Abstract
The sensory innervation of the distal colorectum includes mechanically insensitive afferents (MIAs; ∼25%), which acquire mechanosensitivity in persistent visceral hypersensitivity and thus generate de novo input to the central nervous system. We utilized an optogenetic approach to bypass the process of transduction (generator potential) and focus on transformation (spike initiation) at colorectal MIA sensory terminals, which is otherwise not possible in typical functional studies. From channelrhodopsin2-expressing mice (driven by Advillin-Cre), the distal colorectum with attached pelvic nerve was harvested for ex vivo single-fiber recordings. Afferent receptive fields (RFs) were identified by electrical stimulation and tested for response to mechanical stimuli (probing, stroking, and stretch), and afferents were classified as either MIAs or mechanosensitive afferents (MSAs). All MIA and MSA RFs were subsequently stimulated optically and MIAs were also tested for activation/sensitization with inflammatory soup (IS), acidic hypertonic solution (AHS), and/or bile salts (BS). Responses to pulsed optical stimuli (1–10 Hz) were comparable between MSAs and MIAs whereas 43% of MIAs compared with 86% of MSAs responded tonically to stepped optical stimuli. Tonic-spiking MIAs responded preferentially to AHS (an osmotic stimulus) whereas non-tonic-spiking MIAs responded to IS (an inflammatory stimulus). A significant proportion of MIAs were also sensitized by BS. These results reveal transformation as a critical factor underlying the differences between MIAs (osmosensors vs. inflammatory sensors), revealing a previously unappreciated heterogeneity of MIA endings. The current study draws attention to the sensory encoding of MIA nerve endings that likely contribute to afferent sensitization and thus have important roles in visceral pain.
Keywords: channelrhodopsin2, single fiber, silent afferents, irritable bowel syndrome, afferent sensitization, inflammatory soup
sensory afferents innervating the viscera have drawn significant research interest as clinical and preclinical evidence indicates that persistent afferent drive (e.g., afferent sensitization) contributes to long-lasting organ hypersensitivity, discomfort, and pain, hallmark complaints of patients with visceral pain disorders such as irritable bowel syndrome (IBS) (29, 39, 40) and bladder pain syndrome (27) for which a pathobiology is not apparent. Using an ex vivo colorectum-nerve preparation together with different animal models of persistent colorectal hypersensitivity, we and others have documented long-term sensitization of mechanosensitive colorectal afferents that contribute to prolonged colorectal hypersensitivity (1, 13, 14).
In addition, the colorectum is also innervated by a population of “silent” or “sleeping” nociceptors, more appropriately designated mechanically insensitive afferents (MIA) (26). Unlike most other studies in which MIAs were often recorded fortuitously after organ inflammation, ischemia, or injury (5, 18, 25), we systemically characterized the colorectal innervation using an unbiased electrical stimulation search strategy and discovered that MIAs comprise 25–30% of colorectal afferents in the lumbar splanchnic (LSN) and pelvic nerve (PN) pathways (8). We subsequently found an ∼50% reduction in the proportion of MIAs in the PN pathway in mice with behavioral colorectal hypersensitivity, suggesting a phenotypical switch of those MIAs, which acquire mechanosensitivity and generate de novo sensory input to the central nervous system (CNS) (13, 14, 21). Further understanding of the role of MIAs in visceral sensation and nociception is hindered by the limited knowledge of sensory encoding and processing at the micrometer-thick afferent nerve terminals embedded in the colorectum, prohibiting direct studies by intracellular recordings (for discussion, see Ref. 41).
Sensory afferent terminals contain transducer molecules that respond to specific stimulus modalities (e.g., mechanical, chemical, thermal) by causing a local depolarization known as the generator potential (24), a process referred to as transduction (41). This is immediately followed by the process of transformation, in which the generator potential is transformed into a train of action potential pulses at the spike initiation zone that relays the sensory information to the CNS (15, 38). We hypothesize that unique features of the transduction and transformation processes in MIAs, compared with their mechanosensitive counterparts, are responsible for their insensitivity to mechanical stimuli, subserve their phenotypical switch/sensitization and contribute to their functional heterogeneity. In the present study, we adopted an optogenetic approach to examine the contribution of transformation to colorectal afferent encoding using a light-sensitive, nonselective ion channel channelrohdopsin (ChR2), a method characterized by us and others to assess the excitability of primary afferent endings by driving ChR2 expression in sensory afferent neurons with the Advillin promoter (41). Depolarizing optical current via ChR2 activation allows generating a spatially and temporally controlled generator potential at the afferent ending to permit focused studies of transformation. We found that colorectal MIA endings do not differ from their mechanosensitive counterparts in encoding pulsed optical stimuli and can be about equally divided into two subgroups that have distinct responses to stepped optical stimuli. Importantly, these subgroups are differentially tuned to encode osmotic vs. inflammatory stimuli. Portions of these data have been reported in abstract form (10).
MATERIALS AND METHODS
All experimental protocols were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Transgenic mice.
As described previously(41), male heterozygous Advillin-Cre mice were crossed with homozygous Ai32 female mice (C57BL/6 background). Ai32 mice carry the Channelrhodopsin-2 gene “ChR2(H134R)-EYFP” in the Gt(ROSA)26Sor locus, which is preceded by a LoxP-flanked STOP cassette to prevent its expression. By crossing Ai32 mice with Advillin-Cre mice, the Cre-expressing cell population will have the STOP cassette trimmed, resulting in expression of ChR2-EYFP. Male mice carrying both heterozygous Cre and ChR2-EYFP alleles (Cre+/−ChR2+/−) were used for all experiments with optical stimulation. Some additional experiments were done on male C57BL/6 mice (Taconic, Hudson, NY) to assess responses of MIAs to chemical stimuli.
Immunohistochemistry.
