Quality-of-life scores improved in patients with Lyme disease to the national average; scores increased over follow-up time across all stages and manifestations of the disease. Lower scores were associated with presence of unrelated comorbidities.
Keywords: Borrelia burgdorferi, Lyme disease, epidemiology, quality of life, longitudinal analysis
Abstract
Background. Lyme disease is the most common vector-borne disease in the United States. Some patients report persistent or intermittent subjective symptoms of mild to moderate intensity after antibiotic treatment for Lyme disease. We sought to evaluate trends in clinical and quality-of-life (QOL) measures in a cohort of patients with Lyme disease enrolled in a natural history study at the National Institutes of Health from 2001–2014.
Methods. QOL was measured using the self-administered 36-item Short Form Health Survey (SF-36) during study follow-up. Primary outcomes included mean physical (PCS) and mental (MCS) health QOL composite scores and reporting long-term (≥2 years) symptoms, adjusted for Lyme disease stage and severity at diagnosis.
Results. Overall, 101 patients with an average follow-up time of 3.9 years (range, 0.5–11.3 years) were included. At first visit, overall mean QOL scores were below the US population mean for both PCS (45.6 ± 10.4) and MCS (47.3 ± 11.5) but increased to just above the national average after 3 years of follow-up for both PCS (50.7 ± 9.6) and MCS (50.1 ± 10.0). Baseline QOL scores were lowest in those with late disease (P < 0.01) but also increased by the end of follow-up to national averages. In multivariate analysis, the only factors significantly associated with long-term symptoms or lower QOL scores were other comorbidities unrelated to Lyme disease.
Conclusions. Comorbid conditions can play a role in the reporting of long-term symptoms and overall QOL of Lyme disease patients and should be considered in the evaluation of these patients.
Clinical Trials Registration. NCT00028080.
Lyme disease, caused by Borrelia burgdorferi, is the most common vector-borne disease in the United States [1]. The majority of Lyme disease cases occur in the mid-Atlantic, northeast, and upper Midwest regions [2], with more than 95% coming from 14 states [3]. Evidence suggests that both incidence and geographic range are increasing [4].
For clinical purposes, Lyme disease is classified into early localized, early disseminated, and late stages. Early localized disease is characterized by a primary erythema migrans (EM) lesion and occurs in more than 80% of patients [5–7]. After several days to weeks, untreated B. burgdorferi can disseminate hematogenously, and patients can develop multiple EM lesions, as well as neurologic, cardiac, and rheumatologic involvement [8–11].
Lyme disease is usually successfully treated with antimicrobial therapy [12–15], but patients with late-stage manifestations, such as Lyme arthritis or neuroborreliosis, may be slower to recover [16–24]. A minority of treated patients continue to report persistent or relapsing nonspecific symptoms (ie, fatigue, musculoskeletal pain, and cognitive issues). It is estimated that up to 20% of patients with EM have persistent or intermittent subjective symptoms of mild to moderate intensity 12 months after therapy completion [25]. For these patients, a substantial reduction in quality of life (QOL) may result [26].
Here, we present outcome data from a cohort of Lyme disease patients enrolled in a natural history study at the National Institutes of Health (NIH), Bethesda, Maryland. We aimed to describe trends in Lyme disease symptoms, laboratory results, and QOL to better understand the impact of infection over time and to better inform clinical practices for managing Lyme disease patients.
METHODS
Study Population
From 2001–2014, patients diagnosed with Lyme disease were enrolled into the NIH natural history protocol NCT00028080, which was approved by the National Institute of Allergy and Infectious Diseases institutional review board. Written informed consent was obtained from all patients. For this analysis, only patients aged ≥18 years with confirmed Lyme disease (based on the Centers for Disease Control and Prevention case definition [27]) diagnosed at the NIH or within 6 weeks prior to enrolling and with ≥6 months of follow-up were included.
Data Collection
Patients were evaluated at NIH at the following approximate time points: baseline; 1, 3, 6, and 12 months; and yearly thereafter. At baseline, demographic and clinical data were collected, including Lyme disease–specific manifestations, treatment history, and preexisting comorbidities. At each follow-up visit, patients self-reported current symptoms from a standardized list and completed a QOL survey using the 36-Item Short Form Health Survey (SF-36), version 2. Data were analyzed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Clinical Definitions
Lyme disease presentations were classified into early localized (single EM), early disseminated (multiple EM, early neuroborreliosis, carditis), and late disease (Lyme arthritis and late Lyme neuroborreliosis) [15]. Clinical manifestations were also categorized as dermatological (single and multiple EM), neurological, arthritic, and cardiovascular.
