Significance
Humans express nearly 80 K+ channels. This large diversity of K+ channels (compare 10 Na+ channels and 10 Ca2+ channels) reflects their importance in tying many different biochemical processes to electrical signaling and to shaping and fine-tuning the electrical waveforms produced by excitable cells. We understand the biological roles of only a tiny fraction of the K+ channels because, for many of them, good pharmacological tools do not exist. To remedy this situation and thus open up a new avenue to study K+ channels’ biological roles, we developed a new cell-free assay that enables the discovery of inhibitors and activators for almost any K+ channel. It also provides a new high-throughput human ether-à-go-go-related gene safety assay.
Keywords: liposome flux assay, LFA, K+ channel screening, hERG safety assay
Abstract
K+ channels, a superfamily of ∼80 members, control cell excitability, ion homeostasis, and many forms of cell signaling. Their malfunctions cause numerous diseases including neuronal disorders, cardiac arrhythmia, diabetes, and asthma. Here we present a novel liposome flux assay (LFA) that is applicable to most K+ channels. It is robust, low cost, and high throughput. Using LFA, we performed small molecule screens on three different K+ channels and identified new activators and inhibitors for biological research on channel function and for medicinal development. We further engineered a hERG (human ether-à-go-go-related gene) channel, which, when used in LFA, provides a highly sensitive (zero false negatives on 50 hERG-sensitive drugs) and highly specific (zero false positives on 50 hERG-insensitive drugs), low-cost hERG safety assay.
The K+ channel superfamily can be divided into five subfamilies, including Kir (inwardly rectifying K+ channels), K2P (tandem-pore-domain K+ channels), Kv1–9 (voltage-gated K+ channels), Kv10–12 [including the hERG (human ether-à-go-go-related gene) channel], and KCa (Ca2+-activated K+ channels) (1, 2) (Fig. 1A). Their varied structures and gating mechanisms reflect their diverse and important roles in biology. Kir and K2P channels are major regulators of the resting membrane potential. Among Kir channels, Kir3.2 [G protein-activated inwardly-rectifying K+ channel member 2 (GIRK2)] regulates the electrical excitability of many different neurons in response to inhibitory G protein coupled receptor (GPCR) stimulation (3). Kir6.2 (K-ATP) controls insulin secretion in β cells and is a well-established drug target for diabetes (3). The biological functions of K2P channels remain mostly unknown due to the lack of pharmacological tools. For example, TRAAK (TWIK-related arachidonic acid-stimulated K+ channel) is biophysically mechanosensitive, but little is known about its biology, especially regarding its mechanosensitivity (4–7). Kv channels repolarize action potentials. Mutations in Kv7.1 (KCNQ1) cause congenital long QT syndrome (2). The hERG channel is the dangerous off-target of many drugs that, by inadvertently inhibiting hERG, cause drug-induced long QT syndrome with the potential of torsades de pointes and sudden death (8). High-conductance Ca2+-activated K+ (Slo1) channel of the KCa subfamily regulates smooth muscle contraction, and activators of the Slo1 channel are drug candidates for asthma, over-reactive bladder, and hypertension (9–15). These channels are just a small subset of examples illustrating the important roles of K+ channels to different physiological functions.
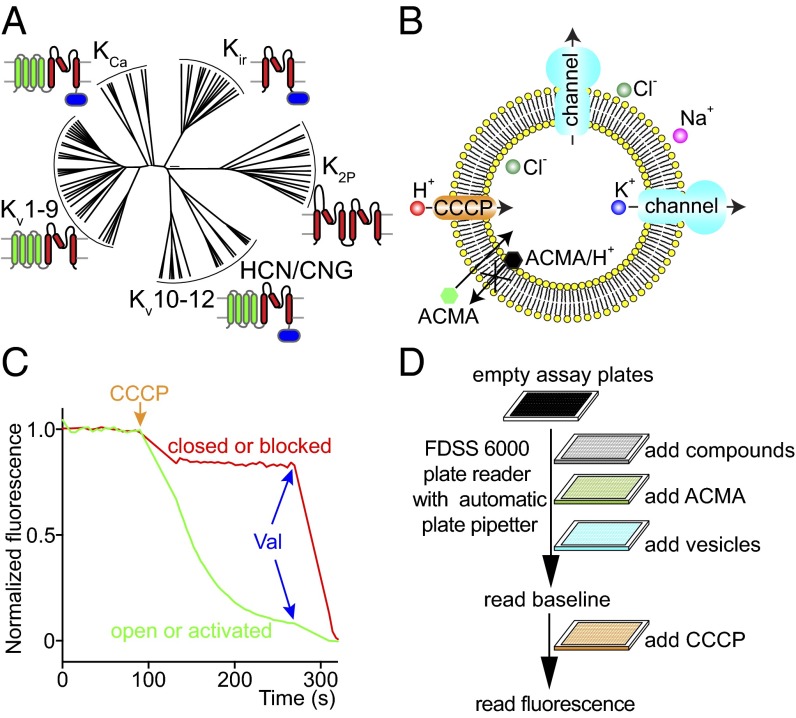
Fig. 1.
Introduction to the LFA. (A) A phylogenetic tree of human K+ channels adapted from ref. 4. The K+ channel superfamily can be divided into five subfamilies, including Kir (inwardly rectifying K+ channels), K2P (tandem-pore-domain K+ channels), Kv1–9 (voltage-gated K+ channels), Kv10–12 (including the hERG channel), and KCa (Ca2+-activated K+ channels). Kv10–12 subfamily is related to HCN/CNG channels (hyperpolarization-activated cyclic nucleotide-gated channels/cyclic nucleotide-gated channels). Red highlights the conserved pore domain, green highlights the voltage sensor domain, and blue highlights the intracellular ligand-binding domain. (B) A cartoon of the LFA. Purified K+ channels are reconstituted into lipid vesicles in the presence of high KCl. To assay, thawed vesicles are diluted into a high NaCl solution, which creates a strong gradient for the efflux of K+. Potassium efflux is initiated by the addition the H+ ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), which allows influx of H+ to counterbalance the efflux of K+. The H+ influx is monitored by the H+-dependent quenching of a fluorescent dye, 9-amino-6-chloro-2-methoxyacridine (ACMA). (C) A representative trace of the LFA. K+ efflux leads to dye quenching when K+ channels are open (green trace), but not when they are closed or inhibited (red trace). The K+ ionophore valinomycin (Val) is finally added to allow K+ channel-independent efflux, to exclude false-positive compounds with strong auto-fluorescence or causing vesicle lysis. (D) A cartoon of the cell-free HTS screen procedure. All flux components were added and mixed automatically by FDSS6000 plate reader in sequence as depicted in the figure. Valinomycin, used to induce channel-independent K+ efflux, was not used in the primary screen on FDSS6000 because it was difficult to wash out and caused contamination among plates.
Potassium channel pharmacology has contributed greatly to our understanding of K+ channel mechanisms. In particular, the discovery of naturally occurring peptide toxins isolated from venomous animals, including scorpions, spiders, insects, and snakes, has advanced our understanding of the ion conduction pore and regulatory domains such as voltage sensors (16). Although these toxins have been instrumental to our understanding of certain K+ channels, there are many K+ channels—including the entire subfamily of K2P channels—that are insensitive to peptide toxins and for which pharmacological agents are almost nonexistent.
