Abstract
The undesired destruction of healthy cells, either endogenous or transplanted, by the immune system results in the loss of tissue function or limits strategies to restore tissue function. Current therapies typically involve non-specific immunosuppression that may prevent the appropriate response to an antigen, thereby decreasing humoral immunity and increasing the risks of patient susceptibility to opportunistic infections, viral reactivation, and neoplasia. The induction of antigen-specific immunological tolerance to block undesired immune responses to self- or allogeneic antigens, while maintaining the integrity of the remaining immune system, has the potential to transform the current treatment of autoimmune disease and serve as a key enabling technology for therapies based on cell transplantation.
Keywords: tolerance, immunomodulation, cell therapies, nanotechnology, inverse vaccines
1. INTRODUCTION
The undesired destruction of healthy cells by the immune system, either endogenous or transplanted, results in the loss of tissue function or limits strategies to restore tissue function. In autoimmune disease, cell killing is carried out by activated T and B cells that are inappropriately responding to self-antigens (1, 2). Autoimmune diseases affect a large number of people, with estimates approaching 23.5 million Americans and rising (3). Between 80 and 100 diseases have an autoimmune basis, with at least another 40 additional diseases suspected. In contrast to the role of endogenous cells in autoimmune disease, transplanted cells elicit an undesired immune response due to the presence of allogeneic or xenogeneic antigens, limiting the development of novel therapies for tissue restoration. Currently, there are nine US Food and Drug Administration (FDA)-approved cell therapies and many more in development (4). Applications include cellular immunotherapies and hematopoietic progenitor cells for autoimmunity and cancers, chondrocytes for cartilage repair, and keratinocytes for wound healing. Although autologous cells may be ideal for this purpose, allogeneic sources are often necessary for diseases in which autologous cells are no longer available. Transplantation of allogeneic cells from deceased donors is a promising option (5, 6), but universal stem cell banks provide a potentially unlimited source of progenitor cells and have significant economic advantages relative to autologous sources (7, 8). Regardless of whether the cell source is deceased donors or universal donor stem cell banks, the cells are allogeneic to the recipients, and a strategy for tolerance to avoid lifelong immunosuppression could greatly expand opportunities for cell-based therapies.
Current approaches for addressing the undesired destruction of healthy cells involve immunosuppression (9). Immunosuppression nonspecifically inhibits immune responses by deleting or inactivating entire T or B cell subsets or by nonselectively inhibiting antigen presentation, proinflammatory cytokine production, or lymphocyte trafficking (10–12), and it is the most common strategy for treating autoimmune disease or transplant rejection (Table 1). The T cell clones mediating the undesired response contain a T cell receptor (TCR) that recognizes the antigen presented by an antigen-presenting cell (APC) on the major histocompatibility complex (MHC) (Figure 1); thus, strategies targeting T cells often rely on targeting the interaction between the APC and the antigen-specific T cell, which is necessary for complete T cell activation. The costimulatory factor CD40L is necessary to activate T cells, and programmed death 1 (PD-1) and cytotoxic T lymphocyte–associated protein 4 (CTLA-4) are necessary to inhibit T cell activity (Figure 1). Infusion of soluble CTLA-4- and/or CD40L-blocking antibodies can reduce proinflammatory cytokine expression and T cell activation while increasing anti-inflammatory cytokine expression and inhibitory T cell costimulatory factors. Despite their benefits, these strategies are not completely efficacious and may prevent appropriate responses to infections and environmental antigens, thereby decreasing both cellular and humoral immunity, which carries inherent risks of patient susceptibility to opportunistic infections, viral reactivation, and neoplasia [as illustrated by the triggering of progressive multifocal leukoencephalopathy following natalizumab (anti-VLA-4) or rituximab (anti-CD20) therapy in a variety of clinical trials]. Whereas short-term nonmitogenic anti-CD3 and anti-interleukin-2 (IL-2) therapies do not result in widespread deletion, clinically they have a poor profile in the context of transplant rejection (5).
Table 1.
Current therapeutics for immune suppression
| Type of immune suppression | Target | Drug(s) |
|---|---|---|
| MHC/TCR interaction blockade | TCR | Anti-abTCR mAb T10B9 |
| Nonselective depleting agents | CD3e | Anti-CD3 mAb (OKT3), ATG |
| Calcineurin inhibitors | FKBP12 | Cyclosporine, tacrolimus |
| Costimulatory signal blockade | CTLA-4 | Anti-CTLA-4 mAb, abatacept, ipilimumab |
| PD-1 | Anti-PD-1 (pembrolizumab) | |
| PD-L1 | Anti-PD-L1 (MPDL3280A; clinical trials) | |
| CD154 | Anti-CD154 mAb (belatacept) | |
| CD40 | Anti-CD40 mAb | |
| ICOS | Anti-ICOS mAb | |
| Cytokine blockade | IL-2 | Anti-CD25 mAb (daclizumab, basiliximab) |
| TNF-α | Anti-TNF (infliximab) | |
| IL-6 | Anti-IL-6 mAb (ALD518) | |
| IL-7 | Anti-IL-7 mAb | |
| Lymphocyte depletion | CD2 | Anti-CD2 mAb, fusion protein with IgG1 (alefacept) |
| CD20 | Anti-CD20 mAb (rituximab) | |
| CD52 | Anti-CD52 mAb (alemtuzumab) | |
| Cell adhesion inhibitor | α-4 integrin | Anti-VLA4 (natalizumab) |
| IL-2 signaling inhibitor | mTOR | Sirolimus (rapamycin), everolimus |
| Cell-cycle blockers | DNA synthesis | MMF, azathioprine |
Abbreviations: ATG, antithymocyte globulin; CTLA, cytotoxic T lymphocyte–associated protein; FKBP, FK506-binding protein; ICOS, inducible costimulator; IgG, immunoglobulin G; IL, interleukin; mAb, monoclonal antibody; MHC, major histocompatibility complex; MMF, mycophenolate mofetil; mTOR, mechanistic target of rapamycin; PD, programmed death; TCR, T cell receptor; TNF, tumor necrosis factor; VLA, very late antigen.
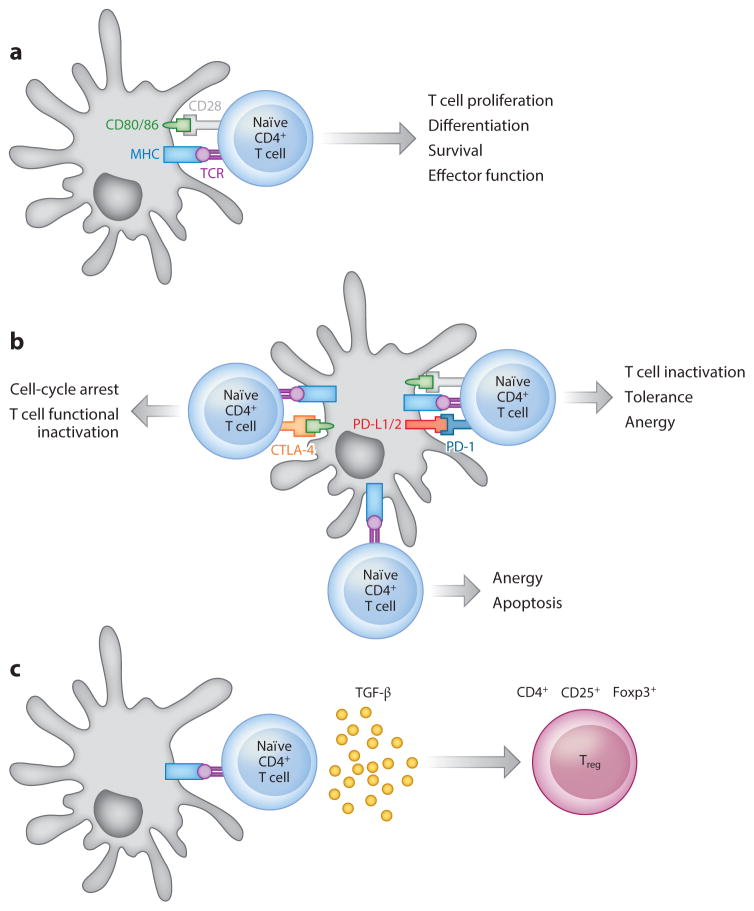
Figure 1.
Tolerance by antigen (Ag) presentation without costimulation. Antigens are internalized by antigen-presenting cells (APCs), and after processing, a peptide of 7–10 amino acids or 14–20 amino acids can be presented on major histocompatibility complex (MHC) I or II, respectively, for recognition by the T cell receptor (TCR) on T cell subsets. The plasticity of T cell subsets enables their response resulting in either immunity or the induction of tolerance. (a) Immunity. The T cell clones mediating the undesired response contain a TCR that recognizes the Ag presented by an APC presenting the MHC/Ag complex (signal 1). The interaction between costimulatory factors present on APCs and cognate receptors on T cells is also needed to cause T cell activation (signal 2). The final stage of T cell activation involves the interaction of soluble mediators (such as interleukins or other factors) with receptors on the T cell surface (signal 3). (b) Blockade of the positive costimulatory CD28/B7 signaling axis between T cells and APCs by the cytotoxic T lymphocyte antigen 4 (CTLA-4)–Ig fusion protein serves as a constitutive regulatory control switch, and it has been exploited as a tolerogenic regimen for autoimmunity treatment. Programmed death 1 (PD-1) signaling on T cells by its ligands PD-L1 and PD-L2 represents another regulatory mechanism of immune responses, and it has been extensively studied in the context of autoimmunity and T cell exhaustion and deletion. Selective blockade of the PD-1/PD-L1 signaling axis can disrupt tolerance induction by blocking T cell receptor stop signals, thereby decreasing T cell motility and increasing physical interactions with APCs, resulting in enhanced immune responses. (c) Induction of regulatory T cells (Tregs) as a mechanism to achieve Ag-specific immunoregulation. In the presence of transforming growth factor β (TGF-β) and interleukin-2 (IL-2), naïve T cells can be differentiated into Tregs that release immunosuppressive cytokines such as IL-10 to diminish the induction and proliferation of effector T cells.
