Abstract
Despite major advances in recent years, graft-versus-host disease (GVHD) remains a major life-threatening complication of allogeneic hematopoietic cell transplantation (allo-HCT). To improve our therapeutic armory against GVHD, preclinical evidence is most frequently generated in mouse and large animal models of GVHD. However, because every model has shortcomings, it is important to understand how predictive the different models are and why certain findings in these models could not be translated into the clinic. Weaknesses of the animal GVHD models include the irradiation only-based conditioning regimen, the homogenous donor/recipient genetics in mice, canine or non-human primates (NHP), anatomic site of T cells used for transfer in mice, the homogenous microbial environment in mice housed under specific pathogen-free conditions, and the lack of pharmacologic GVHD prevention in control groups. Despite these major differences toward clinical allo-HCT, findings generated in animal models of GVHD have led to the current gold standards for GVHD prophylaxis and therapy. The homogenous nature of the preclinical models allows for reproducibility, which is key for the characterization of the role of a new cytokine, chemokine, transcription factor, microRNA, kinase, or immune cell population in the context of GVHD. Therefore, when carefully balancing reasons to apply small and large animal models, it becomes evident that they are valuable tools to generate preclinical hypotheses, which then have to be rigorously evaluated in the clinical setting. In this study, we discuss several clinical approaches that were motivated by preclinical evidence, novel NHP models and their advantages, and highlight the recent advances in understanding the pathophysiology of GVHD.
Introduction
Our understanding of the roles of the innate immune system, the adaptive immune system, and different epithelial and antigen-presenting cell types in graft-versus-host disease (GVHD) pathogenesis has made major advances over the last 2 decades. Despite these advances and the prophylactic treatment with a wider array of immunosuppressive medication, ∼50% of the patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) develop grade 2-4 acute GVHD (aGVHD).1 aGVHD patients who are refractory to standard steroid treatment have a dismal long-term prognosis with only 5% to 30% overall survival (OS).2-4 Chronic GVHD (cGVHD) causes high morbidity, reduces the quality of life, and is associated with a significantly higher risk of treatment-related mortality and inferior OS.5 Clinical experience teaches that aGVHD and cGVHD in humans are multilayer diseases, which are hard to treat once they are fully established. The immunologic complexity of the disease and the role of donor and recipient cell types has been the focus of intensive research.6-10
In this review we discuss different prophylactic and therapeutic approaches against aGVHD and cGVHD that have been developed in preclinical models and analyze how successful these approaches were later in clinical trials. We divide the preclinical approaches into pharmacologic and cellular therapy strategies and connect them to the resulting clinical studies. Additionally, promising novel approaches in mice and non-human primate (NHP) models of GVHD that have not yet entered clinical studies will be discussed.
Pharmacologic prophylaxis and therapy of aGVHD
Basics of aGVHD prophylaxis and therapy
The basic pharmacologic aGVHD prophylaxis with cyclosporine A (CyA) and methotrexate (MTX) that is still used in a large proportion of the currently applied immunosuppressive regiments following allo-HCT was first studied in the dog model, where it showed potent inhibitory effects on aGVHD.11 The studies in dogs were followed by a clinical study that revealed that the combination of CyA and MTX was superior to CyA alone with respect to protection from GVHD and survival, and therefore the calcineurin inhibitor CyA became the gold standard for GVHD prophylaxis.12 Later, the combination of tacrolimus and MTX after unrelated allo-HCT was shown to significantly decrease the risk for aGVHD, but not OS and relapse-free survival rates compared with CyA and MTX,13 and therefore both CyA and tacrolimus are the backbone of most immunosuppressive regimens for patients currently undergoing allo-HCT worldwide. Another established component for aGVHD prophylaxis is anti-thymocyte globulin (ATG), which was initially reported to be protective against GVHD in the canine model.14 Different types of ATG exist and for rabbit anti-thymocyte globulin Fresenius (ATG-F), a randomized, open-label, multicenter phase 3 trial was performed that showed a decreased incidence of aGVHD and cGVHD without an increase in relapse or nonrelapse mortality (NRM) when ATG-F was added to the standard GVHD prophylaxis.15 This was extended recently by a multicenter trial showing that the rate of a composite end point of cGVHD-free survival and relapse-free survival was higher with ATG.16 Besides ATG-F, thymoglobulin was also shown to be protective against GVHD.17 Steroids currently represent the gold-standard treatment of aGVHD based on multiple prospective trials.18,19 Early evidence supporting the use of corticosteroids against aGVHD was provided in the 1990s in a haploidentical parent into F1 mouse models of GVHD.20,21
Cytokine and chemokine inhibition as acute aGVHD prophylaxis and therapy
To avoid the broad spectrum of side effects caused by corticosteroids and to be able to offer a therapeutic option for patients with GVHD that had failed corticosteroids, the role of multiple cytokines in the pathophysiology of aGVHD was investigated in the mouse model. Interleukin-11 (IL-11),22,23 IL-1β,24 tumor necrosis factor-α (TNF-α),25,26 and IL-627,28 among others were targeted in mouse models of GVHD leading to later clinical studies. In the mouse model of GVHD, IL-11 promoted T-cell polarization toward a T helper 2 (Th2) phenotype, was associated with a lower level of IL-12, and reduced GVHD-related mortality.22,23 Consequently, recombinant human IL-11 was then investigated in a phase 1/2 double-blind, placebo-controlled study for mucositis and aGVHD prevention.29 Of 10 evaluable patients who received IL-11 in this trial, 4 died by day 40 and 1 died on day 85 due to transplant-related toxicity.29 The major adverse side effect in patients receiving IL-11 was severe fluid retention that caused pulmonary edema.29 This trial was not able to determine whether IL-11 given in this schedule can reduce the rate of GVHD. The unexpected high mortality showed that a cytokine that was well-tolerated by the mice induced severe side effects in humans, sounding a note of caution for investigators translating findings from preclinical models into a trial on humans. IL-1β was shown to be a proinflammatory cytokine in intestinal inflammation,30 to be released upon tissue damage causing Nlrp3 inflammasome activation,31,32 which is connected to impaired suppressor function of myeloid-derived suppressor cells33 and to promote the severity of aGVHD in the mouse model.24 Conversely, other studies in major histocompatibility complex (MHC) disparate mouse models showed only a very modest effect of IL-1 antagonists.34 Although initial studies using IL-1 antagonism in the therapeutic setting suggested a benefit for patients suffering from GVHD,24,35 the later prospective, randomized, controlled trial failed to show a benefit from IL-1 blockade administered from day −4 to day +10 relative to allo-HCT.36 In different mouse models of GVHD, TNF-α antagonism reduced GVHD severity.37-39
The murine studies showed that TNF-α was derived from donor T cells, regulated by microRNA-146a/TRAF638 and myeloid cells.25 Mechanistically, TNF-α was shown to directly damage the intestinal epithelium25,26 and to downmodulate the function of regulatory T cells (Tregs),39 which protect against GVHD.40-42 Consequently, clinical studies using TNF-α antagonism with etanercept43 or infliximab44 in the therapeutic setting against GVHD were performed. Infliximab in addition to steroids reduced GVHD severity; however, the reported NRM was unexpectedly high.44 Etanercept given as a combination therapy with inolimomab (anti–IL-2Rα) for the treatment of steroid-refractory aGVHD yielded a response rate of 48%.43 However, the estimated rates of 6-month and 2-year OS were 29% and 10%, respectively, leading the authors to conclude that the combination failed to improve the dismal prognosis of severe steroid-refractory aGVHD.43 These unfavorable results of TNF-α blockade are connected to a high NRM and relapse, which is consistent with reports showing a high incidence of fungal infections45 and reduced graft-versus-leukemia (GVL) effects46 when TNF-α is antagonized. These findings could have been predicted by mouse studies as TNF antagonism reduced GVL effects against P815 cells.25 IL-6 blockade was shown to potently reduce aGVHD in mice27,28 and with the availability of the IL-6R antagonist tocilizumab, a prospective single-institution phase 1/2 aGVHD prophylaxis trial was performed.47 This study showed an incidence of grade 2-4 aGVHD in patients treated with tocilizumab at day 100 of 12%, which is lower than expected.47 These results are very promising, and several controlled trials assessing tocilizumab in addition to standard GVHD prophylaxis or as GVHD therapy are currently active (www.clinicaltrials.gov: #NCT01757197, #NCT02447055, #NCT02206035, and #NCT02057770).