Clinical Data Analysis
Visit dates with associated laboratory, clinical, and QOL data were categorized into the following standardized intervals for data analysis: baseline (initial study evaluation), 1- to 3-month visit (≥1.5 to <3.5 months), 4- to 6-month visit (≥3.5 to <6.5 months), 6- to 12-month visit (≥6.5 to <12.5 months), 12- to 24-month visit (≥12.5 to <24.5 months), and ≥24.5 months. Laboratory data were additionally evaluated for a 12- to 18-month visit (≥12.5 to <18.5 months), 18- to 24-month visit (≥18.5 to <24.5 months), and ≥24.5 months.
Comorbidity Analysis
Self-reported preexisting comorbidities were recorded for all patients at baseline during medical history and physical review; comorbidities were validated by clinical staff or by prescribed medications. Preexisting comorbidities were categorized as chronic pain, mental/behavioral health (and, separately, “mental/behavioral health medication use”), and high-risk conditions for cardiovascular disease and metabolic syndrome, including obesity (body mass index >29.5). Presence of a preexisting comorbidity at baseline, number of comorbidities, and comorbidity categories were evaluated.
QOL Analysis
QOL was measured using the SF-36, a 36-item self-administered questionnaire used to measure QOL in patients with a variety of medical conditions [28, 29]. Scores from individual questions are aggregated into 8 subscales. These subscales are physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. Scores from the subscales are aggregated into the following 2 distinct, higher-order summary scores: physical component summary (PCS) and mental component summary (MCS). QOL scores were tabulated using Quality Metric, Inc. software. All scores were measured on a scale of 0–100 and were compared with the US population mean (50 ± 10) [30].
QOL scores were summarized and compared across various patient demographic, clinical, and laboratory factors at baseline and at each time point. Differences in mean scores and changes over time were assessed by stage of Lyme disease at diagnosis, symptom manifestations, and comorbidities (QOL-1). Patients with baseline QOL scores available (within 30 days of study enrollment; QOL-2) were also categorized as having a low PCS or MCS at baseline (defined as ≤45) to compare differences in baseline characteristics and long-term follow-up data.
Statistical Analyses
Descriptive statistics were used to summarize data and describe trends at baseline and over time. Student t tests (2-sided) and χ2 analyses were used to identify significant differences (P < .05). Univariate generalized linear models (with a log-link and Poisson distribution) were used to estimate risk ratios (RRs) and to evaluate demographic and clinical factors significantly (P < .05) associated with the following 2 outcomes: ≥1 long-term (≥24.5 months) symptom (including fatigue, impaired memory/recall/concentration, sleep disturbances, arthralgia, feeling mentally slow/foggy, joint/neck pain, facial weakness, and/or numbness/tingling) and having a low (≤45) baseline QOL score. Significant variables in univariate models were evaluated in multivariate models controlling for sex, age, treatment status at enrollment, and stage of Lyme disease at diagnosis. Adjusted RRs (aRR) and 95% confidence intervals (CIs) were reported for significant variables. Among patients with baseline QOL scores, changes in QOL over time were modeled using linear longitudinal mixed-effects regression models to account for repeated longitudinal measures.
RESULTS
From 2001–2014, 157 patients were enrolled in the study, of whom 101 (64%) met inclusion criteria (Supplementary Figure 1). Of these, 97 (96%) completed ≥1 SF-36, including 60 (59%) with baseline forms available, for a total of 357 questionnaires. Median study follow-up time was 3.4 years (range, 0.5–11.3 years). Of the 101 included patients, 85% were white, 51% female, and mean age was 49 ± 13 years (Table 1). At enrollment, 35 (35%) presented with early localized disease, 46 (46%) with early disseminated disease, and 20 (20%) with late disease (14 [70%] with arthritis, 5 [25%] neurological, and 1 [5%] dermatological). Most patients were untreated (58%) at study enrollment. Among those treated, 20% received <2 weeks of antibiotic treatment, whereas 22% of patients received antibiotic treatment 2–6 weeks prior to enrollment. Most patients (87%) did not report a prior Lyme disease diagnosis, nor did they recall finding a tick (85%).