As previously described (14), mice (Cre+/−ChR2+/−) were killed via CO2 inhalation. The distal colon and L6 dorsal root ganglion (DRG) were harvested and fixed with 4% paraformaldehyde in 0.16 M phosphate buffer containing 14% picric acid (Sigma-Aldrich). After cryoprotection in 20% sucrose, fixed tissue was embedded in OCT compound (Sakura Finetek, Tokyo, Japan), frozen, and sectioned at 20 μm. Tissue sections were incubated with a goat antibody against YFP (1:1,000; Abcam, Cambridge, MA) and the signals were further amplified by Alexa Fluor 488-conjugated anti-goat IgG (1:200; Molecular Probes, Eugene, OR).
In vitro mouse colorectum-nerve preparation.
As described previously (8, 11), male mice (Cre+/−ChR2+/−) 6- to 8-wk old and 20–30 g were killed via CO2 inhalation followed by exsanguination after perforating the right atrium. The distal colorectum (∼2 cm) along with PN was dissected in ice-cold Kreb's solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO47H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, 20 acetate, 0.004 nifedipine, and 0.003 indomethacin bubbled with carbogen (95% O2-5% CO2). The distal colorectum was transferred to a tissue chamber superfused with 32–34°C Krebs solution, opened longitudinally, and pinned flat mucosal side up. The attached PN was placed in the adjacent recording chamber filled with mineral oil (Fisher Scientific) and teased into fine bundles of ∼10-μm thick for single-fiber electrophysiological recordings by a platinum-iridium recording electrode (FHC, Bowdoin, ME).
Afferent identification and classification.
A round-tipped concentric electrode (external diameter: 0.55 mm; internal diameter: 0.125 mm; FHC) was placed perpendicular to the colorectal surface to electrically excite afferent endings (0.5-ms current pulse duration at 0.3 Hz) (8). The tip of the electrode surveys the entire colorectal surface in ∼1.5-mm steps at a suprathreshold stimulus intensity (10 mA) sufficient to excite all afferent endings within a, ∼2-mm radius. The afferent receptive field (RF) was identified as the site of activation requiring minimum stimulus intensity (i.e., stimulus threshold). After location by electrical stimulation, each RF was tested with three distinct mechanical stimuli: probing with calibrated von Frey-like nylon monofilaments (0.4 and 1-g force), mucosal stroking with calibrated nylon filaments (10-mg force), and circumferential stretch (0–170 mN at 5 mN/s, Aurora Scientific, Ontario, Canada).
Optical stimulation of colorectal afferents.
Optical stimulation was applied using a 473-nm Laser (Laserglow Technologies, Toronto, Canada) focused to a point ∼1 mm in diameter on the colorectal mucosal surface, an area comparable to a typical afferent RF. Stepped light stimulation (10 s, constant intensity) was applied to the RF, followed by 5 trains of 20 light pulses (20-ms wide) in ascending frequency (1, 2, 3, 5, and 10 Hz). Light intensity was 6 mW/mm2, well in the saturation range for afferent activation (41). Spike2 software was used to control the timing of the optical stimuli (Cambridge Electronic Design, Cambridge, UK).
Chemical stimulation of MIAs.
MIAs that responded to optical stimulation were tested by sequential exposure of their afferent endings to an acidic hypertonic solution (AHS) and an inflammatory soup (IS), each of which has been used previously on colorectal afferent endings (7, 8, 12, 20, 21). Brass tubing of 1-cm high × 4-mm square, which has been shown to be chemically inert to AHS, IS, and bile salts (BS), was placed over the receptive ending on the mucosal surface, the Krebs was solution removed, and 150 μl of AHS solution were applied directly to the receptive ending for 5 min. Subsequently, the solution and the tubing were removed and responses monitored for up to 30 min. The endings were then tested with IS using the same protocol followed by tests of responses to mechanical probing (1 g) and stepped optical stimulation. Electrical stimulation was repeated at the end of testing to verify viability of the afferent ending. Additional MIAs harvested from naïve C57BL/6 mice were tested by exposing their endings to IS, AHS, and a BS solution.
AHS (pH 6.0, 800 mosM) was prepared by adding d-mannitol and hydrochloric acid (1 N) to freshly oxygenated Krebs solution on the day of an experiment. IS was prepared in aliquots of 20 μl by combining bradykinin, serotonin, and histamine dissolved in distilled water with prostaglandin E2 dissolved in dimethylsulfoxide, frozen, and stored at −20°C. On the day of an experiment, an aliquot was diluted to 10 μM (each mediator) in freshly oxygenated Krebs solution and adjusted to pH 6.0 by hydrochloric acid. BS solution (0.5%) were prepared by dissolving the salts in freshly oxygenated Krebs solution (8). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Data recording and analysis.
Electrical signals from afferent endings were filtered (0.3–10 kHz), differentially amplified (×10,000; DAM 80; World Precision Instruments, New Haven, CT), digitized at 20 kHz using a 1401 interface (Cambridge Electronic Design), stored on a PC, and monitored online by an audio monitor. Action potentials were discriminated based on principal component analysis using Spike2 software (Cambridge Electronic Design). Data are presented as means ± SE. One- or two-way ANOVA and Bonferroni post hoc pairwise multiple comparisons were performed with repeated measures as appropriate using SigmaPlot v11.0 (Systat Software, San Jose, CA). Differences in means and proportions were assessed by t-tests and χ2-tests, respectively. P < 0.05 was considered significant.
RESULTS
Optogenetic activation of colorectal afferents.
