Abstract
The tubulin heterodimer consists of one α- and one β-tubulin polypeptide. Neither protein can partition to the native state or assemble into polymerization competent heterodimers without the concerted action of a series of chaperone proteins including five tubulin-specific chaperones termed TBCA-TBCE. TBCA and TBCB bind to and stabilize newly synthesized quasi-native β- and α-tubulin polypeptides following their generation via multiple rounds of ATP-dependent interaction with the cytosolic chaperonin, CCT. There is free exchange β-tubulin between TBCA and TBCD, and of α-tubulin between TBCB and TBCE, resulting in the formation of TBCD/β and TBCE/α, respectively. The latter two complexes interact, forming a supercomplex (TBCD/α/TBCD/β). Discharge of the native α/β heterodimer occurs via interaction of the supercomplex with TBCC, which results in the triggering of TBC-bound β-tubulin-bound (E-site) GTP hydrolysis. This reaction acts as a switch for disassembly of the supercomplex and the release of GDP-bound heterodimer, which becomes polymerization competent following spontaneous E-site exchange with GTP. The tubulin-specific chaperones thus function together as a tubulin assembly machine, marrying the α- and β-tubulin subunits into a tightly associated heterodimer. The existence of this evolutionarily conserved pathway explains why it has never proved possible to isolate α- or β-tubulin as stable independent entities in the absence of their cognate partners, and implies that each exists and is maintained in the heterodimer in a non-minimal energy state. Here we describe methods for the purification of recombinant TBC’s as biologically active proteins following their expression in a variety of host/vector systems.
I. INTRODUCTION
The α/β -tubulin heterodimer was originally thought to assemble spontaneously via association of the two constituent polypeptides, with a binding constant in the micromolar range (Detrich & Williams, 1978). More recent measurements based on plasmon resonance suggest a dissociation constant in the range 10−11 M (Caplow & Fee, 2002). In any event, it has never proved possible to purify α- or β-tubulin in native form free from its counterpart. Moreover, expression of α- or β-tubulin in E. coli, either alone or together, leads to their deposition within the host cells as completely insoluble inclusion bodies. Attempts to recover native tubulin from these insoluble materials have consistently proved futile, in spite of the well-established principle that all the information required for a given protein to assume its correct three-dimensional structure is contained within its primary structure (Anfinsen, 1973). On the other hand, in vitro translation in a eukaryotic cell extract (such as that derived from rabbit reticulocyte lysate) of the same sequences that yield insoluble material in E. coli results in the generation of soluble tubulin that is functional in terms of its ability to polymerize into microtubules (Cleveland, Kirschner, & Cowan, 1978). This posed the following paradox: tubulin translated in a prokaryotic cell context does not fold and leads to the production of inclusion bodies, while translation of the identical sequences in eukaryotic cells leads to the generation of functional tubulin heterodimers.
The deposition of insoluble α- and β-tubulin in E. coli cells has been successfully exploited in order to develop an in vitro folding assay for these proteins (Cowan, 1998). The method depends on the ability to label the recombinant protein in the prokaryotic host without labeling any host cell proteins. This is done using a vector in which the expression of recombinant sequences is driven by a T7 promotor: in the presence of 35S-methionine and rifampicin (a drug which inhibits E. coli RNA polymerase, but not T7 polymerase), only the recombinant protein is labeled (Studier, Rosenberg, Dunn, & Dubendorff, 1990). The labeled inclusion bodies can be relatively easily purified because of their extreme insolubility, and the recombinant proteins unfolded in 8 M urea. This procedure yields probes of sufficiently high purity and specific activity (i.e. > 106 cpm/µg) that they can be used in in vitro folding assays to identify factors that are required for productive folding. The products of such in vitro folding reactions can be readily identified by their characteristic mobility on native polyacrylamide gels.
Our development and use of this assay led to the discovery and purification of the cytosolic chaperonin (Gao, Thomas, Chow, Lee, & Cowan, 1992) (termed CCT, for Cytosolic Chaperonin containing T-complex polypeptide 1; also termed TriC, for T-ring Complex). This is a large, ribosome-sized multimolecular complex assembled from eight different (though related) polypeptides into a structure that is readily visible in the electron microscope as a double toroid. CCT polypeptides are distantly related to those of GroEL, the chaperonin that is present in E. coli and that functions in the facilitated folding of a significant proportion (estimated to be at least 5%) of newly synthesized proteins (Hartl & Hayer-Hartl, 2002; Lorimer, 1996; Young, Agashe, Siegers, & Hartl, 2004). All chaperonins, including CCT, function by providing a sequestered environment within the toroidal cavity where folding can take place in the absence of non-productive interactions with other proteins. Cycles of ATP hydrolysis and ADP/ATP exchange result in allosteric changes in the chaperonin that govern the binding and release of the target protein (Spiess, Meyer, Reissmann, & Frydman, 2004; Valpuesta, Martin-Benito, Gomez-Puertas, Carrascosa, & Willison, 2002). In the case of α- and β-tubulin, interaction with CCT is an obligatory part of the folding reaction (Cowan and Lewis, 2001). Moreover, while GroEL participates in the facilitated folding of a wide range of proteins in E. coli cells, it cannot facilitate the productive folding of actins or tubulins, the two principle targets of CCT (Tian, Vainberg, Tap, Lewis, & Cowan, 1995b). This explains why it is not possible to produce soluble tubulins via their expression in E. coli.