Table 1.
Demographic and Clinical Characteristics by Stage of Lyme Disease at Diagnosis for All Patients
| Characteristic | All Patients | Early Localized | Early Disseminated | Late Disease |
|---|---|---|---|---|
| (n = 101) n (%) | (n = 35) n (%) | (n = 46) n (%) | (n = 20) n (%) | |
| Age (y) and follow-up time | ||||
| Age at enrollment (mean ± SD) | 49 ± 13 | 47 ± 15 | 49 ± 12 | 51 ± 13 |
| Follow-up months (mean ± SD) | 47 ± 30 | 40 ± 29 | 55 ± 33 | 40 ± 22 |
| Range of follow-up months | 6–136 | 6–120 | 8–136 | 10–89 |
| Gender | ||||
| Female | 52 (51) | 23 (66) | 21 (46) | 8 (40) |
| Treatment status at enrollment | ||||
| Treated | 42 (42) | 12 (66) | 24 (52) | 6 (30) |
| Treatment duration prior to enrollment: | ||||
| <1 wk | 9 (9) | 5 (14) | 2 (4) | 2 (10) |
| 1–2 wk | 11 (11) | 1 (3) | 10 (22) | 0 |
| >2 wk | 22 (22) | 6 (17) | 12 (26) | 4 (20) |
| Untreated | 59 (58) | 23 (34) | 22 (48) | 14 (70) |
| Manifestation | ||||
| Arthritis | 15 (15) | 0 (0) | 1 (2) | 14 (70) |
| Cardiovascular | 3 (3) | 0 (0) | 3 (7) | 0 (0) |
| Dermatological | 55 (54) | 35 (100) | 19 (41) | 1(5) |
| Neurological | 28 (28) | 0 (0) | 23 (50) | 5 (25) |
| Previous Lyme infection | ||||
| No | 88 (87) | 29 (83) | 41 (89) | 18 (90) |
| Yes | 13 (13) | 6 (17) | 5 (11) | 2 (10) |
| History of tick bite | ||||
| No | 86 (85) | 24 (69) | 42 (91) | 20 (100) |
| Yes | 15 (15) | 11 (31) | 4 (9) | 0 (0) |
| Baseline comorbidity | ||||
| No | 43 (43) | 17 (51) | 21 (46) | 5 (25) |
| Yes | 58 (57) | 18 (51) | 25 (54) | 15 (75) |
Total number of patients: 101.
Abbreviation: SD, standard deviation.
Symptom Data
Among all patients (n = 101), 92 (91%) reported ≥1 symptom at baseline (mean number of baseline symptoms per person was 7; range, 0–21). The 5 most common symptoms reported at baseline were fatigue (66%), muscle soreness (55%), joint pain (51%), sleep disturbances (38%), and neck pain (37%; Supplementary Table 1). Of the 75 (74%) patients with ≥24.5 months of follow-up data, 42 (57%) continued to report long-term symptoms (mean number among those reporting long-term symptoms was 4; range, 1–8). Of these 42 patients, 27 (64%) reported fatigue, 28 (67%) muscle soreness, 23 (55%) joint pain, 19 (45%) sleep disturbances, 16 (38%) poor memory, and 14 (33%) problems finding words and poor concentration/memory (Supplementary Table 2).
Comorbidity Data
Most patients (57%) had 1 or more active preexisting comorbidities at enrollment, and of those, 72% reported more than 1 condition (mean number among those reporting preexisting comorbidities was 3; range, 1–6). The most frequent comorbidities reported included obesity (15%), hypercholesterolemia/hyperlipidemia (14%), hypothyroidism (12%), environmental allergies (10%), depression (7%), and gastroesophageal reflux disease (6%). Next, we categorized comorbidities into 3 groups (Supplementary Table 3). When categorized into these comorbidity groups, 25 (25%) had a high-risk condition for cardiovascular disease, 22 (22%) had a condition associated with chronic pain, 16 (16%) reported a mental/behavioral health condition, and 9 (9%) were on medication for a mental/behavioral health condition.