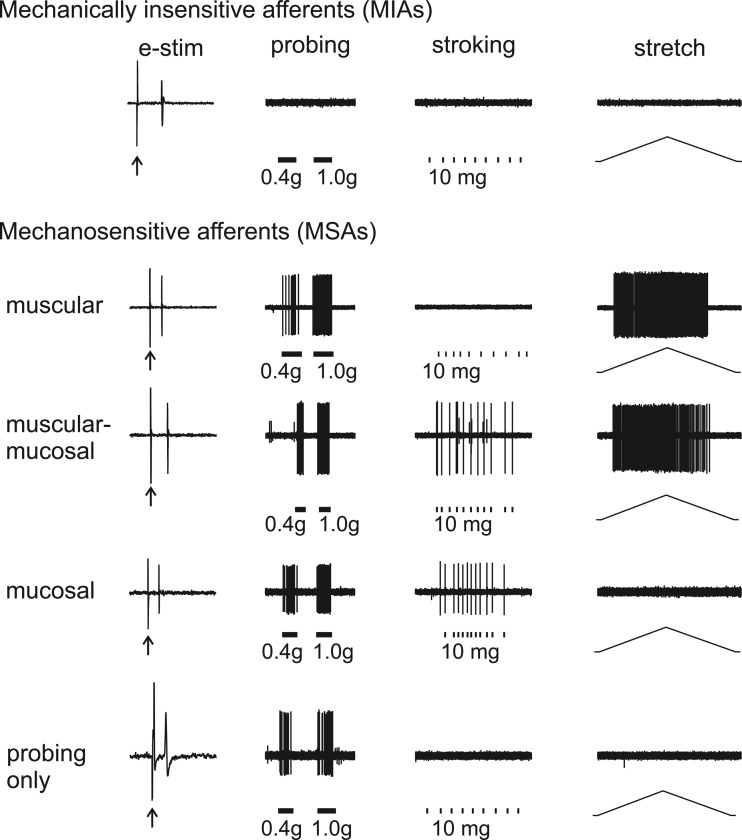
As shown in Fig. 1, colorectal afferents in the PN innervation in male Cre+/−ChR2+/− mice were localized by electrical stimulation of their receptive endings and classified into one MIA class and four mechanosensitive afferent (MSA) classes: muscular, muscular-mucosal, mucosal, and those responding only to blunt probing (formerly termed “serosal”) afferents. MIAs do not respond to any of the three mechanical stimuli (punctate probing, mucosal stroking, or circumferential stretch). In contrast, all four MSA classes respond to blunt probing of their RF; in addition, muscular and muscular-mucosal afferents also respond to circumferential stretch and mucosal and muscular-mucosal afferents also respond to mucosal stroking. The terms “mucosal” and “muscular” are suggestive of histological localization of RFs in the colorectum, for which histological support exists (36). However, they have not yet been extensively validated by studies that couple functional characterization with afferent ending localization. Colorectal afferents that respond only to punctate probing of their RF were previously denoted “serosal” afferents, suggestive of a RF in the serosa. However, a recent morphological study of the mouse colorectum failed to identify sensory endings in the colorectal serosa (36), prompting the use of “probing only” to represent this class of afferent here.
Fig. 1.
Classification of pelvic nerve colorectal afferents into 5 classes based on responses to 3 mechanical stimuli applied to their receptive fields (RF): probing (1 g), stroking (10 mg), and ramped circumferential stretch (1–170 mN at 5 mN/s). Leftmost column: responses and response latency of afferents to the electrical search stimulus (e-stim). All mechanosensitive afferents (MSAs) classes respond to blunt probing of their receptive field and include afferents designated: muscular (responding also to circumferential stretch); muscular-mucosal (responding also to mucosal stroking and circumferential stretch); mucosal (responding also to mucosal stroking); and those responding only to blunt probing (formerly designated as “serosal”). MIAs, mechanically insensitive afferents.
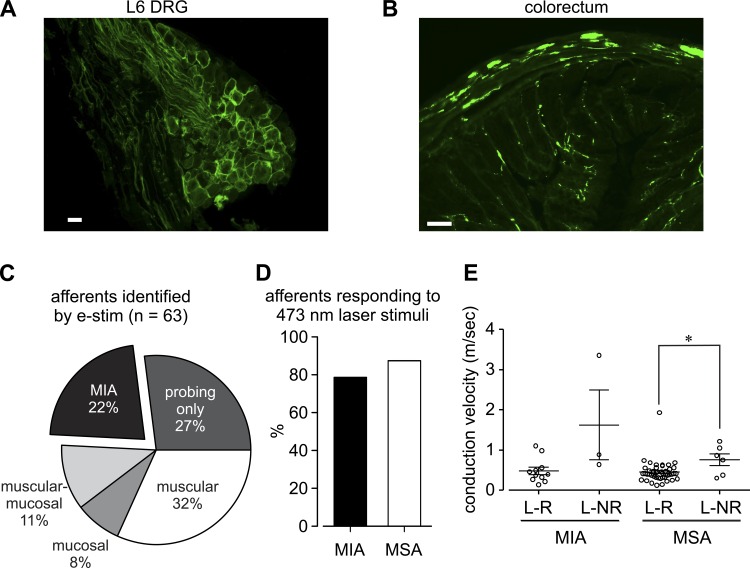
Confirming our previous findings (41), ChR2 fused with a YFP protein, detected in male Cre+/−ChR2+/− mice using a YFP antibody, was expressed in PN primary afferent neurons in both the L6 dorsal root ganglion (Fig. 2A) and colorectum (Fig. 2B). Sixty-three colorectal afferents were identified from 14 male Cre+/−ChR2+/− mice and the proportions of the five afferent classes (Fig. 2C) were comparable to those reported previously from naïve C57BL/6 mice(8) (χ2-test, P = 0.12). Optical stimulation was equally effective in exciting MIAs and MSAs, activating >79% of RFs in both groups (Fig. 2D). Laser-responsive (L-R) afferents had significantly slower mean conduction velocities than their laser nonresponsive (L-NR) counterparts in MSA groups (t-test, P = 0.02) as shown in Fig. 2E. Laser-responsive MIAs and MSAs have comparable mean conduction velocities (t-test, P = 0.75).