The discovery of small molecule pharmacological agents directed against K+ channels depends on the availability of a robust, affordable, high-throughput assay. None of the methods currently used—electrophysiology (17, 18), cell-based assays such as Tl+ flux assays/membrane potential dye assays/yeast growth assays (19–21), and binding/inhibitor competition assays (22, 23)—fulfill all three of the above criteria. We developed and present here an assay that does fulfill these criteria. The assay is based on new methods that allow us to express, purify, and reconstitute virtually any mammalian K+ channel. Vesicles reconstituted with these channels can be stored frozen for assay at a later date. The assay records K+ flux across the vesicle membranes, monitored optically with a high signal-to-noise ratio. In this paper, we describe the assay and how it fulfills the criteria of robustness, affordability, and speed. To demonstrate the assay’s efficacy we apply it to four K+ channels, each from a different subfamily. In three channels we identify novel small molecule inhibitors and/or activators. In a fourth channel, hERG, we present a new hERG safety assay and demonstrate its high speed and fidelity.
Results
Assay Description.
The principle of the assay is illustrated in Fig. 1B. Purified K+ channels are reconstituted into lipid vesicles in the presence of KCl ranging in concentration from 150 to 300 mM. The channel-containing vesicles are usually frozen for storage at this stage. To assay, thawed vesicles are diluted into a NaCl solution, which creates a strong gradient for the efflux of K+. Potassium efflux is initiated by the addition of the H+ ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), which allows influx of H+ to counterbalance the efflux of K+. The H+ influx is monitored by the H+-dependent quenching of a fluorescent dye, 9-amino-6-chloro-2-methoxyacridine (ACMA). In the example shown in Fig. 1C, K+ efflux leads to dye quenching when K+ channels are active (green trace), but not when they are inhibited (red trace). The K+ ionophore valinomycin is finally added to allow K+ channel-independent efflux. For many K+ channels, assay conditions under which the channels are inactive can be found to screen for activators that will initiate K+ efflux. Fig. 1D illustrates the sequence of additions used in a multiwell plate format and Fig. 1C shows an example of the data readout.
GIRK2.
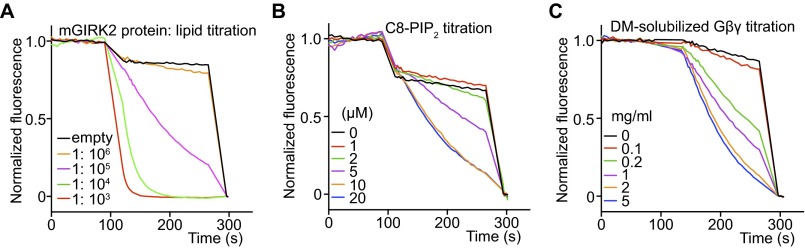
GIRK2 is a K+ channel that mediates neural inhibition in response to stimulation of Gαi-coupled GPCRs. On neurotransmitter stimulation, G protein subunits (Gβγ) are released from the receptor, bind to GIRK2, and cause it to open (Fig. 2A). The open K+ channel drives the cell membrane voltage toward the K+ reversal potential and thus inhibits cellular excitation. In addition to Gβγ, GIRK2 activation also requires PIP2, a signaling lipid, and activation is also modulated by Na+ (Fig. 2 A and B). Because GPCR stimulation and multiple intracellular ligands are required to activate GIRK2, cell-based assays will naturally identify many off-target effectors. LFA permits strong biochemical control and is more directed at the channel rather than upstream components of the signaling pathway. Fig. 2 illustrates the biochemical control. When reconstituted in vesicles, GIRK2 channels will incorporate in both directions, inside out and outside out (Fig. 2B). By applying Gβγ subunits and a soluble version of PIP2 outside the vesicles, only inside-out channels become activated. LFA replicates electrophysiological data showing that both Gβγ and PIP2 are required to robustly activate GIRK2 (Fig. 2C). The rate of optical signal response can be controlled to an extent through the GIRK2 protein-to-lipid ratio of vesicles (Fig. S1A). This level of fine control enables the use of rate (i.e., slope of the optical signal) as a measure of relative channel activity during inhibitor or activator titrations. Furthermore, titrations of natural ligands such as Gβγ and PIP2 can be performed with LFA (Fig. S1 B and C).
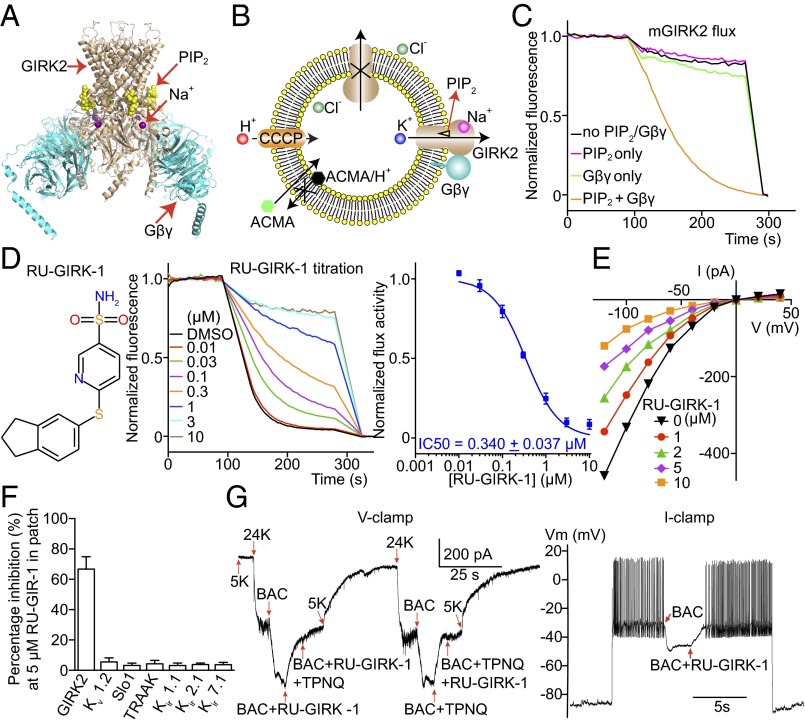
Fig. 2.
