Abstract
Matrix vesicles (MVs) are membrane organelles found in the extracellular matrix of calcifying cells, which contain matrix processing enzymes and regulate the extracellular environment via action of these enzymes. It is unknown whether MVs are also exosomic mediators of cell-cell communication via transfer of RNA material, and specifically, microRNA (miRNA). We investigated the presence of RNA in MVs isolated from cultures of costochondral growth zone chondrocytes. Our results showed that the average yield of MV RNA was 1.93 ± 0.78 ng RNA/104 cells, which was approximately 0.1% of the parent cell's total RNA. MV RNA was well-protected from RNase by the lipid membrane and was highly enriched in small RNA molecules compared to cells. Moreover, coding and non-coding small RNAs in MVs were in proportions that differed from parent cells. Enrichment of specific miRNAs was consistently observed in all three miRNA detection platforms that we used, suggesting that miRNAs are selectively packaged into MVs. MV-enriched miRNAs were related to different signaling pathways associated with bone formation. This study suggests a significant role for MVs as “matrisomes” in cell-cell communication in cartilage and bone development via transfer of specific miRNAs.
Keywords: matrix vesicles, miRNA, cell membrane, chondrocyte, bone formation, exosomes, endochondral calcification
Introduction
Endochondral bone development is a coordinated process that involves calcification of growth plate cartilage, which is subsequently replaced by cancellous bone. A hallmark of the process is the presence of small membrane-bound microvesicles (≤100 nm in diameter), termed “matrix vesicles” (MV), located in the extracellular matrix of the cartilage (1,2). The classical theory behind MV function in endochondral bone development is as a biochemical hub for calcification. MVs are enriched in alkaline phosphatase specific activity compared to the chondrocyte plasma membrane, they possess a hydroxyapatite nucleation core consisting of amorphous calcium phosphate, phosphatidylserine and calcium binding proteins including annexin family members, and they contain enzymes that diminish inhibitors of calcium phosphate formation (3-10). There is increasing evidence that MV function is not limited to mineralization. MVs are also present in the growth plate resting zone (11,12), an area that is not mineralized, and in non-calcifying tissues including articular cartilage (13), and at the epithelial/mesenchymal transition zone in various neoplasms (14).
A number of cell types produce MVs in culture, as defined by a greater than 2-fold enrichment in alkaline phosphate specific activity compared to plasma membranes isolated from the cells (15). However, their composition varies with the type of cell. MVs produced by resting zone chondrocytes contain neutral metalloproteinases, whereas MVs produced by growth zone (prehypertrophic and upper hypertrophic zones) chondrocytes contain acidic matrix metalloproteinases (MMPs) (16-18). These studies indicate that the composition is controlled genetically and support the hypothesis that MVs are heterogeneous and multi-functional. One function of MVs is to convey information to the extracellular matrix, which is particularly important in cartilage where cell responses are separated in both time and space. MVs directly regulate the availability of growth factors in the extracellular environment by enzymes such as MMP-3 (19) and MMP-13 (20). Not only is MV composition regulated during production (21,22), but once in the extracellular matrix, MVs also are regulated in a differential manner by hormones secreted by the cells (23). In addition to activation of growth factors stored in the matrix by MV MMPs, MVs are themselves reservoirs of growth factors, including bone morphogenetic proteins (BMP)-2, -4, -7 and vascular endothelial growth factor (VEGF) (24).
These observations suggest that MVs also may participate in intercellular communications. Recently, extracellular RNA, and in particular microRNA (miRNA), has been reported to be a conserved cell-cell communication mechanism in species from C. elegans to Homo sapiens (25,26). miRNAs are short (20-22-nucleotide), endogenous, single-stranded, non-coding RNA molecules that regulate gene expression at the post-transcriptional level (27). Selective miRNAs are packed into exosomes, small vesicles secreted by most cells, transferred to recipient cells both proximal and distal to the cells, and regulate gene expression (28). This horizontal gene transmission is involved in a variety of cellular behaviors in the body including angiogenesis, stem cell proliferation and differentiation and tumorigenesis through an autocrine or paracrine manner (28-31).
MVs share commonalities with exosomes with regard to size, structure and composition (32,33). Although they had been reported to possess nucleic acid (34), scientific technology at that time was not adequate to establish whether intact RNA constructs were present. We hypothesized that MVs may also play a role as mediators of intercellular crosstalk during bone formation by the transfer of RNA material. In the past, our group established an in vitro model that showed growth zone chondrocytes derived from costochondral cartilage were able to produce MVs with highly enriched alkaline phosphatase specific activity (15). The morphology (15), lipid membrane components (15,35), enzymatic activity (8,19,36,37), membrane receptors (38), response to vitamin D metabolites (16,36,39), response to growth factors (40,41) and mineralization potential of these MVs (9) have been intensively investigated by our group. Here, using this well-characterized model, we investigated whether RNA, particularly miRNA, is present in MVs from growth zone chondrocytes. We further characterized the miRNA profiles of these MVs and compared them to those of the cells producing them.
Material and Methods
Key experimental methods and statistical analyses are described below. Supplementary files contain details information regarding chondrocyte cultures, MV isolation and bioinformatics analysis of miRNA PCR array and RNA-Seq.
Chondrocyte cultures and MV isolation
Costochondral cartilage cells were isolated by enzymic digestion from 125g male Sprague Dawley rats (∼5-weeks-old) and cultured as described by Boyan et al.(15) using exosome-free fetal bovine serum (FBS, Life Technologies). Approximately 1 million growth zone cells were isolated from the costochondral cartilage of 8 rats. These cells were plated and expanded. MVs were isolated from cultures in their fourth passage. Cells from at least 3 different dissections (8 rats per dissection) were used in the experiments. Exosome-free FBS was prepared by centrifuging FBS at 184,000×g for 4h at 4°C to remove serum exosomes. MVs were isolated from chondrocyte cultures by differentiation centrifugation of the trypsin digested cell layer (15). The same centrifugation protocol was used to pellet the microvesicles in cell culture media. MV isolation was validated by comparing its alkaline phosphatase specific activity to that of the plasma membranes isolated from the cells (36). The MVs were shown to be enriched 5-fold in enzyme activity compared to plasma membrane (Supplemental Table 1), consistent with our previous studies (11,23,36,42).