Fig. 2.
Proportions of pelvic nerve colorectal afferent classes in male Cre+/−ChR2+/− mice and their responses to 473-nm laser stimuli. Immunohistological staining confirmed the presence of ChR2-EYFP in most L6 dorsal root ganglion (DRG) neural membranes (A) and at nerve endings (B) in the colorectum; the proportions of afferent classes in Cre+/−ChR2+/− mice (C) were comparable with proportions previously established in C57BL/6 mice (8). D: of the 63 pelvic nerve afferent endings located by electrical stimulation of the colorectum, 11 of 14 MIAs (79%) and 43 of 49 MSAs (88%) were also activated by blue laser (473 nm). E: laser-responsive (L-R) afferents had significantly slower conduction velocities (CV) than their laser-non-responsive (L-NR) counterparts in both MIA (P = 0.02) and MSA groups (P = 0.02). Laser-responsive MIAs and MSAs had comparable CVs (P = 0.75). Scale bar in A = 20 μm. Scale bar in B = 100 μm. *P < 0.05 by t-tests.
Responses of MSAs and MIAs to optical stimuli.
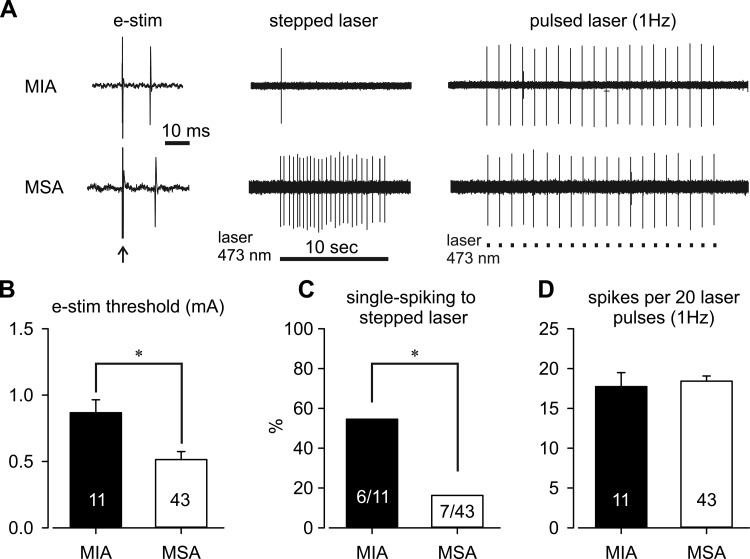
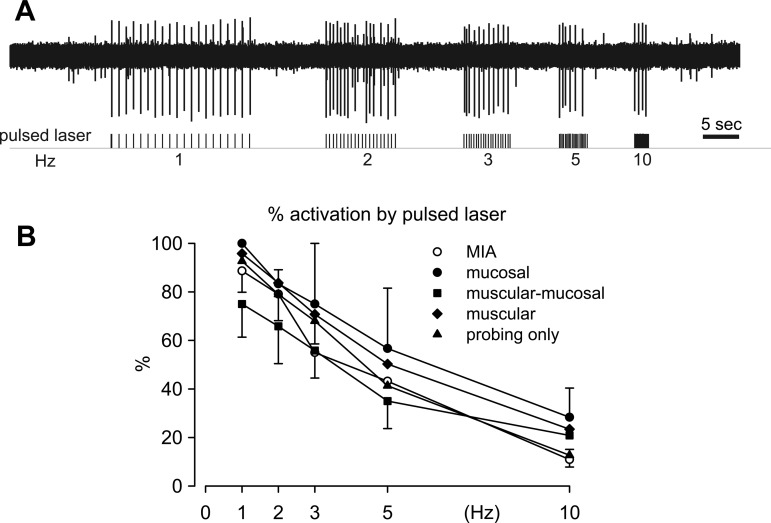
As shown in Fig. 3A, after identification of afferent RFs by electrical stimulation, colorectal afferents were further tested with stepped (10-s duration) and pulsed (20-ms duration, 1 Hz) laser stimulation of their RFs on the colorectum. Consistent with our previous findings in naïve C57BL/6 mice, MIAs in the present study were found to have a significantly higher threshold for activation by electrical stimulation than MSAs (Fig. 3B, t-test, P = 0.004). As displayed in Fig. 3C, 57% of MIAs responded to a stepped laser stimulus with a single spike (single-spiking; e.g., Fig. 3A, top trace), a proportion significantly greater than of MSAs (14%, Fisher's exact, P = 0.015). The majority of MSAs (86%) were tonic-spiking, responding to a stepped laser stimulus with multiple spikes (e.g., Fig. 3A, bottom trace). We stimulated 16 single-spiking afferents (4 MIAs and 12 MSAs) at three different light intensities (threshold, 5 times threshold, and 6 mW/mm2) which uniformly evoked single spiking responses from all afferents, validating the optical intensity of 6 mW/mm2 for testing the stepped response. When stimulated for 20 pulses at 1 Hz, pulsed laser stimulation evoked a comparable number of action potentials in MIA and MSA groups (Fig. 3D, t-test, P = 0.66). Comparing MSA classes with MIAs stimulated with a range of frequency pulses (1, 2, 3, 5, and 10 Hz, 20 pulses each; Fig. 4A), low-frequency stimulation reliably evoked action potentials from all classes of colorectal afferents with >70% activation by laser pulses. The proportions of activation to pulsed laser stimulation were reduced significantly and to an equivalent extent in MIAs and all four classes of MSAs to <30% as the stimulus frequency increased progressively to 10 Hz (Fig. 4B, two-way repeated-measures ANOVA, F4,49 = 0.667, P = 0.618).
Fig. 3.