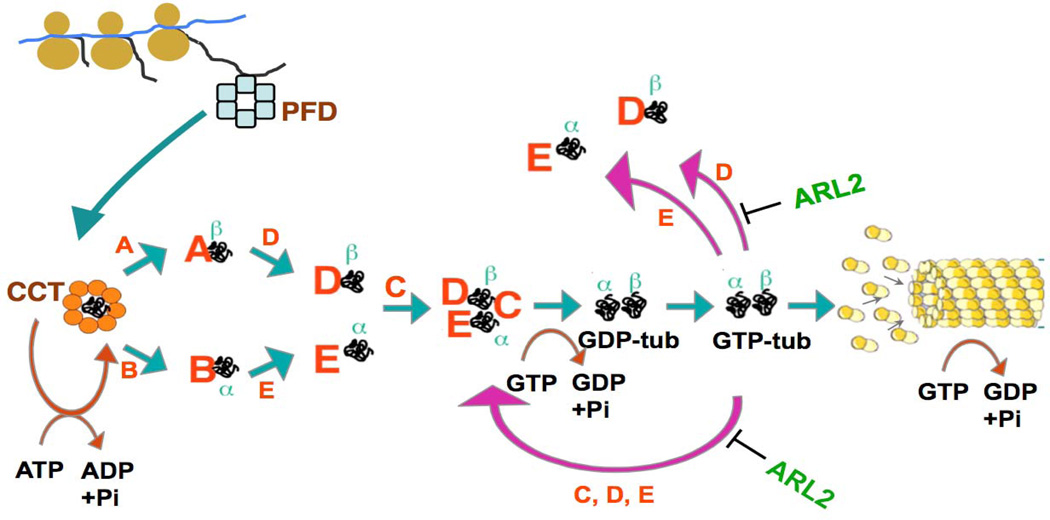
Unlike actin and other obligate targets of CCT, neither α- nor β-tubulin can partition to the native state as a result of ATP-dependent interaction with CCT alone (Gao, Vainberg, Chow, & Cowan, 1993). Rather, tubulin subunits released from CCT are assembled into α/β heterodimers by interaction with several proteins known as cofactors or tubulin-specific chaperones in a reaction that depends on GTP hydrolysis by the chaperone-bound β-tubulin (Lewis, Tian, & Cowan, 1997; Tian et al., 1997; Bhamidipati, Lewis, & Cowan, 2000; Cowan and Lewis, 2002). The overall pathway whereby tubulin heterodimers are formed de novo in higher eukaryotes is depicted in Figure 1. Quasi-native intermediates (defined as containing a native GTP-binding pocket) generated as a result of multiple cycles of ATP-dependent interaction with CCT (Tian, Vainberg, Tap, Lewis, & Cowan, 1995a) are captured and stabilized by TBCA or TBCD (in the case of β-tubulin) or by TBCB and TBCE (in the case of α-tubulin). There is free exchange of β-tubulin between TBCA and TBCD, and of α-tubulin between TBCB and TBCE. TBCE/α and TBCD/β interact with each other, forming a multi-molecular complex; TBCC enters this complex, forming a supercomplex that releases native tubulin heterodimers upon E-site GTP hydrolysis. The hydrolysis of GTP by β-tubulin in the supercomplex may be thought of as a switch for the release of heterodimers if cofactors have a much lower affinity for GDP-tubulin than for GTP-tubulin. This notion has been borne out experimentally (Tian, Bhamidipati, Cowan, & Lewis, 1999). Importantly, in addition to participating in the generation of de novo tubulin heterodimers, TBCC, TBCD and TBCE can act together as a GTPase activator (GAP) for native tubulin. The biological significance of this activity has not been clearly established in vivo, but it may serve as a quality control mechanism that continually checks for the ability of native heterodimers to hydrolyze GTP. It may also contribute towards modulating microtubule dynamics by influencing the size of the pool of GTP-bound tubulin (Tian, Bhamidipati, Cowan, & Lewis, 1999).
Fig. 1.
The chaperone-dependent tubulin folding and heterodimer assembly pathway. Nascent α- and β-tubulin polypeptides are bound by the chaperone protein prefoldin (blue) (Vainberg et al., 1998) and transferred to the cytosolic chaperonin CCT (orange). As a result of multiple rounds of ATP-dependent interaction with the chaperonin, the tubulin target proteins partition to a quasi-native state defined by the acquisition of a native GTP binding pocket. Quasi-native folding intermediates are captured and stabilized by TBCB (in the case of α-tubulin) or TBCA (in the case of β-tubulin). The tubulin target proteins are transferred by free exchange to TBCD and TBCE, forming TBCD/β and TBCE/α; these complexes associate to form a supercomplex. Entry of TBCC into this supercomplex triggers hydrolysis of GTP in the E-site, destabilizing it and releasing newly formed α/β tubulin heterodimers. Following free exchange of E-site guanine nucleotide, these heterodimers are competent for entry into the microtubule polymer. Note that TBCD and TBCE are each capable of disrupting the heterodimer in the back-reaction (shown as purple arrows in the Figure); in the presence of TBCC and GTP, this results in a perpetual cycling of tubulin polypeptides through the supercomplex. The activity of TBCD is modulated via its interaction with the small Ras family GTPase Arl2 (green). Based on (Tian et al., 1997;Tian et al., 1999; Vainberg et al., 1998; Tian, Huang, Parvari, Diaz, & Cowan, 2006; Bhamidipati, Lewis and Cowan, 2000; Cowan and Lewis, 2001.