QOL Data
Among patients with QOL data available, 35 (36%) had early localized, 43 (44%) early disseminated, and 19 (20%) late disseminated disease. At first visit (QOL-1), overall mean QOL scores were below the SF-36 US population mean for both physical health (mean PCS = 45.6 ± 10.4; range, 21.4–61.4) and mental health (mean MCS = 47.3 ± 11.5; range, 19.7–62.7; Supplementary Figure 2). However, overall mean scores increased to just above the US national average after 3 years of follow-up time for both PCS (50.7 ± 9.6) and MCS (50.1 ± 10.0). SF-36 subdomain scores at first visit were highest for general health (52.0 ± 9.3) and mental health (49.3 ± 10.5) and lowest for role-physical (40.9 ± 12.2) and social functioning (43.6 ± 12.1). At last visit, subdomain scores were highest for general health (52.4 ± 9.2) and physical functioning (51.4 ± 7.4) and lowest for role-physical (48.4 ± 9.8) and role-emotional (47.8 ± 10.8). All subdomain scores across all groups increased over time (Supplementary Table 4).
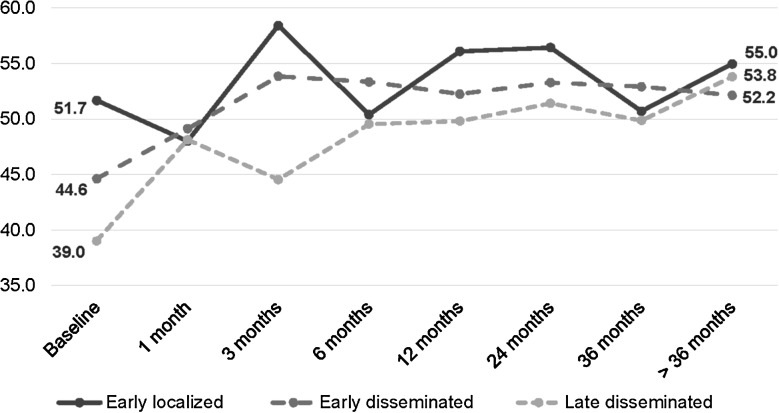
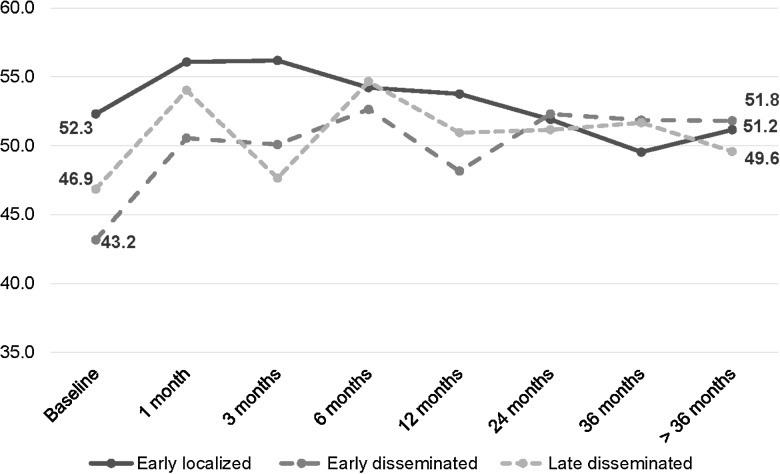
At baseline (QOL-2), when evaluated by Lyme disease stage, QOL scores were higher for patients with early localized disease than for those with disseminated disease (mean PCS: 51.7 ± 8.6 vs 42.7 ± 10.4, P = .0019; mean MCS: 52.3 ± 8.0 vs 44.5 ± 12.5, P = .02). However, both scores increased over time across all groups to approximately 50 (Figures 1 and 2). Similarly, patients with more severe manifestations of Lyme disease (ie, neurological, arthritic, and/or cardiac vs only dermatological) compared with those with dermatological manifestations only had lower PCS (41.0 ± 10.7 vs 50.9 ± 7.8, P = .0005) and similar MCS (44.9 ± 11.6 vs 50.3 ± 11.2, P = .1) scores at baseline, though scores for this group also increased over time. By >36 months of follow-up, PCS (55.1 ± 5.5 vs 51.1 ± 9.4, P = .2) and MCS scores (54.5 ± 7.4 vs 48.6 ± 10.0, P = .1) from those with initially severe manifestations were not significantly different when compared with scores from those with dermatological manifestations. Patients with baseline QOL data did not differ significantly from those without baseline QOL data with respect to age, treatment status at enrollment, or by preexisting comorbidities; they were more likely to be female (P = .001). However, gender was not associated with any outcomes of interest and was controlled for in all subsequent models.