The induction of antigen-specific immunological tolerance to block undesired immune responses to self- or allogeneic antigens, while maintaining the integrity of the remaining immune system, has been the focus of translational research for several decades, as it has the potential to transform the current treatment of numerous autoimmune disease and serve as a key enabling technology for therapies based on cell transplantation. The specific induction of T cell activity occurs when an APC becomes activated and presents the antigen along with multiple costimulatory signals (Figure 1a). This process has formed the basis of vaccination, which has saved and continues to save countless lives by preparing the recipient for an encounter with a pathogen. A comparable approach for attenuating a specific immune response to a target tissue or environmental antigen has not yet become standard in the clinic. Silencing the activity of specific T cell clones is challenging because they do not have distinguishing molecular markers relative to other T cell clones; thus, strategies are aimed at altering the signals between T cells and APCs rather than blocking the interaction. For antigen-specific tolerance, the antigen is presented in the absence of costimulatory signals (Figure 1b), which can lead to deletion or anergy (i.e., nonreactivity) of effector T cells and/or the induction of regulatory T cells (Tregs) that can modulate the activity of other T cells (Figure 1c). The strategies for promoting one or more of these tolerogenic responses are described in the following sections in the context of autoimmune disease and allogeneic cell transplantation. Key parameters that affect the efficacy of these strategies include the route of administration, the dosing schedule, and the codelivery of antigens with tolerogenic agents aimed at either inducing tolerance or inducing deviation of the response toward an anti-inflammatory cytokine profile (1).
2. AUTOIMMUNE DISEASE AND ANTIGEN-SPECIFIC TOLERANCE
The recognition of an autoantigen by the TCR is the feature that distinguishes autoreactive T cells from other T cell subsets. The TCR repertoire is generated in immature T cells in a relatively random manner, and some TCRs recognize self-antigens. The majority of T cell clones with high-affinity TCRs that recognize self are deleted as a consequence of self-antigen presentation by thymic epithelial cells (13). Thymic selection is imperfect; therefore, autoreactive T cells are present in the peripheral T cell repertoire of healthy individuals (14). T cells that escape negative selection in the thymus must be held in check by additional peripheral tolerance mechanisms, and the ability to tightly control and avoid the activation of peripheral self-reactive T cells is crucial for avoiding autoimmunity. The following sections describe peptide and protein delivery that aims to interface with natural peripheral tolerance mechanisms in order to influence the activity of T cell clones that can recognize a specific antigen. Multiple strategies are being investigated that employ various routes of delivery, each with distinct challenges and opportunities (Figure 2).
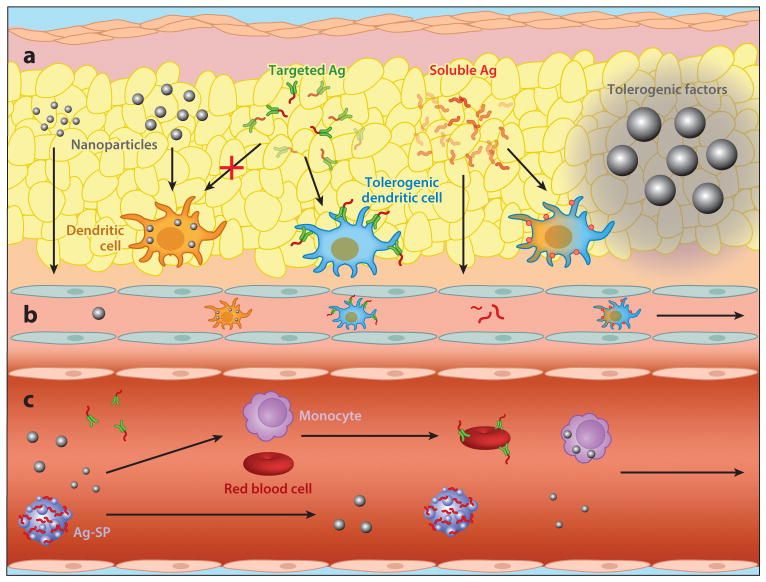
Figure 2.
Schematic illustrating tolerogenic approaches being developed for delivery (a) subcutaneously, (b) to the lymphatics, or (c) intravenously. Factors delivered subcutaneously can release factor locally, be internalized by cells within the subcutaneous space, or travel to the lymphatics. Intravenous delivery can involve soluble antigen (Ag) or modified Ag or nanoparticles that can associate with cells in the blood, or it can be internalized by antigen-presenting cells in organs such as the liver or spleen. Abbreviation: Ag-SP, antigen coupled to splenocytes.
2.1. Intravenous Peptide Delivery
The intravenous delivery of peptides has been investigated for the induction of immune tolerance. These studies date to the 1920s and demonstrate that intravenously delivered peptides induce antigen-specific anergy (15). Similarly, intravenous soluble protein or peptide therapy has been a potent strategy for inducing peripheral tolerance in experimental settings (16). Although the precise mechanisms underlying the unresponsiveness induced by the intravenous administration of antigen are unknown, this unresponsiveness may be due in part to the short availability of antigens, as well as the immature or tolerogenic phenotype of the APCs that encounter, acquire, and present the antigens to T cells. Peptide delivery can block T cell proliferation and/or IL-2 production, cause activation-induced cell death (AICD) after T cell restimulation with the cognate peptide (17–20), and block disease in animal models. Despite the demonstration that intravenous administration can provide long-lasting tolerance in experimental mouse models, concerns exist relating to the translation of these observations to the treatment of established disease. Intravenous administration of peptides induced a fatal anaphylactic response in multiple mouse strains (21) and in a primate model of multiple sclerosis (22). This variability in response, combined with the expectation that tolerance will require multiple treatments, means that anaphylactic responses remain a significant safety concern for soluble peptide intravenous delivery.
Recently, peptides have been engineered that, upon intravenous administration, bind to circulating erythrocytes (23). Large numbers of erythrocytes are cleared each day after apoptosis-like programmed cell death (eryptosis), which has been implicated in tolerance induction (see the next section). Eryptotic erythrocytes are characterized by phosphatidylserine asymmetry, membrane heterogeneity, and annexin V binding, analogous to apoptotic nucleated cells. These cells are cleared by APCs, and the bound peptides are displayed over the ensuing days. Apoptotic cells have molecular markers associated with a tolerogenic polarization (24–26), such as phosphatidylserine, which is recognized by phagocytic cells through either direct ligation to receptors or indirect bridging by proteins. Two molecular designs were developed: (a) a protein antigen conjugated to multiple copies of a glycophorin A–binding peptide and (b) a peptide or protein antigen fused to a single-chain variable fragment (scFv) antibody fragment specific for the same erythrocyte-specific target. Dendritic cell (DC) populations within the spleen were observed to have uptaken the antigen, and these cell types have been implicated in tolerogenesis (27, 28). These peptides were also associated with cells in the liver, such as hepatocytes and stellate cells, which were suggested to contribute to cross-presentation of exogenous antigens (29–32). Ultimately, these molecular conjugates led to deletion of both CD8+ and CD4+ T cells, and the results indicate that these erythrocyte-binding antigens may have induced PD-1 signaling to drive deletional proliferation of clonal T cell populations (33).
2.1.1. Ex vivo antigen coupling to cells
The peptide association with erythrocytes builds on decades of research on the coupling of peptides to donor splenocytes (SPs) using the chemical cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (ECDI) (34). ECDI is employed to affix antigens to the donor cells in order to minimize the amount of free antigen in the blood that may lead to anaphylaxis; it also induces apoptosis of the donor splenic leukocytes. Antigen coupled to splenocytes (Ag-SP) delivers the antigen to APCs that can mediate immune tolerance. The nonspecific cross-linking of antigen to the cell surface during the induction of apoptosis allows the donor cells to be perceived by the host in a noninflammatory (nonimmunogenic) manner. Ag-SPs have been employed to prevent and treat the relapsing experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS) (35) and type 1 diabetes (T1D) in the nonobese diabetic (NOD) mouse (36).
The mechanism of action of Ag-SP-induced tolerance has yet to be fully elucidated; however, the route of administration, the dosage used, the levels of costimulatory molecules expressed (the two-signal hypothesis), and the extent of T helper (Th) cell polarization and Treg induction are all probable factors that contribute to the efficacy of the treatment. Tolerance by this strategy involves splenic marginal zone APCs, which clear the body of apoptotic cells and debris and can process the infused Ag-SPs with subsequent presentation of the coupled antigen in a tolerance-inducing manner (26, 37). The tolerogenic host APCs upregulate negative costimulatory molecules (e.g., PD-L1), with subsequent phasic tolerogenic effects on antigen-specific T cells, including anergy, deletion, and induction of Tregs (37–41). In vitro studies have suggested that engagement of the TCR in the absence of costimulation contributes to the inability to activate Th1 cell clones and leads to the induction of anergy (40). Tregs appear to have a role in antigen-coupled cell-induced tolerance, and are required for the long-term maintenance of tolerance, but are not necessary for tolerance induction (39). T cells retrieved from tolerized mice produce higher amounts of the anti-inflammatory cytokines IL-10 and transforming growth factor β (TGF-β) than do T cells from control mice, and this pattern suggests an increase in natural Treg function. The induction of tolerance with Ag-SPs appears to work by several distinct synergistic mechanisms, increasing their therapeutic efficacy.
A recent publication presented the results of a Phase I trial in MS patients in Germany that used apoptotic ECDI-fixed peripheral blood mononuclear cells (PBMCs) pulsed with a cocktail of myelin peptides. The results illustrate the safety and efficacy of this procedure for the treatment of human autoimmune disease (42). Importantly, the mechanistic aspects of this study provided an important proof of principle that induced peripheral tolerance can be successfully employed to induce unresponsiveness in human autoreactive T cells; responses to four of the seven tolerated myelin epitopes were significantly reduced (with no effect on tetanus responses) in the four MS patients treated with >109 autologous antigen-coupled PBMCs (Ag-PBMCs), and there was no effect on the nine patients receiving <5 × 108 Ag-PBMCs. These studies provided the first definitive demonstration of induced tolerance to autoantigens in humans.