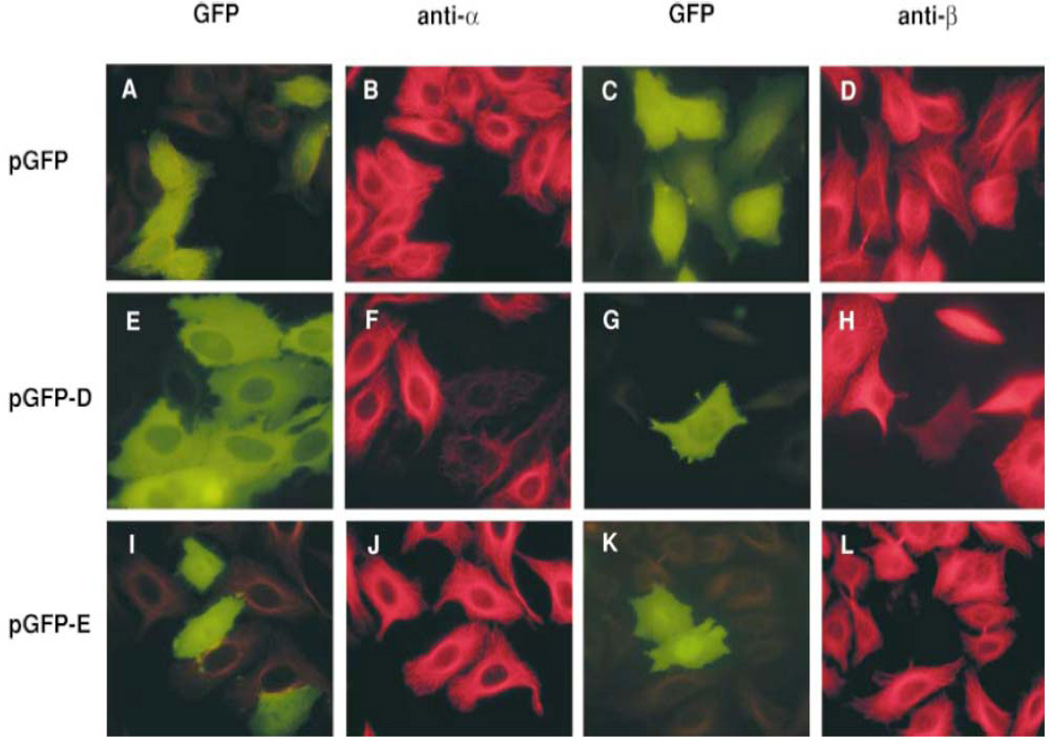
Not surprisingly, the tubulin specific chaperone-dependent reaction that assembles the heterodimer can be driven in reverse: incubation in vitro of native heterodimers with a molar excess of TBCD or TBCE results in heterodimer disruption and (at least in the case of TBCD) the formation of the TBCD/β complex (the TBCE/α complex does not appear to exist as a stable entity). These reactions (which we refer to as the back reaction, shown as purple arrows in Fig. 1) are also apparent in vivo: when cells are transfected with plasmids engineered for the overexpression of TBCD or TBCE, their microtubule network is destroyed (Fig. 2). A noteworthy feature of tubulin destruction by TBCD is that it is modulated by interaction of TBCD with the small Ras family member GTPase Arl2 (Bhamidipati et al., 2000).
Fig. 2.
Microtubule destruction by overexpression of TBCD or TBCE in cultured cells. HeLa cells were transfected with constructs engineered for the expression of GFP alone (as a control; A–D), GFP-TBCD (E–H) or GFP-TBCE (I–L). Microtubules are shown in red, detected with either an anti-α-tublin antibody (B, F and J) or an anti-β-tubulin antibody (D, H and L). From (Bhamidipati, Lewis & Cowan, 2000).
This chapter describes methods for the preparation and purification of the tubulin specific chaperones. All five of these proteins were originally isolated from a tissue source (bovine testis) via multiple chromatographic dimensions in which the protein of interest was assayed via CCT-driven in vitro tubulin folding reactions. These laborious and time-consuming protocols have now been superseded by the production of the corresponding biochemically active recombinant proteins in a variety of host/vector systems, and it is these methods that are described below. The sequences of TBCA-E are well conserved among mammals, so the protocols we provide should be applicable for their purification via expression using cloned cDNAs from any mammalian species. The methods we describe are for the proteins in unmodified form. This is because the effect on biological activity (if any) of the addition of a tag to facilitate affinity purification is uncertain. The yield of recombinant protein in the host systems we have developed is variable (depending on the chaperone in question), but the chromatographic methods we describe are for the most part straightforward. The in vitro CCT-dependent folding assay used to demonstrate TBC activity has been described in detail elsewhere (Cowan, 1998).
II. METHODS
A. cDNA clones and Vectors
Full-length cDNA clones encoding TBCA, TBCB, TBCC, TBCD and TBCE are available a number of commercial sources (e.g. Origene Inc., Cambridge Biosciences Inc., Invitrogen Inc., GeneCopoeia, Inc.). For expression, the vector of choice depends on the tubulin specific chaperone: TBCA, TBCB and TBCC, for example, can be expressed in E. coli in good yield as biologically active proteins. The preferred expression vector for these cloned cDNAs is one belonging to the pET series (Invitrogen, Inc.), in which expression is driven from an inducible T7 promoter. Unfortunately, although TBCD and TBCE can also be expressed in E. coli, the recombinant protein is completely insoluble and cannot be recovered as biologically active material. Resort must therefore be made to eukaryotic host/vector systems. In the case of TBCE, the biologically active protein can be expressed and purified from insect sf9 cells via infection with recombinant baculovirus. A number of commercially available vectors and kits are available for this purpose. Although TBCD can also be expressed as a soluble protein in insect sf9 cells, the yield is relatively poor and we have not found it possible to purify the protein in active form. The reason for this finding is not clear, but it may reflect the absence of some post-translational modification that is required for activity. In any event, we have found that expression of TBCD in cultured human cells via the use of recombinant adenovirus yields material that can be purified with relative ease and is biologically active (Tian, Thomas & Cowan, 2010).
B. Expression of TBCA, TBCB and TBCC
TBCA, TBCB and TBCC can be expressed as soluble, biologically active recombinant proteins in good yield in E. coli host cells. Note that in the case of TBCC, the protein exists as two isoforms, short (TBCC) and long (TBCC-L). The biological significance of these two isoforms is unknown; both are equally active in in vitro heterodimer assembly assays. The protocol below is for the expression and purification of the long form.
1. Bacterial Cell Growth and Induction
The following protocol is for the preparation of bacterial host cells expressing either TBCA, TBCB or TBCC.
Transform the T7 expression plasmid into E. coli BL21DE3 cells using antibiotic selection appropriate for the pET vector in use. Incubate at 37°C until the resulting bacterial colonies are easily visible to the naked eye (typically overnight).
Transfer the colonies en masse into a flask containing 1 l of sterile Luria broth containing the selection antibiotic. This can be conveniently done by pipetting 5ml of broth directly onto the plate, scraping up the colonies with a sterile spreader, and transferring the bacterial suspension to the growth flask. It is not necessary to pick a single colony; indeed, in addition to the greatly extended time required for growth, doing so may lead to diminished yield because of the larger number of division cycles with an accompanying enhanced chance of plasmid loss.