Besides cytokines that promote the activation of T cells, chemokines that guide the migration of T cells toward GVHD target organs were identified as a pharmacologic target in mouse models of aGVHD.48,49 However, this strategy is seen controversial as high radiation can break the principles of chemokine-mediated selected tissue migration and trapping. For example, CCR5 inhibition was protective against GVHD in a non-irradiated GVHD mouse model,48 whereas in the presence of total body irradiation (TBI) an earlier time to onset and a worsening of GVHD was observed when CCR5−/− T cells were used.50 This knowledge was later applied in a GVHD prophylaxis setting where a single institution phase 1 trial reported that CCR5 inhibition prevents aGVHD of the liver and gut before day 100,51 which has to be confirmed in a randomized prospective multicenter study. However, recent data using CCR5 inhibition in the setting of reduced-intensity conditioning revealed no protection from GVHD.52 Therefore, the potential efficacy of CCR5 inhibition may be context dependent and has yet to be fully tested. Inhibition of T-cell egress from the lymph node53,54 and dendritic cell (DC) migration55 was potently inhibited by the sphingosine 1-phosphate receptor agonist FTY720 in the mouse model of GVHD, a therapeutic concept that is currently investigated by using KRP-203, the sphingosine 1-phosphate receptor type 1 agonist,56 in a clinical study on patients undergoing allo-HCT (www.clinicaltrials.gov: #NCT01830010) with the advantage that upon discontinuation of the drug, T-cell effector function can be unleashed from suppression,57 as has been demonstrated for rodent T-effector responses in allo-bone marrow (BM) transplantation.18
Besides blocking T-cell migration, the co-stimulation of T cells was recognized as a potential powerful target during aGVHD. In the 1990s, it was shown that CTLA4-immunoglobulin (Ig) reduces lethal murine GVHD,58 which later motivated a trial showing that CD28:CD80/86 co-stimulation blockade with abatacept leads to low GVHD rates.59 The opponent of CTLA4-Ig, the CTLA-4 blocking antibody ipilimumab was recently applied in the posttransplant setting for patients with refractory malignancies and did not lead to an unacceptable high GVHD rate when given at a median of 1 year (range, 125-2368 days) after the last allogeneic cell infusion.60 Recent studies on another negative regulator of T-cell activation, namely programmed death-1 using checkpoint inhibition showed promising results in the mouse model61,62 that should be further investigated in the clinic.
The multiple approaches developed from the mouse model into a clinical application for aGVHD are summarized in Figure 1 and the summary of translation of each application is provided in Tables 1 and 2.
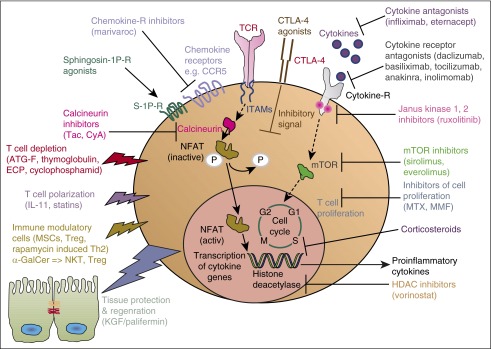
Figure 1.
aGVHD. Simplified sketch showing the mode of action of multiple immunosuppressive strategies that were all developed from animal models into a clinical application for aGVHD. Ab, antibody; ECP, extracorporal photophoresis; ITAM, immunoreceptor tyrosine-based activation motif; MMF, mycophenolate mofetil; MSC, mesenchymal stroma cells; mTOR, mammalian target of rapamycin complex; NKT, natural killer T cells.
Table 1.