2.1.2. Antigen on synthetic carriers
The translational challenges associated with Ag-SP-based therapy have motivated the development of nanoparticles for antigen-specific tolerance. Nanoparticles with properties similar to apoptotic cell debris may function as an alternative antigen carrier for tolerance induction. Intravenous injection of 500-nm “nonbiodegradable” carboxylated polystyrene (PS) particles coupled with peptides prevented the onset of disease in the EAE mouse model, prevented epitope spreading, and ameliorated progression of preestablished EAE (43). Interestingly, the studies using PS nanoparticles demonstrated that particles with diameters ranging from 500 to 1,000 nm were most effective, and tolerance depended on particle uptake by the macrophage receptor with collagenous structures (MARCO) scavenger receptor (44, 45). MARCO is responsible for uptake of PS beads that have an anionically charged surface (46).
More recently, biodegradable particles made from the copolymers of lactide and glycolide (PLG) induced antigen-specific tolerance for prevention and treatment of EAE (43, 47). Administration of particles results in significantly reduced central nervous system infiltration of encephalitogenic Th1 [interferon-γ (IFN-γ)] and Th17 [IL-17a, granulocyte macrophage colony–stimulating factor (GM-CSF)] cells, as well as inflammatory monocytes/macrophages. Tolerance was most effectively induced by intravenous infusion of Ag-PLG (43, 48), although intraperitoneal and subcutaneous delivery was able to attenuate disease scores. The intravenous route likely has greater efficacy due to direct trafficking to the liver and spleen, whereas the alternative routes have distinct patterns of trafficking (39). The liver, in particular, plays a significant role in tolerance induction with nanoparticles, as splenectomized mice could be tolerized with PLG nanoparticles (D. McCarthy, W.T. Yap, C.T. Harp, W.K. Song, J. Chen, et al., manuscript in revision).
Upon intravenous delivery, particles associate with a range of APCs, including macrophages and Kupffer cells, and conventional and plasmacytoid DCs. Whereas the smallest particles reported by Getts et al. (48) were less efficient at tolerance induction, recent research with smaller particles loaded with rapamycin were employed to promote tolerance via intravenous delivery (49). Similarly, tolerance induction was demonstrated using gold nanoparticles loaded with 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE, a ligand for the aryl hydrocarbon receptor) and antigen (50). Differences in cellular association between small and large particles may underlie the need for a pharmaceutical agent along with the smaller particles (49, 50). More recently, nanoparticles were employed to target liver sinusoidal endothelial cells (51), which can induce CD4+Foxp3+ Tregs (52). This nanoparticle-based antigen delivery prevented the onset of clinical EAE and, when delivered therapeutically, improved clinical scores following a single dose. The Treg frequencies in the spleens of mice treated with autoantigen peptide–loaded nanoparticles were significantly higher than those in vehicle-treated mice. Moreover, nanoparticle-mediated disease control was abrogated after Treg depletion by repeated administration of Treg-depleting anti-CD25 antibody. Other groups have also investigated particles for the induction of Tregs in vitro and in vivo by delivering combinations of immune-modifying agents such as IL-2, TGF-β, or rapamycin (53, 54). Understanding the cell types associated with particles in vivo will enable the development of more effective tolerizing therapies that focus on optimizing cell-specific tolerogenic responses.
2.2. Oral Delivery
The gut mucosa is regularly exposed to foreign antigens and has developed the ability to protect the body from harmful pathogens while becoming and remaining unresponsive to food antigens and commensal microbes (55). For more than 100 years, this ability to develop tolerance to specific antigens has motivated the development of strategies to exploit this mechanism (56). Tolerance via the oral route is desirable due to its lack of toxicity, ease of administration, and antigen-specific mechanism (57, 58). Sites of antigen uptake in the intestines can be separated into inductive and effector sites that affect the outcome of oral tolerance (59). Inductive sites include the gut-associated lymphoid tissue (GALT) that comprises Peyer’s patches, isolated lymphoid follicles, and the mesenteric lymph nodes (mLNs). The effector sites that house large numbers of T cells and plasma cells are composed of the lamina propria (LP) and epithelium. The GALT is crucial for the recognition of particulate antigens, including viruses and bacteria, whereas antigen uptake by CD103+ DCs in the LP are important for the induction of immune oral tolerance to soluble antigens in the intestine.
Delivery of multiple low doses of antigen favors the induction of regulatory cells, whereas high doses of antigen result in anergy or deletion of specific T cells (57, 60, 61). At high doses, antigen can transfer across the gastrointestinal wall and enter the systemic circulation, where it can lead to tolerance, as described above for intravenous delivery of peptides. Low doses of antigen, however, are internalized by mucosa-associated APCs that activate Tregs to secrete suppressive cytokines, such as TGF-β, IL-4, and IL-10 (62–64). Oral administration of antigen suppresses the initiation of autoimmune disease in multiple animal models, although attempts to treat disease after the onset of symptoms by peptide delivered through oral routes have been less successful (65). Approaches are being developed on the basis of combinatorial delivery with other tolerogenic factors (66); nevertheless, despite the attractive features of oral tolerance, degradation of the peptide prior to reaching APCs within the intestines remains a significant challenge.
Whereas many soluble antigens and food proteins achieve tolerance through the LP, larger molecules or particles may aim to deliver antigen to the GALT (M cells) (67). The use of particles as delivery vehicles for oral administration is a topic of interest due to these particles’ stability in the gastrointestinal tract, modular physicochemical properties, payload encapsulation and release properties, and tunable biological interactions (68). Oral delivery of PLG particles with encapsulated type II collagen accumulated in Peyer’s patches and coincided with significantly reduced incidence and severity of arthritis in comparison to particles with unencapsulated collagen (69). Additionally, oral peptide delivery within PLG particles in a single dose resulted in the production of IL-4 and IL-10 in the Peyer’s patches, which could contribute to a tolerogenic response (70). The design of particles capable of targeting delivery to specific receptors on M cells may have the potential to enhance oral tolerance (67).
An alternative approach to facilitate delivery of the peptides to the appropriate cells within the intestines involves engineering food, such as lettuce, to contain the peptide of interest (71). This method overcomes the need for cold storage and transport, does not require expensive purification, and does not have the short shelf life of current protein drugs (72). Additionally, it should eliminate injections, increase patient compliance/convenience, and significantly lower costs. Lettuce leaf cells contain chloroplasts, which have their own DNA that is separate from genomic DNA; these were engineered to express a protein. The protein is protected from the enzymes in the stomach by the plant cell walls, yet enzymes available from microorganisms within the gut can degrade the cell walls and release the protein. Animal models have demonstrated the potential of inducing tolerogenic responses by feeding animals these engineered foods. Ma et al. (73) demonstrated that oral tolerance to prevent diabetes with transgenic plants required glutamic acid decarboxylase (GAD) and IL-4. Both human GAD65 and IL-4 were expressed in transgenic plants for feeding trials; both were required to protect NOD mice from diabetes.
The packaging of proteins to protect against degradation is widely used for gene delivery, which has also been employed for immune tolerance through oral delivery. In this strategy, DNA encoding for the antigen is delivered. Chitosan is a positively charged carbohydrate, isolated from crustaceans, that can form a complex with negatively charged DNA, which protects the DNA within the stomach and delivers the DNA into the gut (74). Cells are able to internalize the plasmid and express the protein without activating the immune system, and the presence of the protein in the gut mucosa can promote reduced immune responses toward the target protein. Nanoparticles with ovalbumin (OVA)-encoding DNA mitigated the OVA-specific delayed-type hypersensitivity response and reduced the formation of anti-OVA antibody as well as spleen cell proliferation following OVA stimulation (75). Cytokine expression following OVA stimulation showed a shift from a Th1 cell response to a Th2/Th3 cell response. Tregs mediated the tolerance, and the tolerance was transferable to naïve mice.
2.3. Subcutaneous Delivery
Subcutaneous immunization is standard in clinical vaccination and thus would be desirable for administering tolerogenic therapies. Physiologically, the subcutaneous space contains immune cells prepared to mount an immune response to pathogens that pass across the skin (76). In vaccination, DCs are the primary target for the antigen and adjuvant that initiate the immune response. Nevertheless, subcutaneous tolerance has been achieved in mouse models by administering subimmunogenic doses of antigen by osmotic pumps (77) and has been employed to desensitize patients to allergens. The past 20 years have uncovered new and distinct DC subsets, which have become targets for immunomodulation. However, it is unclear whether the tolerogenic phenotype of DCs is attributed to the DC subtype inherently or determined by the experience-based functional state of the DCs (78). Therefore, tolerance strategies have focused on both targeting of distinct DC subsets and codelivery of factors that skew the interaction of DCs with T cells.
The physical properties of the antigen carrier influence the antigen distribution upon delivery, which involves transport of the antigen to the draining lymph node by passive diffusion or active transport via migratory DCs (79, 80). Small particles (20–200 nm) passively diffuse to the lymph nodes, whereas larger particles (500–2,000 nm) are associated with DCs at the injection site and require their migration to reach the draining lymph node (81). Furthermore, surface properties, such as the density of PEG on particles, influence access and uptake of particles following subcutaneous delivery (82). Increased PEG densities on 200-nm particles generally lead to an increased number of particles draining to the lymph nodes. Additionally, the PEG density affects the cell types interacting with the particles. Greater PEG densities result in greater associations with DCs and reduced interactions with B cells.