Figure 1.
Change in mean physical component summary by follow-up time period and stage of Lyme disease at diagnosis (n = 60*). *Sample sizes: early localized/early disseminated/late disseminated—baseline (19/24/13), 1 month (2/9/5), 3 months (5/12/3), 6 months (6/12/12), 12 months (14/19/7), 24 months (11/16/8), 36 months (4/15/10), >36 months (6/14/4).
Figure 2.
Change in mean mental component summary by follow-up time period and stage of Lyme disease at diagnosis (n = 60*). *Sample sizes: early localized/early disseminated/late disseminated—baseline (19/24/13), 1 month (2/9/5), 3 months (5/12/3), 6 months (6/12/12), 12 months (14/19/7), 24 months (11/16/8), 36 months (4/15/10), >36 months (6/14/4).
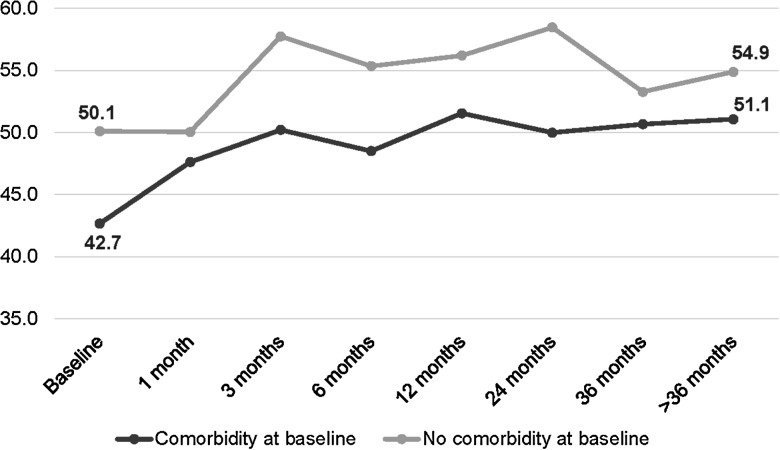
Among patients with an active comorbidity reported at baseline, PCS scores at baseline were lower in those without comorbidities (42.7 ± 10.4 vs 50.1 ± 9.6, P = .0087); although PCS remained lower over time, this difference was no longer significant at the end of follow-up (51.1 ± 5.6 vs 54.9 ± 8.1, P = .2; Figure 3). Similarly, patients with mental/behavioral health comorbidities had lower but not significantly different MCS scores compared with patients with no mental/behavioral health conditions reported (42.8 ± 12.0 vs 48.1 ± 11.6, P = .2). However, this difference increased and, by the end of follow-up, was significantly lower in those reporting mental/behavioral health comorbidities (38.5 ± 7.4 vs 52.4 ± 8.02, P = .03; Supplementary Figure 3).
Figure 3.
Change in mean physical component summary in a cohort of Lyme disease patients by follow-up time period and comorbidity at baseline (n = 60*). *Sample sizes: comorbidity/no comorbidity—baseline (33/23), 1 month (9/7), 3 months (11/9), 6 months (18/12), 12 months (26/14), 24 months (19/16), 36 months (19/10), >36 months (11/13).
Among patients with baseline PCS and MCS scores (n = 56), 24 (43%) were categorized as having low (≤45) PCS and 19 (24%) as having low (≤45) MCS at baseline. Patients with low MCS and low PCS at baseline continued to have significantly lower QOL scores than those with baseline scores >45 after 24.5 months of follow-up (PCS: 47.7 ± 8.2 [low] vs 55.1 ± 4.3, P = .005; MCS: 43.1 ± 9.0 [low] vs 53.2 ± 6.0 [high], P = .0042).
Regression Analyses
Multivariate analyses were conducted to evaluate risk factors associated with reporting long-term (≥24.5 months) symptoms and with having low (≤45) baseline QOL scores; all models controlled for gender, age, Lyme treatment status at enrollment, and stage of Lyme disease at diagnosis. Patients with long-term symptoms were more likely to report mental/behavioral health conditions (aRR = 1.7; 95% CI, 1.2–2.5; P = .006), mental health medications (aRR = 1.6; 95% CI, 1.0–2.5; P = .05), and obesity (aRR = 1.6; 95% CI, 1.1–2.4; P = .02). Further, for each additional comorbidity reported at baseline, a patient was 13% more likely to report long-term symptoms (aRR = 1.13; 95% CI, 1.0–1.3; P = .04). Patients with disseminated or more severe stage of Lyme disease at diagnosis were not more likely to report long-term symptoms (P = .9).