RNA extraction and detection
RNA was isolated from MVs or cells using TRizol (Life Technologies). RNA was eluted with RNase-free water and RNA quantity was determined using a NanoDrop spectrophotometer (Thermo Scientific). For agarose gels, 100ng RNA was run and visualized on a 2.2% agarose gel FlashGel™ System (LONZA). Bioanalyzer analysis was performed using 300 ng RNA with an RNA 600 Nano Kit (Agilent) and an Agilent 2100 Bioanalyzer (Agilent).
miRNA PCR profiling in MVs
MV and cell RNA were reverse transcribed to cDNA using the miRCURY LNA™ Universal RT microRNA PCR, Polyadenylation and cDNA synthesis kit (EXIQON) (n=3 for each sample type). microRNAs were then profiled in the miRCURY LNA Universal RT microRNA PCR array, which consisted of 223 rat miRNAs. All data were normalized to the average of assays detected in all samples. miRNAs with cycle number (Cq) <37 for all three independent samples of MVs or cells were included for further analysis. Student's t-test was performed across all groups (p<0.05). Normalization was performed based on the average of the assays detected in all samples, as this has shown to be the best normalization for qPCR studies involving numerous assays (43). The stability of these miRNAs was higher than any single miRNA in the data set as measured by the NormFinder software (44).
Reverse transcription and quantitative real-time PCR (RT-qPCR)
50 ng RNA was reverse transcribed to cDNA with miScript II RT kit (Qiagen). The resulting cDNA was used for real-time qPCR with miRNA specific primers using the StepOnePlus Real-time PCR System and miScript SYBR Green PCR Kit (Qiagen). Fluorescence values were quantified as starting quantities using known dilutions of standard control. The reverse primer was the universal primer in the kit. The sequences for the forward primers are listed in the supplementary materials.
MVs treated with RNase before isolation
To confirm that RNA was inside and not outside the MVs, the vesicles were treated with RNase A (10mg/ml, Qiagen) and results were measured on the FlashGel™ System. To assess resistance of MV-associated RNA to ribonucleases, 0.1μg RNase A was added to MVs with or without addition of 500ng cell total RNA isolated from the cells and incubated at 37°C for 15min. Cell total RNA in nuclease-free water was used as a control. TRIzol was added to extract RNA, which was then analyzed on a 2.2% agarose gel FlashGel™ System. RT-PCR was also used to detect specific miRNAs in these samples. In some experiments, MVs were incubated with 0.05% Triton-X 100 in order to break down the membrane.
RNA sequencing (RNA-Seq)
RNA was isolated from MVs or cells using TRizol and eluted with RNase-free water. For each sample type, RNA harvested from 3 independent experiments was pooled for sequencing. After initial evaluation and quantitation of total RNA, small RNA libraries were prepared using NEBNext Multiplex Small RNA Library Set for Illumina (NEB). Based on qPCR data, libraries were pooled together and sequenced on the Illumina HiSeq 2500 (Illumina, San Diego, CA) in a single lane using TruSeq Rapid SBS (Illumina) and Single Read Cluster Kit (Illumina). Details of the bioinformatics analysis of RNA-Seq data are described in the supplementary materials.
Pathway analysis of potential miRNA target genes
BLASTN was used to obtain the human homologs of the top 10 miRNAs highly enriched in MVs from sequences that matched 100% (and the highest e-value) to the human miRNA sequence. Tarbase was used to collect experimentally determined target genes (45). For miRNA with no experimentally determined target genes, Diana microT-CDS was used (46). KEGG Pathway enrichment was performed using a hypergeometric test for significance (47). Pathways were determined using the union of all target genes given and a p-value threshold of <0.05. Disease specific pathways were not considered in the present study since our primary focus was in proteins in signaling pathways. In addition, most of the disease specific pathways identified in our analysis were associated with cancer, which was not related to our study.
Statistical analysis
Alkaline phosphatase specific activity is presented as mean±standard error of the mean (SEM) for 6 independent MV samples per variable. RT-qPCR is presented as mean±SEM for 3 independent samples per variable. Data were examined by ANOVA with post-hoc Bonferroni's modification of Student's t-test. A p value of less than 0.05 was considered statistically significant. Statistical analyses for miRNA array and RNA-Seq data are reported in the respective section.
Results
RNA is present in MVs derived from growth zone chondrocytes
Purification of MVs was demonstrated by a 5-fold enrichment of alkaline phosphatase specific activity compared to plasma membranes (Table S1), consistent with previous studies (11,23,36,47). We were able to obtain 240±66 ng RNA from MVs in two T-175 flasks of cells, resulting in an average yield of 1.9±0.8 ng RNA/104 cells. MV RNA was ∼0.1% of the total RNA in parent cells. Agarose gel electrophoresis (Fig. 1A) and Bioanalyzer data (Fig. 1B) demonstrated that MV RNA was heterogeneous in size but contained little or no large ribosomal RNA species (18S and 28S rRNA). Instead, MVs were enriched in small RNAs.
Fig. 1. Different RNA expression patterns between MVs and the parent cells.
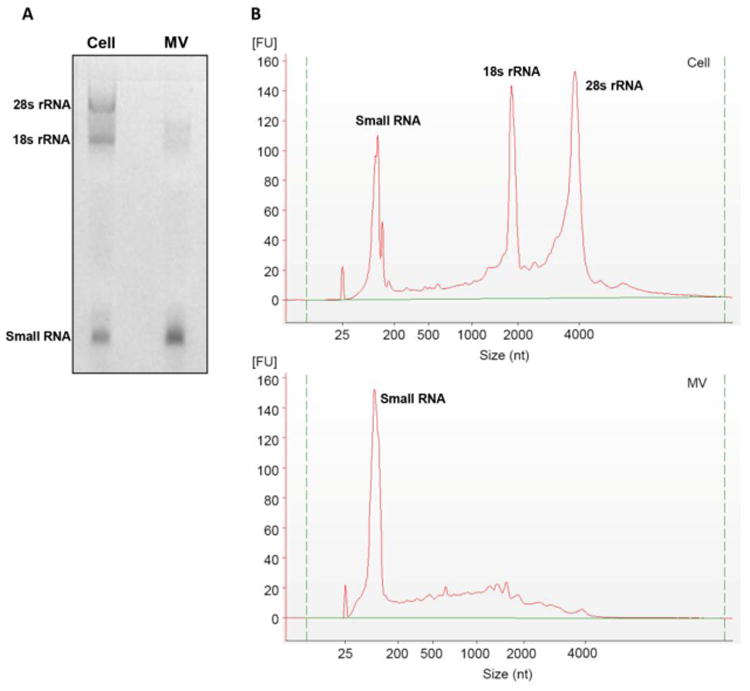
(A) RNA from MVs derived from growth zone chondrocyte cultures was compared to that from parent cells. In a 2% agarose gel, MV RNA was heterogeneous in size but contained little or no large ribosomal RNA species (18S- and 28S- rRNA) compared to parent cells. Enriched small RNAs were observed in MVs. (B) A similar pattern was observed in the Bioanalyzer.