Translation of immunosuppressive strategies from animal models of GVHD into clinical trials
| Main conclusion from the preclinical model of GVHD (y) | Reference | Main conclusion from the clinical trials (y) | Reference |
|---|---|---|---|
| CyA and MTX reduce GVHD in the canine GVHD model (1982) | 11 | MTX and CyA is superior to CyA alone for GVHD prophylaxis (1986). Phase 3 prospective, randomized trial | 12 |
| ATG prevents GVHD in DLA haplotype mismatched littermate dogs (1979) | 14 | Different types of ATG reduce the risk of acute and cGVHD in patients (1979, 2009, 2016). Phase 3 prospective, randomized trials (2009, 2016) | 15,16,127 |
| Corticosteroids reduce GVHD severity in mice (1990, 1995) | 20,21 | Corticosteroids are effective as first-line therapy for aGVHD in patients (1998, 2009). Phase 3 multicenter randomized trial (1998); retrospective analysis (2009) | 18,19 |
| IL-11 downregulated IL-12, and reduced aGVHD-related mortality (1998, 1999) | 22,23 | IL-11 leads to increased mortality in patients (2002). Phase 1/2 double-blind, placebo-controlled study | 29 |
| IL-1 blockade reduces GVHD in mice in some but not all models (1991) | 24 | IL-1 antagonist is not effective in the GVHD prophylaxis setting (2002). Phase 3 prospective placebo-controlled study | 36 |
| TNF-α antagonism reduces GVHD (1999, 2003) | 25,26 | Infliximab and corticosteroids are effective as initial treatment of GVHD. Prospective phase 3 study (2009); retrospective analysis (2011) | 44,128 |
| IL-6 blockade reduces aGVHD in mice (2009, 2011) | 27,28 | Early IL-6 inhibition with tocilizumab leads to a low risk of aGVHD (2014). Phase 1/2 single institution trial | 47 |
| Anti-CCR5 antibody treatment protects against aGVHD-related mortality (1999, 2003) | 48,49 | CCR5 inhibition prevents aGVHD of liver and gut before day 100 (2012). Phase 1/2 single institution trial | 129 |
| The sphingosine 1-phosphate receptor agonist FTY720 reduces GVHD (2003, 2007) | 53,54 | Active clinical study on KRP203 in patients undergoing allo-HCT (2016). Randomized, open-label phase 1/2 study | 57 |
| CTLA4-Ig reduces lethal murine GVHD (1994) | 58 | CD28:CD80/86 co-stimulation blockade with abatacept leads to low GVHD rates (2013). Single-arm feasibility study | 59 |
| KGF reduces but does not uniformly eliminate GVHD lethality in mice (1998, 1999) | 63,64 | Palifermin does not reduce aGVHD severity (2012) but there is a need for parenteral nutrition after TBI (2013). Radomized, double-blind, placebo-controlled trial (2012); retrospective analysis (2013) | 65,66 |
| Memory CD4+ T cells cause less aGVHD and cGVHD (2003, 2007, 2009) | 68,69,113 | Naive T-cell–depleted stem cell graft transfer is connected to less cGVHD and no change aGVHD incidence though more steroid responsiveness (2015). Single-arm, 2 site clinical trial | 70 |
| Photoinactivation of T-cell function with psoralen and UV radiation suppresses GVHD in mice (1991) | 71 | ECP is an effective therapy for aGVHD (2000, 2015). Pilot study (2000); meta-analysis of prospective studies for ECP (2015) | 72,73 |
| Statins reduce GVHD severity (2007, 2009) | 75,76 | Statin intake is connected to reduced GVHD incidence in patients (2010, 2010, 2013). Retrospective analysis (2010); prospective phase 2 trial, donor and recipient treatment (2013) | 77-79 |
| HDAC inhibition reduced GVHD severity in mice (2008) | 81 | Vorinostat in combination with standard GVHD prophylaxis is associated with a low incidence of severe aGVHD (2014). | 82 |
| Phase 1/2 trial | |||
| JAK1/2 inhibition reduces aGVHD (2014, 2015) | 83,84 | JAK1/2 inhibition reduces aGVHD in patients refractory to multiple therapies (2015). Retrospective analysis | 85 |
| Proteasome inhibition with bortezomib reduces GVHD (2004) | 87 | Short-course, bortezomib-based GVHD prophylaxis yields low aGVHD rates (2009, 2012). Phase 1 trial (2009); prospective phase 1/2 trial (2012) | 88,89 |
| α-GalCer reduces GVHD (2005) | 92 | RGI-2001 is tested for GVHD prevention (2016). Phase 1/2 trial | 51 |
| CP can induce tolerance toward skin allografts (1989)93 and posttransplant CP reduced GVHD severity in mice (2014)95 | 93,95 | Posttransplantation CP is effective as single-agent aGVHD prophylaxis (2014). Open label multi-institutional trial | 97,98 |
CP, cyclophosphamide; DLA, dog leukocyte antigen.
Table 2.
Translation of cellular therapies from aGVHD mouse models into clinical trials
| Main conclusion from the mouse model of GVHD | Reference | Clinical trial | Reference |
|---|---|---|---|
| Treg transfer reduces GVHD (2002, 2003) | 40,42,100 | Treg transfer is associated with low GVHD rates (2011, 2011, 2016). Open label and phase 1 trials | 101-103 |
| Th2 cells generated by rapamycin exposure cause GVHD protection (2005) | 107 | Rapamycin-resistant donor CD4+ Th2/Th1 transfer after allo-HCT is well-tolerated and connected to low aGVHD day 100 (2013). Phase 2 clinical trial | 108 |
| MSCs reduce GVHD (2012) | 130 | MSC reduces GVHD in open-label studies and phase 2 trials (2004, 2014) | 110,111 |
Targeting multiple layers of aGVHD
In contrast to the approaches against GVHD that aim at targeting T cells or their cytokines, other studies were performed in the 1990s that aimed at improving the regeneration of the epithelial barrier by using a growth factor called keratinocyte growth factor (KGF).63,64 KGF reduced aGVHD in mouse models as shown by different groups.63,64 However, the survival benefit did vary between the different reports, raging from a modest improvement of the survival63 to highly protective effects.64 The drug palifermin did not reduce aGVHD in patients, but it did reduce the need for parenteral nutrition after TBI.65,66 The concept of enhancing epithelial regeneration via stimulation of intestinal stem cells, eg, via R-spondin-167 is still actively investigated and may have the advantage of sparing GVL effects as donor T cells are not blocked.