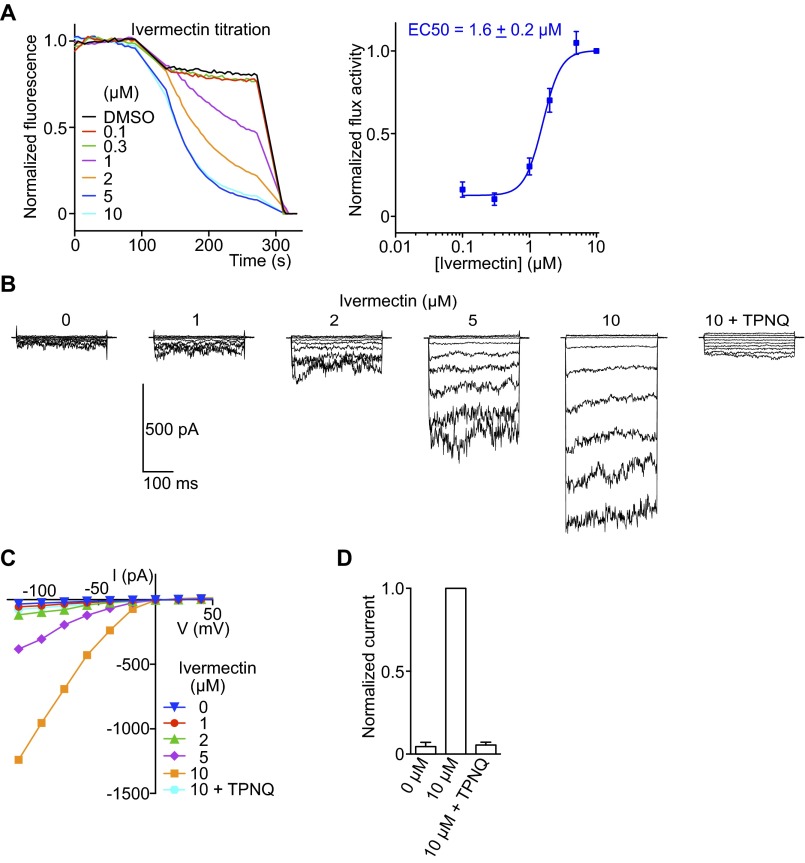
Mouse GIRK2 screens and characterization of a GIRK2 inhibitor. (A) The crystal structure of GIRK2 with PIP2, Gβγ, and Na+ bound [Protein Data Bank (PDB) ID code 4KFM]. (B) A cartoon showing that GIRK2 ligands applied outside vesicles conveniently activate inside-out facing channels. (C) Normalized GIRK2 flux in response to PIP2 and Gβγ recapitulating electrophysiological recordings. (D) (Left) RU-GIRK-1 chemical structure. (Center) mGIRK2 mediated flux at various concentrations of RU-GIRK-1. (Right) Dose–response of RU-GIRK-1 inhibition (n = 9). (E) Current-voltage curves from mGIRK2 expressed in HEK293 cells at various RU-GIRK-1 concentrations (n = 3). (F) RU-GIRK-1 inhibition of different K+ channels expressed in cultured cells and recorded using the patch-clamp recording method (n = 3 each). (G) (Left) A representative voltage-clamp recording of cultured mouse hippocampal neurons with RU-GIRK-1. Extracellular K+ was increased from 5 to 24 mM to increase K+ current. GIRK-mediated current was activated by baclofen (BAC), a GABAB receptor agonist; 5 μM RU-GIRK-1 selectively blocked BAC-induced current similar to Tertiapin-Q (TPNQ), a specific peptide toxin for GIRK2. (Right) A representative current-clamp recording. Current was injected to depolarize the membrane voltage and induce action potentials. Application of BAC induced hyperpolarization and stopped neuron firing by activating GIRK K+ current. Subsequent addition of 5 μM RU-GIRK-1 inhibited GIRK current, depolarized the membrane voltage, and restored firing. All data are mean ± SEM.
Fig. S1.
Optimization of mouse GIRK2 screens. (A) Protein-lipid ratio titrations of GIRK2 reconstituted with both brain PIP2 and lipidated Gβγ. Low protein lipid ratio ∼1: 104-1:105 produced robust flux signals sensitive to blockage and ideal for inhibitor screening. (B) C8-PIP2 titration of GIRK2 reconstituted with lipidated Gβγ only. In the absence of brain PIP2, GIRK2, and Gβγ containing vesicles showed little flux. Additions of soluble short-chain C8-PIP2 induced robust flux by activating the channels whose cytoplasmic domain face the outside of the vesicles. (C) DM-solubilized Gβγ titration of GIRK2 reconstituted with brain-PIP2 only. Without lipidated Gβγ, GIRK2 and brain-PIP2 containing vesicles showed weak flux. Addition of DM-solubilized Gβγ evoked robust flux by activating inside-out facing channels.
To test the ability of LFA to identify small molecules that modify GIRK2 activity, we screened a 100,000 member compound library under two different conditions. First, in the presence of Gβγ and PIP2 we sought to identify inhibitors. Second, in the absence of Gβγ, we sought to identify compounds that could activate GIRK2 by possibly short-circuiting the G protein pathway. Four inhibitors and one activator were identified (Fig. 2D and Figs. S2 and S3).
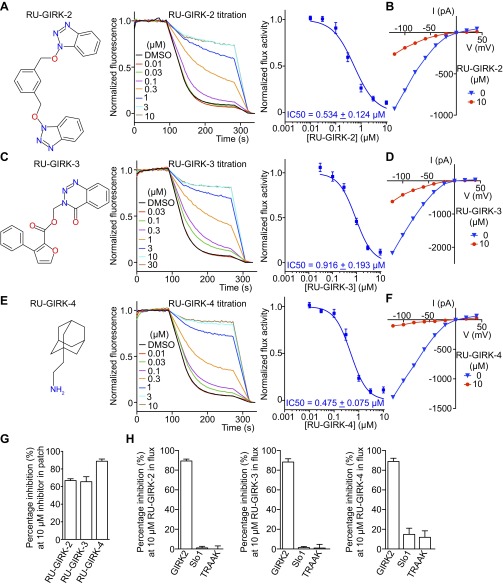
Fig. S2.
Characterization of other GIRK2 inhibitors. (A, C, and E) Chemical structures, normalized flux titrations, and curve fitting (Hill function) of dose–response curves of other GIRK2 inhibitors (n = 9 for RU-GIRK-2, n = 5 for RU-GIRK-3, and n = 9 for RU-GIRK-4). (B, D, and F) Representative whole-cell recordings of GIRK2 expressed in HEK293 cells in response to other GIRK2 inhibitors (n = 3 each). Pipette solution contained 20 mM Na+ to potentiate basal current and 10 μM drugs were applied. (G) Quantifications of recordings in B, D, and F (n = 3). (H) Selectivity test of GIRK2 inhibitors on different K+ channels using LFA (n = 3 each). All data are mean ± SEM.
Fig. S3.
Characterization of a mouse GIRK2 activator, ivermectin (IVM). (A) (Left) GIRK2 was reconstituted with brain PIP2 only and addition of IVM induced robust flux, short-circuiting the need for Gβγ. (Right) Dose–response curve of GIRK2 flux with IVM (n = 3). (B) A representative whole-cell recording of GIRK2 expressed in HEK293 cells in response to IVM (n = 3). Pipette solution contained no Na+ and the basal activities of GIRK2 was low. Addition of IVM to the bath activated large inward currents which were sensitive to TPNQ, indicating the currents being GIRK2 in origin. Membrane voltage was held at 0 mV, stepped from \x{2212}120 to +40 mV in 20-mV increments, and returned to 0 mV. (C) Current-voltage plot from recordings in B. (D) Summary of IVM activation; 10 μM IVM-activated current was normalized to 1. All data are mean ± SEM.
The first inhibitor, RU-GIRK-1, is shown to inhibit GIRK2-mediated flux with an IC50 of about 0.35 μM (Fig. 2D). The activity of RU-GIRK-1 was confirmed in an electrophysiological assay in which GIRK2 channels were expressed in cultured cells (Fig. 2E). The specificity of this compound for GIRK2 among several K+ channels, including several other members of the Kir channel subfamily, was also examined (Fig. 2F). The compound is also active in a more native context. RU-GIRK-1 inhibits baclofen-stimulated K+ currents in cultured mouse hippocampal neurons. The compound thus directly confirms the importance of GIRK2 to GPCR-mediated firing of these neurons (Fig. 2G).