Differential miRNA profiles exist for MVs and parent cells
miRCURY LNA microRNA PCR array profiling of MV and cell miRNA transcriptomes showed that 175 and 182 of 223 rat miRNAs in the arrays were detected in MVs and cells, respectively. 9 miRNAs were uniquely expressed in MVs and 16 were unique to cells (Fig. 2A and Table S2). Cluster analysis showed distinct differences in expression patterns (Fig. 2B). 62 (37%) of miRNAs found in both MVs and cells were significantly increased or decreased in MVs (p<0.05). 19 of these miRNAs passed a Benjamin-Hochberg correction for multiple testing at the significance level of 0.05 (red dots in Fig. 2C). 20 miRNAs demonstrated at least a 2-fold difference (Table S2).
Fig. 2. Characterization of MV miRNAs.
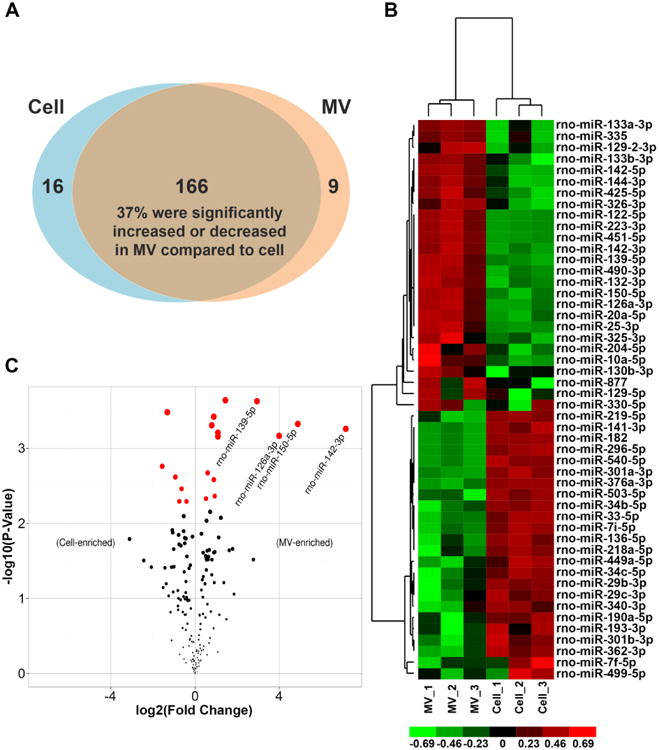
PCR arrays were used to quantitatively profile the MV miRNA transcriptome and compare it to that of the parent cells. N=3 in each group. (A) 182 and 185 of 372 miRNAs in the arrays were detected in MVs and cells, respectively. 9 miRNAs were uniquely expressed in vesicles and 16 were unique to cell RNA. For those miRNAs found in both MVs and cells, 62 (37%) were relatively increased or decreased in MVs compared to in cells. (B) Clustered heatmap showed distinct miRNA expression patterns in cells and MVs. (C) The volcano plot shows the relation between the p-values and the fold change (ddCq). 19 miRNAs passed a Benjamin-Hochberg correction for multiple testing at the significance level of 0.05 (labelled in red), 4 of which had at least 4-fold (log2(2)) difference.
Specific miRNAs are enriched in MVs relative to cells
The top three miRNAs that were detected only in MVs (miR-451-5p, miR-223-3p, miR-122-5p) and the top three miRNAs demonstrating the most significant increases in MVs compared to cells (miR-142-3p, miR-150-5p and miR-126a-3p) were validated by RT-qPCR (highlighted in a yellow box in Table S2). It is still arguable which endogenous control should be used to normalize RT-qPCR results for exosomal miRNAs. Stability of miR-652-3p was closest to the global expression level in our array and we used it as our endogenous control. Consistent with the array, the selected miRNAs were all significantly higher in MVs compared to cells (Fig. 3). For instance, miR-451-5p showed more than a 1500-fold enrichment in MVs v. cells, and levels of miR-223-3p in MVs was more than 100-fold higher. Expression of RNU6-2, a small RNA commonly used as an endogenous control for miRNA studies, was much higher in cells compared to MVs (Fig. S2). That a group of miRNAs tended to remain inside the cell instead of being exported into MVs (Table S2), suggests the packaging of RNA into MVs is selective.
Fig. 3. Further validation of MV miRNA expression.
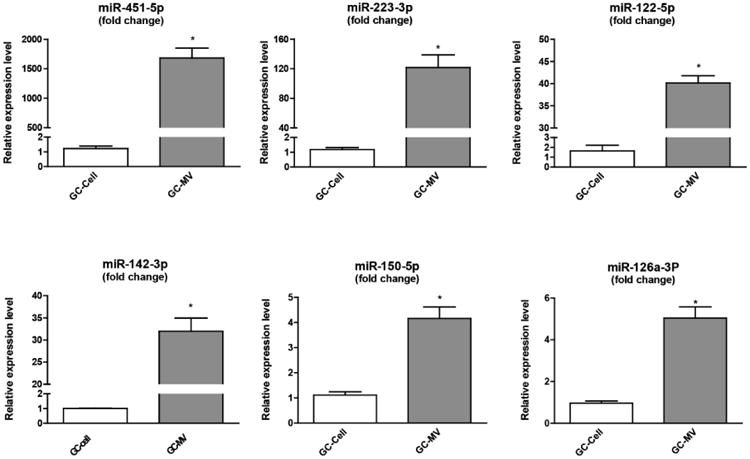
Based on array results, six miRNAs (miR-451-5p, miR-223-3p, miR-122-5p, miR-142-3p, miR-150-5p, miR-126a-3p) were selected for further RT-qPCR assays. Consistent with previous array results, the expression of all these miRNAs were significantly higher in MVs compared to in cells. These results suggest that certain miRNAs are selectively exported into MVs. *: p<0.001 in t-test. (n=3)
A complex population of small RNAs was found in MVs
In order to obtain a global view of the small RNA in MVs, we employed high throughput, unbiased next generation sequencing technology to sequence the small RNA from MVs, and compared the results to parent chondrocytes. We obtained 17,528,550 clean reads that represented 2,568,971 small RNA species in the MVs, and 35,329,606 clean reads that represented 2,840,918 small RNAs in cells (Table S3). A majority of the reads (68.23%) in MVs were unannotated, which was a much higher percentage than that in cells (27.01%) (Fig. 4A). The annotated small RNAs in MVs included rRNA (11.53%), repeat associated sequence (8.08%), miRNAs (6.08%), transfer RNA (tRNA) (3.33%), and protein coding sequence (0.68%), whereas the donor chondrocytes demonstrated different small RNA profiles with significantly more rRNA (27.65%) and miRNAs (22.65%). In addition, the length distribution of unique small RNA reads (15-30 nt) in cells and MVs varied remarkably. In cells, a striking peak was seen between the lengths of 22 to 24 nt, into which the majority of the miRNAs fell (Fig. 4B). However, this peak was not prominent in MVs, in which the length distribution of the sequencing reads skewed toward smaller sizes (15-22nt), suggesting an abundance of other non-miRNA small RNAs.
