 2013;225:201–208. doi: 10.1007/s00213-012-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–544. doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. Novel alpha3beta4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. Journal of medicinal chemistry. 2010;53:8187–8191. doi: 10.1021/jm1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando OJ, Martin GE, George O, Gardner PD, Tapper AR. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nature communications. 2015;6:6770. doi: 10.1038/ncomms7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, Gardner PD, Tapper AR. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr Biol. 2013;23:2327–2335. doi: 10.1016/j.cub.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.