Three additional inhibitors were identified and characterized (Fig. S2 A, C, and E). Graphs of the slope of the optical signal (relative to the slope in the absence of inhibitor) as a function of compound concentration generated inhibition curves that follow a rectangular hyperbola form. Each of these compounds also inhibited GIRK2 channels in an electrophysiological assay (Fig. S2 B, D, F, and G). LFA was also used to assess specificity of these compounds for the GIRK2 channel (Fig. S2H).
In the absence of Gβγ, LFA identified the antiparasitic agent ivermectin as an apparently G protein-independent activator of GIRK2 (Fig. S3A). This activity was confirmed in a whole-cell electrophysiology assay (Fig. S3 B–D). Although a cell assay alone could not exclude the possibility that ivermectin enhances the action of small quantities of Gβγ, LFA was carried out in the complete absence of Gβγ. Thus, ivermectin truly appears to short-circuit the G protein signaling pathway.
G protein-independent activation of GIRK2 by ivermectin is interesting because this drug is used in the treatment of parasitic infections, and its major side effect is CNS depression (24, 25). The mechanism of depression is thought to occur through activation of mammalian GABA and glycine receptor Cl− channels (24, 26–28). However, direct activation of GIRK2 channels in the nervous system could equally contribute to this side effect.
TRAAK.
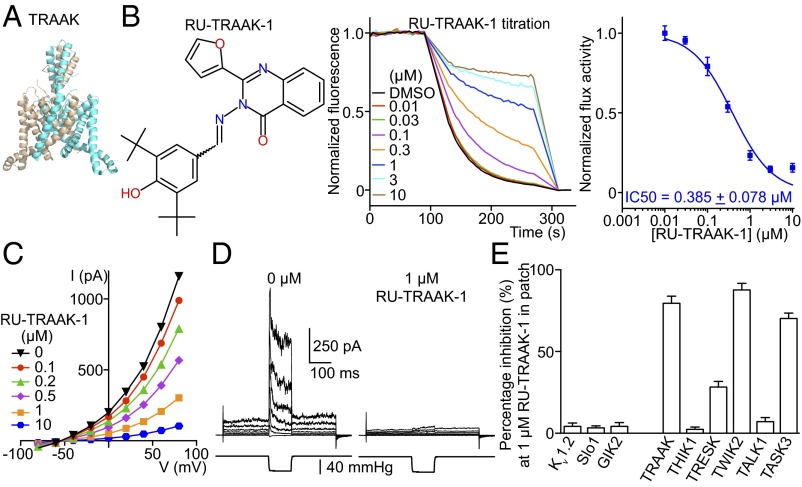
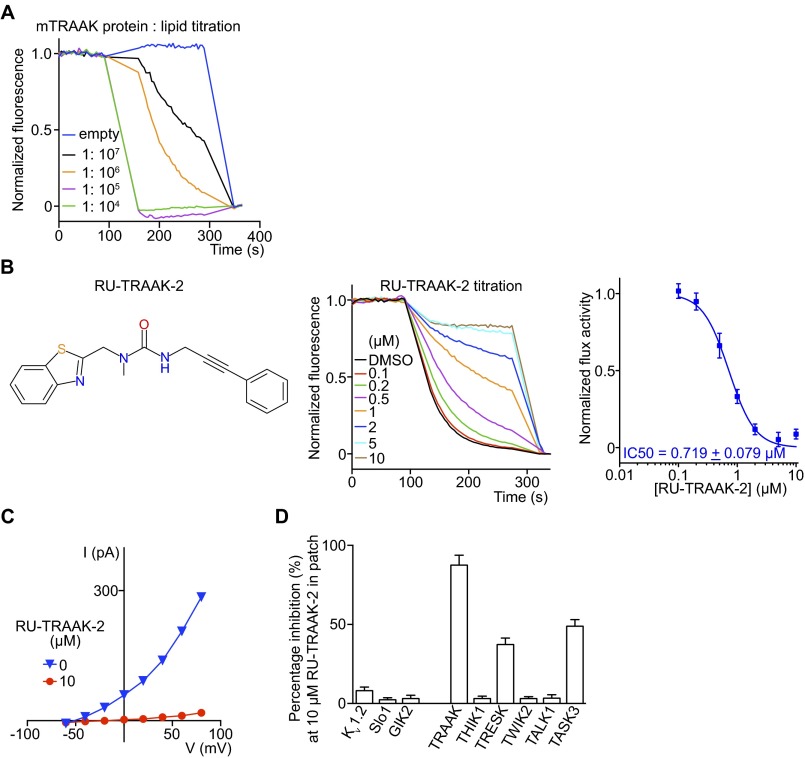
K2P channels are the most extreme structural outliers in the K+ channel family. Instead of containing four subunits they contain only two, but each has an internal repeat with two K+ channel signature sequences, providing the required complement of four such sequences to build a K+ selectivity filter (Figs. 1A and 3A). K2P channels as a subfamily are the least understood in terms of their biological function. This lack of understanding is due in large part to the paucity of specific K2P channel inhibitors. Our laboratory is working to understand in particular a K2P channel called TRAAK, a mechanosensitive K+ channel for which no inhibitors have been identified. We identified conditions under which TRAAK yields a robust LFA signal (Fig. S4A). We screened 300,000 compounds and identified two inhibitors: RU-TRAAK-1 (Fig. 3B) and RU-TRAAK-2 (Fig. S4B). These compounds also inhibit TRAAK in electrophysiological assays (Fig. 3 C–E and Fig. S4 C and D). RU-TRAAK-1 is poorly reversible, whereas RU-TRAAK-2 is completely reversible. Neither compound inhibits non-K2P channels tested (Kv1.2, Slo1 and GIRK2), but both show some cross-reactivity with certain other members of the K2P subfamily (Fig. 3E and Fig. S4D). TRAAK’s relative resistance to inhibition (only two inhibitors of 300,000 compounds tested) might reflect its unusual structure. Compared with most other eukaryotic K+ channels, TRAAK is relatively small and does not have structured regulatory domains outside the membrane that would create opportunity for binding small inhibitory molecules. We anticipate that these inhibitors, applied in well-controlled experiments, will offer new information on the biological roles of TRAAK channels.
Fig. 3.
Characterization of a mouse TRAAK inhibitor. (A) A crystal structure of TRAAK (PDB 4I9W). (B) (Left) RU-TRAAK-1 chemical structure. (Center) Normalized LFA efflux curves in the presence of different concentrations of RU-TRAAK-1. (Right) Dose–response curve of TRAAK-mediated efflux in the presence of RU-TRAAK-1 (n = 9). (C) Whole-cell current-voltage curves from TRAAK expressed in CHO cells in the presence of RU-TRAAK-1 (n = 3). (D) One of five recordings of TRAAK in excised patches in the absence and presence of RU-TRAAK-1. Membrane voltage was held at −50 mV, stepped from −80 to +80 mV in 20-mV increments, and returned to −50 mV. In the middle of the voltage protocol, −40 mmHg pressure was applied to the pipette using a pressure clamp to mechanically activate TRAAK; 1 μM RU-TRAAK-1 inhibited both basal current and pressure-stimulated current. (E) Channel inhibition by 1 μM RU-TRAAK-1 to test its selectivity in whole-cell recordings (n = 3 each). All data are mean ± SEM.
Fig. S4.