Antigen-delivery strategies focused on targeting lymphoid-resident and migratory DCs aim to polarize the cells toward a tolerogenic phenotype, which is accomplished through subimmunogenic doses of antigen or codelivery of an immunomodulatory factor to promote a tolerogenic response. Paralleling traditional immunization, diabetogenic antigen GAD65 administered subcutaneously with Th2 cell–skewing adjuvant aluminum hydroxide has been tested in Phase II and III clinical trials for the treatment of early-onset T1D. The Phase II trial reported an increase in type 2 cytokine production and Foxp3+ cells, as well as a decreased rate of fasting C-peptide loss compared with placebo. However, the Phase III trial was unable to show an advantage of GAD-alum over placebo (83, 84). Alternatively, strongly agonistic antigen mimotopes have prevented T1D onset in NOD mice in the absence of adjuvants and cofactors (85). The authors of this study posit that whereas moderately agonistic T cell stimulation by self-peptide stimulates autoreactive T cells, low-dose delivery of highly agonistic peptides causes Treg expansion in the periphery. Osmotic minipumps delivering low doses of insulin mimotope over a 2-week period prevented diabetes and increased the Treg population in the pancreas (85).
Strategies have also been developed to conjugate antigen onto larger polymers or proteins (86). Hyaluronic acid polymers grafted with antigen- and B7-inhibiting peptides decreased disease scores in the EAE model with three subcutaneous injections, a result that is hypothesized to result from inhibition of costimulation in the context of TCR engagement. Similarly, myelin oligodendrocyte glycoprotein (MOG) peptide has been cloned into antibodies to target specific migratory and lymphoid-resident DCs subsets (87). Subcutaneous delivery of anti-DEC205-MOG and anti-Langerin-MOG monoclonal antibodies resulted in de novo expansion of Tregs as well as decreased clinical scores in EAE mice treated prophylactically. These findings implicate migratory DCs as crucial for inducing tolerance subcutaneously.
Nanoparticles have been employed to target cells in the proximal draining lymph nodes (88–91). PLG particles loaded with antigen and rapamycin delivered subcutaneously into the hind feet of mice had rapid and selective accumulation in the popliteal, iliac, and renal lymph nodes (49). Particle delivery reduced EAE disease scores and relapse, and was associated with a decrease in antigen-specific T cell proliferation and an increase in Tregs. (50). PLG particles have also been employed to codeliver antisense oligonucleotides targeting CD40, CD80, and CD86, reversing hyperglycemia in NOD mice. After subcutaneous abdominal injection, particles trafficked to the pancreas and pancreatic draining lymph nodes in mice and cynomolgus monkeys. Interestingly, normoglycemia was achieved in mice injected with and without antigen, which was attributed to the presence of β cell antigens draining to the lymph nodes (92).
Particle systems have been used to codeliver multiple payloads to the cells in the subcutaneous space. MOG35–55 peptide was encapsulated in 200-nm PLG particles and delivered subcutaneously, along with a second particle containing IL-10, for the treatment of EAE (93). Interestingly, IL-10 was required in order to significantly mitigate the EAE clinical scores. The particles significantly inhibit IL-17 and IFN-γ production. The authors of this study (93) suggested that the particles induced immune deviation rather than anergy. A combination of four particle types was employed to simultaneously deliver multiple signals to achieve T1D protection in 40% of NOD mice (94). Large particles (~30 μm, unphagocytosable) delivered TGF-β and GM-CSF, whereas mesoparticles (~1 μm, phagocytosable) were loaded with insulin and vitamin D3. More recently, a subcutaneous hydrogel containing GM-CSF, CpG ODN1826 (CpG), and insulin-encapsulated PLG microparticles was used to recruit and polarize cells toward a tolerogenic phenotype (95). This combinatorial hydrogel protected 40% of NOD mice from T1D onset when implanted subcutaneously at three time points. The implantation sites formed granulomas rich in macrophages, T cells, and B cells. The authors of this study suggested that this temporary microenvironment served as a site for reeducating cells toward a tolerogenic phenotype. More generally, these studies collectively represent only one example of the opportunities associated with localized delivery of factors as a means to recruit, expand, and direct cell fate (96).
3. TOLERANCE IN CELL TRANSPLANTATION
T1D illustrates the opportunity and need for cell-based transplant therapies. In T1D, the body’s insulin-producing β cells are destroyed by autoimmunity. Although transplantation of allogeneic islets from deceased donors is a promising form of β cell replacement for T1D (5, 6), stem cell–derived β cells represent an alternative and potentially unlimited source of β cells (7, 8). In this case, establishing universal donors of stem cells for β cell differentiation would have significant advantages over individually derived β cells due to reduced costs from product testing for large-scale manufacturing. However, both deceased donor islets and universal donor stem cell–derived β cells are allogeneic to the recipients and require lifelong immunosuppression to prevent rejection. Achieving transplant tolerance to avoid immunosuppression is therefore highly desirable. In this section, we review currently available approaches and discuss the potential of bioengineering methods to control the complex immune process of rejection, involving activation of both innate immunity and adaptive immunity (97, 98).
3.1. Introduction to Allogeneic Tolerance
Achieving transplant tolerance will specifically constrain antidonor immunity and yet leave an otherwise intact immune system to avoid deleterious situations such as emergent pathogen infections and malignancies associated with immunosuppressive therapies. Ways to achieve immune tolerance are multifactorial, representing the complexity of the process. The process of immune recognition and immune destruction of transplanted cells comprises multiple steps: (a) inflammation, (b) maturation and migration of DCs to draining lymph nodes, (c) T cell activation by DCs, resulting in expansion of antidonor T cells, and (d ) migration of T cells to the graft, where they mediate cytotoxicity (98). Furthermore, the scope of tolerance in transplantation from nonself is broader than that of tolerance in autoimmunity, due to the many, sometimes redundant pathways of transplant immunity.
3.1.1. Breadth of antigens
The primary antigens that trigger the host rejection immune response are the MHCs; in humans, these are called human leukocyte antigens (HLAs). The HLA genes exhibit extreme polymorphism, and thousands of new alleles have been and are continuing to be identified. However, the immunogenicity of HLA mismatches has recently been suggested to stem from individual alloreactive “determinants” or “epitopes” within each HLA antigen (99). Every HLA antigen has a unique set of such epitopes, although many are shared between different HLA antigens. Consequently, each HLA mismatch, in essence, could be viewed as a set of multiple epitope mismatches. In any given donor–recipient pair, the number of HLA mismatches multiplied by the number of different epitopes in these HLA antigens results in a large number of potentially immunogenic epitope mismatches. To further complicate the situation, as evidenced in rejection in HLA–identically matched transplants, non-HLA or minor histocompatibility antigens (mHAs) have also been implicated in eliciting strong cellular immune responses. Although the Y chromosome–encoded male-specific antigens were the first identified mHAs, based on the known abundance of functional variants in the human genome and recent rapid genomic advances, the number of mHA mismatches between any given donor–recipient pair is expected to be large (100). Two important aspects of the potentially large numbers of HLA and mHA mismatches should be considered when assessing their importance in transplant rejection and tolerance. First, it is likely that different mismatches elicit immunogenicity of a wide range of strength, and the same mismatch may elicit different immunogenicity depending on recipient antigen processing and presenting HLAs. Second, when considering antigen-specific tolerance strategies (as detailed in Section 3.2, below), engineered tolerance to one epitope may result in cotolerance (bystander regulation) to other epitopes that are expressed by the same cells, a situation that has previously been described as linked suppression (101). The latter possibility may be exploited to reduce the complexity of the target transplant antigens.
3.1.2. Redundant effector pathways
Transplant immunity is uniquely robust because it can be triggered by several parallel antigen presentation pathways (97): direct antigen presentation by donor-derived APCs presenting donor HLAs, indirect antigen presentation by recipient-derived APCs presenting processed donor HLA peptides, and semidirect antigen presentation by recipient-derived APCs that have acquired and now present intact donor HLAs. The subsequent effector mechanisms triggered by these antigen presentation pathways are also varied. Whereas classical Th1 CD4+ T cells and cytotoxic CD8 T cells are thought to be mainly responsible for rejection, recent studies have implicated a whole spectrum of other effector cells in this process, including Th2 cells, Th17 cells, memory CD8 T cells, and cells of the innate immune system such as monocytes and natural killer cells. Which effector pathway(s) dominates in any given rejection process varies depending on the specific tissue/organ transplanted and the host immune composition (e.g., microbiota, presence or absence of other inflammatory signals). In addition, suppression of one effector pathway may lead to the induction of an alternative effector pathway to promote rejection (102). The challenge resulting from this redundancy is that a robust tolerance strategy will likely need to effectively control multiple pathways. At the same time, effective tolerance approaches will likely need to be personalized on the basis of best-predicted effector pathways involved in a given patient and for the transplant of a specific tissue.
3.1.3. Prior sensitization
Transplant recipients are frequently sensitized to alloantigens because of prior blood transfusions, pregnancies, and/or transplantation. Sensitized recipients may manifest preexisting anti-HLA antibodies, which may fix complement and mediate cytotoxicity upon binding to the recognized HLA antigens on the transplanted organ, leading to hyperacute rejection of the transplanted organ. This situation can now be effectively avoided by ensuring pretransplant elimination of such antibodies by desensitization (103), a process that usually involves plasmapheresis. However, in addition to such humoral sensitization, cellular sensitization is also a significant barrier. Allospecific memory T cells can mount robust antidonor responses even with minimal costimulation signals, and memory B cells may be capable of rapidly developing into antibody-secreting plasma cells even in the absence of T cell help (104, 105). These “shortcuts” frequently evade and nullify conventional tolerance mechanisms, and may additionally turn a donor cell–based tolerance therapy into an exacerbating event. Consequently, the design of tolerance therapy in presensitized recipients will need to (a) eliminate the possibility of the tolerogen turning into an immunogen and (b) effectively tolerize memory T and B cells, the latter of which requires an improved understanding of the precise cellular pathways involved in memory T and B cell activation and their effector functions.
3.2. Current Approaches for Clinical Transplant Tolerance
This section summarizes currently available approaches to transplant tolerance induction in the clinical setting.