Grow with shaking at 37°C until A600 = 1.0 – 1.2. Remove 1 ml of culture as an uninduced control. Induce expression in the bulk culture by the addition of isopropyl thiogalactoside (IPTG) to 1 mM. Continue growth with shaking at 37°C for a further 2.5 hr; remove a 1 ml aliquot of the culture for use in determining the efficiency of expression of the recombinant protein. Harvest the bacteria in these small aliquots by centrifugation in 1.5 ml Eppendorf tubes, discard the supernatant, and suspend directly in SDS PAGE loading buffer. If necessary, the pellet can be efficiently dispersed by brief sonication using a micro tip powered by an ultrasonic generator.
Harvest the bulk of the bacterial culture by centrifugation at 10,000 × g.
Resuspend the bacterial pellet in 10 ml 10 mM Tris-HCl, pH 7.2, 1 mM EDTA (T10E1) by vigorous vortexing. Dilute to 500ml with T10E1 and repeat step d. At this stage, the bacterial pellet may be stored frozen at −20°C.
Analyze the bacterial protein content on a 10% SDS-PAGE gel. In each case, the recombinant protein should be readily visible as a Coomassie stained band that is absent from the corresponding uninduced control.
2. Preparation of Bacterial Lysates
Resuspend the washed bacterial pellet in 10 ml of T10E1 containing a cocktail of protease inhibitors and transfer to a French Pressure Cell. Lyse the bacteria under 1,500 PSI with minimally two passes or until the lysate has no detectable viscosity when discharged drop-wise from a Pasteur pipet.
Centrifuge the lysate at 30,000 × g for 15 mins at 4°C. Decant the supernatant and centrifuge at 200,000 × g for 15 mins at 4°C in an ultracentrifuge.
The particle-free supernatant can either be applied directly to the first chromatographic dimension as described below, or stored by flash freezing. The latter is most conveniently accomplished by adding the cleared supernatant in a drop-wise stream from a pipet directly into a small Dewar flask containing liquid nitrogen. The resulting frozen pellets can be stored indefinitely in a −70°C freezer.
C. Purification of TBCA, TBCB and TBCC
1. Chromatography on either Sepharose Q (TBCA and TBCB) or Sepharose S High Performance (TBCC) Ion Exchange Resins
Thaw the particle-free extracts prepared as described in Section B2c in a 37°C water bath, taking care not to let the temperature of the melt rise above 4°C. Filter the extract through a 0.4 µ membrane to ensure removal of all particulate material.
Measure the total protein using (for example) the Bio-Rad Protein Assay reagent (BioRad, Inc.). The purification procedures described below are for yields of less than 200 mg of total protein in the starting material. Larger yields require a prorated increase in the size of the ion exchange columns used.
Apply the filtrate to a 10/10 (10 mm × 10 cm) column of Sepharose Q High Performance anion exchange (QHP) resin equilibrated in 20 mM Tris-HCl, pH 7.2, 0.5 mM MgCl2, 1 mM EDTA) (in the case of TBCA and TBCB) or an equivalent column of Sepharose S High Performance cation exchange resin (SHP) equilibrated in 10 mM NaPO4, pH 6.8, 1 mM EDTA (in the case of TBCC). In either case this can be done either using the 10 ml or 50 ml FPLC super-loop. Wash the column with at least 2 column volumes of equilibration buffer, or until the absorbance at 280 nm declines to a value of 0.2 or less.
Develop the column with a 100 ml linear gradient of equilibration buffer containing 0.5 M MgCl2, (in the case of TBCA and TBCB) or equilibration buffer containing 0.25 M NaPO4, pH 6.8 (in the case of TBCC), collecting fractions of 2 ml. The columns can be run at room temperature, but the fractions should be stored on ice as soon as they emerge.
Assay an aliquot (2 – 5 µl) of the fractions emerging from the ion exchange column by SDS-PAGE (12%). The approximate expected conductivity ranges for the elution positions of TBCA, TBCB and TBCC are 7.8 – 9.0 mS/cm, 10.4 – 12.8 mS/cm and 9.3 – 10.0 mS/cm, respectively. The recombinant proteins should be readily visible as prominent Coomassie stained bands migrating at 12, 40 and 38 kDa, respectively.
Pool the fractions identified as containing the recombinant protein of interest and concentrate to 2 ml using a Millipore Centriprep device (Millipore, Inc.) or equivalent. The pooled concentrated material can be stored at −70°C following the addition of glycerol to 10%.
2. Anion exchange chromatography on MonoQ
Exchange the protein obtained by the ion exchange steps described above into 10 mM NaP04 buffer, pH 7.5, 0.1 mM MgCl2, 1 mM DTT using a PD10 desalting column (GE Healthcare Inc).
Apply the sample to a 0.5 × 5.0 cm (5/5) MonoQ column (GE Healthcare Inc.) equilibrated in desalting buffer.
Wash the column with 2 column volumes of desalting (start) buffer and develop with a 50 ml linear gradient containing start buffer plus 0.25 M NaP04 buffer, pH 7.5. TBCB and TBCC emerge from this dimension as major peaks in the range 13.7 – 15.6 mS/cm and 4.3 – 5.6 mS/cm, respectively.
Pool the fractions contained in this peak and concentrate to 0.5 ml in a Millipore Centricon 10 concentration device.
3. Gel filtration Chromatography (Optional)
Apply the material obtained in Step 2 to a 22 ml Superdex 75 gel filtration column (GE Healthcare Inc.) equilibrated in 20 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 1mM EDTA, 1 mM DTT. TBCA, TBCB and TBCC emerge as symmetrical peaks with apparent molecular masses of approximately 30, 50 and 55 kDa, respectively.
Pool the fractions contained in the peak, concentrate them as described in Section 2d and store as multiple small aliquots at −70°C after flash freezing in liquid nitrogen.
D. Expression of TBCE
1. Construction of Recombinant Baculovirus
We have found the BakPak kit sold by Clontech Inc. to be relatively straightforward and convenient for the purpose of recombinant virus production; similar kits are available from other manufacturers (e.g. Invitrogen Inc.). As complete materials and comprehensive instructions are given with these commercial products, including the provision of vectors for the insertion of the sequences to be expressed, no details will be provided here. We recommend that both the titer of the amplified recombinant viruses and the time of infection be optimized for maximum yield of recombinant protein production, as described in the commercially provided user’s manuals.