Another approach aimed at leaving effector T cells that mediate GVL effects intact was based on studies in the mouse model of aGVHD showing that memory CD4+ T cells cause less or almost no aGVHD but mediate GVL effects.68,69,113 The clinical study showed that the use of naive T-cell–depleted stem cell grafts with tacrolimus only suppression was connected to the same incidence of but more steroid responsive aGVHD, along with a markedly reduced cGVHD incidence, the latter consistent with rodent studies of Tn depletion in a cGVHD model.70,113 An early study performed in 1991 in mice showed that photoinactivation of T-cell function with psoralen and UV radiation suppresses aGVHD.71 Meanwhile, extracorporal photophoresis has become an important treatment option for patients with aGVHD.72,73 Statins that inhibit the rate-limiting enzyme of the l-mevalonate pathway were shown to reduce farnesyl- and geranylgeranyl-residues that are required for the correct attachment of different small GTPases to the cell membrane and thereby modulate the allogeneic immune response.74 Consistently different independent groups could show that statins reduce aGVHD in mouse models.75,76 In patients undergoing allo-HCT, some studies showed that statin intake by the donor77 or host78 was connected to a reduced GVHD incidence,77-79 whereas another trial showed that the addition of atorvastatin to standard aGVHD prophylaxis did not provide a benefit with respect to GVHD rates.80 Comparable to statins that have a broad range of inhibitory effects, histone deacetylase (HDAC) inhibitors were shown to modify multiple layers of the allogeneic immune response. Mechanistically, it was shown that not only the phenotype of T cells was polarized toward Tregs but also that DCs treated ex vivo with HDAC inhibitors displayed increased expression of indoleamine 2,3-dioxygenase, which reduces both DC and T-cell function.81 Consistently, administration of the HDAC inhibitor vorinostat in combination with standard aGVHD prophylaxis in a phase 1/2 study after related-donor reduced-intensity conditioning allo-HCT was associated with a relatively low incidence of grade 2-4 aGVHD by day 100 of 22%.82
Signaling of multiple cytokine receptors requires intact Janus kinase 1/2 (JAK1/2) activity (Figure 1) and different groups could show that pharmacologic inhibition of JAK1/2 reduced aGVHD in the mouse.83,84 In a retrospective survey, 19 stem cell transplant centers in Europe and the United States reported their data on the use of the JAK1/2 inhibitor ruxolitinib for steroid refractory GVHD.85 The overall response rate was 81.5% (44/54) in steroid refractory aGVHD including 25 complete responses (46.3%), whereas for steroid refractory cGVHD the overall response rate was 85.4% (35/41), consistent with data in a cGVHD mouse model.85 Ruxolitinib will be investigated in a prospective trial in Germany (#NCT02396628) and a clinical trial using the JAK1 selective inhibitor INCB39110 has begun for the treatment of GVHD (#NCT02614612). Potential differences between JAK1 and JAK2 inhibition include a potentially lower risk of cytopenia when only JAK1 is inhibited, although this may come with a reduced efficacy as JAK2 inhibition alone was shown to reduce GVHD. Also, a recent study suggests that topical ruxolitinib suppresses GVHD and protects skin follicular stem cells, whereas topical corticosteroids inhibit skin stem cells and niche pre-adipocytes.86 A promising approach to reduce aGVHD is the proteasome inhibitor bortezomib that was shown to reduce aGVHD in the mouse model.87 Mechanistically bortezomib inhibits nuclear factor-κB, thereby reducing inflammatory protein production. Clinical studies using a short-course, bortezomib-based GVHD prophylaxis yielded low aGVHD rates.88,89
α-GalCer is a glycolipid that functions as a CD1d ligand. Because α-GalCer was found to expand and activate natural killer T cells, and subsequently Tregs,90 it was developed as an immune modulator. Preclinical models have demonstrated efficacy of α-GalCer in many autoimmune disorders and GVHD.91,92 Although inhibition was found when the N-acyl variant C20:2 was used, another form of α-GalCer exacerbated GVHD.91 Based on these preclinical studies, the liposomal formulation of α-GalCer named RGI-2001 is currently investigated in a phase 1/2a, open-label, multicenter, dose-escalation study for patients undergoing allo-HCT (www.clinicaltrials.gov: #NCT01379209).51
An important observation made in murine BM chimera was that CP given on day 2 after transplantation induced tolerance toward skin allografts.93 The authors concluded that the form of CP posttransplantation conditioning most likely decreased the number of MHC-alloreactive T cells.93 Consistent with that concept, analyses of T-cell receptor Vβ subunits that recognize endogenous super-antigens in disparate murine allo-combinations showed that CP deletes alloreactive T cells.94 Posttransplant CP reduced aGVHD severity in mice in a Treg-dependent manner, which was shown by using transgenic mice in whom Foxp3+ Tregs can be selectively depleted.95 In patients, it was shown that posttransplant CP is highly effective in preserving human Tregs, and in aGVHD prophylaxis96 when used in combination with sirolimus or as a single agent.97-99 The multiple approaches developed from the mouse model into a clinical application for aGVHD are summarized in Figure 1.
A goal of preclinical models is to develop new approaches to prevent and treat GVHD. Although numerous reagents and cell therapies have progressed from preclinical studies into clinical trials, these were predominantly phase 1 and 2 studies. Alternatively, some approaches have been moved into the clinic based upon biological underpinnings and targets without in vivo preclinical testing. Although both approaches have merit and the true predictive value of either for successfully completing phase 3 studies or changing practice has yet to be determined, there are as yet a paucity of examples in which uniformly negative data in preclinical models have proven to be robustly positive in clinical studies. However, it is also true that well-designed phase 1 and 2 clinical trials are fundamentally important in deciding whether and how to best move forward new advances in GVHD prevention and therapy.
Cellular approaches to prevent or treat aGVHD translated from the mouse into the clinic
In contrast to pharmacologic approaches against aGVHD, that are by their nature short lived unless a state of deep tolerance is acquired during drug therapy, the transfer of a tolerogenic cell population that persists in the body could ideally lead to long-term tolerance. To exploit this concept, the transfer of tolerogenic Foxp3+ Tregs in mice was performed and led to an impressive reduction of aGVHD.40,42,100 Clinical studies using Treg transfer in the prophylactic setting was found to be connected to low aGVHD rates and adequate immune reconstitution.101-103
The administration of mogamulizumab besides reducing adult T-cell leukemia cells also induced prolonged suppression of normal Tregs.104 Consistent with a suppressive role of Tregs against GVHD in patients undergoing allo-HCT, pretransplant use of mogamulizumab induces severe aGVHD.105 This observation supports the clinical relevance of the finding in the mouse model that Tregs are potent suppressors of GVHD.
Rapamycin (sirolimus) was shown to be more potent in suppressing conventional T-cell expansion compared with Treg expansion due to differential dependence on mammalian target of rapamycin complex/Akt expanded Treg cells106 and to polarize T cells toward a Th2 cytokine profile that was protective against aGVHD in mice.107 Motivated by these and other preclinical murine studies, a phase 2 multicenter clinical trial of ex vivo expanded rapamycin-resistant donor CD4+ Th2/Th1 cells after allogeneic-matched sibling donor HCT was performed.108 The cumulative incidence probability of aGVHD was 20% and 40% at days 100 and 180 post–allo-HCT indicating a potential benefit of this strategy that will have to be compared with other immunosuppressive interventions in future studies. Another cell population that holds promise to protect from aGVHD are MSCs, based on findings in a humanized mouse model of T-cell activation.109 However, the mouse data are controversial and differ between groups, which was also the case for the later studies in humans, as MSCs reduced aGVHD in some trials and a randomized trial failed to show a benefit against aGVHD for patients undergoing allo-HCT.110,111 The reported controversies could be due to the differences in the preparation process of the MSCs, as well as the time point of transfer.