3.2.1. Mixed chimerism
Mixed chimerism is a state in which donor-derived hematopoietic cells cocirculate with recipient cells without being rejected. When mixed chimerism is achieved, recipients become tolerant to the donor MHC antigens and consequently readily accept a transplanted allograft from the same donor. Induction of mixed chimerism with donor hematopoietic stem cell infusion has recently been achieved in clinical kidney transplantation in humans (106–110). The number of donor-derived hematopoietic cells could be high (>1% of total leukocytes, known as macrochimerism) and readily detectable by conventional methods such as fluorescence-activated cell sorting (FACS), or it could be low (<1% of total leukocytes, known as microchimerism) and detectable only by highly sensitive methods such as polymerase chain reaction (PCR). The induced chimerism could be durable or transient. The predominant mechanism thought to underlie tolerance by macrochimerism is deletional, in which high numbers of donor APCs continuously delete newly developing alloreactive T and B cells in primary lymphoid organs such as the thymus and the bone marrow. Thus, the stability of such tolerance relies on the persistence of donor chimerism. Conversely, the predominant mechanism thought to underlie tolerance by microchimerism is regulatory, in which Tregs are present in peripheral tissues. Clinically, mixed chimerism has been achieved in humans by several existing protocols involving total lymphocyte irradiation (TLI) or localized thymic irradiation, as well as various cytoreductive therapies (e.g., rabbit antithymocyte globulin, cyclophosphamide, anti-CD2 monoclonal antibody, fludarabine), in combination with donor hematopoietic stem cell infusion and, in one case, novel tolerogenic “facilitating cells.” Such mixed chimerism has been achieved in both HLA-matched and HLA-mismatched donor–recipient pairs. Although these groundbreaking successes have led to immunosuppression-free graft survival in a substantial percentage of recipients, their immune reconstitution and long-term immune competence, the stability of tolerance in macro- versus microchimerism, and the long-term risk of graft-versus-host disease (GVHD) remain to be defined.
3.2.2. Donor negative vaccines
The concept of donor negative vaccines stems from the observation that T cell activation requires two signals (Figure 1). Experimental protocols blocking the CD28/CD80/CD86 axis or the CD40/CD40L axis (signal 2), in combination with providing signal 1 (e.g., donor-specific transfusion), can successfully induce long-term tolerance. However, this approach has yet to be translated to clinical practice. Treatment with the fusion protein CTLA-4–Ig (belatacept) in clinical kidney transplantation to block the CD28/CD80/CD86 pathway is associated with a higher frequency of acute rejection, although long-term graft function is not impaired (111). This adverse effect may be due to the escape of memory T cells from the need for positive costimulation, a reduction in the number of the suppressive Tregs due to their dependence on positive costimulation, and/or blockade of negative costimulation by this agent. Treatment with anti-CD154 to block the CD40/CD40L pathway in humans resulted in the development of unexpected thrombotic events due to the expression of CD154 on human platelets (112). With the development of newer costimulation blockade agents that have improved safety profiles in humans, there is a renewed interest in further exploration of the two-signal hypothesis for transplant tolerance induction in the clinic.
3.2.3. Suppressor cells
Several suppressor cell populations have been demonstrated to play important roles in mitigating transplant rejection. The best-studied suppressor cell population is the CD4+CD25+Foxp3+ Tregs. These cells are critical for the induction and maintenance of peripheral tolerance. In transplantation, they migrate to the graft and the graft-draining lymph nodes, where they suppress donor-stimulated effector T cell function (113–115). Since the first report of human Treg expansion, many investigators have experimented with protocols for non-specific or donor-specific expansion of natural Tregs, as well as with protocols for conversion and expansion of induced Tregs. The multicenter international ONE study, involving eight clinical centers in Europe and the United States, is currently under way to test the safety and feasibility of seven different Treg populations in living donor kidney transplantation (116). With such concerted efforts, defining the optimal source, type, number, in vivo stability, and efficacy of Treg therapy will hopefully occur soon.
The second suppressor cell population is mesenchymal stem cells (MSCs). MSCs are multipotent progenitor cells that inhibit innate immunity as well as adaptive immune responses associated with transplant rejection. They suppress the activation and proliferation of both naïve and effector T cells and promote the expansion of Tregs. MSCs were recently demonstrated to have the unique ability to control the expansion of memory T cells, a major barrier in clinical transplantation, as described above. Both autologous and allogeneic MSCs have been studied in animal models in organ or cell transplantation and have shown potential tolerogenic characteristics. The first inhuman study of MSCs in kidney transplantation was published by Tan et al. (117) in 2012; in this experiment, infusion of autologous MSCs at the time of surgery and 10 days later demonstrated superior early graft function, reduced the frequency of acute rejection, and allowed reduction of maintenance calcineurin inhibitor in comparison to conventional therapy. However, clinical application of allogeneic and/or third-party MSCs awaits further studies.
The third suppressor cell population is myeloid-derived suppressor cells (MDSCs). These cells are typically CD11b+Gr1+ in mouse models of transplantation and are considerably less well defined in human recipients of transplantation; they were only recently identified in renal transplant patients as CD11b+CD33+HLA-DR− cells with variable levels of expression of CD14 and CD16, underscoring their phenotypic heterogeneity (118–121). They mediate suppression by several mechanisms, including inhibition of cytotoxic T cell responses by generation of reactive oxygen species and IL-10, depletion of L-arginine via arginase 1 and inducible nitric oxide synthase, depletion of tryptophan via indoleamine 2,3-dioxygenase (IDO), and promotion of Tregs via IFN-γ-dependent pathways. The use of MDSC adoptive transfer cell therapy in modulating allograft rejection has, however, been demonstrated only in animal models of transplantation. Clinical applications that harness the potential of this suppressor cell population will depend on the availability of in vitro protocols that can reproducibly generate well-defined MDSC populations that suppress human alloimmune responses.
3.3. Localized Protection of Transplanted Cells
The concept of local protection by immune privilege has been promoted since the recognition of maternal–fetal tolerance. It later became clear that such a privilege extends to many other tissues, such as the anterior chamber of the eye, the testis, the brain, and to some degree the gut and liver (122). Tumors, by contrast, may be an example of such an immune privilege going awry, allowing malignant cells to evade immune surveillance. Although overlapping mechanisms may be operating for immune privilege or immune tolerance, it is clear that immune privilege involves “local” immune regulation that is dependent on specific tissue-based regulatory mechanisms, which may vary at different sites (123). Nonetheless, these naturally existing mechanisms provide an intriguing opportunity for converting nonprivileged sites to privileged sites for therapeutic purposes such as protecting the transplanted cells or organs. In this regard, immune engineering of the transplantation site for cells or organs is a potentially powerful tool that can exploit local regulatory mechanisms for creating such an immune-privileged environment.
Several soluble immunomodulatory factors have emerged from studies of immune-privileged sites. These include cytokines (TGF-β, IL-10), chemokines (monocyte chemotactic protein 1, stromal cell–derived factor 1), cellular enzymes (IDO, L-arginase), and prostaglandins (prostaglandin E2, leukotriene B4), among others. Biological scaffolds can be used as a vehicle for cell transplantation and are engineered to release factors for dampening local inflammation and creating immune-privileged sites (reviewed in Reference 124). In this regard, we have developed microporous PLG scaffolds for transplantation of islets into an extrahepatic site that is the equivalent of the omentum in the abdomen. These scaffolds can release growth factors that support survival, vascularization, and long-term function of the cells engrafted on/within them—such as release of exendin 4, a glucagon-like protein 1 (GLP-1) receptor agonist that promotes β cell survival and function—and promote superior function of the transplant islets (125). In addition, these scaffolds can deliver immunomodulatory factors, such as TGF-β, CXCL12, or CCL22. TGF-β-releasing scaffolds had fewer infiltrating inflammatory immune cells and promoted longer survival of transplant islet allografts (126). The localized delivery of CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression (127). CCL22-releasing scaffolds attracted plasmacytoid DCs and activated Tregs to promote the ability of DCs to express IDO (128). Scaffolds can be further engineered to release additional factors alone or in combination so as to maximally attract and/or induce suppressor cell phenotypes and suppress inflammatory phenotypes. To further increase suppressor cell populations locally, scaffolds could be directly loaded with cells with immune-inhibitory capacity. In this regard, loading of ex vivo–expanded antigen-specific Tregs onto the scaffolds provided a significant survival advantage for transplanted islet grafts (129).
Various cell surface molecules have been associated with the establishment of immune privilege by manipulating T cell function at the local site. These include Fas ligand (FasL), tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), and CD200. Ligand–receptor ligation and subsequent engagement of cell death pathways induce AICD, playing a pivotal role in immune homeostasis and self-tolerance. Of these cell death pathways, inducing expression of FasL has been the most extensively studied for local immunomodulation for attempted tolerance induction to the transplanted tissue by eliminating pathogenic T cells. Modification of transplanted cells with FasL, when accompanied with short-term rapamycin treatment, yielded long-term engraftment of allogeneic and xenogeneic islets for the treatment of T1D (130–133). The genetic modification of cells to express FasL has been reported to prevent CD4+ T cell–mediated rejection in cardiomyocyte and hematopoietic cell transplants (131, 132). Additionally, FasL-overexpressing myoblasts have been cotransplanted with islets, in either the ipsilateral or contralateral kidney, and induced site-specific and systemic tolerance to restore normoglycemia (130). More recently, FasL was attached to biomaterials to promote T cell apoptosis (134), and it has been adapted to biomaterial carriers used for cell transplantation.
