Abstract
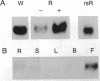
The p34 protein kinase encoded by the cdc2 gene is a key component of the eukaryotic cell cycle required for the G1- to S-phase transition and entry into mitosis. To study the regulation of plant meristem activity and cell proliferation, we have examined the tissue-specific accumulation of cdc2 transcripts in Arabidopsis thaliana and the related crucifer radish (Raphanus sativus) by in situ hybridization using A. thaliana cdc2 cDNA sequences as a probe. cdc2 transcripts accumulated in leaf primordia and within the vegetative shoot apical meristem. During flower development, high levels of expression were observed in meristems, in the basal regions of developing organs, in the developing vasculature, and associated with rib meristems elaborated late in the development of some floral organs. In root tips, cdc2 transcripts accumulated in the meristematic region and adjacent daughter cells but were not detected in the quiescent center. There was strong hybridization throughout the pericycle, and a further localized accumulation of cdc2 transcripts was observed in the initial stages of the activation of a new meristem at sites of lateral root development. We conclude that cdc2 expression is a critical factor in the regulation of meristem activity and establishment of proliferative competence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow P. W. Towards an understanding of the behaviour of root meristems. J Theor Biol. 1976 Apr;57(2):433–451. doi: 10.1016/0022-5193(76)90014-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J., Tyers M., Sundaresan V. Isolation and characterization of cDNA clones encoding a functional p34cdc2 homologue from Zea mays. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3377–3381. doi: 10.1073/pnas.88.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992 May;11(5):1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. N., Bowman J. L., Meyerowitz E. M. Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell. 1991 Jun 14;65(6):991–1002. doi: 10.1016/0092-8674(91)90551-9. [DOI] [PubMed] [Google Scholar]
- Feiler H. S., Jacobs T. W. Cell division in higher plants: a cdc2 gene, its 34-kDa product, and histone H1 kinase activity in pea. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5397–5401. doi: 10.1073/pnas.87.14.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P. C., Hemerly A. S., Villarroel R., Van Montagu M., Inzé D. The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell. 1991 May;3(5):531–540. doi: 10.1105/tpc.3.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Imajuku Y., Anai T., Matsui M., Oka A. Identification of two cell-cycle-controlling cdc2 gene homologs in Arabidopsis thaliana. Gene. 1991 Sep 15;105(2):159–165. doi: 10.1016/0378-1119(91)90146-3. [DOI] [PubMed] [Google Scholar]
- Hirt H., Páy A., Györgyey J., Bakó L., Németh K., Bögre L., Schweyen R. J., Heberle-Bors E., Dudits D. Complementation of a yeast cell cycle mutant by an alfalfa cDNA encoding a protein kinase homologous to p34cdc2. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1636–1640. doi: 10.1073/pnas.88.5.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T., Brockman L. L., Meyerowitz E. M. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992 Feb 21;68(4):683–697. doi: 10.1016/0092-8674(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Structure and developmental expression of the chicken CDC2 kinase. EMBO J. 1989 Oct;8(10):3071–3078. doi: 10.1002/j.1460-2075.1989.tb08458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. p34cdc2: the S and M kinase? New Biol. 1990 May;2(5):389–401. [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Groppe J. C. Preliminary characterization of the transcriptional and translational products of the Saccharomyces cerevisiae cell division cycle gene CDC28. Mol Cell Biol. 1982 Apr;2(4):412–425. doi: 10.1128/mcb.2.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. W., Benfey P. N. The development of plant roots: new approaches to underground problems. Plant Cell. 1991 Nov;3(11):1147–1154. doi: 10.1105/tpc.3.11.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. R., Bowman J. L., Meyerowitz E. M. Early flower development in Arabidopsis. Plant Cell. 1990 Aug;2(8):755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M. F., Ma H., Bowman J. L., Drews G. N., Feldmann K. A., Meyerowitz E. M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990 Jul 5;346(6279):35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]