Pharmacologic prophylaxis and therapy of cGVHD translated from the mouse into the clinic
In a mouse model of cGVHD, it was shown that animals lacking Bruton tyrosine kinase (BTK) in B cells or IL-2 inducible kinase in T cells did not develop cGVHD, indicating that these molecules play a central role in the pathophysiology of cGVHD.134 In addition to the findings in the cGVHD mouse model, activation of T and B cells from patients with active cGVHD was inhibited by BTK and IL-2 inducible kinase blockade by ibrutinib.134 Based on these data, a multicenter open-label phase 1b/2 study of ibrutinib in GVHD is being performed.112 Based on a potent inhibitor effect of ruxolitinib in an aGVHD mouse model,83 patients with cGVHD having failed multiple previous therapies were treated with the JAK1/2 inhibitor and yielded a response rate of more than 80%85; however, these results need to be confirmed in a prospective trial. Studies in mice showed that IL-2 is critical for Treg expansion, activity, and survival during GVHD,41 which was later followed by a phase 1/2 study showing that exogenous IL-2 increases Treg numbers and improves disease in patients with cGVHD.114 A central role for B cells in the pathogenesis of cGVHD was shown by studies in a mouse model of non-sclerodermatous cGVHD,115 which motivated the successful use of the B-cell–depleting antibody rituximab in patients with cGVHD.116,117 Posttransplant CP applied as described above was shown to reduce rates of cGVHD. Additionally, the synthetic retinoid tamibarotene (AM80G) was found to reduce skin scores and pathology of cGVHD in a mouse model,118 and has been tested consecutively in a phase 2 trial for cGVHD (#UMIN 000020363) in Japan. The multiple approaches developed from the mouse model into a clinical application for cGVHD are summarized in Figure 2 and the summary of translation of each approach is provided in Tables 3 and 4.
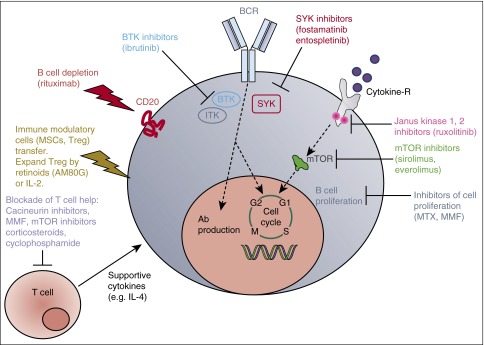
Figure 2.
cGVHD. Simplified sketch showing the mode of action of multiple immunosuppressive strategies that were all developed from animal models into a clinical application for cGVHD. BCR, B-cell receptor; M, M phase; S, S phase; SYK, spleen tyrosine kinase.
Table 3.
Translation of immunosuppressive strategies from animal models of cGVHD into clinical trials
| Main conclusion from the mouse model of cGVHD | Reference | Clinical trial | Reference |
|---|---|---|---|
| BTK inhibition reduces cGVHD in mice (2014) | 134 | BTK inhibition reduces cGVHD in patients (2016). Phase 1b/2 study | 112 |
| IL-2 is critical for Treg expansion, activity, and survival during GVHD (2004, 2006) | 41,131 | Exogenous IL-2 increases Treg numbers and improves disease in patients with cGVHD (2011). Phase 1/2 study | 114 |
| B cells play a central role in the pathogenesis of cGVHD (2006) | 115 | The B-cell–depleting antibody rituximab is effective in patients with cGVHD. Retrospective analysis (2003); phase 1/2 study (2006) | 116,117 |
| JAK1/2 inhibition reduces cGVHD in the mouse (2015, Supplemental Figures) | 85 | JAK1/2 inhibition reduces cGVHD in patients refractory to multiple therapies (2015). Retrospective analysis | 85 |
| Syk inhibition reduces cGVHD in the mouse (2014, 2015) | 124,125,132 | Open clinical trials for Syk inhibitors (fostamatinib, entospletinib) (2016) | 135 |
Table 4.
Translation of cellular therapies from cGVHD mouse models into clinical trials
Cellular therapy approaches against cGVHD
Because Tregs were shown to reduce aGVHD in the mouse model,40,42,100 investigators used human donor Tregs that were cultivated for 7 to 12 days and then given to patients with cGVHD.118 Two of 5 patients showed a clinical response with improvement of cGVHD symptoms and 3 patients showed stable cGVHD symptoms for up to 21 months.119
Trials are in progress and planned to extend these studies, and to incorporate low-dose IL-2 to treat cGVHD resistant to conventional therapies.
Novel promising targets and approaches
In order to generate hypotheses that match the human situation as closely as possible, recent studies on novel targets for GVHD, the RNA expression profiles of CD3+ T cells from NHP with aGVHD were analyzed.20 The study included cohorts of allo-HCT as an untreated control and recipients were given autologous HCT or allo-HCT with no immunoprophylaxis, sirolimus monotherapy, or tacrolimus-MTX.20 The authors found that aurora kinase A was more abundant in the GVHD group, and then directly applied this knowledge in the mouse model of GVHD where they could show that pharmacologic inhibition of aurora kinase A reduced GVHD severity. One strength of this study lies in the fact that the NHP used were evolutionary closer to resembling humans and therefore, the identified targets will most likely match the human situation much better than targets found in mice developing GVHD. Another potent strategy of GVHD prevention is the common γ-chain blockade,120 as this chain is a subunit of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. However, although GVHD is reduced, the effect on T cells that reject allogeneic leukemia cells needs to be considered, making this approach most interesting for patients undergoing allo-HCT for nonmalignant diseases. Other lines of promising future translation for aGVHD are: besides others, natural killer T-cell therapy,121 IL-22 proteins that help to protect the intestinal stem cell mice,122 and modifications of the microbiome.123 cGVHD promising approaches include among others: Syk inhibitors that were shown to reduce disease severity in the mouse model124,125 and are currently developed into a clinical trial, BTK inhibition, targeting of IL-21, and B-cell activating factor that may help to control this major complication after allo-HCT.