Mouse TRAAK flux optimization and characterization of the second TRAAK inhibitor. (A) Protein-lipid-ratio titration revealed that a low ratio (∼1:106) was necessary to obtain a robust flux signal that is responsive to inhibitors. (B) (Left) RU-TRAAK-2 chemical structure. (Center) Normalized flux titration of RU-TRAAK-2. (Right) Dose–response curve of TRAAK flux in the presence of RU-TRAAK-2 (n = 6). (C) Whole-cell current-voltage curves from TRAAK expressing CHO cells in the absence and presence of 10 μM RU-TRAAK-2 (n = 3). (D) RU-TRAAK-2 inhibition of different K+ channels expressed in cultured cells and recorded with whole-cell patch clamp (n = 3 each). All data are mean ± SEM.
High Conductance Ca2+-Activated K+ Channel.
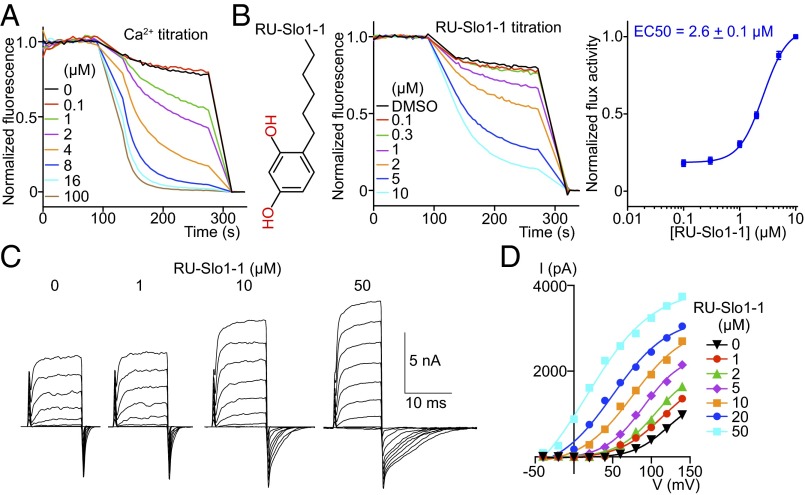
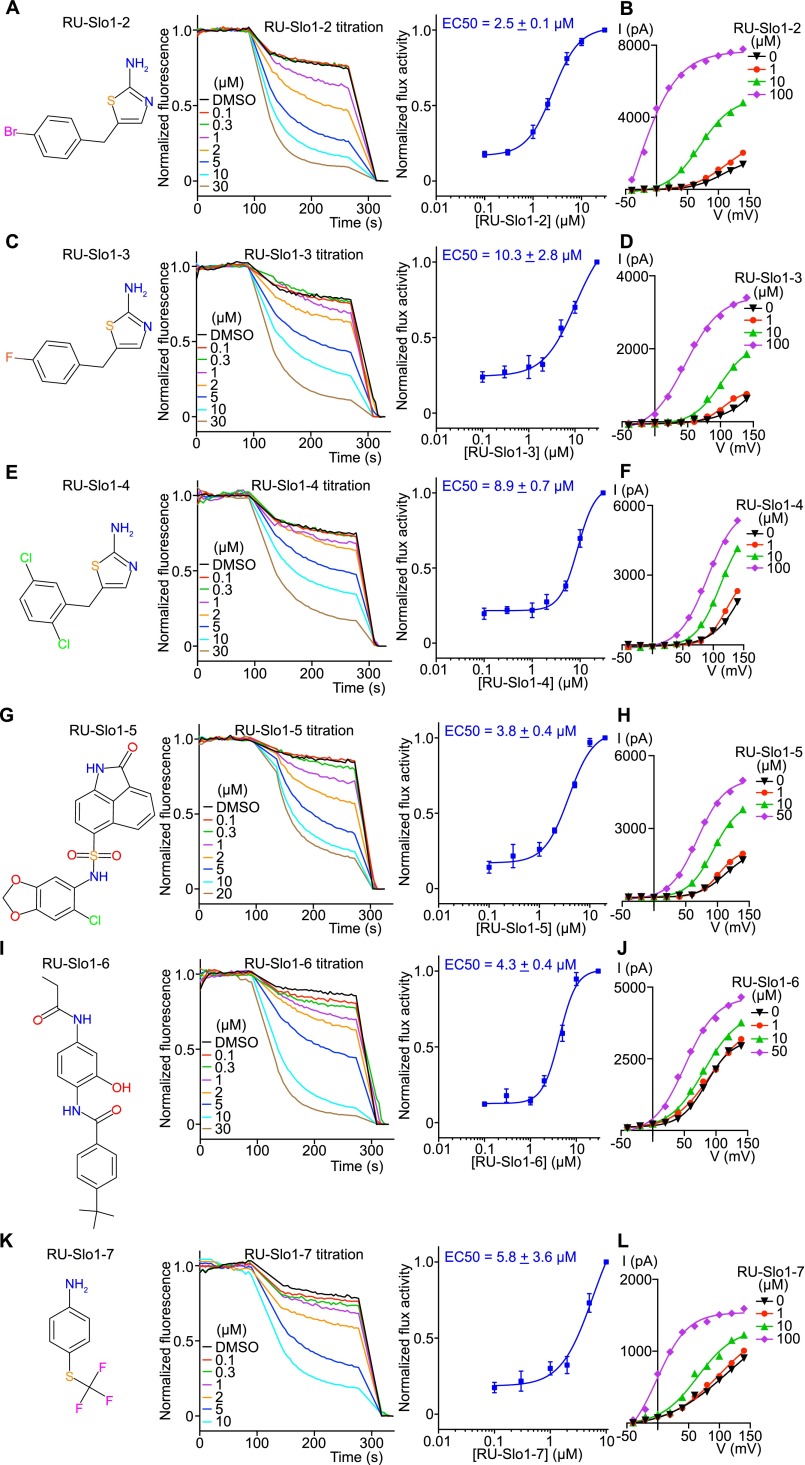
High conductance Ca2+-activated K+ channel, here referred to as Slo1, regulates smooth muscle tone and several other cellular processes related to feedback inhibition by intracellular Ca2+. Human mutations that cause asthma and mouse genetic and pharmacological studies suggest that Slo1 activators might provide benefit in the treatment of asthma, hypertension and over-reactive bladder (9–15). We thus applied LFA to the Slo1 channel to identify molecules that would open the channel. A control experiment shows that the natural intracellular ligand, Ca2+, opens the channel and induces K+ flux in LFA (Fig. 4A). A channel opener (i.e., activator) screen was run at subthreshold Ca2+ concentrations (1.0 mM EDTA); 300,000 compounds were assayed and seven activators belonging to five distinct chemical categories were identified (Fig. 4B and Fig. S5 A, C, E, G, I, and K). One of these—RU-Slo1-2—was previously identified as a Slo1 activator (29). Each of these compounds were further examined in an electrophysiology assay for their ability to activate Slo1 at very low Ca2+ concentrations (Fig. 4 C and D and Fig. S5 B, D, F, H, J, and L). Because Slo1 is also a voltage-dependent K+ channel, it can open at low Ca2+ concentrations as long as that the membrane is sufficiently depolarized (i.e., voltage on the inside is made very positive relative to outside; Fig. 4C). We found that all activators promote channel opening at less depolarizing voltages (Fig. 4 C and D and Fig. S5 B, D, F, H, J, and L). Ca2+ is known to have precisely this effect (30). Therefore, the activators appear to mimic the effect of Ca2+ on the Slo1 channel. Such compounds have the potential to induce smooth muscle relaxation by opening Slo1 channels and hyperpolarizing the membrane.