Examples of responses of MIAs and MSAs to stepped and pulsed (1 Hz) laser stimulation (A). Compared with MSAs, MIAs had a greater mean threshold for activation by electrical stimulation (e-stim; B) and a significantly greater proportion of single-spiking responses to stepped laser stimuli (C); 86% (36/43) of MSAs responded tonically to stepped laser stimulation (see text). D: responses to 1-Hz pulsed laser were comparable between MIAs and MSAs. Numbers within the bars indicate the numbers of afferents in each group. *P < 0.05 by t-tests in B (P = 0.004) and D (P = 0.66) and Fisher's exact test in C (P = 0.015).
Fig. 4.
Responses to pulsed laser stimulation at different stimulus frequencies. Illustration (A) of responses of a MIA to different frequencies of pulsed laser stimulation and summary (B) of responses of MIAs and the 4 classes of MSAs to pulsed laser stimulation at frequencies of 1, 2, 3, 5, and 10 Hz. There were no differences between responses of MIAs and any of the 4 classes of MSAs to pulsed laser stimulation (two-way repeated measures ANOVA, F4,49 = 0.667, P = 0.618).
Responses of MIAs to chemical stimuli.
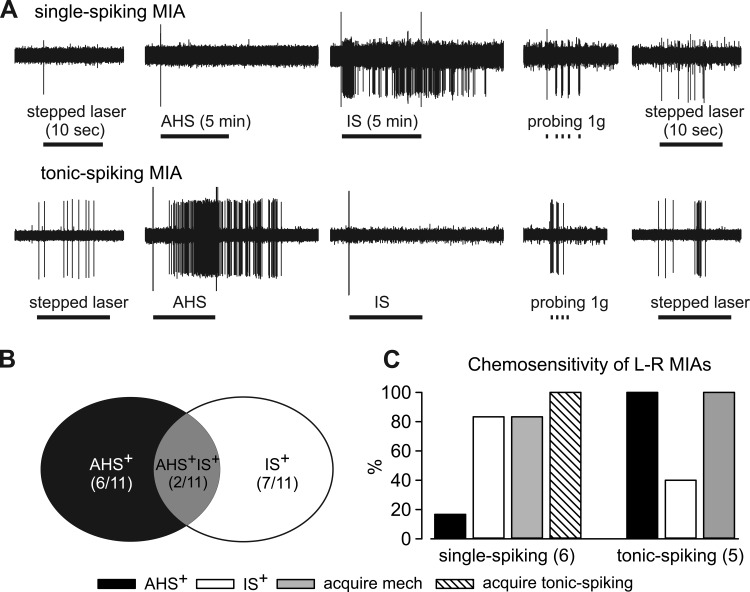
Differential responses to stepped laser stimuli provided direct evidence for heterogeneity within the MIA population. We hypothesized that tonic-spiking MIAs are regular sensors of another stimulus modality, capable of informing the CNS about the quality of that modality, whereas single-spiking MIAs are putative nociceptors that require sensitization to spike tonically. We thus exposed individual MIA RFs to AHS (an osmotic stimulus; 5-min application) and, after a 30-min washout, IS (an inflammatory/sensitizing stimulus; 5-min application), followed by punctate probing (1 g) of the RF and repeated stepped laser stimulation. Eleven laser-responsive (6 single-spiking and 5 tonic-spiking) MIAs from Cre+/−ChR2+/− mice were tested in this manner. Figure 5A illustrates characteristic responses of single- and tonic-spiking MIAs, and Fig. 5, B and C, summarize sthe results. Single-spiking MIAs (5/6) were activated by IS, but not AHS whereas 5/5 tonic-spiking MIAs responded to AHS, two of which also responded to IS (Fig. 5C). Virtually all MIAs (10/11) were sensitized by exposure to AHS and/or IS and acquired mechanosensitivity (responded to blunt probing), and all single-spiking MIAs became tonic spiking to stepped laser stimuli (6/6) after exposure to chemical stimuli. Results presented in Fig. 5, B and C, collectively suggest the segregation of MIAs into two subgroups, one capable of spiking tonically and responsive to AHS whereas the other being single-spiking and responsive to IS.
Fig. 5.
Responses of light-sensitive MIAs to chemical acidic hypertonic solution (AHS) and inflammatory soup (IS) exposure. Representative responses are shown in A. All laser-responsive MIAs were activated by either AHS or IS (B); 2 were activated by both chemical stimuli. C: single-spiking MIAs were more likely to be activated by IS whereas tonic-spiking MIAs were preferentially activated by AHS. After AHS and IS application, most (10/11) MIAs acquired responses to mechanical probing (1 g) and all 6 single-spiking MIAs spiked tonically to stepped laser stimulus (6/6).