Conclusion
It is unlikely that the mouse model will ever fully reflect the human situation even if chemotherapy based conditioning, minor antigen mismatch models, and granulocyte colony-stimulating factor mobilized peripheral blood stem cells will be used in humanized mouse models, because the situation in humans is much more complex, in particular with respect to genetic differences that lie outside the MHC loci and environmental conditions. Additionally, a major reason why certain novel pharmacologic approaches against GVHD that were shown to be successful in the mouse model then failed in the clinical setting is that they were applied to patients as treatment of steroid-refractory GVHD, which shares some similarities with severe murine GVHD but is per se a different disease. Moreover, generally, mouse models use BM that contains few T cells and are supplemented typically by splenocytes or purified T cells from secondary lymphoid organs. In contrast, patients will receive BM grafts that are “contaminated” with peripheral blood T cells, mobilized peripheral blood grafts, or cord blood grafts. T cells in each instance may have distinct functional characteristics compared with mouse T cells situated in secondary lymphoid organs. Therefore, it may be advisable to directly search for correlates in patient samples when a finding in the mouse model of GVHD has been made. This can be done by a prospective quantification of the potential target (eg, a cytokine or a kinase such as BTK) in human material, and a correlation with the incidence and severity of GVHD. Conversely, the novel “omics”-driven approaches (eg, proteomics, genomics) applied to human samples that are now increasingly used in many areas of medicine will still need a counterpart to functionally address the role of the identified candidate molecules before a translation into the clinic is possible. Additionally, there is now increasing use of “omics”-based discovery approaches in animal models that may provide new mechanisms and insights for interrogation and potential validation in GVHD patients. There are multiple examples for proteomics- or genomics-based approaches in mice driving human studies.20,21,126 Finally, another important aspect to potentially improve the predictive value of preclinical GVHD models may be to incorporate GVHD treatment models into testing rather than GVHD prevention models alone, because GVHD treatment has been and continues to be an important research field in the future, in particular with the high medical need for GVHD patients who have failed conventional therapies such as steroids. By combining insights from small and large animal models, as well as human clinical laboratory and interventional studies, we believe that this collective approach will have the highest likelihood for success in improving the outcome for patients who are in need of new therapies.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft Germany (DFG); Heisenberg professorship to R.Z. (DFG ZE 872/3-1); a DFG individual grant to R.Z. (DFG ZE 872/1-2); a European Research Council consolidator grant to R.Z. (681012 GVHDCure); grants from the National Institutes of Health, National Heart, Lung and Blood Institute (R01 HL56067, HL11879), National Institute of Allergy and Infectious Diseases (AI 34495 and AI 056299), and National Cancer Institute (P01 CA142106); and a Leukemia and Lymphoma Translational Research grant (6458). The authors apologize to those investigators whose work could not be cited due to space restrictions.
Authorship
Contribution: Both R.Z. and B.R.B. collected literature, discussed the studies, and contributed equally to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Zeiser, Department of Hematology and Oncology, Freiburg University Medical Center, Albert-Ludwigs University, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de; and Bruce R. Blazar, Department of Pediatrics, Division of Blood and Marrow Transplantation, MMC 366, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
- 1.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2:35–44. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412–5417. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 3.Westin JR, Saliba RM, De Lima M, et al. Steroid-refractory acute GVHD: predictors and outcomes. Adv Hematol. 2011;2011:601953. doi: 10.1155/2011/601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 8.Alpdogan O, van den Brink MR. Immune tolerance and transplantation. Semin Oncol. 2012;39(6):629–642. doi: 10.1053/j.seminoncol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of intolerance. Transplantation. 1982;34(1):30–35. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 13.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 14.Kolb HJ, Rieder I, Rodt H, et al. Antilymphocytic antibodies and marrow transplantation. VI. Graft-versus-host tolerance in DLA-incompatible dogs after in vitro treatment of bone marrow with absorbed antithymocyte globulin. Transplantation. 1979;27(4):242–245. [PubMed] [Google Scholar]
- 15.Finke J, Bethge WA, Schmoor C, et al. ATG-Fresenius Trial Group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 16.Kröger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43–53. doi: 10.1056/NEJMoa1506002. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Zhao X, Tian Y, et al. Outcomes of peripheral blood stem cell transplantation patients from HLA-mismatched unrelated donor with antithymocyte globulin (ATG)-thymoglobulin versus ATG-fresenius: a single-center study. Med Oncol. 2015;32(2):465–473. doi: 10.1007/s12032-014-0465-y. [DOI] [PubMed] [Google Scholar]
- 18.Taylor PA, Kelly RM, Bade ND, Smith MJ, Stefanski HE, Blazar BR. FTY720 markedly increases alloengraftment but does not eliminate host anti-donor T cells that cause graft rejection on its withdrawal. Biol Blood Marrow Transplant. 2012;18(9):1341–1352. doi: 10.1016/j.bbmt.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113(13):2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss L, Reich S, Slavin S. Effect of cyclosporine A and methylprednisolone on the graft-versus-leukemia effects across major histocompatibility barriers in mice following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1990;6:229–233. [PubMed] [Google Scholar]
- 21.You-Ten KE, Seemayer TA, Wisse B, Bertley FM, Lapp WS. Induction of a glucocorticoid-sensitive F1-anti-parental mechanism that affects engraftment during graft-versus-host disease. J Immunol. 1995;155:172–180. [PubMed] [Google Scholar]
- 22.Hill GR, Cooke KR, Teshima T, et al. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J Clin Invest. 1998;102(1):115–123. doi: 10.1172/JCI3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teshima T, Hill GR, Pan L, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest. 1999;104(3):317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy PL, Jr, Abhyankar S, Neben S, et al. Inhibition of interleukin-1 by an interleukin-1 receptor antagonist prevents graft-versus-host disease. Blood. 1991;78(8):1915–1918. [PubMed] [Google Scholar]
- 25.Hill GR, Teshima T, Gerbitz A, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104(4):459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmaltz C, Alpdogan O, Muriglan SJ, et al. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101(6):2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17(1):77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antin JH, Lee SJ, Neuberg D, et al. A phase I/II double-blind, placebo-controlled study of recombinant human interleukin-11 for mucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29(5):373–377. doi: 10.1038/sj.bmt.1703394. [DOI] [PubMed] [Google Scholar]
- 30.Coccia M, Harrison OJ, Schiering C, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209(9):1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jankovic D, Ganesan J, Bscheider M, et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210(10):1899–1910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Smith BA, Iype J, et al. MicroRNA-155-deficient dendritic cells cause less severe GVHD through reduced migration and defective inflammasome activation. Blood. 2015;126(1):103–112. doi: 10.1182/blood-2014-12-617258. [DOI] [PubMed] [Google Scholar]
- 33.Koehn BH, Apostolova P, Haverkamp JM, et al. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood. 2015;126(13):1621–1628. doi: 10.1182/blood-2015-03-634691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallera DA, Taylor PA, Vannice JL, Panoskaltsis-Mortari A, Blazar BR. Interleukin-1 or tumor necrosis factor-alpha antagonists do not inhibit graft-versus-host disease induced across the major histocompatibility barrier in mice. Transplantation. 1995;60(11):1371–1374. [PubMed] [Google Scholar]
- 35.McCarthy PL, Jr, Williams L, Harris-Bacile M, et al. A clinical phase I/II study of recombinant human interleukin-1 receptor in glucocorticoid-resistant graft-versus-host disease. Transplantation. 1996;62(5):626–631. doi: 10.1097/00007890-199609150-00015. [DOI] [PubMed] [Google Scholar]
- 36.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100(10):3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 37.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175(2):405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stickel N, Prinz G, Pfeifer D, et al. MiR-146a regulates the TRAF6/TNF-axis in donor T cells during GVHD. Blood. 2014;124(16):2586–2595. doi: 10.1182/blood-2014-04-569046. [DOI] [PubMed] [Google Scholar]
- 39.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 41.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 43.van Groningen LF, Liefferink AM, de Haan AF, et al. Combination therapy with inolimomab and etanercept for severe steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2016;22(1):179–182. doi: 10.1016/j.bbmt.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 44.Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(12):1555–1562. doi: 10.1016/j.bbmt.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahn J, Erdmann A, Grube M, et al. High incidence of invasive aspergillosis after treatment of acute GvHD with the combination of OKT3 and infliximab [abstract]. Bone Marrow Transplant. 2001;27:203–208. [Google Scholar]
- 46.Veeraputhiran M, Mangan K. Sudden loss of the GVL effect following use of the TNF inhibitor infliximab in a chronic myelogenous leukemia patient with chronic GVHD. Bone Marrow Transplant. 2010;45(6):1113–1114. doi: 10.1038/bmt.2009.288. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy GA, Varelias A, Vuckovic S, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15(13):1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 48.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104(1):49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murai M, Yoneyama H, Ezaki T, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4(2):154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 50.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, et al. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol. 2004;173(2):845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 51.National Institutes of Health. A phase 1/2a, open-label, multicenter, dose-escalation study to evaluate the safety and tolerability of intravenous administration of RGI-2001 in patients undergoing allogeneic hematopoietic stem cell transplantation (AHSCT). Available at: https://clinicaltrials.gov/show/NCT01379209. Accessed March 10, 2016.