Fig. 4.
Characterization of a human Slo1 activator. (A) Normalized efflux mediated by Slo1 in response to Ca2+; 1 mM EDTA used for Slo1 small-molecule activator titrations was omitted in the Ca2+ titration experiments. (B) (Left) RU-Slo1-1 chemical structure. (Center) Normalized RU-Slo1-1 titration using LFA. (Right) Dose–response of RU-Slo1-1 activation (n = 15); 1 mM EDTA was present in RU-Slo1-1 titration. (C) A representative inside-out recording of Slo1 expressed in CHO cells at different concentrations of RU-Slo1-1. Channels were held at −40 mV, stepped from −40 to 140 mV in 20-mV increments, and returned to −40 mV. RU-Slo1-1 activated Slo1 by reducing the channel deactivation rate (n = 3). (D) A representative current-voltage plot of Slo1 tail current fitted with a Boltzmann equation (n = 3). All data are mean ± SEM.
Fig. S5.
Characterization of other human BK activators. (A, C, E, G, I, and K) Chemical structures and normalized efflux curves in the presence of Slo1 activators (n = 9 for RU-Slo1-2, n = 6 for RU-Slo1-3, n = 6 for RU-Slo1-4, n = 3 for RU-Slo1-5, n = 6 for RU-Slo1-6, n = 5 for RU-Slo1-7). (B, D, F, H, J, and L) Current-voltage plots from whole-cell patch recordings of Slo1 activators (n = 3 each). All activators induced leftward shifts to different degrees, indicating activation of Slo1 at less depolarizing voltages. All data are mean ± SEM.
hERG.
The hERG channel is present in cardiac cells where it participates in repolarization of the action potential. When hERG function is impeded, a characteristic lengthening of the Q-T interval is detectable on the electrocardiogram of mammals. This phenomenon is associated in humans with a lethal arrhythmia called torsade de pointes. For a reason that is not well understood, the hERG channel is highly susceptible to block by many structurally diverse small molecules. Our ability to predict which small molecules will block hERG is poor and therefore all compounds under consideration for drug development are tested in a hERG activity assay. Several assays exist: cell-based functional assays, such as thallium and/or rubidium flux, nonfunctional assays, such as ligand binding and/or fluorescence polarization, and electrophysiology (22, 23, 31, 32). The gold standard is electrophysiology, but it can be slow and expensive when the need exists for evaluating many compounds.
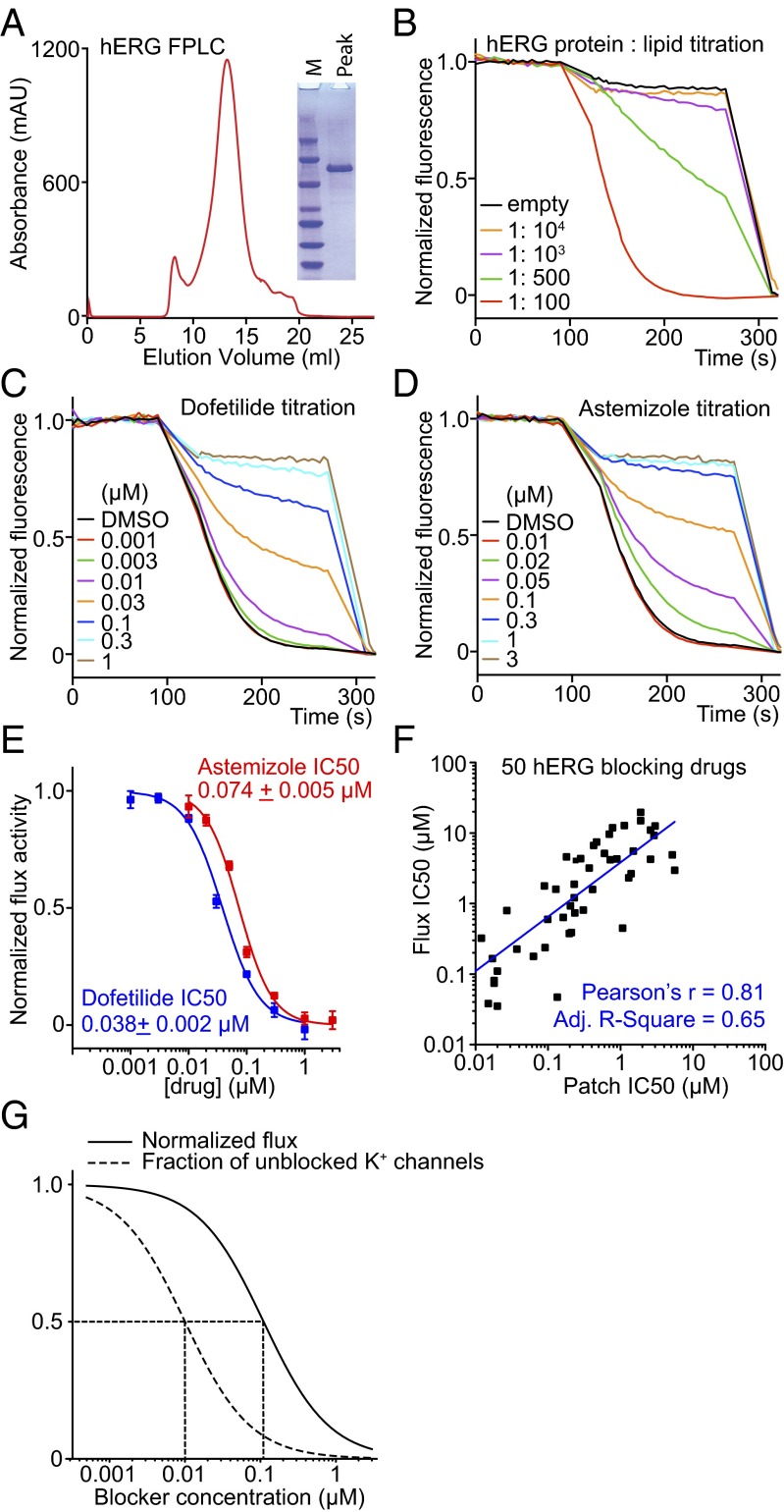
We applied LFA to the hERG channel. We first had to solve how to produce sufficient quantities of a stable version of this human K+ channel because the native, full-length channel aggregates when purified. Through trial and error we discovered that if we modified the amino acid sequence by deleting two different intracellular loops we could purify a biochemically well-behaved hERG channel with good function and correct pharmacology (Fig. 5). The behavior in LFA of two well-known hERG channel blockers, dofetilide and astemizole, is shown (Fig. 5 C–E). These drugs inhibit flux as predicted on the basis of electrophysiology assays (31, 33).
Fig. 5.
Development and validation of a new hERG assay. (A) A hERG mutant channel with internal deletions of unstructured cytoplasmic loops (residues 141–380 and 871–1005) was engineered. This mutant channel protein ran as a monodisperse FPLC peak on gel filtration and as a single band on SDS/PAGE. (B) Protein-to-lipid ratio titration of hERG in LFA showing that 1:100 provided a good signal for drug safety testing. (C and D) Normalized titration of dofetilide and astemizole, two well-characterized hERG blockers. The LFA IC50 values correlate well with electrophysiological recordings, although are around 10-fold higher (Methods). (E) Dose–response curves derived from LFA (n = 3 each). (F) LFA IC50 values were plotted against patch recording IC50 values reported in the literature (the lowest affinity IC50 values were used if a range of IC50 values were reported by patch clamp). No false negatives were found. The plot was fitted with linear regression in OriginPro. All data are mean ± SEM. (G) Simulation of LFA recorded normalized flux (solid curve) and fraction of unblocked K+ channels (dashed curve) as a function of blocker concentration (Methods).