E. Purification of TBCE
1. Preparation of Insect Cell Lysates
Dislodge cells that remain attached to the dishes containing infected sf9 cells with a polypropylene scraper. Pool the suspensions into 500 ml conical polypropylene centrifuge bottles and centrifuge at 2,000 × g in a Beckman J6 centrifuge or equivalent. Remove the supernatant by aspiration with a Pasteur pipet linked to a vacuum flask, taking care not to disturb the cell pellet.
Resuspend the cell pellet by gently tapping the centrifuge bottle.
Gently add 50 ml of cold T10E1, swirl to generate a homogeneous suspension, centrifuge at 2,000 × g at 4°C and remove and discard the supernatant as before.
Resuspend the cell pellet by gently tapping the centrifuge bottle.
Add 5 ml of ice-cold T10E1 containing protease inhibitor cocktail (Roche Inc.).
Transfer to a 15 ml glass Dounce homogenizer with a type A (tight fitting) pestle. Lyse the cells with 20 full strokes of the homogenizer at 0°C.
Centrifuge the lysate at 500 × g at 4°C for 5 mins. Carefully aspirate the supernatant and transfer to a clean centrifuge tube.
Resuspend the nuclear pellet in half the original volume of ice-cold T10E1 by persistent tapping, centrifuge at 500 × g as before, and combine the supernatant with that obtained in step g.
Centrifuge the combined supernatants at 200,000 × g at 4°C for 15 mins in an ultracentrifuge (such as a Beckman Optima).
Collect the supernatants and store flash frozen in liquid nitrogen as described in Section B2c.
2. Anion Exchange Chromatography
Thaw the flash-frozen sf9 cell extracts prepared as described in Section 1 above in a 37°C water bath, taking care that the melt does not warm above 4°C.
Filter through a 0.4 µ Millipore membrane to ensure removal of any aggregated material that may have formed.
Measure the total protein content of the sample using (for example) the Bio-Rad Protein Assay Reagent. The total soluble protein recovered from 15–20 dishes (20 cm × 20cm) of infected sf9 cells should be in the range 80 – 100 mg.
Apply the mixture to an FPLC column of Q High Performance anion exchange resin (GE Healthcare Inc.) (1 cm × 10 cm) equilibrated in 10 mM Tris-HCL, pH 8.0, 1 mM EDTA and 1 mM DTT. For volumes in the range 5–10 ml, it is most convenient to use the 10 ml superloop for this purpose.
Wash the column after sample loading with 2 column volumes of equilibration buffer, and develop with a 100 ml linear gradient of equilibration buffer containing 0.25M NaCl, collecting fractions of 2 ml. The column may be run at room temperature, but it is advisable to transfer the fractions to an ice bucket as soon as they emerge from the column.
Analyze a small proportion (e.g. 5 µl) of the relevant fractions by SDS-PAGE. As a guide, TBCE emerges from the column in the conductivity range 7.3 – 10.6 mS/cm. The recombinant protein should be visible as a conspicuous band migrating with an apparent mass of 62 kDa.
3. Chromatography on Hydroxylapatite
Pool the fractions containing TBCE from the anion exchange column and concentrate them to 2 ml using a Millipore Centriprep device or equivalent.
Exchange the protein into 10 mM NaP04 buffer, pH 7.5, 0.1 mM MgCl2, 1 mM DTT using a PD10 desalting column (GE Healthcare Inc).
Apply the desalted sample to an FPLC column of hydroxylapatite (Pentax, American Chemical Co. Inc.) (0.5 × 2.0 cm) equilibrated in 10 mM NaP04 buffer, pH 7.5, 0.1 mM MgCl2, 1 mM DTT using the 2 ml loop.
Wash the column after sample loading with 2 ml of equilibration buffer, and develop with a 20 ml linear gradient of equilibration buffer containing 0.25M NaP04, pH 7.5. Collect fractions of 1 ml. The column may be run at room temperature, but it is advisable to transfer the fractions to an ice bucket as soon as they emerge from the column.
TBCE emerges from the Pentax column as a symmetrical peak in the conductivity range 2.4 – 4.7 mS/cm.
Analyze a small proportion (e.g. 2.5 µl) of the samples contained within this conductivity range by SDS-PAGE to determine the purity and yield of the product.
4. Size Exclusion Chromatography (Optional)
Pool the fractions containing TBC from the hydroxylapatite column and concentrate them to 0.5 ml at 4°C using a Millipore Centricon device or equivalent.
Apply this material to a Superdex 200 gel filtration column (22 ml) equilibrated in 10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 1 mM EDTA, 1 mM DTT. Collect 0.5 ml fractions.
TBCE emerges from the column as a symmetrical peak at 13 – 14 ml and should have a purity of 98% or better as judged by SDS-PAGE. This material can be stored flash frozen in small aliquots at −70°C.
F. Expression of TBCD
TBCD expressed in E. coli is insoluble and cannot be recovered by renaturation. We have also attempted to express TBCD as a recombinant protein in insect sf9 cells. While this results in a modest yield of recombinant protein that is soluble, we found this material to be unstable (for a reason that is unclear) in the sense that attempts to purify it by any chromatographic procedure resulted in massive losses. We therefore adopted a procedure that depends on the use of recombinant adenovirus engineered for the expression of TBCD in HeLa cells. We have expressed both human and bovine TBCD via adenovirus vectors, and both are biologically active, although they have distinctive properties upon expression in cultured cells (Tian, Thomas & Cowan, 2010). We have found the AdEasy kit sold by Stratagene, Inc. to be relatively straightforward and convenient for the purpose of recombinant adenovirus production; similar kits are available from other manufacturers. As complete and detailed instructions are given with these commercial products, including the provision of vectors for the insertion of the sequences to be expressed and the cell lines required for recombinant viral construction and propagation, no description will be provided here. However, as in the case of recombinant baculovirus, we recommend that both the titer of the amplified recombinant viruses and the length of time of infection be optimized for production of recombinant TBCD. Even under optimal conditions, the yield of recombinant TBCD is rarely better than 1–5% of the total protein. Nonetheless, even at the low end of this range, it is possible to purify this material to homogeneity as described below.