Fig. 4. Differences in the complex populations of small RNAs between MVs and parent cells.
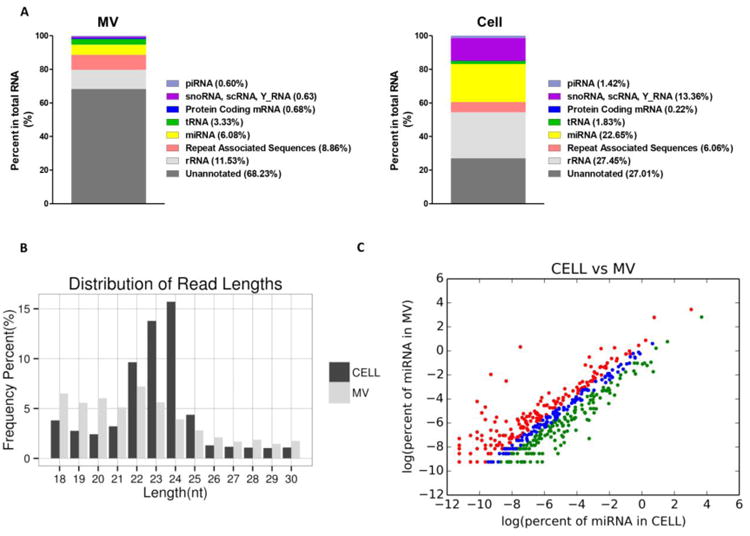
(A) Complex populations of coding and non-coding small RNAs were found in MVs in proportions that were distinct from those in the cells from which they were derived. (B) Distribution of small RNA lengths in MVs and growth zone chondrocytes. (C) miRNAs in MVs and cells identified by RNA-seq were plotted. Red dots represent miRNAs with at least a 2-fold expression level increase in MVs than those in cells. Green dots represent miRNAs with more than a 2-fold expression level increase in cells than that in MVs. Blue indicates the overlap between these two groups.
Although miRNAs were in a relatively smaller proportion of the total small RNA pool in MVs compared with those in cells, they were still the third most abundant annotated small RNA species in MVs. RNA-Seq identified 452 miRNAs in MVs, 289 of which were not found in previous PCR arrays (Fig. S3). To our surprise, the entire MV miRNA pool was dominated by a small number of miRNAs. The most abundant miRNA in MVs was miR-143-3p, which accounted for 31.58% of the total miRNA reads. The top 3 most abundant miRNAs occupied over 60% of the miRNA pool (Table S4), which was similar to that of the parent cell miRNA pool (Table S5), indicating that these highly abundant miRNAs may be critical background components of gene expression regulation in chondrocytes. Despite the similarities, the differences in miRNA profile between MVs and donor cells were distinct. 131 (28.9%) miRNAs were found to be enriched in MVs by at least two-fold (Fig. 4C). The difference between MVs and cells was far more dramatic than that between cells harvested from different zones of cartilage (data not shown). The most differentially expressed miRNAs identified from RNA-Seq (including miR-122-5p, miR-451-5p, miR-144-3p, miR-142-3p, miR-133a-3p) were also reported by PCR arrays (Table 1 and Table S6). In addition, we found a number of miRNA processing variants in MVs v. cells. For example, miR-376a-5p was the dominant form of miR-376a in cells with more than a 3-fold higher expression level than miR-376a-3p. However, the dominant form was miR-376a-3p in MVs. Other examples are listed in Table S7. Differential packaging of miRNA variants derived from the same precursor further supported that the packaging of miRNAs into MV is a selective process.
Table 1.
Top 20 selectively packaged miRNAs in MVs in RNA-Seq. Gray indicates the enriched miRNAs confirmed by PCR arrays. Most of the rest miRNAs were not included in the PCR arrays.
| miRNA | % in MVs | % in cells | Fold enrichment in MVs |
|---|---|---|---|
| rno-miR-122-5p | 1.40E+00 | 5.57E-04 | 2504.17 |
| rno-miR-451-5p | 1.42E-01 | 9.10E-05 | 1560.37 |
| rno-miR-144-3p | 1.49E-02 | 3.90E-05 | 382.85 |
| rno-miR-486 | 8.12E-02 | 2.33E-04 | 347.82 |
| rno-miR-142-3p | 9.34E-03 | 6.50E-05 | 144.13 |
| rno-miR-133a-3p | 5.74E-03 | 5.20E-05 | 110.73 |
| rno-miR-328a-5p | 6.80E-04 | 1.30E-05 | 52.55 |
| rno-miR-363-3p | 3.31E-03 | 6.50E-05 | 51.05 |
| rno-miR-375-3p | 8.80E-04 | 2.60E-05 | 33.78 |
| rno-miR-142-5p | 3.99E-03 | 1.30E-04 | 30.78 |
| rno-miR-193b-5p | 1.17E-03 | 3.90E-05 | 30.03 |
| rno-miR-144-5p | 3.90E-04 | 1.30E-05 | 30.03 |
| rno-miR-23a-5p | 2.72E-03 | 1.17E-04 | 23.35 |
| rno-miR-92b-5p | 2.90E-04 | 1.30E-05 | 22.52 |
| rno-miR-1298 | 2.90E-04 | 1.30E-05 | 22.52 |
| rno-miR-1b | 6.80E-04 | 3.90E-05 | 17.52 |
| rno-miR-1224 | 8.80E-04 | 5.20E-05 | 16.89 |
| rno-miR-423-5p | 5.98E-01 | 3.90E-02 | 15.33 |
| rno-miR-320-3p | 1.22E+00 | 8.09E-02 | 15.02 |
| rno-miR-92a-l-5p | 1.36E-03 | 9.10E-05 | 15.01 |
Functional annotation of MV-enriched miRNAs
miRNAs function by binding to target mRNAs and inhibiting their translation or promoting degradation. In order to explore the functions of MV miRNA, we identified the potential target genes of the top 10 most enriched miRNAs in MVs. Based on results from KEGG signaling pathway analysis, key functions of target genes included roles in the phosphoinositide 3-kinase (PI3K)–AKT signaling pathway, focal adhesion, endocytosis, ubiquitin mediated proteolysis, the hypoxia-inducible factors (HIF-1) signaling pathway, the WNT signaling pathway and the transforming growth factor beta (TGFB) signaling pathway, many of which have been shown to play important roles in cartilage development and extracellular matrix regulation (Table 2).