- 52.Hammond WA, Heckman M, Finn L, et al. No evidence of impact of maraviroc on outcome after allogeneic hematopoietic stem cell transplant with reduced intensity conditioning (RIC). Biol Blood Marrow Transplant. 2016;22(3):S396–S397. [Google Scholar]
- 53.Hashimoto D, Asakura S, Matsuoka K, et al. FTY720 enhances the activation-induced apoptosis of donor T cells and modulates graft-versus-host disease. Eur J Immunol. 2007;37(1):271–281. doi: 10.1002/eji.200636123. [DOI] [PubMed] [Google Scholar]
- 54.Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest. 2003;111(5):659–669. doi: 10.1172/JCI16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor PA, Ehrhardt MJ, Lees CJ, et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD). Blood. 2007;110(9):3480–3488. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potì F, Gualtieri F, Sacchi S, et al. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R-/- mice. Arterioscler Thromb Vasc Biol. 2013;33(7):1505–1512. doi: 10.1161/ATVBAHA.113.301347. [DOI] [PubMed] [Google Scholar]
- 57.National Institutes of Health. A two-part study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of KRP203 in patients undergoing stem cell transplant for hematological malignancies. Available at: https://clinicaltrials.gov/ct2/show/NCT01830010. Accessed March 10, 2016.
- 58.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83(12):3815–3825. [PubMed] [Google Scholar]
- 59.Koura DT, Horan JT, Langston AA, et al. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: a first-in-disease trial. Biol Blood Marrow Transplant. 2013;19(11):1638–1649. doi: 10.1016/j.bbmt.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asakura S, Hashimoto D, Takashima S, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Invest. 2010;120(7):2370–2378. doi: 10.1172/JCI39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michonneau D, Sagoo P, Breart B, Garcia Z, Celli S, Bousso P. The PD-1 axis enforces an anatomical segregation of CTL activity that creates tumor niches after allogeneic hematopoietic stem cell transplantation. Immunity. 2016;44(1):143–154. doi: 10.1016/j.immuni.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR. Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood. 1998;92(10):3960–3967. [PubMed] [Google Scholar]
- 64.Krijanovski OI, Hill GR, Cooke KR, et al. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999;94(2):825–831. [PubMed] [Google Scholar]
- 65.Jagasia MH, Abonour R, Long GD, et al. Palifermin for the reduction of acute GVHD: a randomized, double-blind, placebo-controlled trial. Bone Marrow Transplant. 2012;47(10):1350–1355. doi: 10.1038/bmt.2011.261. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg JD, Zheng J, Castro-Malaspina H, et al. Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2013;48(1):99–104. doi: 10.1038/bmt.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takashima S, Kadowaki M, Aoyama K, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208(2):285–294. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109(7):3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125(7):2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ullrich SE. Photoinactivation of T-cell function with psoralen and UVA radiation suppresses the induction of experimental murine graft-versus-host disease across major histocompatibility barriers. J Invest Dermatol. 1991;96(3):303–308. doi: 10.1111/1523-1747.ep12465134. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Chen R, Cheng J, Jin N, Chen B. Systematic review and meta-analysis of prospective studies for ECP treatment in patients with steroid-refractory acute GVHD. Patient Prefer Adherence. 2015;9:105–111. doi: 10.2147/PPA.S76563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greinix HT, Volc-Platzer B, Kalhs P, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96(7):2426–2431. [PubMed] [Google Scholar]
- 74.Hechinger AK, Maas K, Dürr C, et al. Inhibition of protein geranylgeranylation and farnesylation protects against graft-versus-host disease via effects on CD4 effector T cells. Haematologica. 2013;98(1):31–40. doi: 10.3324/haematol.2012.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeiser R, Youssef S, Baker J, Kambhan N, Steinman L, Negrin RS. Preemptive HMG-CoA reductase inhibition provides graft-versus-host disease protection by Th-2 polarization while sparing graft-versus-leukemia activity. Blood. 2007;110(13):4588–4598. doi: 10.1182/blood-2007-08-106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Li D, Jones D, et al. Blocking LFA-1 activation with lovastatin prevents graft-versus-host disease in mouse bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(12):1513–1522. doi: 10.1016/j.bbmt.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rotta M, Storer BE, Storb RF, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood. 2010;115(6):1288–1295. doi: 10.1182/blood-2009-08-240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rotta M, Storer BE, Storb R, et al. Impact of recipient statin treatment on graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(10):1463–1466. doi: 10.1016/j.bbmt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamadani M, Gibson LF, Remick SC, et al. Sibling donor and recipient immune modulation with atorvastatin for the prophylaxis of acute graft-versus-host disease. J Clin Oncol. 2013;31(35):4416–4423. doi: 10.1200/JCO.2013.50.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Efebera YA, Geyer S, Andritsos L, et al. Atorvastatin for the prophylaxis of acute graft-versus-host disease in patients undergoing HLA-matched related donor allogeneic hematopoietic stem cell transplantation (allo-HCT). Biol Blood Marrow Transplant. 2016;22(1):71–79. doi: 10.1016/j.bbmt.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118(7):2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi SW, Braun T, Chang L, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15(1):87–95. doi: 10.1016/S1470-2045(13)70512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 84.Carniti C, Gimondi S, Vendramin A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21(16):3740–3749. doi: 10.1158/1078-0432.CCR-14-2758. [DOI] [PubMed] [Google Scholar]
- 85.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graftversus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahashi S, Hashimoto D, Hayase E, Teshima T. Topical ruxolitinib protects LGR5+ stem cells in the hair follicle and ameliorates skin graft-versus-host disease. Biol Blood Marrow Transplant. 2016;22(3):S21–S22. [Google Scholar]