G. Purification of TBCD
1. Preparation of HeLa Cell Lysates
The preparation of a particle-free lysate of adenovirus-infected HeLa cells is identical to that used for the preparation of lysates from infected insect sf9 cells. Follow the steps described in Section IIG1, a–j.
2. Anion Exchange Chromatography on Q Sepharose High Performance
Purification of recombinant TBCD on Q Sepharose High Performance (QHP) follows the same procedure as that described for TBCE (Section IIG2). TBCD emerges from the QHP column in the conductivity range 17.5 – 21.5 mS/cm. The relatively low yield of the recombinant protein in adenovirus-infected HeLa cells make it advisable to locate it as a 116 kDa band by Western blotting using an anti-TBCD antibody..
3. Anion Exchange Chromatography on MonoQ
Pool the fractions identified as containing TBCD and concentrate to 2 ml using a Millipore Centricon device (Millipore Inc.) or equivalent.
Exchange the protein into 20 mM NaP04 buffer, pH 7.5 using a PD10 desalting column (GE Healthcare, Inc.).
Apply the sample to a 0.5 × 5.0 cm (5/5) MonoQ column (GE Healthcare Inc.). Wash and elute the column as described in Section C2c. TBCD emerges from this column in the conductivity range 19.0 – 22.0 mS/cm, and can be detected either by Western blotting with an anti-TBCD antibody or as a Coomassie stained band migrating at 116 kDa.
4. Chromatography on Hydroxylapatite
Pool the fractions identified as containing TBCD and concentrate to 2 ml using a Millipore Centricon device (Millipore Inc.) or equivalent.
Exchange the protein into NaP04 buffer, apply this material to a hydroxylapatite column, wash and develop as described in Section IIE3b-d. TBCD emerges as an identifiable absorbance peak in the conductivity range 1.5 – 6.5 mS/cm.
Analyze a small proportion of the fractions contained within this conductivity range by 8% SDS-PAGE to determine the purity and yield of the product.
4. Size Exclusion Chromatography
A final purification step is done as described in Section IIE4, except that the optimal gel filtration column for TBCD is Superose 6 rather than Superdex 200. TBCD emerges from this column as a symmetrical peak with an apparent molecular mass of about 120 kDa. This material can be concentrated using a Millipore Centricon 30 concentration device, flash frozen in small aliquots and stored at −70°C.
DISCUSSION
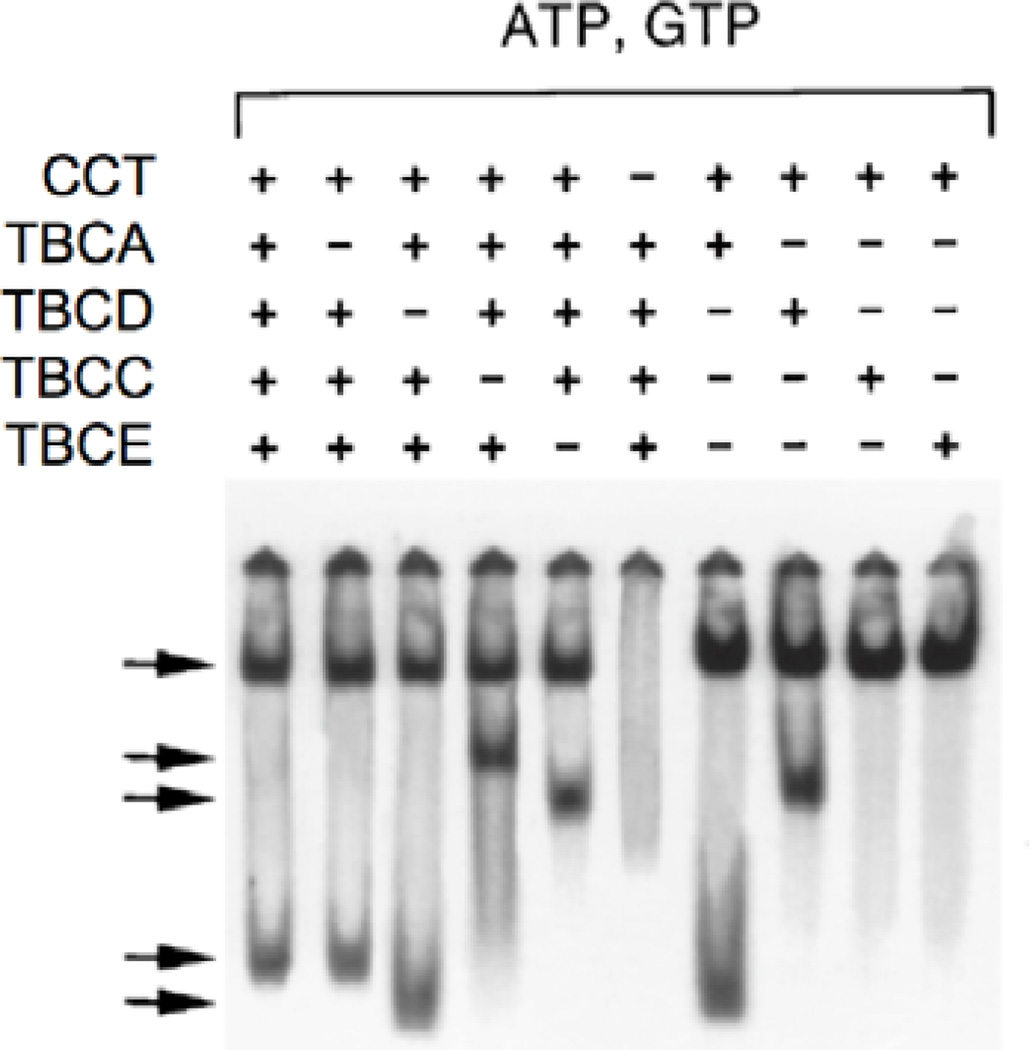
The ultimate test of the quality of the tubulin specific chaperones prepared by the methods described here is their performance in in vitro folding reactions. In these reactions, highly labeled unfolded α- or β-tubulins made by expression in E. coli are presented by sudden dilution from denaturant into an aqueous reaction containing CCT, ATP, GTP and one or more of the tubulin specific chaperones prepared as described above. Detailed protocols describing the preparation of labeled tubulin probes, the purification of CCT and the assembly of the folding reaction are described in (Cowan, 1998). Alternatively, in the case of TBCD and TBCE, the activity of the purified proteins may be tested via their ability to disrupt the native tubulin heterodimer in the back-reaction. In either case, the reaction products are analyzed by electrophoresis on non-denaturing (ND) polyacrylamide gels, a procedure described in detail by (Fanarraga et al., 2010). An example of the complexes formed by TBCA, TBCC, TBCD and TBCE either alone or in combination and their migration properties is shown in Figure 3. Such analyses (among others) were essential for the elucidation of the overall tubulin heterodimer assembly pathway shown in Fig. 1, but they have also proved useful in the analysis of spontaneously occurring human mutations in TUBA1A and TUBB2B. These mutations result in devastating defects in the neuronal migration events that accompany normal brain development during late embryogenesis. For example, some of the disease-causing mutations result in defective interactions of CCT-generated folding intermediates with either TBCB (in the case of TUBA1A) (Tian et al., 2008) or TBCA (in the case of TUBB2B) (Jaglin et al., 2009). Mutations in the gene encoding TBCE are also known to cause the rare inherited disease HRD (hypoparathyroidism, mental retardation and facial dysmorphism) (Parvari et al., 2002; Tian, Huang, Parvari, Diaz & Cowan, 2006).