Table 2. KEGG pathways targeted by the top 10 MV enriched miRNAs.
| KEGG pathway | P value | Genes | # of miRNAs |
|---|---|---|---|
| PI3K-Akt signaling pathway | 2.53E-08 | 23 | 8 |
| Endocytosis | 4.88E-02 | 18 | 8 |
| Focal adhesion | 3.75E-04 | 21 | 7 |
| Ubiquitin mediated proteolysis | 3.17E-09 | 20 | 7 |
| Viral carcinogenesis | 2.54E-03 | 19 | 7 |
| HIF-1 signaling pathway | 5.67E-06 | 17 | 7 |
| Adherens junction | 2.83E-02 | 8 | 7 |
| Regulation of actin cytoskeleton | 2.80E-04 | 22 | 6 |
| Cholinergic synapse | 6.89E-05 | 17 | 6 |
| Wnt signaling pathway | 8.86E-03 | 17 | 6 |
| Dopaminergic synapse | 1.17E-02 | 15 | 6 |
| Osteoclast differentiation | 2.02E-02 | 14 | 6 |
| TGF-beta signaling pathway | 1.56E-04 | 12 | 6 |
| Progesterone-mediated oocyte maturation | 2.40E-04 | 11 | 6 |
| mTOR signaling pathway | 2.24E-06 | 10 | 6 |
| Gastric acid secretion | 4.82E-02 | 6 | 6 |
| Neurotrophin signaling pathway | 5.20E-04 | 16 | 5 |
| Axon guidance | 5.21E-03 | 15 | 5 |
| Insulin signaling pathway | 2.27E-02 | 14 | 5 |
| T cell receptor signaling pathway | 6.93E-04 | 13 | 5 |
RNA in MVs was resistant to RNase
MV RNA was confined inside MVs and was not due to cell RNA contamination during isolation, nor was it bound to external structures or macromolecules. RNase treatment alone could not digest the RNA component in MVs (Fig. 5A, lane 2), but exogenous cellular RNA added into the MV suspension was completely degraded by RNase (Fig. 5B, lane 3). These results showed that the RNase was functional and no RNase inhibitors were in the MV samples. Trypsin was used to release the MVs from the extracellular matrix during harvesting; thus the exosomal RNA was also resistant to trypsin digestion. However, when we treated the MVs with membrane detergent Triton X-100, RNA degradation was observed (Fig. 5B, lane 5 and 6). Pretreatment of MVs with RNase before RNA extraction did not result in a significant decrease in the RNA:protein ratio of 0.3ng RNA/μg protein (Fig. 5C). Pretreatment with detergent had no effect on detection of miRNAs. miR-451-5p, miR-223-3p, miR-142-3p and miR-652-3p all required similar cycle numbers regardless of RNase treatment (Fig. 5D). After normalization, RNase treatment did not lead to significant differences in the levels of these miRNAs, which indicates that these miRNAs were resistant to RNase and present inside the MVs (Fig. 5E).
Fig. 5. MV RNA is resistant to RNase.
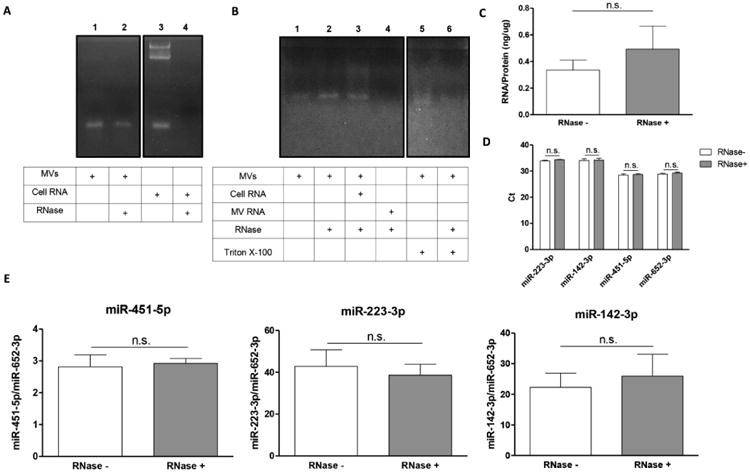
(A) MVs were treated by RNase A before RNA extraction by TRIzol. Total cell RNA was also treated with RNase A to confirm enzymatic activity. RNase was not able to digest the RNA component in MVs. However, it was able to digest the total cell RNA. (B) When the MVs were pre-treated with membrane detergent Triton X-100, RNA degradation was observed, suggesting that the intact lipid membrane was important for protection of MV RNA. (C) No significant differences in the RNA:protein ratios of MVs with or without RNase pretreatment (n=6) were observed. (D) The detection of miR-451-5p, miR-223-3p, miR-142-3p and miR-652-3p by RT-PCR occured at similar cycle numbers regardless of RNase treatment (no significant difference, n=6). (E) After normalization, no significant differences were observed for expression levels of miR-451-5p, miR-223-3p and miR-142-3p between MV samples with and without RNase pretreatment.
Discussion
Although Slavkin et al. first reported the presence of RNA bases in extracellular membrane-bound electron dense bodies in tooth germ organ cultures, which accounted for 0.4-0.5% by dry weight of the extracellular matrix at the epithelial/mesenchymal interface (34), genetic material was not considered to exist in MVs because these extracellular organelles lack protein synthesis activity. Very recently, Mitton et al. detected certain mRNAs in MVs harvested from articular cartilage, but did not address the overall profile of the MV RNA or its relationship to cellular RNA (48). Our results demonstrate RNA in MVs in the endochondral bone formation system, confirming these earlier indications that it is present in extracellular vesicles. RNA was still present in MVs after RNase treatment of intact vesicles but was susceptible to RNase following disruption of the membrane with detergent. This indicates MV RNAs are not due to non-specific adsorption of cellular RNA during isolation and the majority of the RNA we obtained is inside the MVs. Similar observations have been found in other extracellular microvesicles such as exosomes (28-30,49).