To test the sensitivity of an LFA-based hERG safety assay, we examined 50 known inhibitors at a concentration of 10−5 M and obtained zero false negatives (Fig. S6 and Table S1). A complete dose–response relation was determined for all 50 hERG channel blockers (Fig. S6 and Table S1). A plot showing IC50 values determined using LFA against IC50 values using electrophysiology reported in the literature shows that LFA correlates well with electrophysiology-determined values (Fig. 5F). We note that the IC50 determined by LFA for hERG blockers is, on average, approximately 10 times higher (i.e., lower affinity) than the IC50 determined by electrophysiology. The origin of this offset is due mainly to the following property of the LFA assay: K+ efflux is coupled to H+ influx and for some channels such as hERG the latter is rate limiting until K+ channels are mostly inhibited. Therefore, IC50 in LFA corresponds to a percentage of channel inhibition much higher than 50%, which leads to the IC50 offset observed (Fig. 5 F and G. Also see Methods for a detailed explanation). Thus, we consider LFA a semiquantitative high throughput assay for rapid identification of hERG blockers. Once an inhibitor is identified, the IC50 can then be confirmed using an electrophysiology assay.
Fig. S6.
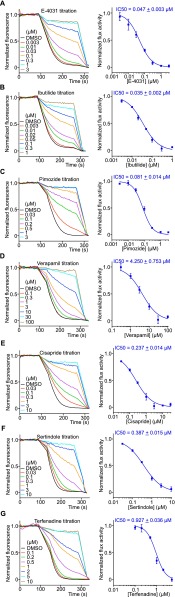
Characterization of hERG positive control drugs. (A–D) Efflux curves and dose–response curves of well-characterized hERG blockers, E-4031, ibutilide, pimozide, and verapamil (n = 3 each). (E–G) Efflux curves and dose–response curves using drugs that were withdrawn from the market because of risk of serious cardiac arrhythmias and increased risk of sudden death. These drugs have been shown to inhibit hERG activities from electrophysiological recordings. The LFA IC50 values correlate well to electrophysiological recordings with an approximate 10-fold IC50 offset. All data are mean ± SEM.
Table S1.
IC50 values of 50 hERG positive control drugs determined using LFA and compared with electrophysiology-determined values reported in the literature (n = 3 each)
| hERG positive controls | Target | IC50 patch (μM) | IC50 flux (μM) |
| Amiodarone | Class III antiarrhythmic | 0.015–1.071 | 0.447 ± 0.029 |
| Amitriptyline | Tricyclic antidepressant | 1.7–3.0 | 12.531 ± 1.320 |
| Amsacrine | Antineoplastic | 0.209–0.230 | 1.208 ± 0.036 |
| Aprindine | Class 1b antiarrhythmic | 0.23 | 1.881 ± 0.221 |
| Astemizole | Antihistamine | 0.001–0.018 | 0.074 ± 0.005 |
| Azimilide | Class ΙΙΙ antiarrhythmic | 0.891 | 4.275 ± 1.027 |
| Bepridil | Calcium channel blocker | 0.023–0.099 | 0.595 ± 0.066 |
| Chlorpromazine | Dopamine antagonist | 0.37 | 3.179 ± 0.297 |
| Cisapride | Gastroprokinetic agent | 0.007–0.091 | 0.237 ± 0.014 |
| Clemastine | Antihistamine | 0.012 | 0.323 ± 0.018 |
| Clomiphene | Estrogen receptor modulator | 0.18 | 4.582 ± 0.598 |
| Cloperastine | Cough suppressant | 0.027 | 0.796 ± 0.119 |
| Clotrimazole | Antifungal | 1.13 | 12.774 ± 0.304 |
| Cyamemazine | Antipsychotic | 0.468 | 7.451 ± 0.308 |
| Dofetilide | Class III antiarrhythmic | 0.003–0.015 | 0.038 ± 0.002 |
| Domperidone | Anti-dopamine | 0.162 | 0.635 ± 0.069 |
| Doxazosin | Alpha blocker | 0.6 | 5.128 ± 0.797 |
| Droperidol | Antidopaminergic | 0.100–0.307 | 0.806 ± 0.064 |
| E-4031 | Class III antiarrhythmic | 0.008–0.134 | 0.047 ± 0.003 |
| Eliprodil | NMDA antagonist | 0.02 | 0.110 ± 0.009 |
| Escitalopram | Serotonin reuptake inhibitor | 2.6 | 11.005 ± 0.959 |
| Fluoxetine | Antidepressant | 0.500–0.720 | 4.179 ± 0.237 |
| Fluspirilene | Antipsychotic | 0.003 | 0.164 ± 0.010 |
| GBR-12909 | Dopamine reuptake | 0.007 | 0.233 ± 0.022 |
| Halofantrine | Antimalaria | 0.022–0.197 | 0.376 ± 0.018 |
| Haloperidol | Antipsychotic | 0.015–0.063 | 0.178 ± 0.014 |
| Ibutilide | Class III antiarrhythmic | 0.02 | 0.035 ± 0.002 |
| Ifenprodil | NMDA antagonist | 0.41 | 1.589 ± 0.071 |
| Imipramine | Tricyclic antidepressant | 1.9 | 14.934 ± 1.618 |
| KB-R7943 | Na/Ca exchanger inhibitor | 0.089 | 1.775 ± 0.140 |
| Ketanserin | Antihypertensive | 0.121–0.128 | 1.590 ± 0.247 |
| Ketoconazole | Antifungal | 1.9 | 19.653 ± 2.394 |
| Lidoflazine | Calcium channel blocker | 0.017–0.037 | 0.225 ± 0.021 |
| Maprotiline | Tetracyclic antidepressants | 3.1–5.2 | 4.882 ± 0.314 |
| Mefloquine | Antimalaria | 2.6–5.6 | 2.969 ± 0.421 |
| Mesoridazine | Piperidine neuroleptic | 0.426 | 6.647 ± 1.393 |
| Mibefradil | Ca channel blocker | 1.4 | 2.650 ± 0.141 |
| Pimozide | Antipsychotic | 0.001–0.018 | 0.081 ± 0.014 |
| Quinidine | Class I antiarrhythmic | 0.320–1.5 | 5.520 ± 1.254 |
| Risperidone | Antipsychotic | 0.282 | 4.317 ± 0.447 |
| Sertindole | Antipsychotic | 0.003–0.210 | 0.387 ± 0.015 |
| Tamoxifen | Estrogen receptor | 0.777 | 11.874 ± 0.678 |
| Terfenadine | Antihistamine | 0.007–0.204 | 0.927 ± 0.036 |
| Terodiline | Antispasmodic | 0.375–0.700 | 9.640 ± 1.592 |
| Thioridazine | Antipsychotic | 0.116–1.3 | 2.311 ± 0.160 |
| Tolterodine | Antimuscarinic | 0.017 | 0.166 ± 0.008 |
| Trazadone | Antidepressant | 0.690–2.9 | 9.248 ± 0.529 |
| Trifluoperazine | Antipsychotic | 0.234 | 0.734 ± 0.078 |
| Verapamil | Calcium channel blocker | 0.143–2.6 | 4.250 ± 0.753 |
| Ziprasidone | Antipsychotic | 0.120–0.240 | 4.061 ± 0.475 |
Measured IC50 values were plotted against the upper limit of IC50 values in electrophysiology in Fig. 5F. No false-negative drugs were found. All data are mean ± SEM.