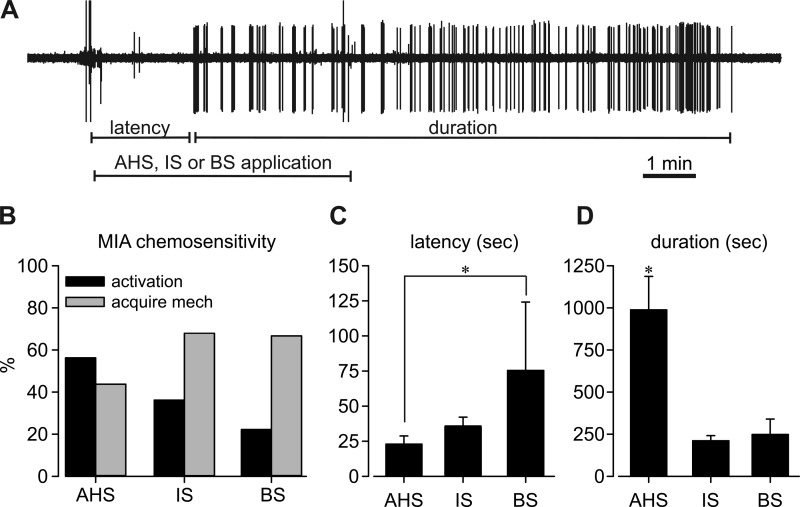
Responses to chemical stimuli were tested in a larger population of MIAs from C57BL/6 control mice. As illustrated by the typical example in Fig. 6A, MIA endings were exposed to either AHS, IS, or a solution of BS for 5 min to assess direct activation; activation latency (time to response) and duration of response were quantified for comparison between chemical applications. Similar to findings from Cre+/−ChR2+/− mice, about half the MIAs tested (56%, 9/16) were activated directly by AHS, and 7 of the 16 MIAs tested (44%) acquired mechanosensitivity (Fig. 6B). About one-third of MIAs tested (37%, 15/41) were directly activated by IS and 68% acquired mechanosensitivity. BS activated a comparatively smaller proportion of MIAs (22%, 2/9), but most MIAs acquired mechanosensitivity afterwards (67%, 6/9). BS had the longest activation latency among the three chemical stimuli (Fig. 6C, F2,23 = 3.7, P = 0.04, post hoc comparison, P = 0.037 vs. AHS), and activation by AHS had the longest duration (Fig. 6D, F2,23 = 13.5, P < 0.001, post hoc comparison P < 0.05 vs. IS or BS).
Fig. 6.
Chemosensitivity of MIAs recorded in colorectums from naive C57B/L6 mice. An additional 66 MIA endings were tested with chemical stimuli: either AHS (n = 16), IS (n = 41), or 0.5% bile salts (BS; n = 9). A representative example is shown in A. More than 56% of MIAs were directly activated by chemical stimuli and/or acquired responses to mechanical probing (B). BS application was associated with a significantly longer latency for activation (C), whereas AHS application produced a significantly longer duration of activation (D). *P < 0.05 by one-way ANOVA tests in C (F2,23 = 3.7, P = 0.04, post hoc comparison, P = 0.037 vs. AHS) and D (F2,23 = 13.5, P < 0.001, post hoc comparison P < 0.05 vs. IS or BS).
DISCUSSION
The current study offers novel insights into the encoding properties of MIAs innervating the colorectum, namely their chemosensitivity. The chemosensitivity of presumed MIAs innervating the viscera was suggested in earlier reports in which afferent nerve activity “appeared” in the record after chemical or ischemic insults of visceral organs (5, 18, 25). Using an unbiased electrical search strategy to localize MSAs and MIAs (8, 9), we assessed MIA sensitization by in vitro application of an IS to the RF. We found that 14/42 (33%) of colorectal MIAs in the pelvic pathway were activated by IS, establishing chemosensitivity, and that they and 16 additional MIAs (total 71%) became sensitized, acquiring mechanosensitivity after IS application. To further understand MIA chemosensitivity, several challenges have to be overcome, including 1) the absence of a known “universal” chemical ligand that binds to chemo-transducers and activates MIA endings; 2) often slow onset and typically long-lasting effects of chemical stimuli; 3) indirect effect of chemicals on surrounding, nonneural tissues; and 4) inability to tease apart chemically induced transduction (e.g., ligand binding) from transformation (modulation of spike initiation). Accordingly, we used in the present study optogenetic activation of afferent endings that contained ChR2. We previously characterized the onset and termination of blue light activation of ChR2 in DRG neurons and documented that the gating of ChR2 itself was not significantly affected by inflammatory mediators (41). By providing a temporarily and spatially controlled depolarizing photo-activated current at the terminal ending, the present study investigated the activation and encoding of colorectal MIAs via combined optogenetic and chemical stimuli.
Advillin-Cre mice were used to drive the expression of ChR2 in DRG neurons because, compared with other Cre-expressing mouse lines, Advillin induces expression in a greater proportion of DRG neurons and apparently does not bias expression in only a subset(s) of DRG neurons (17, 28). We confirmed by immunohistochemistry abundant expression of ChR2 in most L6 DRG neurons as well as at terminal endings in the colorectum, consistent with previous findings (41). It has been suggested anecdotally that Advillin-Cre may drive expression in muscular tissue, but we did not observe signs of expression in colorectal muscle layers. Nonetheless, use of the L-type Ca2+ blocker nifedipine in the ex vivo bathing solution eliminated any influence of smooth muscle activation on afferent fiber characterization. The Cre+/−ChR2+/− male mice used here have comparable visceral afferent innervations to C57BL/6 control mice as the proportions of the five afferent classes identified by electrical stimulation in Cre+/−ChR2+/− mice were not different from proportions reported previously in C57BL/6 mice (8). We observed that >79% of all MSAs and MIAs were activated by optical stimulation, confirming that ChR2 expression is not biased towards any one colorectal afferent class. In addition, MIAs in Cre+/−ChR2+/− mice had significantly greater thresholds for electrical activation than their mechanosensitive counterparts and virtually all colorectal afferents were unmyelinated (conduction velocities: <1 m/s), consistent with previous findings in C57BL/6 mice (8). Collectively, these findings suggest that expression of ChR2 does not affect the baseline properties of the PN innervation of the colorectum. That almost all electrically identified afferent endings responded to optical stimulation validates the study of photo-sensitive colorectal afferents as representative of the general afferent population.