Our results support the hypothesis that RNA is packaged in MVs in a regulated manner. The profile of MV RNA was distinct from that of parent cells. Large RNA species such as 18S and 28S rRNA were lacking in MVs. Instead, small RNAs less than 200nt were highly enriched, similar to other cell-derived microvesicles (28,50,51). Cells contained more rRNAs, miRNAs, snoRNAs and scRNAs than MVs, whereas more than two thirds of the small RNAs in MVs were unannotated in current databases. Together with the difference in read size distribution, this suggests that other unknown small RNAs are present in MVs. Similar observations were also reported in glioma microvesicles and exosomes secreted by human esophageal cancer cells (52,53).
It is likely that the RNA content of extracellular vesicles is tissue specific. MVs exhibited a complex population of annotated small RNAs, of which the majority was rRNA followed by repeat-associated small RNA, which is similar to small RNAs reported in the literature (28,50,54,55). However, miRNA only comprised 6.1% of the total reads from MVs, whereas miRNAs were the most abundant small RNA species in plasma-derived exosomes, accounting for over 42.3% of the total RNA(55). 17% of genome-matching sequences in monocyte-derived exosomes were miRNAs (50) but only about 1% of the small RNA pool in neurons included miRNAs (56).
It is possible that MVs are a unique type of microvesicle compared to exosomes. MVs have a strong affinity to collagen and stay in the extracellular matrix (10), while exosomes are released into the culture media. Chondrocytes also released membrane vesicles into the medium but they differed from MVs with respect to alkaline phosphatase activity, as noted previously (8). MVs differ from exosomes in other ways as well. MVs are formed by a cell “budding” mechanism (6,21), but exosomes are formed through membrane invagination (32). Such differences suggest that cells may secret different microvesicle species and their cargos may vary from each other in order to achieve a variety of biological functions.
Many MV miRNAs found in this study were differentially expressed between MVs and cells raising an interesting question: is exportation of miRNAs into MVs a random result of membrane shedding, or an actively regulated selection process? Using a somatic cell hybrid model, we previously showed that MVs are produced as distinct organelles (21). They have a distinct phospholipid composition (11,15), protein kinase C isoforms are differentially incorporated into MVs and plasma membranes under hormonal regulation (57), and they contain MMPs and growth factors that are not found in the cytoplasm (18,24).
Certain miRNAs, e.g. miR-122-5p, miR-223-3p and miR-451-5p, were enriched hundreds or even thousands of times in MVs whereas other miRNAs tended to remain in parent cells. This was consistently observed in all miRNA detection platforms that we used: miRCURY LNA miRNA PCR array, next generation RNA sequencing and individual miRNA qRT-PCR. Hence, it is unlikely that enrichment of specific miRNAs in MVs was a technical artifact. Other evidence also demonstrated the specificity in the MV miRNA cargo. Generally, only one strand of the hairpin structure in the precursor transcript will be incorporated into and stabilized by the RNA-induced silencing complex (RISC), and is considered the dominant/mature functional miRNA (58). However, the dominant form of some miRNAs in MVs was present on opposite strands of the predominant miRNAs in cells, as has been reported in glioma microvesicles (52). Therefore, MV RNA is unlikely only a simple, small copy of intracellular RNA. Selective mechanisms may exist in chondrocytes to determine which miRNAs will be packed into MVs. These mechanisms may intersect with the miRNA biogenesis pathways to preserve and export a certain strand of the miRNA hairpin precursors. Despite the discrepancies in the biogenesis process between MVs and exosomes, it is possible that miRNA sorting systems used to sort miRNAs for exosomes (59,60), also are used by MVs for selective miRNA packaging.
miRNAs have been shown to play important roles in bone formation and homeostasis (61). Through RNA-Seq, we found that miR-143-3p and miR-21-5p were the two most abundant miRNAs in growth zone chondrocytes as well the MVs they produce, dominating over 60% of the miRNA pools. Previous studies have shown that miR-143 has high levels of expression in the superficial zone of articular cartilage (62). miR-21 was also reported to control the development of osteoarthritis by targeting GDF-5 in chondrocytes (63). Overexpression of miR-21-5p promotes chondrocyte proliferation and matrix synthesis (64). Therefore, our data suggest that these miRNAs may be critical components in chondrocyte gene regulation networks. It will be interesting to determine whether such miRNAs also exert their functions through MVs, at least partially.
At this time, little is known about the function of MV miRNAs in bone formation. Extracellular miRNAs have been shown to play an important role in cell-cell communication in physiological and pathological processes (65-68). It is reasonable to assume that MV miRNAs can also be transferred to other cells in the local environment in an autocrine or paracrine manner, and thereby, regulate their gene expression and biological behavior. In our study, geneontology analysis of the predicted targets of miRNAs enriched in MVs identified several signaling pathways including PI3K-AKT, focal adhesion, HIF-1, TGFβ, insulin and WNT signaling, which have all been shown to play important roles chondrogenesis and bone formation (69-73). It has been shown that PI3K-Akt-mTOR axis is central to insulin and insulin like growth factor (IGF) signaling, which are major anabolic factors for musculoskeletal cells including chondrocytes and osteoblasts. The strong association between MV miRNAs and PI3K-Akt-mTOR/IGF/WNT signaling molecules suggests that MVs may act as timed release agents to affect neighbor cell metabolism in the musculoskeletal system.
Most of the miRNAs enriched in chondrocyte MVs have also been shown to be regulators in cell proliferation. For example, overexpression of miR-451-5p, one of the most highly enriched MV miRNAs, inhibited the proliferation of osteosarcoma cells by modulating the expression of prostaglandin E2 (PGE2) and cyclin D1 (74). Transfection of miR-223-3p has been shown to greatly block osteosarcoma Saos-2 cells from proliferation (75). miR-122-5p inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R (76). We also observed that some of the MV-enriched miRNAs were involved in the regulation of chondrocyte proliferation (data not shown). These examples indicate that MVs may carry a group of miRNAs that are capable of regulating the proliferation and differentiation of recipient cells through modification of their gene expression. These also increase the complexity of communication among cells in the mineralization environment. These communications may be important for the regulation of biological cascades, including matrix remodeling and angiogenesis.
miRNAs are 10 times more stable than mRNAs and have an average half-life of 5 days make them a perfect mediator to survive the stringent extracellular environment and function in cell-cell communications (77). How MVs transfer their miRNAs to recipient cells, is not clear, however. Future studies will provide more details about the trafficking of MV miRNAs.
In conclusion, we demonstrated that RNA is present in MVs, and we further characterized the miRNA profile of this RNA. Compared to parent cells, MVs possessed a different miRNA expression pattern, and specific miRNAs were highly enriched. Our study suggests a potential role of MVs as “matrisomes” in cell-cell communication during bone formation and healing through the transfer of miRNAs. It may be possible to engineer MVs to contain specific miRNA to be delivered to cells in the mineralization environment to facilitate bone formation.