However, the multifaceted nature of this molecule confounds the utility of this approach in several ways, although it also provides clues about the future engineering of this pathway to maximize the desired effects. The first issue is direct tissue toxicity mediated by FasL. The degree of toxicity is dependent on the level of FasL expression and the intrinsic antiapoptotic threshold of the transplanted cells. The second issue involves the potential inflammatory characteristics of the FasL molecule. For example, released soluble FasL can compete with membrane-bound FasL to antagonize its apoptotic effect. In addition, soluble FasL can be chemotactic for neutrophils, further exacerbating local inflammation. Therefore, strategies for FasL-mediated local immunomodulation will likely need to be specifically tailored for the specific tissue/cells to be protected. One such approach is to engineer FasL to be resistant to metalloproteinase cleavage, thereby inhibiting the release of soluble FasL (135). Another approach is to target FasL delivery to specific bystander cell types adjacent to the transplanted tissue/cells to be protected (e.g., chimeric streptavidin–FasL targeted only to biotinylated APCs cotransplanted with allogeneic islets) (136). With advances in nanotechnology, future approaches may involve delivering these apoptosis-inducing molecules along with donor antigens in a single platform to promote transplant tolerance.
3.4. Antigen Delivery in Allogeneic Tolerance
This section summarizes the current approaches for delivering donor antigen as a negative vaccine for transplantation tolerance.
3.4.1. ECDI-treated cells
Within the context of transplant tolerance, intravenous delivery of donor SPs chemically treated with ECDI (ECDI-SPs) have shown robust efficacy in allogeneic islet, heart, and xenogeneic islet transplantation in mouse models by taking advantage of mechanisms similar to those of Ag-SP treatment of autoimmunity (37, 137, 138). However, no additional coupling of antigens is necessary, because donor cells contain the full spectrum of allogeneic or xenogeneic antigens. This tolerance strategy is donor specific (139). This approach is also flexible because B cells of donor origin can be expanded in vitro and subsequently treated with ECDI to induce tolerance. Additionally, the lysate from allogeneic donor cells can be conjugated to syngeneic SPs with ECDI to induce tolerance with no loss of efficacy (140). These findings support the study of the clinical applicability of this approach.
Tolerance mediated by donor ECDI-SPs depends on low APC expression of positive costimulatory molecules such as CD80 and CD86 and enhanced expression of negative costimulatory molecules such as PD-L1 and PD-L2 (141, 142). The immunoregulatory cytokine milieu induced by recognition of apoptotic cells is likely the reason underlying the establishment of an environment wherein negative costimulation is the favored outcome. Supporting this hypothesis are the findings that splenic macrophages express IL-10 following infusion of ECDI-treated antigen coupled leukocytes and that inhibition of IL-10 following infusion of ECDI-SPs prevents tolerance in the context of both autoimmunity and allogeneic islet transplantation (39, 137, 143). A second cytokine implicated in donor ECDI-SP-induced tolerance is IFN-γ. This cytokine is initially produced by CD4 T cells that indirectly recognize donor antigens presented by self-APCs, and it later mediates depletion of such donor-specific T cells and establishment of transplant tolerance (144). Recent experiments indicate that another soluble factor, IDO, can be induced by infusion of apoptotic donor ECDI-SPs (143, 145). The critical role of this factor in transplanting tolerance induced by ECDI-SPs is demonstrated by the abolishment of tolerance when an inhibitor of IDO, 1-methyl-D-tryptophan, is administered at the time of ECDI-SP infusion (145).
Two regulatory cell populations are induced by infusion of donor ECDI-SPs and play a critical role in mediating its tolerogenic effects. In vivo, Tregs are preferentially expanded in frequency in the secondary lymphoid organs and grafts of ECDI-SP-tolerized transplant recipients, and depletion of CD25+ Tregs at the time of donor ECDI-SP treatment prevents the establishment of transplant tolerance. Furthermore, the requirement for TGF-β at the time of tolerance induction by donor ECDI-SPs supports the idea that these Tregs are induced from naïve T cells rather than expanded from existing Tregs (143). Another suppressor cell population induced by donor ECDI-SPs is the MDSCs. Treatment with allo-ECDI-SPs induced a splenic population of MDSCs that produced significant levels of IFN-γ-dependent IDO and suppressed CD8+ T cell proliferation when compared with control mice. Moreover, MDSCs were present in the cardiac allograft and suppressed the infiltration of CD8+ T cells and other effectors. MDSCs present in the graft also produced IL-10 and recruited Tregs in a CCL4-dependent manner, and depletion of MDSCs restored CD8+ T cell infiltration and graft rejection (121).
3.4.2. Nanoparticles
The need for many fresh donor cells in the manufacturing of tolerance cell products (i.e., ECDI-SPs) may be logistically challenging and may also introduce variability and opportunities for pathogen transmission. These issues could be overcome through the use of PLG particles as carriers for delivering donor antigens. PLG particles used for autoimmune tolerance have been adapted to the delivery of alloantigens (146). Given the antigenic diversity, allogeneic tolerance was expected to be inherently more challenging than tolerance to autoimmunity (43, 47, 48). However, our data show that PLG particles linked to solubilized donor antigens (PLG-dAg) can promote long-term islet allograft function (146). Interestingly, we now know that, in addition to modifying adaptive immunity, PLG particles can also modify innate immunity by targeting inflammatory monocytes via MARCO, leading to sequestration in the spleen of monocytes that are incapable of migrating to injured tissues to induce inflammation (48).
We have developed nanoparticles loaded with donor cell lysates and tested their ability to provide long-term protection to islet allografts in a BALB/c → B6 transplant model (146). Donor cell lysate containing donor antigens was coupled to PLG particles through ECDI chemistry to generate PLG-dAg. Delivery of PLG-dAg alone provided a slight advantage to graft survival compared with treatment with empty PLG particles. This is in sharp contrast to the high efficiency of tolerance induction to autoimmunity by peptide-coupled PLG particles (43, 47), highlighting the differences in alloimmune versus autoimmune tolerance by antigen-delivering PLG particles. However, when PLG-dAg particles were combined with a low-dose (0.1 mg/kg) short course (4 days total from day −2 to day +1) of rapamycin, significant long-term graft protection was observed in ~60% of recipients (146). These studies demonstrate the potential of PLG particles for donor antigen–negative vaccination for allogeneic tolerance induction.
Antigen-coupled nanoparticles are likely to be more versatile than ECDI-SPs because they can be engineered to potentially target different host cell populations and consequently elicit a wide spectrum of desired in vivo effects. For the induction of transplantation tolerance using PLG-dAg, our early studies demonstrated that PLG-dAg displayed a similar biodistribution profile to ECDI-SP and accumulated primarily in the liver, spleen, and lungs following intravenous injection (146). Within the spleen, PLG-dAg appears to be internalized by F4/80+ macrophages, CD11c+CD11b+ cells, CD11c+B220+ cells, CD11c+CD8α+ DC subsets, and B220+ B cells, again similar to internalization of ECDI-SPs (146). Importantly, ECDI-PLG-dAg modulates T cells with indirect donor specificity through initial clonal expansion followed by clonal contraction. However, in contrast to ECDI-SPs, PLG-dAg does not seem to anergize T cells with direct donor specificity (146). These findings provide us with initial ideas for improving PLG designs in order to maximize tolerance efficacy, including the route of administration, properties of the nanoparticle platform (size and surface charge), and codelivery immunotherapeutics.
3.4.3. Codelivery of immunotherapeutics
Codelivery of immunotherapeutics along with donor antigens using nanoparticles is an attractive engineering option that may result in significant enhancement of tolerance efficacy. One possibility is codelivery of immunosuppressive/immunomodulatory agents by nanoparticles that may further attenuate the response of T cells interacting with the targeted APCs internalizing the nanoparticles via donor-specific TCRs. Choices include encapsulation of pharmacological agents such as rapamycin and suppressive cytokines such as IL-10, TGF-β, and IDO. Another option is the engineering of tolerogenic ligands into the nanoparticles. For example, incorporating surface phosphatidylserine may promote recognition of the nanoparticles as “apoptotic,” thereby facilitating uptake of the nanoparticles by host phagocytes via specific pathways that lead to “tolerogenic” antigen presentation (147). Other tolerogenic ligands include the cationic polymer polyamine polyethylenimine and ITE, which exploit the immunomodulatory pathways of IDO and aryl hydrocarbon receptor in interacting DCs (148, 149). Lastly, targeted delivery of the donor antigen cargo only to specialized DC populations may preferentially amplify the desired tolerant response. One such DC target is DEC205+ DCs. PLG particles functionalized with anti-DEC205 antibodies have been reported to cross-link the DEC205 receptor, resulting in enhanced production of IL-10 (150).
4. OPPORTUNITIES AND CONCLUSIONS
Owing to the adverse effects of antibody therapy, antigen-specific tolerance strategies have the best therapeutic potential, although more precise knowledge of the antigen(s) and epitope(s) involved in the ongoing pathogenic process in a particular autoimmune disease will be required. Furthermore, a central need will be to extend our understanding of tolerance to a single antigen to the ability to tolerize for multiple antigens. Upon presentation with an autoimmune disease, most patients will have had the disease progress across multiple antigens (epitope switching). As mentioned for allogeneic tolerance, multiple major and minor antigens are responsible for rejection. Thus, strategies must be developed to fully characterize the epitopes recognized by the TCRs on the T cells. Furthermore, we must identify appropriate strategies for delivering multiple antigens capable of suppressing the activity of a broad range of T cell responses.
Mechanistic studies associated with antigen delivery will give rise to particles with improved efficacy and may extend their utility to applications for allergic responses or prevent the development of resistance in patients receiving protein replacement or antibody therapies. Additionally, development and/or identification of therapeutics that either inhibit signaling intermediates of T cell activation or promote anergy-associated signaling intermediates, when used in combination with peptide-specific tolerance therapies, presents a possible combinatorial strategy that may increase therapeutic efficacy while maintaining antigen specificity. Therefore, continued research to enhance specificity and efficacy of treatment in all these strategies is necessary both in animal models and in the clinic with advanced patient screening using modern genomic and pharmacogenomic techniques. The use of these tolerogenic approaches in combination with non-antigen-specific therapies could eventually provide “tailored therapy” to deal with the complexity of the human immune system.
Acknowledgments
The authors are grateful to Ryan Pearson and Liam Casey for their contributions to this manuscript and the figures. The writing of this review was supported by funding from the National Institutes of Health (R01 EB013198, R01 EB09910).