We used a blue laser (473 nm) of 6 mW/mm2 to stimulate afferent RFs in the colorectum, an optical intensity sufficient to activate all ChR2 within the thin (0.1–0.2 mm) layer of the flattened mouse colorectum (41). Because the open probability of ChR2 increases exponentially with increased optical intensity, we used stepped or pulsed laser stimulation at a constant intensity to ensure consistent stimulation of all afferents across different depths of the colorectum. The intensity used was sufficient at a low frequency (1 Hz) to reliably evoke action potentials from both MIAs and MSAs. Falloff of laser-evoked action potentials started to occur when the stimulus frequency exceeded 3 Hz, and stimulation at 10 Hz evoked significantly less repetitive firing in both MIAs and MSAs, suggesting that the spike initiation zone of colorectal afferents is not capable of high-frequency firing. This is consistent with previous findings by us and others that mouse colorectal afferents are generally low firing (<5 Hz), even when stimulated at noxious intensities (2, 3, 7). Consistent with unpublished observations (B. Feng and G. F. Gebhart), the peak frequency of chemically induced discharges seldom exceed 5 Hz, comparable with the range of laser-evoked peak firing frequencies in Cre+/−ChR2+/− mice. We also found no difference between MIAs and MSAs in responses to pulsed laser stimulation, suggesting that colorectal MIAs are capable of relaying sensory information as trains of action potentials like their mechanosensitive counterparts.
A noteworthy finding was the differential response of MSAs and MIAs to stepped laser stimulation. Most MSAs (86%) responded tonically with multiple spikes whereas MIAs were about equally divided into two groups; one MIA group had single-spike responses to stepped laser stimulation and the other gave tonic-spiking responses. Stepped laser stimulation caused persistent opening of ChR2 at the afferent ending, a nonselective cation channel with small maximum conductance (34). This mimics transduction at MIA sensory terminals (i.e., activation of transducer molecules intrinsic to the afferent ending followed by a slow depolarization in the form of a generator potential). That tonic-spiking MIAs are able to encode these generator potentials into repetitive action potentials in the transformation process strongly suggests a sensory role for those MIAs in normal physiological conditions. In contrast, MIAs with a transformation process incapable of encoding trains of action potentials are likely silent under normal conditions.
The most revealing finding was that the transformation properties of light-sensitive MIAs (tonic- vs. single-spiking) are associated with differential chemosensitivity (osmotic vs. inflammatory). All tonic-spiking MIAs responded to AHS whereas 80% of single-spiking MIAs did not. Conversely, 80% of single-spiking MIAs were activated directly by IS and all spiked tonically to stepped laser stimuli afterwards. AHS was used in previous studies as a physiologically relevant stimulus because it recapitulates the common situation of carbohydrate malabsorption in the large intestine (21). The virtue of IS is its rapid onset and relative ease of wash out, leading to widespread use to assess afferent sensitization in visceral and cutaneous tissues (4, 9, 11, 19, 20, 35). We documented previously and here as well that significant proportions of MIAs from C57BL/6 mice are AHS or IS sensitive. The present study revealed that AHS-sensitive and IS-sensitive MIAs represent discrete afferent groups with distinct transformation characteristics. The effects of BS on colorectal MIAs were briefly reported previously and also studied here; BS enhances permeability of the colorectal mucosa (37) and may act on afferents via TRPA1 channels (23). However, MIAs did not appear to directly encode BS stimulation because only a small proportion of MIAs gave direct responses. BS appeared to have an indirect effect that resulted in MIA sensitization to mechanical stimuli afterwards, likely via enhanced mucosal permeability. Conducting combined optogenetic and chemical stimuli on MIAs is not without challenges. First, male Cre+/−ChR2+/− mice comprise only 25% of litters of breeding pairs of heterozygous Advillin-Cre and homozygous Ai32 mice. Second, each electrically and optically identified MIA undergoes an hours-long protocol, including two sequential exposures to chemical stimuli with necessary wash-out periods between, thus limiting study to a single MIA per mouse. In the present report, 11 MIAs were tested with electrical, optical, and chemical stimuli, which, collectively with our prior studies on MIAs in naïve c57bl/6 mice, adequately support the proposed functional segregation of MIAs based on transformation properties.
The functional role of colorectal MIAs in pain and hypersensitivity was studied previously in the context of long-term models of visceral hypersensitivity induced by intracolonic instillation of trinitrobenzene sulfonic acid (TNBS) (14) or zymosan (13). In both studies, the proportions of MIAs in mice that developed prolonged behavioral colorectal hypersensitivity were reduced by ∼50%. This is consistent with the current findings that single-spiking MIAs, making up ∼50% of the MIA population, were all sensitized and started to spike tonically following an inflammatory stimulus, suggesting a nociceptive role of single-spiking MIAs in pathophysiological conditions. Tonic-spiking MIAs behave as osmosensors responsive to AHS, which also has implications related to colorectal hypersensitivity as repeated intracolonic AHS instillation induced long-lasting visceral hypersensitivity in mice (21). The exact roles of different MIA subtypes in long-term visceral hypersensitivity will require additional studies of MIAs in Cre+/−ChR2+/− mice after intracolonic TNBS, zymosan, or AHS treatments.
Recent advances in identifying the molecular identities of osmosensers have added to a growing list of ion channels and membrane proteins gated by osmotic stimuli (6, 22, 30–32). Rather than encoding hyperosmotic challenge, most channels are activated by hypotonic stimuli and presumed cell swelling, including TrpV4 that is widely expressed in visceral sensory neurons (6, 22, 32). In contrast, hypertonic stimuli cause hyperpolarization in some cells (16, 33) and presumably result in cell membrane shrinkage, contributing to an increased activation threshold for stretch-activated ion channels, consistent with a previous finding that AHS significantly reduced colorectal afferent response frequency and increased response threshold to mechanical stretch (21). The underlying mechanism(s) for short-latency, long-lasting responses of tonic-spiking MIAs and most probing-responsive afferents to AHS remain unresolved and await further study.