Supplementary Material
Highlights.
RNA is present in matrix vesicles (MVs) secreted by chondrocytes, in which small RNA is enriched.
MV RNA is well-protected from degradation by the lipid membrane.
Enrichment of specific miRNAs is consistently observed, suggesting selective packaging.
MV-enriched miRNAs are related to major bone formation and metabolic signaling pathways.
MVs as “matrisomes” are involved in cell-cell communication in cartilage and bone development.
Acknowledgments
We thank Alice Cheng for her help in editing the manuscript. This paper is dedicated to Harold Slavkin in honor of his original finding of extracellular RNA in calcifying tissue.
Grant: This work was funded by Virginia Commonwealth University Startup Support (ZL), Shanghai Jiao Tong University School of Medicine 085 Project (JZ), and Department of Defense W81XWH-11-1-0306 (BDB).
Footnotes
Disclosure Statement: All authors state that they have no conflicts of interest.
Author Contributions: Z.L., Z.S. and B.D.B. conceived and designed the experiments; Z.L., J.Z., and A.N.R. performed the experiments; N.R. and Z.L. performed bioinformatics analysis, Z.L, J.Z., N.E.R., Z.S. and B.D.B. analyzed the data; S.L.H. contributed reagents/materials/analysis tools; Z.L., J.Z., N.E.R., Z.S. and B.D.B. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20(1):33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- 3.Ali SY, Anderson HC, Sajdera SW. Enzymic and electron-microscopic analysis of extracellular matrix vesicles associated with calcification in cartilage. Biochem J. 1971;122(5):56P. doi: 10.1042/bj1220056pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970;67(3):1513–20. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majeska RJ, Wuthier RE. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim Biophysica Acta. 1975;391(1):51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- 6.Wuthier RE, Lipscomb GF. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci. 2011;16:2812–902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 7.Dean DD, Schwartz Z, Bonewald L, et al. Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic acid. Calcif Tissue Int. 1994;54(5):399–408. doi: 10.1007/BF00305527. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz JP, Dean DD, Schwartz Z, et al. Chondrocyte cultures express matrix metalloproteinase mRNA and immunoreactive protein; stromelysin-1 and 72 kDa gelatinase are localized in extracellular matrix vesicles. J Cell Biochem. 1996;61(3):375–91. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C375::AID-JCB5%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Boskey AL, Boyan BD, Schwartz Z. Matrix vesicles promote mineralization in a gelatin gel. Calcif Tissue Int. 1997;60(3):309–15. doi: 10.1007/s002239900234. [DOI] [PubMed] [Google Scholar]
- 10.Wu LN, Sauer GR, Genge BR, Wuthier RE. Induction of mineral deposition by primary cultures of chicken growth plate chondrocytes in ascorbate-containing media. Evidence of an association between matrix vesicles and collagen. J Biol Chem. 1989;264(35):21346–55. [PubMed] [Google Scholar]
- 11.Boyan BD, Schwartz Z, Carnes DL, Jr, Ramirez V. The effects of vitamin D metabolites on the plasma and matrix vesicle membranes of growth and resting cartilage cells in vitro. Endocrinology. 1988;122(6):2851–60. doi: 10.1210/endo-122-6-2851. [DOI] [PubMed] [Google Scholar]
- 12.Buckwalter JA, Mower D, Schaeffer J. Differences in matrix vesicle concentration among growth plate zones. J Orthop Res. 1987;5(2):157–63. doi: 10.1002/jor.1100050202. [DOI] [PubMed] [Google Scholar]
- 13.Derfus BA, Kurtin SM, Camacho NP, Kurup I, Ryan LM. Comparison of matrix vesicles derived from normal and osteoarthritic human articular cartilage. Connect Tissue Res. 1996;35(1-4):337–42. doi: 10.3109/03008209609029209. [DOI] [PubMed] [Google Scholar]
- 14.Anderson HC. Osteogenetic epithelial-mesenchymal cell interactions. Clin Othop Relat Res. 1976;(119):211–23. [PubMed] [Google Scholar]
- 15.Boyan BD, Schwartz Z, Swain LD, Carnes DL, Jr, Zislis T. Differential expression of phenotype by resting zone and growth region costochondral chondrocytes in vitro. Bone. 1988;9(3):185–94. doi: 10.1016/8756-3282(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 16.Dean DD, Boyan BD, Muniz OE, Howell DS, Schwartz Z. Vitamin D metabolites regulate matrix vesicle metalloproteinase content in a cell maturation-dependent manner. Calcif Tissue Int. 1996;59(2):109–16. doi: 10.1007/s002239900096. [DOI] [PubMed] [Google Scholar]
- 17.Dean DD, Schwartz Z, Schmitz J, et al. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Connect Tissue Res. 1996;35(1-4):331–6. doi: 10.3109/03008209609029208. [DOI] [PubMed] [Google Scholar]
- 18.Dean DD, Schwartz Z, Muniz OE, et al. Matrix vesicles are enriched in metalloproteinases that degrade proteoglycans. Calcif Tissue Int. 1992;50(4):342–9. doi: 10.1007/BF00301632. [DOI] [PubMed] [Google Scholar]
- 19.Maeda S, Dean DD, Gay I, Schwartz Z, Boyan BD. Activation of latent transforming growth factor beta1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 2001;16(7):1281–90. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- 20.D'Angelo M, Billings PC, Pacifici M, Leboy PS, Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Bioll Chem. 2001;276(14):11347–53. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- 21.Leach RJ, Schwartz Z, Johnson-Pais TL, Dean DD, Luna M, Boyan BD. Osteosarcoma hybrids can preferentially target alkaline phosphatase activity to matrix vesicles: evidence for independent membrane biogenesis. J Bone Miner Res. 1995;10(11):1614–24. doi: 10.1002/jbmr.5650101103. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz Z, Sela J, Ramirez V, Amir D, Boyan BD. Changes in extracellular matrix vesicles during healing of rat tibial bone: a morphometric and biochemical study. Bone. 1989;10(1):53–60. doi: 10.1016/8756-3282(89)90147-6. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz Z, Schlader DL, Swain LD, Boyan BD. Direct effects of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 on growth zone and resting zone chondrocyte membrane alkaline phosphatase and phospholipase-A2 specific activities. Endocrinology. 1988;123(6):2878–84. doi: 10.1210/endo-123-6-2878. [DOI] [PubMed] [Google Scholar]
- 24.Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab. 2008;26(5):514–9. doi: 10.1007/s00774-008-0859-z. [DOI] [PubMed] [Google Scholar]
- 25.Jose AM, Garcia GA, Hunter CP. Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Nat Struct Mol Biol. 2011;18(11):1184–8. doi: 10.1038/nsmb.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110(3):483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 27.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2(5):606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: Are they anchored exosomes? Bone. 2015;79:29–36. doi: 10.1016/j.bone.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slavkin HC, Bringas P, Bavetta LA. Ribonucleic acid within the extracellular matrix during embryonic tooth formation. J Cell Physio. 1969;73(3):179–90. doi: 10.1002/jcp.1040730304. [DOI] [PubMed] [Google Scholar]