Patients with low PCS at baseline were twice as likely to report a comorbidity associated with chronic pain at baseline (aRR = 2.1l; 95% CI, 1.3–3.6; P = .005), and for each additional comorbidity reported, a patient was 24% more likely to have low baseline PCS (aRR = 1.2; 95% CI, 1.0–1.5; P = .01). While those with low MCS were twice as likely as those with MCS > 45 to have a mental/behavioral health condition at baseline (RR = 2.0; 95% CI, 1.0–4.1; P = .05) and 3 times more likely to be taking medications for a mental health condition (RR = 3.0; 95% Cl, 1.6–5.3; P = .0007) in univariate analyses, neither factor remained significant after adjusting for gender, age, Lyme treatment status at enrollment, and Lyme disease stage.
DISCUSSION
In a cohort of patients with diverse manifestations of Lyme disease and detailed clinical follow-up, we found that both mental health and physical health scores increased to be at or above the national average over time, regardless of Lyme disease stage or severity at diagnosis. However, patients with comorbidities unrelated to Lyme disease at baseline had lower QOL scores throughout follow-up and were significantly more likely to report long-term subjective symptoms.
The mean overall PCS and MCS scores at baseline were >45 for those with early localized disease but <45 for those with disseminated disease. QOL scores by stage of Lyme disease increased over the course of the study to the US population mean. Among patients with baseline QOL scores available, 43% had PCS and 24% MCS scores that were at least half a standard deviation below average. Patients with low initial scores were more likely to report other comorbidities and have greater numbers of comorbidities at baseline; QOL scores in this group remained significantly lower even after 2 years of follow-up. Since low QOL can directly impact health outcomes, it represents a critical measure for identifying subgroups that might be at risk of worse outcomes as a result of perceived poor QOL [31].
Few studies have measured QOL among patients with Lyme disease, and most have been cross-sectional evaluations that categorized long-term effects by measuring QOL at a time distant from the initial diagnosis. Our findings are similar to those from a recent study where patients with EM were evaluated for a median of 16 years after initial diagnosis. In this study, mean PCS, MCS, and subscale scores were also identical to those for the general population [32]. In another cross-sectional study, patients with an initial or second episode of EM, evaluated 2–4 years post-diagnosis, had QOL scores and symptom profiles that were similar to those of non-Lyme controls [33]. Our results expand on these findings, as we also included patients with extracutaneous manifestations of B. burgdorferi infection, such as neuroborreliosis and Lyme arthritis. In our study, these patients achieved good long-term outcomes that were similar to those of patients with EM.
While patients with more severe manifestations of Lyme disease initially presented with lower PCS scores in our cohort, this difference did not persist beyond 2 years of follow-up. In another US study that compared patients with early Lyme neuroborreliosis (facial nerve palsy) with patients with EM 4.5 years post-illness, they found that only the social functioning subscale score was significantly lower [34]. Similarly, in a study of patients with EM, facial palsy, or arthritis, evaluated 10–20 years after disease, facial palsy patients who did not receive antibiotic therapy had more pain and lower PCS scores [19]. In a European study, patients with Lyme neuroborreliosis scored lower than controls for both PCS and MCS, but those who reported complete recovery had scores that were similar to those of controls [35]; incomplete recovery at 4 months was associated with lower PCS and MCS scores 30 months post-treatment. In a related study, the same authors found that patients with a baseline comorbidity had significantly lower PCS and fatigue scale scores [36].
Unlike the studies described above, our study uniquely followed patients for an extended period of time, providing serial information about the health impact of Lyme disease. Only 1 other study prospectively evaluated patients with Lyme disease (EM) using the SF-36 questionnaire, though this study was limited to just 6 months of follow-up and was focused on comparing patients who did and did not develop post-treatment Lyme disease syndrome (PTLDS). In this study, baseline scores for PTLDS patients tended to be lower and were significantly lower than those for non-PTLDS patients by 6 months. While participants reported an average of 1 medical diagnosis at baseline, participants with preexisting comorbidities and symptoms similar to those with PTLDS were excluded from enrollment; therefore, comparability between studies is limited [37].