In summary, this study provided novel insights into the heterogeneity of colorectal MIAs using an optogenetic approach that stimulated MIA afferent endings with a temporally and spatially controlled depolarizing generator potential. A significant portion of MIAs (45%) spike tonically to optogenetic stimuli and all are osmosensitive to AHS. In contrast, 55% MIAs are single spiking, all of which become tonic spiking after IS application to their RF; 80% are activated directly by IS. As a result, two distinct chemosensitive populations of colorectal MIAs (osmo- and inflammation sensitive) linked to distinct neural encoding characteristics (tonic- vs. single spiking) were identified. In conjunction with prior study of colorectal MIAs, the present study opens a new avenue by focusing on the micrometer-thick afferent nerve terminals for further understanding the contributions of different MIA subtypes to prolonged colorectal pain and hypersensitivity. In particular, the optogenetic approach, either via rhodopsin activation/inhibition or calcium signal detection (e.g., GCaMP), has proved to be a powerful tool to allow focused studies on the micrometer-thick afferent terminals.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K01-DK-100460 (to B. Feng) and R01-DK-093525 (to G. F. Gebhart).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.F. and G.F.G. conception and design of research; B.F. and S.C.J. performed experiments; B.F., S.C.J., and G.F.G. analyzed data; B.F., S.C.J., and G.F.G. interpreted results of experiments; B.F. and G.F.G. prepared figures; B.F. and S.C.J. drafted manuscript; B.F., S.C.J., and G.F.G. edited and revised manuscript; B.F., S.C.J., and G.F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michael Burcham for assistance in preparation of figures.
REFERENCES
- 1.Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Brierley SM, Carter R, Jones W III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol 297: G1250–G1258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho SV, Su X, Sengupta JN, Gebhart GF. Role of sensitized pelvic nerve afferents from the inflamed rat colon in the maintenance of visceral hyperalgesia. Prog Brain Res 129: 375–387, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Feetham CH, Nunn N, Lewis R, Dart C, Barrett-Jolley R. TRPV4 and K(Ca) ion channels functionally couple as osmosensors in the paraventricular nucleus. Br J Pharmacol 172: 1753–1768, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, Gebhart GF. In vitro functional characterization of mouse colorectal afferent endings. J Vis Exp 95: 52310, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng B, Gebhart GF. Mo2044 studying mechanically-insensitive colorectal afferents via optogenetic activation of sensory nerve terminals. Gastroenterology 148: S-778, 2015. [Google Scholar]
- 11.Feng B, Kiyatkin ME, La JH, Ge P, Solinga R, Silos-Santiago I, Gebhart GF. Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci 33: 9831–9839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng B, Kiyatkin ME, La JH, Gebhart GF. Activation of guanylate cyclase C receptors attenuates peripheral drive from mouse colorectal afferents. Neuroscience Meeting Planner, 47317/HH7. New Orleans, LA: Soc Neurosci, 2012. [Google Scholar]
- 13.Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302: G676–G683, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF. Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol 303: G817–G824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng B, Zhu Y, La JH, Wills ZP, Gebhart GF. Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J Neurophysiol 113: 2618–2634, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferenczi EA, Fraser JA, Chawla S, Skepper JN, Schwiening CJ, Huang CL. Membrane potential stabilization in amphibian skeletal muscle fibres in hypertonic solutions. J Physiol 555: 423–438, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci 27: 14404–14414, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jänig W, Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst 30, Suppl: S89–S96, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Kessler W, Kirchhoff C, Reeh PW, Handwerker HO. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp Brain Res 91: 467–476, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Kiyatkin ME, Feng B, Schwartz ES, Gebhart GF. Combined genetic and pharmacological inhibition of TRPV1 and P2X3 attenuates colorectal hypersensitivity and afferent sensitization. Am J Physiol Gastrointest Liver Physiol 305: G638–G648, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La JH, Feng B, Schwartz ES, Brumovsky PR, Gebhart GF. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 303: G802–G809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, Haerteis S, Korbmacher C, Steinhoff MS, Nassini R, Materazzi S, Geppetti P, Corvera CU, Bunnett NW. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology 147: 1417–1428, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loewenstein WR, Rathkamp R. Localization of generator structures of electric activity in a Pacinian corpuscle. Science 127: 341, 1958. [DOI] [PubMed] [Google Scholar]
- 25.Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol 518: 271–282, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res 561: 252–261, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Nickel JC, Jain P, Shore N, Anderson J, Giesing D, Lee H, Kim G, Daniel K, White S, Larrivee-Elkins C, Lekstrom-Himes J, Cima M. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Sci Transl Med 4: 143ra100, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Pagadala P, Park CK, Bang S, Xu ZZ, Xie RG, Liu T, Han BX, Tracey WD Jr, Wang F, Ji RR. Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: involvement of Ca(2+)-activated small conductance potassium channels. J Neurosci 33: 13425–13430, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47: 995–1001, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157: 447–458, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quallo T, Vastani N, Horridge E, Gentry C, Parra A, Moss S, Viana F, Belmonte C, Andersson DA, Bevan S. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat Commun 6: 7150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, Krizaj D. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci 34: 15689–15700, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez JC, Lopez-Zapata DF. The role of BKCa channels on hyperpolarization mediated by hyperosmolarity in human articular chondrocytes. Gen Physiol Biophys 30: 20–27, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Schoenenberger P, Scharer YP, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol 96: 34–39, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer NJ, Kyloh M, Duffield M. Identification of different types of spinal afferent nerve endings that encode noxious and innocuous stimuli in the large intestine using a novel anterograde tracing technique. PLoS One 9: , 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenman LK, Holma R, Eggert A, Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 304: G227–G234, 2013. [DOI] [PubMed] [Google Scholar]
- 38.van Brederode JF, Seagard JL, Dean C, Hopp FA, Kampine JP. Experimental and modeling study of the excitability of carotid sinus baroreceptors. Circ Res 66: 1510–1525, 1990. [DOI] [PubMed] [Google Scholar]
- 39.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain 139: 218–224, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Feng B, Schwartz ES, Gebhart GF, Prescott SA. Novel method to assess axonal excitability using channelrhodopsin-based photoactivation. J Neurophysiol 113: 2242–2249, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]