Footnotes
DISCLOSURE STATEMENT
S.D.M. and L.D.S. have a financial interest in Cour Pharmaceuticals.
Contributor Information
Xunrong Luo, Email: xunrongluo@northwestern.edu.
Stephen D. Miller, Email: s-d-miller@northwestern.edu.
Lonnie D. Shea, Email: ldshea@umich.edu.
LITERATURE CITED
- 1.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–77. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 2.Zakrzewski JL, van den Brink MR, Hubbell JA. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol. 2014;32:786–94. doi: 10.1038/nbt.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobias LD. A Briefing Report on Autoimmune Diseases and AARDA: Past, Present, and Future. Eastpointe, MI: AARDA; 2010. [Google Scholar]
- 4.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: the next pillar of medicine. Sci Transl Med. 2013;5:179ps7. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–38. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–69. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 7.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Jiang W, Liu M, Sui X, Yin X, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–38. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 9.Weiner HL. The challenge of multiple sclerosis: How do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–48. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- 10.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 11.Kobashigawa JA, Patel JK. Immunosuppression for heart transplantation: Where are we now? Nat Clin Pract Cardiovasc Med. 2006;3:203–12. doi: 10.1038/ncpcardio0510. [DOI] [PubMed] [Google Scholar]
- 12.Van Gelder H, Charles-Schoeman C. The heart in inflammatory myopathies. Rheum Dis Clin N Am. 2014;40:1–10. doi: 10.1016/j.rdc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–32. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 14.Hafler DA, Slavik JM, Anderson DE, O’Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–31. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 15.Sulzberger MB. Experiments in prevention and in desensitization. Arch Dermatol Syphilol. 1929;20:669–697. [Google Scholar]
- 16.Chiller JM, Weigle WO. Cellular events during induction of immunologic unresponsiveness in adult mice. J Immunol. 1971;106:1647–53. [PubMed] [Google Scholar]
- 17.Burstein HJ, Shea CM, Abbas AK. Aqueous antigens induce in vivo tolerance selectively in IL-2-and IFN-γ-producing (Th1) cells. J Immunol. 1992;148:3687–91. [PubMed] [Google Scholar]
- 18.Critchfield JM, Racke MK, Zúñiga-Pflücker JC, Cannella B, Raine CS, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–43. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 19.Gaur A, Wiers B, Liu A, Rothbard J, Fathman CG. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide–induced anergy. Science. 1992;258:1491–94. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 20.Racke MK, Critchfield JM, Quigley L, Cannella B, Raine CS, et al. Intravenous antigen administration as a therapy for autoimmune demyelinating disease. Ann Neurol. 1996;39:46–56. doi: 10.1002/ana.410390108. [DOI] [PubMed] [Google Scholar]
- 21.Smith CE, Eagar TN, Strominger JL, Miller SD. Differential induction of IgE-mediated anaphylaxis after soluble versus cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. PNAS. 2005;102:9595–600. doi: 10.1073/pnas.0504131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, et al. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996;274:2054–57. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- 23.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. PNAS. 2013;110:e60–68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith TS, Ferguson TA. Cell death in the maintenance and abrogation of tolerance: the five Ws of dying cells. Immunity. 2011;35:456–66. doi: 10.1016/j.immuni.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–30. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. J Immunol. 2013;191:5341–46. doi: 10.4049/jimmunol.1302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson TA, Choi J, Green DR. Armed response: how dying cells influence T-cell functions. Immunol Rev. 2011;241:77–88. doi: 10.1111/j.1600-065X.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–33. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357–65. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190:2009–16. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burghardt S, Erhardt A, Claass B, Huber S, Adler G, et al. Hepatocytes contribute to immune regulation in the liver by activation of the Notch signaling pathway in T cells. J Immunol. 2013;191:5574–82. doi: 10.4049/jimmunol.1300826. [DOI] [PubMed] [Google Scholar]
- 32.Ichikawa S, Mucida D, Tyznik AJ, Kronenberg M, Cheroutre H. Hepatic stellate cells function as regulatory bystanders. J Immunol. 2011;186:5549–55. doi: 10.4049/jimmunol.1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, et al. In vivo–generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci Transl Med. 2014;6:241ra78. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- 34.Wetzig R, Hanson DG, Miller SD, Claman HN. Binding of ovalbumin to mouse spleen cells with and without carbodiimide. J Immunol Methods. 1979;28:361–68. doi: 10.1016/0022-1759(79)90201-1. [DOI] [PubMed] [Google Scholar]
- 35.Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol Rev. 2009;229:337–55. doi: 10.1111/j.1600-065X.2009.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen-coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud. 2012;9:319–27. doi: 10.1900/RDS.2012.9.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–20. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 38.Karpus WJ, Peterson JD, Miller SD. Anergy in vivo: down-regulation of antigen-specific CD4+ Th1 but not Th2 cytokine responses. Int Immunol. 1994;6:721–30. doi: 10.1093/intimm/6.5.721. [DOI] [PubMed] [Google Scholar]
- 39.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187:2405–17. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandenbark AA, Barnes D, Finn T, Bourdette DN, Whitham R, et al. Differential susceptibility of human Th1 versus Th2 cells to induction of anergy and apoptosis by ECDI/antigen-coupled antigen-presenting cells. Int Immunol. 2000;12:57–66. doi: 10.1093/intimm/12.1.57. [DOI] [PubMed] [Google Scholar]
- 41.Vandenbark AA, Celnik B, Vainiene M, Miller SD, Offner H. Myelin antigen-coupled splenocytes suppress experimental autoimmune encephalomyelitis in Lewis rats through a partially reversible anergy mechanism. J Immunol. 1995;155:5861–67. [PubMed] [Google Scholar]
- 42.Lutterotti A, Yusef S, Sputtek A, Sturner K, Stellmann J-P, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5:188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZH, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jing J, Yang IV, Hui L, Patel JA, Evans CM, et al. Role of macrophage receptor with collagenous structure in innate immune tolerance. J Immunol. 2013;190:6360–67. doi: 10.4049/jimmunol.1202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–34. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 46.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci. 2007;97:398–406. doi: 10.1093/toxsci/kfm050. [DOI] [PubMed] [Google Scholar]
- 47.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8:2148–60. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. PNAS. 2015;112:e156–65. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. PNAS. 2012;109:11270–75. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carambia A, Freund B, Schwinge D, Bruns OT, Salmen SC, et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol. 2015;62:1349–56. doi: 10.1016/j.jhep.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Carambia A, Freund B, Schwinge D, Heine M, Laschtowitz A, et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594–99. doi: 10.1016/j.jhep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 53.McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–81. doi: 10.1016/j.biomaterials.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jhunjhunwala S, Chen LC, Nichols EE, Thomson AW, Raimondi G, Little SR. All-trans retinoic acid and rapamycin synergize with transforming growth factor β1 to induce regulatory T cells but confer different migratory capacities. J Leukoc Biol. 2013;94:981–89. doi: 10.1189/jlb.0312167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–39. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells HG, Osborne TB. The biological reactions of the vegetable proteins. I Anaphylaxis. J Infect Dis. 1911;8:66–124. [Google Scholar]
- 57.Faria AMC, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizrahi M, Ilan Y. The gut mucosa as a site for induction of regulatory T cells. Curr Pharm Des. 2009;15:1191–202. doi: 10.2174/138161209787846784. [DOI] [PubMed] [Google Scholar]
- 59.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 60.Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell Immunol. 1988;112:364–70. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 61.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III Evidence for clonal anergy. J Immunol. 1991;147:2155–63. [PubMed] [Google Scholar]
- 62.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 64.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor β after antigen-specific triggering. PNAS. 1992;89:421–25. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy KJ, Smith WS, Miller SD, Karpus WJ. Induction of antigen-specific tolerance for the treatment of ongoing, relapsing autoimmune encephalomyelitis: a comparison between oral and peripheral tolerance. J Immunol. 1997;159:1036–44. [PubMed] [Google Scholar]
- 66.Slavin AJ, Maron R, Weiner HL. Mucosal administration of IL-10 enhances oral tolerance in autoimmune encephalomyelitis and diabetes. Int Immunol. 2001;13:825–33. doi: 10.1093/intimm/13.6.825. [DOI] [PubMed] [Google Scholar]
- 67.Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLOS Pathog. 2010;6:e1001147. doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, et al. Oral delivery of bioencapsulated proteins across blood–brain and blood–retinal barriers. Mol Ther. 2014;22:535–46. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee WK, Park JY, Jung S, Yang CW, Kim WU, et al. Preparation and characterization of biodegradable nanoparticles entrapping immunodominant peptide conjugated with PEG for oral tolerance induction. J Control Release. 2005;105:77–88. doi: 10.1016/j.jconrel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in β-TC6 cells. Plant Biotechnol J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su J, Zhu L, Sherman A, Wang X, Lin S, et al. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015;70:84–93. doi: 10.1016/j.biomaterials.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma S, Huang Y, Yin Z, Menassa R, Brandle JE, Jevnikar AM. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. PNAS. 2004;101:5680–85. doi: 10.1073/pnas.0307420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowman K, Sarkar R, Raut S, Leong KW. Gene transfer to hemophilia A mice via oral delivery of FVIII-chitosan nanoparticles. J Control Release. 2008;132:252–59. doi: 10.1016/j.jconrel.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldmann K, Ensminger SM, Spriewald BM. Oral gene application using chitosan–DNA nanoparticles induces transferable tolerance. Clin Vaccine Immunol. 2012;19:1758–64. doi: 10.1128/CVI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 77.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–8. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–77. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szczepanik M. Skin-induced tolerance as a new needle free therapeutic strategy. Pharmacol Rep. 2014;66:192–97. doi: 10.1016/j.pharep.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Ma W, Chen M, Kaushal S, McElroy M, Zhang Y, et al. PLGA nanoparticle–mediated delivery of tumor antigenic peptides elicits effective immune responses. Int J Nanomedicine. 