Although more than half of patients reported long-term symptoms at 2 years post-enrollment, the only significant predictor of long-term symptoms was the presence of other comorbidities. In our longitudinal analysis, the association between self-reported comorbidities and long-term symptoms suggests a contributory relationship. Patients with mental/behavioral health and/or cardiovascular-related risk factors, especially obesity, may be more sensitive to symptoms of fatigue, joint pain, and sleep disturbances, which may be exacerbated and/or prolonged in this group. It is possible that patients with post-Lyme disease symptoms may have other comorbidities present that have either gone undiagnosed or were not reported over concerns of stigmatization.
This study represents the first to longitudinally measure QOL in patients with Lyme disease using the standardized SF-36, providing hundreds of QOL questionnaires for analysis in conjunction with extensive clinical data. Limitations of this analysis include variable follow-up time due to patient attrition, which is inherent in natural history study designs, as patients who contributed more follow-up time may differ from those with less follow-up time due to unmeasured confounders. Additionally, in the QOL analysis, one-third of patients did not complete surveys within 30 days of enrollment, though no significant differences were detected between those with and without earlier QOL measures with respect to the outcomes evaluated. Similarly, while there were only 47 patients with more than 2 years of follow-up data, they did not differ significantly from patients with less follow-up time.
In conclusion, we characterized the clinical and QOL outcomes of patients with Lyme disease and found that, even among those with more severe manifestations of Lyme disease, both physical health and mental health scores increased to be at or above the national average by the end of follow-up. Further, only preexisting comorbidities—and not Lyme disease stage or severity—were predictive of having lower QOL measures and long-term symptoms. Comorbid conditions should be considered when evaluating patients with Lyme disease who are suffering from long-term symptoms and reduced QoL.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the official views of the National institutes of Health (NIH). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported, in part, by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the NIH. This project was funded, in part, with federal funds from the National Cancer Institute, Center for Cancer Research, NIH (contract number HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. How many people get Lyme disease, 2013. Available at: http://www.cdc.gov/lyme/stats/humanCases.html. Accessed 15 December 2015.
- 2.Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am 2015; 29:187–210. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Lyme disease: data and statistics, 2013. Available at: http://www.cdc.gov/lyme/stats/index.html. Accessed 15 December 2015.
- 4.Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographicdistribution and expansion of human Lyme disease, United States. Emerg Infect Dis 2015; 21:1455–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steere AC, Bartenhagen NH, Craft JE et al. The early clinical manifestations of Lyme disease. Ann Intern Med 1983; 99:76–82. [DOI] [PubMed] [Google Scholar]
- 6.Nadelman RB, Nowakowski J, Forseter G et al. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med 1996; 100:502–8. [DOI] [PubMed] [Google Scholar]
- 7.Strle F, Nadelman RB, Cimperman J et al. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann Intern Med 1999; 130:32–6. [DOI] [PubMed] [Google Scholar]
- 8.Kindstrand E, Nilsson BY, Hovmark A, Pirskanen R, Asbrink E. Peripheral neuropathy in acrodermatitis chronica atrophicans—a late Borrelia manifestation. Acta Neurol Scand 1997; 95:338–45. [DOI] [PubMed] [Google Scholar]
- 9.Halperin JJ, Logigian EL, Finkel MF, Pearl RA. Practice parameters for the diagnosis of patients with nervous system Lyme borreliosis (Lyme disease). Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1996; 46:619–27. [PubMed] [Google Scholar]
- 10.Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med 1990; 323:1438–44. [DOI] [PubMed] [Google Scholar]
- 11.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med 1987; 107:725–31. [DOI] [PubMed] [Google Scholar]