- 35.Boyan BD, Schwartz Z, Swain LD, Khare A. Role of lipids in calcification of cartilage. Anat Rec. 1989;224(2):211–9. doi: 10.1002/ar.1092240210. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz Z, Knight G, Swain LD, Boyan BD. Localization of vitamin D3-responsive alkaline phosphatase in cultured chondrocytes. J Biol Chem. 1988;263(13):6023–6. [PubMed] [Google Scholar]
- 37.Boyan BD, Schwartz Z, Park-Snyder S, et al. Latent transforming growth factor-beta is produced by chondrocytes and activated by extracellular matrix vesicles upon exposure to 1,25-(OH)2D3. J Biol Chem. 1994;269(45):28374–81. [PubMed] [Google Scholar]
- 38.Nemere I, Schwartz Z, Pedrozo H, Sylvia VL, Dean DD, Boyan BD. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13(9):1353–9. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz Z, Sylvia VL, Larsson D, et al. 1alpha,25(OH)2D3 regulates chondrocyte matrix vesicle protein kinase C (PKC) directly via G-protein-dependent mechanisms and indirectly via incorporation of PKC during matrix vesicle biogenesis. J Biol Chem. 2002;277(14):11828–37. doi: 10.1074/jbc.M110398200. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz Z, Bonewald LF, Caulfield K, Brooks B, Boyan BD. Direct effects of transforming growth factor-beta on chondrocytes are modulated by vitamin D metabolites in a cell maturation-specific manner. Endocrinology. 1993;132(4):1544–52. doi: 10.1210/endo.132.4.8462452. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz Z, Semba S, Graves D, Dean DD, Sylvia VL, Boyan BD. Rapid and long-term effects of PTH(1-34) on growth plate chondrocytes are mediated through two different pathways in a cell-maturation-dependent manner. Bone. 1997;21(3):249–59. doi: 10.1016/s8756-3282(97)00123-3. [DOI] [PubMed] [Google Scholar]
- 42.Boyan BD, Schwartz Z, Swain LD. Matrix vesicles as a marker of endochondral ossification. Connect Tissue Res. 1990;24(1):67–75. doi: 10.3109/03008209009152423. [DOI] [PubMed] [Google Scholar]
- 43.Mestdagh P, Van Vlierberghe P, De Weer A, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10(6):R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–50. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 45.Vlachos IS, Paraskevopoulou MD, Karagkouni D, et al. DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015;43(Database issue):D153–9. doi: 10.1093/nar/gku1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paraskevopoulou MD, Georgakilas G, Kostoulas N, et al. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41(Web Server issue):W169–73. doi: 10.1093/nar/gkt393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42(Database issue):D199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitton E, Gohr CM, McNally MT, Rosenthal AK. Articular cartilage vesicles contain RNA. Biochem Biophys Res Commun. 2009;388(3):533–8. doi: 10.1016/j.bbrc.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11(9):1143–9. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 51.Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li CC, Eaton SA, Young PE, et al. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10(8):1333–44. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao J, Liu R, Yin L, Pu Y. Expression profiling of exosomal miRNAs derived from human esophageal cancer cells by Solexa high-throughput sequencing. Int J Mol Sci. 2014;15(9):15530–51. doi: 10.3390/ijms150915530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji H, Chen M, Greening DW, et al. Deep sequencing of RNA from three different extracellular vesicle (EV) subtypes released from the human LIM1863 colon cancer cell line uncovers distinct miRNA-enrichment signatures. PLoS One. 2014;9(10):e110314. doi: 10.1371/journal.pone.0110314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40(21):10937–49. doi: 10.1093/nar/gks832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sylvia VL, Schwartz Z, Ellis EB, et al. Nongenomic regulation of protein kinase C isoforms by the vitamin D metabolites 1 alpha,25-(OH)2D3 and 24R,25-(OH)2D3. J Cell Physiol. 1996;167(3):380–93. doi: 10.1002/(SICI)1097-4652(199606)167:3<380::AID-JCP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 58.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 59.Squadrito ML, Baer C, Burdet F, et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Reports. 2014;8(5):1432–46. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 60.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lian JB, Stein GS, van Wijnen AJ, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212–27. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong E, Reddi AH. Dedifferentiation and redifferentiation of articular chondrocytes from surface and middle zones: changes in microRNAs-221/-222, -140, and -143/145 expression. Tissue Eng Part A. 2013;19(7-8):1015–22. doi: 10.1089/ten.TEA.2012.0055. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Jia J, Yang S, Liu X, Ye S, Tian H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp Mol Med. 2014;46:e79. doi: 10.1038/emm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kongcharoensombat W, Nakasa T, Ishikawa M, et al. The effect of microRNA-21 on proliferation and matrix synthesis of chondrocytes embedded in atelocollagen gel. Knee Surg Sports Traumatol Arthrosc. 2010;18(12):1679–84. doi: 10.1007/s00167-010-1111-7. [DOI] [PubMed] [Google Scholar]
- 65.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 68.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 69.Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci. 2009;122(Pt 5):716–26. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biggs MJ, Dalby MJ. Focal adhesions in osteoneogenesis. Proc Inst Mech Eng H. 2010;224(12):1441–53. doi: 10.1243/09544119JEIM775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wan C, Shao J, Gilbert SR, et al. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci. 2010;1192:322–6. doi: 10.1111/j.1749-6632.2009.05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 74.Xu H, Mei Q, Shi L, Lu J, Zhao J, Fu Q. Tumor-suppressing effects of miR451 in human osteosarcoma. Cell Biochem Biophys. 2014;69(1):163–8. doi: 10.1007/s12013-013-9783-5. [DOI] [PubMed] [Google Scholar]
- 75.Xu J, Yao Q, Hou Y, et al. MiR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferation. Biomed Pharmacother. 2013;67(5):381–6. doi: 10.1016/j.biopha.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Wang B, Wang H, Yang Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS One. 2012;7(10):e47053. doi: 10.1371/journal.pone.0047053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gantier MP, McCoy CE, Rusinova I, et al. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res. 2011;39(13):5692–703. doi: 10.1093/nar/gkr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