- 87.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib [published correction appears in Proc Natl Acad Sci USA. 2004;101(34):12777]. Proc Natl Acad Sci USA. 2004;101(21):8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114(18):3956–3959. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30(26):3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175(12):7898–7904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 91.Kuns RD, Morris ES, Macdonald KP, et al. Invariant natural killer T cell-natural killer cell interactions dictate transplantation outcome after alpha-galactosylceramide administration. Blood. 2009;113(23):5999–6010. doi: 10.1182/blood-2008-10-183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hashimoto D, Asakura S, Miyake S, et al. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J Immunol. 2005;174(1):551–556. doi: 10.4049/jimmunol.174.1.551. [DOI] [PubMed] [Google Scholar]
- 93.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169(1):213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eto M, Mayumi H, Tomita Y, et al. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991;146(5):1402–1409. [PubMed] [Google Scholar]
- 95.Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124(13):2131–2141. doi: 10.1182/blood-2013-10-525873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solomon SR, Sanacore M, Zhang X, et al. Calcineurin inhibitor--free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1828–1834. doi: 10.1016/j.bbmt.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 102.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044–1051. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 105.Inoue Y, Fuji S, Tanosaki R, Fukuda T. Pretransplant mogamulizumab against ATLL might increase the risk of acute GVHD and non-relapse mortality [published online ahead of print December 21, 2015]. Bone Marrow Transplant. doi: 10.1038/bmt.2015.315. [DOI] [PubMed] [Google Scholar]
- 106.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells as compared to conventional CD4+ T cells. Blood. 2008;111(1):453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foley JE, Jung U, Miera A, et al. Ex vivo rapamycin generates donor Th2 cells that potently inhibit graft-versus-host disease and graft-versus-tumor effects via an IL-4-dependent mechanism. J Immunol. 2005;175(9):5732–5743. doi: 10.4049/jimmunol.175.9.5732. [DOI] [PubMed] [Google Scholar]
- 108.Fowler DH, Mossoba ME, Steinberg SM, et al. Phase 2 clinical trial of rapamycin-resistant donor CD4+ Th2/Th1 (T-Rapa) cells after low-intensity allogeneic hematopoietic cell transplantation. Blood. 2013;121(15):2864–2874. doi: 10.1182/blood-2012-08-446872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33(6):597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 110.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 111.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 112. Study of the Bruton’s tyrosine kinase inhibitor in subjects with chronic graft versus host disease. ClinicalTrials.gov Identifier: NCT02195869. Available at: https://clinicaltrials.gov/ct2/show/NCT02195869. Accessed March 10, 2016. [Google Scholar]
- 113.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182(10):5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107(7):2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 116.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9(8):505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 117.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nishimori H, Maeda Y, Teshima T, et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood. 2012;119(1):285–295. doi: 10.1182/blood-2011-01-332478. [DOI] [PubMed] [Google Scholar]
- 119.Theil A, Tuve S, Oelschlägel U, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy. 2015;17(4):473–486. doi: 10.1016/j.jcyt.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Hechinger AK, Smith BA, Flynn R, et al. Therapeutic activity of multiple common γ-chain cytokine inhibition in acute and chronic GVHD. Blood. 2015;125(3):570–580. doi: 10.1182/blood-2014-06-581793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneidawind D, Baker J, Pierini A, et al. Third-party CD4+ invariant natural killer T cells protect from murine GVHD lethality. Blood. 2015;125(22):3491–3500. doi: 10.1182/blood-2014-11-612762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanash AM, Dudakov JA, Hua G, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leonhardt F, Zirlik K, Buchner M, et al. Spleen tyrosine kinase (Syk) is a potent target for GvHD prevention at different cellular levels. Leukemia. 2012;26(7):1617–1629. doi: 10.1038/leu.2012.10. [DOI] [PubMed] [Google Scholar]
- 125.Flynn R, Allen JL, Luznik L, et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood. 2015;125(26):4085–4094. doi: 10.1182/blood-2014-08-595470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwab L, Goroncy L, Palaniyandi S, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014;20(6):648–654. doi: 10.1038/nm.3517. [DOI] [PubMed] [Google Scholar]
- 127.Weiden PL, Doney K, Storb R, Thomas ED. Antihuman thymocyte globulin for prophylaxis of graft-versus-host disease. A randomized trial in patients with leukemia treated with HLA-identical sibling marrow grafts. Transplantation. 1979;27(4):227–230. doi: 10.1097/00007890-197904000-00003. [DOI] [PubMed] [Google Scholar]
- 128.Rager A, Frey N, Goldstein SC, et al. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transplant. 2011;46(3):430–435. doi: 10.1038/bmt.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367(2):135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gregoire-Gauthier J, Selleri S, Fontaine F, et al. Therapeutic efficacy of cord blood-derived mesenchymal stromal cells for the prevention of acute graft-versus-host disease in a xenogenic mouse model. Stem Cells Dev. 2012;21(10):1616–1626. doi: 10.1089/scd.2011.0413. [DOI] [PubMed] [Google Scholar]
- 131.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 132.Le Huu D, Kimura H, Date M, et al. Blockade of Syk ameliorates the development of murine sclerodermatous chronic graft-versus-host disease. J Dermatol Sci. 2014;74(3):214–221. doi: 10.1016/j.jdermsci.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 133.Giorgini A, Noble A. Blockade of chronic graft-versus-host disease by alloantigen-induced CD4+CD25+Foxp3+ regulatory T cells in nonlymphopenic hosts. J Leukoc Biol. 2007;82(5):1053–1061. doi: 10.1189/jlb.0407227. [DOI] [PubMed] [Google Scholar]
- 134.Dubovsky JA, Flynn R, Du J, et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest. 2014;124:4867–4876. doi: 10.1172/JCI75328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. National Institutes of Health. Evaluation of fostamatinib in patients with cGVHD after allogeneic stem cell transplant. Available at: www.clinicaltrials.gov/ct2/show/ NCT02611063. Assessed March 10, 2016.