- 12.Smith RP, Schoen RT, Rahn DW et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002; 136:421–8. [DOI] [PubMed] [Google Scholar]
- 13.Nowakowski J, Nadelman RB, Sell R et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med 2003; 115:91–6. [DOI] [PubMed] [Google Scholar]
- 14.Wormser GP, Ramanathan R, Nowakowski J et al. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2003; 138:697–704. [DOI] [PubMed] [Google Scholar]
- 15.Wormser GP, Dattwyler RJ, Shapiro ED et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 16.Dattwyler RJ, Halperin JJ, Volkman DJ, Luft BJ. Treatment of late Lyme borreliosis—randomised comparison of ceftriaxone and penicillin. Lancet 1988; 1:1191–4. [DOI] [PubMed] [Google Scholar]
- 17.Pfister HW, Preac-Mursic V, Wilske B, Schielke E, Sörgel F, Einhäupl KM. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J Infect Dis 1991; 163:311–8. [DOI] [PubMed] [Google Scholar]
- 18.Steere AC, Levin RE, Molloy PJ et al. Treatment of Lyme arthritis. Arthritis Rheum 1994; 37:878–88. [DOI] [PubMed] [Google Scholar]
- 19.Kalish RA, Kaplan RF, Taylor E, Jones-Woodward L, Workman K, Steere AC. Evaluation of study patients with Lyme disease, 10-20-year follow-up. J Infect Dis 2001; 183:453–60. [DOI] [PubMed] [Google Scholar]
- 20.Kindstrand E, Nilsson BY, Hovmark A, Pirskanen R, Asbrink E. Peripheral neuropathy in acrodermatitis chronica atrophicans—effect of treatment. Acta Neurol Scand 2002; 106:253–7. [DOI] [PubMed] [Google Scholar]
- 21.Berglund J, Stjernberg L, Ornstein K, Tykesson-Joelsson K, Walter H. 5-y follow-up study of patients with neuroborreliosis. Scand J Infect Dis 2002; 34:421–5. [DOI] [PubMed] [Google Scholar]
- 22.Dattwyler RJ, Wormser GP, Rush TJ et al. A comparison of two treatment regimens of ceftriaxone in late Lyme disease. Wien Klin Wochenschr 2005; 117:393–7. [DOI] [PubMed] [Google Scholar]
- 23.Borg R, Dotevall L, Hagberg L et al. Intravenous ceftriaxone compared with oral doxycycline for the treatment of Lyme neuroborreliosis. Scand J Infect Dis 2005; 37:449–54. [DOI] [PubMed] [Google Scholar]
- 24.Oksi J, Nikoskelainen J, Hiekkanen H et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: a double-blind, randomized, placebo-controlled, multicenter clinical study. Eur J Clin Microbiol Infect Dis 2007; 26:571–81. [DOI] [PubMed] [Google Scholar]
- 25.Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am 2008; 22:341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adrion ER, Aucott J, Lemke KW, Weiner JP. Health care costs, utilization and patterns of care following Lyme disease. PLoS One 2015; 10:e0116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi) 2011 Case definition, 2011. Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=752%26DatePub=1/1/2011. Accessed 10 October 2015.
- 28.Ware JE Jr, Kosinski M, Dewey JE. How to score version 2 of the SF-36 Health Survey (standard and acute forms). Lincoln, RI: QualityMetric, Inc., 2005. [Google Scholar]
- 29.Scoggins JF, Patrick DL. The use of patient-reported outcomes instruments in registered clinical trials: evidence from ClinicalTrials.gov. Contemp Clin Trials 2009; 30:289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473–83. [PubMed] [Google Scholar]
- 31.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995; 273:59–65. [PubMed] [Google Scholar]
- 32.Wormser GP, Weitzner E, McKenna D et al. Long-term assessment of health-related quality of life in patients with culture-confirmed early Lyme disease. Clin Infect Dis 2015; 61:244–7. [DOI] [PubMed] [Google Scholar]
- 33.Jares TM, Mathiason MA, Kowalski TJ. Functional outcomes in patients with Borrelia burgdorferi reinfection. Ticks Tick Borne Dis 2014; 5:58–62. [DOI] [PubMed] [Google Scholar]
- 34.Kowalski TJ, Berth WL, Mathiason MA, Agger WA. Oral antibiotic treatment and long-term outcomes of Lyme facial nerve palsy. Infection 2011; 39:239–45. [DOI] [PubMed] [Google Scholar]
- 35.Eikeland R, Mygland A, Herlofson K, Ljøstad U. European neuroborreliosis: quality of life 30 months after treatment. Acta Neurol Scand 2011; 124:349–54. [DOI] [PubMed] [Google Scholar]
- 36.Eikeland R, Mygland Å, Herlofson K, Ljøstad U. Risk factors for a non-favorable outcome after treated European neuroborreliosis. Acta Neurol Scand 2013; 127:154–60. [DOI] [PubMed] [Google Scholar]
- 37.Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res 2013; 22:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.