Fig. 3.
An example of in vitro folding reactions containing purified tubulin specific chaperones used to determine the necessary and sufficient conditions for assembly of the α/β tubulin heterodimer. 35S-labeled, unfolded β-tubulin was suddenly diluted from 8 M urea into buffer containing CCT, ATP, GTP and the tubulin-specific chaperones shown in the Figure. Purified native tubulin heterodimer was included as a source of the α-tubulin subunit. Reaction products were resolved by electrophoresis on non-denaturing PAGE. Arrows (top to bottom) denote the migration positions of the CCT/β-tubulin binary complex, the TBCDβ/TBCEα supercomplex, the TBCD/β complex, native α/β tubulin heterodimers, and the TBCA/β complex, respectively. Note that TBCA and TBCB are not essential for de novo heterodimer assembly in vitro. From (Tian et al., 1996).
It is likely that the tubulin-specific chaperone-dependent reactions that participate in the de novo assembly of the α/β tubulin heterodimer occur in vivo in free solution in a manner that does not depend on microtubules or microtubule dynamics. This is because the heterodimer is assembled upon transcription/ translation of α- or β-tubulin in rabbit reticulocyte lysate, a cell-free cocktail in which the endogenous heterodimer concentration (about 0.1 mg/ml) is well below the critical concentration (CC) required for polymerization into microtubules. However, there is accumulating evidence that at least some of the TBC proteins can modulate microtubule behavior in addition to their involvement in heterodimer assembly. For example, TBCB plays a role in determining microtubule behavior in the neuronal growth cone (Lopez-Fanarraga et al., 2007), and regulates microtubule density in microglia during their transition to reactive states (Fanarraga, Villegas, Carranza, Castano, & Zabala, 2009). Moreover, TBCD is not only distributed throughout the cytosol: it also localizes at centrosomes and the midbody, and is required for proper spindle organization, cell abscission, centriole formation and ciliogenesis (Cunningham & Kahn, 2008; Fanarraga, Belido, Jaen, Villegas, & Zabala, 2010). Both TBCB and TBCE contain conserved CAP-Gly (cytoskeleton-associated protein glycine-rich) domains (Tian et al., 1997), which include a motif (GKNDG) responsible for targeting to the C-terminal EEY/F sequence motifs of CLIP170, EB proteins and microtubules (Weisbrich et al., 2007). Thus, it is conceivable that TBCB and TBCE also influence dynamic behavior by interacting either directly with microtubule plus ends or indirectly via other +TIPs. No clear evidence for such interactions currently exists, but this may reflect equilibrium dissociation constants that are too weak for the formation of stable complexes.
The ability to generate tubulin-specific chaperones as recombinant proteins has been essential for the acquisition of structural data (Table 1), although (with the exception of TBCA) this information is incomplete in the sense that it is currently limited to individual domains, and there is as yet no structural data on TBCD. Nonetheless, the available structures represent an important step towards a mechanistic understanding of the molecular machinery that assembles the α/β heterodimer.
Table 1.
| TBCA* | Whole molecule | 1H7C |
| TBCB** | N-terminal Ubl-domain | 1V6E |
| TBCB** | C-terminal CAP-Gly domain | 1WHG |
| TBCC* | N-terminal domain | 2L3L |
| TBCC* | C-terminal domain | 2YUH |
| TBCE** | C-terminal Ubl-domain | 1WJN |
Accession numbers for structures of human (*) or mouse (**) tubulin-specific chaperones in the PDB database (http://www.rcsb.org/pdb/home/home.do).