2012;7:1475–87. doi: 10.2147/IJN.S29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 82.Zhan X, Tran KK, Shen H. Effect of the poly(ethylene glycol) (PEG) density on the access and uptake of particles by antigen-presenting cells (APCs) after subcutaneous administration. Mol Pharm. 2012;9:3442–51. doi: 10.1021/mp300190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–20. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 84.Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–42. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 85.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501–10. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Northrup L, Sestak JO, Sullivan BP, Thati S, Hartwell BL, et al. Co-delivery of autoantigen and B7 pathway modulators suppresses experimental autoimmune encephalomyelitis. AAPS J. 2014;16:1204–13. doi: 10.1208/s12248-014-9671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Investig. 2013;123:844–54. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clatworthy MR, Aronin CE, Mathews RJ, Morgan NY, Smith KG, Germain RN. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat Med. 2014;20:1458–63. doi: 10.1038/nm.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–99. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, et al. Lymph node–resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–88. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waithman J, Allan RS, Kosaka H, Azukizawa H, Shortman K, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I–restricted self-reactive T cells. J Immunol. 2007;179:4535–41. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 92.Engman C, Wen Y, Meng WS, Bottino R, Trucco M, Giannoukakis N. Generation of antigen-specific Foxp3+ regulatory T-cells in vivo following administration of diabetes-reversing tolerogenic microspheres does not require provision of antigen in the formulation. Clin Immunol. 2015;160:103–23. doi: 10.1016/j.clim.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Cappellano G, Woldetsadik AD, Orilieri E, Shivakumar Y, Rizzi M, et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine. 2014;32:5681–89. doi: 10.1016/j.vaccine.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 94.Lewis JS, Dolgova NV, Zhang Y, Xia CQ, Wasserfall CH, et al. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clin Immunol. 2015;160:90–102. doi: 10.1016/j.clim.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoon YM, Lewis JS, Carstens MR, Campbell-Thompson M, Wasserfall CH, et al. A combination hydrogel microparticle–based vaccine prevents type 1 diabetes in non-obese diabetic mice. Sci Rep. 2015;5:13155. doi: 10.1038/srep13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glowacki AJ, Gottardi R, Yoshizawa S, Cavalla F, Garlet GP, et al. Strategies to direct the enrichment, expansion, and recruitment of regulatory cells for the treatment of disease. Ann Biomed Eng. 2015;43:593–602. doi: 10.1007/s10439-014-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alegre ML, Florquin S, Goldman M. Cellular mechanisms underlying acute graft rejection: time for reassessment. Curr Opin Immunol. 2007;19:563–68. doi: 10.1016/j.coi.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 98.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 99.Glotz D, Tambur A. Stratifying patients based on epitope mismatching: ready for primetime? Am J Transplant. 2015;15:2021–22. doi: 10.1111/ajt.13343. [DOI] [PubMed] [Google Scholar]
- 100.Spierings E. Minor histocompatibility antigens: past, present, and future. Tissue Antigens. 2014;84:374–60. doi: 10.1111/tan.12445. [DOI] [PubMed] [Google Scholar]
- 101.Cobbold S, Waldmann H. Infectious tolerance. Curr Opin Immunol. 1998;10:518–24. doi: 10.1016/s0952-7915(98)80217-3. [DOI] [PubMed] [Google Scholar]
- 102.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D’Addio F, Mfarrej B, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–44. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jordan SC, Choi J, Vo A. Achieving incompatible transplantation through desensitization: current perspectives and future directions. Immunotherapy. 2015;7:377–98. doi: 10.2217/imt.15.10. [DOI] [PubMed] [Google Scholar]
- 104.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17:564–72. doi: 10.1681/ASN.2005030293. [DOI] [PubMed] [Google Scholar]
- 105.Lúcia M, Luque S, Crespo E, Melilli E, Cruzado JM, et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 2015;88:874–87. doi: 10.1038/ki.2015.205. [DOI] [PubMed] [Google Scholar]
- 106.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–68. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 107.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: Durable chimerism predicts outcome. Transplantation. 2013;95:169–76. doi: 10.1097/TP.0b013e3182782fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leventhal JR, Mathew JM, Salomon DR, Kurian SM, Suthanthiran M, et al. Genomic biomarkers correlate with HLA-identical renal transplant tolerance. J Am Soc Nephrol. 2013;24:1376–85. doi: 10.1681/ASN.2013010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talawila N, Pengel LH. Does belatacept improve outcomes for kidney transplant recipients? A systematic review. Transpl Int. 2015;28:1251–64. doi: 10.1111/tri.12605. [DOI] [PubMed] [Google Scholar]
- 112.Li XL, Menoret S, Le Mauff B, Angin M, Anegon I. Promises and obstacles for the blockade of CD40–CD40L interactions in allotransplantation. Transplantation. 2008;86:10–15. doi: 10.1097/TP.0b013e31817c4b97. [DOI] [PubMed] [Google Scholar]
- 113.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, et al. Dendritic cells with TGF-β1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. PNAS. 2007;104:2821–26. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pothoven KL, Kheradmand T, Yang Q, Houlihan JL, Zhang H, et al. Rapamycin-conditioned donor dendritic cells differentiate CD4CD25Foxp3 T cells in vitro with TGF-β1 for islet transplantation. Am J Transplant. 2010;10:1774–84. doi: 10.1111/j.1600-6143.2010.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 116.Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant. 2014;14:750–63. doi: 10.1111/ajt.12647. [DOI] [PubMed] [Google Scholar]
- 117.Tan J, Wu W, Xu X, Liao L, Zheng F, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–77. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 118.Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, et al. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4+ Foxp3+ Treg expansion. Am J Transplant. 2013;13:3123–31. doi: 10.1111/ajt.12461. [DOI] [PubMed] [Google Scholar]
- 119.Ochando J, Conde P, Bronte V. Monocyte-derived suppressor cells in transplantation. Curr Transplant Rep. 2015;2:176–83. doi: 10.1007/s40472-015-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conde P, Rodriguez M, van der Touw W, Jimenez A, Burns M, et al. Immunity. 2015;42:1143–58. doi: 10.1016/j.immuni.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bryant J, Lerret NM, Wang JJ, Kang HK, Tasch J, et al. Preemptive donor apoptotic cell infusions induce IFN-γ-producing myeloid-derived suppressor cells for cardiac allograft protection. J Immunol. 2014;192:6092–101. doi: 10.4049/jimmunol.1302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gibly RF, Graham JG, Luo X, Lowe WL, Jr, Hering BJ, Shea LD. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54:2494–505. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372–81. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 124.Dumont CM, Park J, Shea LD. Controlled release strategies for modulating immune responses to promote tissue regeneration. J Control Release. 2015;219:155–56. doi: 10.1016/j.jconrel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hlavaty KA, Gibly RF, Zhang X, Rives CB, Graham JG, et al. Enhancing human islet transplantation by localized release of trophic factors from PLG scaffolds. Am J Transplant. 2014;14:1523–32. doi: 10.1111/ajt.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu JMH, Zhang J, Zhang X, Hlavaty KA, Ricci CF, et al. Transforming growth factor β1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials. 2015;80:11–19. doi: 10.1016/j.biomaterials.2015.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen T, Yuan J, Duncanson S, Hibert ML, Kodish BC, et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am J Transplant. 2015;15:618–27. doi: 10.1111/ajt.13049. [DOI] [PubMed] [Google Scholar]
- 128.Bischoff L, Alvarez S, Dai DL, Soukhatcheva G, Orban PC, Verchere CB. Cellular mechanisms of CCL22-mediated attenuation of autoimmune diabetes. J Immunol. 2015;194:3054–64. doi: 10.4049/jimmunol.1400567. [DOI] [PubMed] [Google Scholar]
- 129.Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng A. 2013;19:1465–75. doi: 10.1089/ten.tea.2012.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–12. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 131.Pearl-Yafe M, Kaminitz A, Yolcu ES, Yaniv I, Stein J, Askenasy N. Pancreatic islets under attack: cellular and molecular effectors. Curr Pharm Des. 2007;13:749–60. doi: 10.2174/138161207780249155. [DOI] [PubMed] [Google Scholar]
- 132.Plenter RJ, Grazia TJ, Nelson DP, Zamora MR, Gill RG, Pietra BA. Ectopic expression of Fas ligand on cardiomyocytes renders cardiac allografts resistant to CD4+ T-cell mediated rejection. Cell Immunol. 2015;293:30–33. doi: 10.1016/j.cellimm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J Immunol. 2011;187:5901–9. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hume PS, Anseth KS. Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations. Biomaterials. 2010;31:3166–74. doi: 10.1016/j.biomaterials.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–17. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 136.Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 137.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. PNAS. 2008;105:14527–32. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S, Tasch J, Kheradmand T, Ulaszek J, Ely S, et al. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes. 2013;62:3143–50. doi: 10.2337/db12-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. PNAS. 2008;105:14527–32. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, et al. Preemptive tolerogenic delivery of donor antigens for permanent allogeneic islet graft protection. Cell Transplant. 2014;24:1155–65. doi: 10.3727/096368914X681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eagar TN, Karandikar NJ, Bluestone J, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immnol. 2002;32:972–81. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 142.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–10. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189:804–12. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–7. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 145.Chen G, Kheradmand T, Bryant J, Wang S, Tasch J, et al. Intragraft CD11b+IDO+ cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am J Transplant. 2012;12:2920–29. doi: 10.1111/j.1600-6143.2012.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bryant J, Hlavaty KA, Zhang X, Yap WT, Zhang L, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials. 2014;35:8887–94. doi: 10.1016/j.biomaterials.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang L, Lemos HP, Li L, Li M, Chandler PR, et al. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J Immunol. 2012;188:4913–20. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peterson JC, Nordstrom RJ, Long RK. Subtle source of contamination in spectrophones. Appl Opt. 1976;15:2974–76. doi: 10.1364/AO.15.002974. [DOI] [PubMed] [Google Scholar]
- 150.Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32:3094–105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]