REFERENCES
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181(96):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Bartolini F, Tian G, Piehl M, Cassimeris L, Lewis SA, Cowan NJ. Identification of a novel tubulin-destabilizing protein related to the chaperone cofactor E. J Cell Sci. 2005;118(Pt 6):1197–1207. doi: 10.1242/jcs.01719. [DOI] [PubMed] [Google Scholar]
- Bhamidipati A, Lewis SA, Cowan NJ. ADP ribosylation factor-like protein 2 (Arl2) regulates the interaction of tubulin-folding cofactor D with native tubulin. J Cell Biol. 2000;149(5):1087–1096. doi: 10.1083/jcb.149.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplow M, Fee L. Dissociation of the tubulin dimer is extremely slow, thermodynamically very unfavorable, and reversible in the absence of an energy source. Mol Biol Cell. 2002;13(6):2120–2131. doi: 10.1091/mbc.E01-10-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Kirschner MW, Cowan NJ. Isolation of separate mRNAs for alpha- and beta-tubulin and characterization of the corresponding in vitro translation products. Cell. 1978;15(3):1021–1031. doi: 10.1016/0092-8674(78)90286-6. [DOI] [PubMed] [Google Scholar]
- Cowan NJ. Mammalian cytosolic chaperonin. Methods Enzymol. 1998;290:230–241. doi: 10.1016/s0076-6879(98)90022-2. [DOI] [PubMed] [Google Scholar]
- Cowan NJ, Lewis SA. Type II chaperonins, prefoldin and the tubulin-specific chaperones. Advances in Prot Chem. 2001;59:73–104. doi: 10.1016/s0065-3233(01)59003-8. [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Kahn RA. Cofactor D functions as a centrosomal protein and is required for the recruitment of the gamma-tubulin ring complex at centrosomes and organization of the mitotic spindle. J Biol Chem. 2008;283(11):7155–7165. doi: 10.1074/jbc.M706753200. [DOI] [PubMed] [Google Scholar]
- Detrich HWd, Williams RC. Reversible dissociation of the alpha beta dimer of tubulin from bovine brain. Biochemistry. 1978;17(19):3900–3907. doi: 10.1021/bi00612a002. [DOI] [PubMed] [Google Scholar]
- Fanarraga ML, Bellido J, Jaen C, Villegas JC, Zabala JC. TBCD links centriogenesis, spindle microtubule dynamics, and midbody abscission in human cells. PLoS One. 2010;5(1):e8846. doi: 10.1371/journal.pone.0008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanarraga ML, Carranza G, Castano R, Nolasco S, Avila J, Zabala JC. Nondenaturing electrophoresis as a tool to investigate tubulin complexes. Methods Cell Biol. 2010;95:59–75. doi: 10.1016/S0091-679X(10)95005-X. [DOI] [PubMed] [Google Scholar]
- Fanarraga ML, Villegas JC, Carranza G, Castano R, Zabala JC. Tubulin cofactor B regulates microtubule densities during microglia transition to the reactive states. Exp Cell Res. 2009;315(3):535–541. doi: 10.1016/j.yexcr.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992;69(6):1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vainberg IE, Chow RL, Cowan NJ. Two cofactors and cytoplasmic chaperonin are required for the folding of alpha- and beta-tubulin. Mol Cell Biol. 1993;13(4):2478–2485. doi: 10.1128/mcb.13.4.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-Buisson N, et al. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009;41(6):746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Tian G, Cowan NJ. The alpha and beta tubulin folding pathways. Trends Cell Biol. 1997;7:479–485. doi: 10.1016/S0962-8924(97)01168-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Fanarraga M, Carranza G, Bellido J, Kortazar D, Villegas JC, Zabala JC. Tubulin cofactor B plays a role in the neuronal growth cone. J Neurochem. 2007;100(6):1680–1687. doi: 10.1111/j.1471-4159.2006.04328.x. [DOI] [PubMed] [Google Scholar]
- Lorimer GH. A quantitative assessment of the role of the chaperonin proteins in protein folding in vivo. Faseb J. 1996;10(1):5–9. doi: 10.1096/fasebj.10.1.8566548. [DOI] [PubMed] [Google Scholar]
- Parvari R, Hershkovitz E, Grossman N, Gorodischer R, Loeys B, Zecic A, et al. Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet. 2002;32(3):448–452. doi: 10.1038/ng1012. [DOI] [PubMed] [Google Scholar]
- Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 2004;14(11):598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tian G, Bhamidipati A, Cowan NJ, Lewis SA. Tubulin folding cofactors as GTPase activating proteins: GTP hydrolysis and the assembly of the alpha/beta tubulin heterodimer. J. Biol. Chem. 1999;274:24054–24058. doi: 10.1074/jbc.274.34.24054. [DOI] [PubMed] [Google Scholar]
- Tian G, Huang MC, Parvari R, Diaz GA, Cowan NJ. Cryptic out-of-frame translational initiation of TBCE rescues tubulin formation in compound heterozygous HRD. Proc Natl Acad Sci U S A. 2006;103(36):13491–13496. doi: 10.1073/pnas.0602798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Huang Y, Rommelaere H, Vandekerckhove J, Ampe C, Cowan NJ. Pathway leading to correctly folded beta-tubulin. Cell. 1996;86(2):287–296. doi: 10.1016/s0092-8674(00)80100-2. [DOI] [PubMed] [Google Scholar]
- Tian G, Kong XP, Jaglin XH, Chelly J, Keays D, Cowan NJ. A pachygyria-causing alpha-tubulin mutation results in inefficient cycling with CCT and a deficient interaction with TBCB. Mol Biol Cell. 2008;19(3):1152–1161. doi: 10.1091/mbc.E07-09-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Lewis SA, Feierbach B, Stearns T, Rommelaere H, Ampe C, et al. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J Cell Biol. 1997;138(4):821–832. doi: 10.1083/jcb.138.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Thomas S, Cowan NJ. Effect of TBCD and its regulatory interactor Arl2 on tubulin and microtubule integrity. Cytoskeleton. 2010;67:706–714. doi: 10.1002/cm.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ. Quasi-native chaperonin-bound intermediates in facilitated protein folding. J Biol Chem. 1995a;270(41):23910–23913. doi: 10.1074/jbc.270.41.23910. [DOI] [PubMed] [Google Scholar]
- Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ. Specificity in chaperonin-mediated protein folding. Nature. 1995b;375(6528):250–253. doi: 10.1038/375250a0. [DOI] [PubMed] [Google Scholar]
- Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J, Klein HL, et al. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93(5):863–873. doi: 10.1016/s0092-8674(00)81446-4. [DOI] [PubMed] [Google Scholar]
- Valpuesta JM, Martin-Benito J, Gomez-Puertas P, Carrascosa JL, Willison KR. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 2002;529(1):11–16. doi: 10.1016/s0014-5793(02)03180-0. [DOI] [PubMed] [Google Scholar]
- Weisbrich A, Honnappa S, Jaussi R, Okhrimenko O, Frey D, Jelesarov I, et al. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol. 2007;14(10):959–967. doi: 10.1038/nsmb1291. [DOI] [PubMed] [Google Scholar]
- Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5(10):781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]