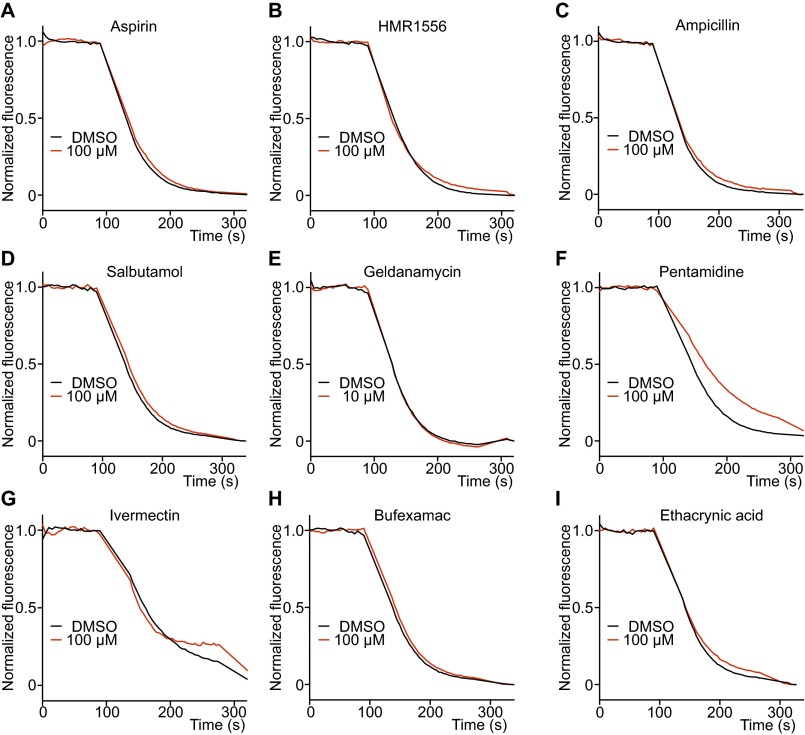
To test the assay’s specificity we examined 50 known noninhibitors (34) at the same concentration and obtained a zero false-positive rate (Fig. S7 and Table S2). On repeat at 10 times higher concentration (approaching the solubility of many drugs), less than 25% inhibition was observed compared with a DMSO control (Table S2).
Fig. S7.
Validation of hERG negative control drugs. (A–D) Four widely used drugs, aspirin, HMR1556, ampicillin, and salbutamol, that are known not to inhibit hERG, did not inhibit hERG-mediated efflux in LFA at a concentration of 100 μM (n = 3 each). (E–I) Five drugs that are reported to inhibit hERG trafficking to plasma membrane were negative in the hERG efflux assay at 10 μM (Geldanamycin) and 100 μM (others) (n = 3 each).
Table S2.
Percent blockage of 50 hERG negative drugs determined by LFA
| hERG negative controls | Target | Percentage inhibition (%) at 100 μM |
| Acetaminophen | Pain medication | 11.6 ± 7.5 |
| Acetazolamide | Carbonic anhydrase inhibitor | 4.4 ± 1.8 |
| Acrivastine | Antihistamine | 16.2 ± 5.7 |
| Amiloride | Enac channel blocker | 15.4 ± 1.0 |
| Amoxillin | Antibiotic | −2.6 ± 9.1 |
| Ampicillin | Antibiotic | 5.6 ± 4.7 |
| Arterenol | Hormonre and neurotransmitter | 3.7 ± 4.3 |
| Aspirin | Pain | −1.6 ± 2.4 |
| Bufexamac | Anti-inflammatory | 1.7 ± 6.8 |
| Captopril | ACE inhibitor | 1.8 ± 2.9 |
| Carbachol | Acetylcholine receptor agonist | 2.2 ± 4.2 |
| Cetirizine | Antihistamine | 11.8 ± 4.3 |
| Cimetidine | Antihistamine | 19.9 ± 3.7 |
| Clindamycin | Antibiotic | 9.2 ± 4.3 |
| Clonidine | Alpha2 agonist | 15.2 ± 5.8 |
| Clozapine N-oxide | DREADD agonist | 2.1 ± 3.0 |
| Doxycycline | Antibiotic | 1.6 ± 8.7 |
| Enalapril | ACE inhibitor | −0.3 ± 6.8 |
| Ethacrynic acid | Loop diuretics | 8.1 ± 4.4 |
| Famotidine | Antihistamine | −11.1 ± 15.8 |
| Furosemide | Hypertension and edema | −3.7 ± 16.1 |
| Geldanamycin | Antitumor antibiotic | −1.5 ± 2.3** (10 μM) |
| Glyburide | Antidiabetic | 4.0 ± 12.0 |
| Guaifenesin | Expectorant | −3.5 ± 7.4 |
| HMR-1556 | KCNQ1 blocker | 6.5 ± 6.9 |
| Ibuprofen | Anti-inflammatory | 13.1 ± 2.1 |
| Indapamide | Diuretic | 4.8 ± 6.7 |
| Ivermectin | Antiparasitic | −21 ± 2.9 |
| Kynurenic acid | Antiexcitotoxic | −8.3 ± 4.8 |
| Lidocaine | Nav blocker | 13.0 ± 2.8 |
| Midodrine | Vasopressor | 8.2 ± 4.8 |
| Minocycline | Antibiotic | −2.5 ± 11.9 |
| N-acetylprocainamide | Class III antiarrhythmic | 14.6 ± 4.2 |
| Naproxen | Cyclooxygenase inhibitor | 3.8 ± 9.9 |
| Oxypeucedanin | Anti-tumor | 4.5 ± 19.3 |
| Penicillin | Antibiotic | −0.4 ± 9.5 |
| Pentamidine | Antimicrobial | 22.8 ± 1.4 |
| Phenylephrine | α1-adrenergic receptor agonist | 0.7 ± 5.9 |
| Picrotoxin | GABAA channel blocker | 3.3 ± 4.0 |
| Pyridoxine | Vitamin B6 | −5.0 ± 3.7 |
| Ranitidine | Antihistamine | 14.9 ± 2.9 |
| Resveratrol | Natural phenol | 21.1 ± 8.6 |
| Salbutamol | Beta2 agonist | −6.6 ± 3.3 |
| Spiramycin | Antibiotic | −5.8 ± 10.7 |
| Sulfamethoxazole | Antibiotic | 11.5 ± 4.3 |
| Sulindac | Anti-inflammatory | 1.1 ± 2.8 |
| Thalidomide | Immunomodulatory | 9.5 ± 8.1 |
| Trimethoprim | Antibiotic | 20.6 ± 1.9 |
| Warfarin | Anticoagulant | 9.1 ± 5.5 |
| Wortmannin | PI3K inhibitor | 1.7 ± 5.8 |
Drugs were tested at a concentration of 100 μM except for geldanamycin (**), which was tested at 10 μM due to its auto fluorescence (n = 3 each). No drugs induced more than 25% difference compared with DMSO controls at this high concentration and were all considered negative. No false-positives were found. All data are mean ± SEM.
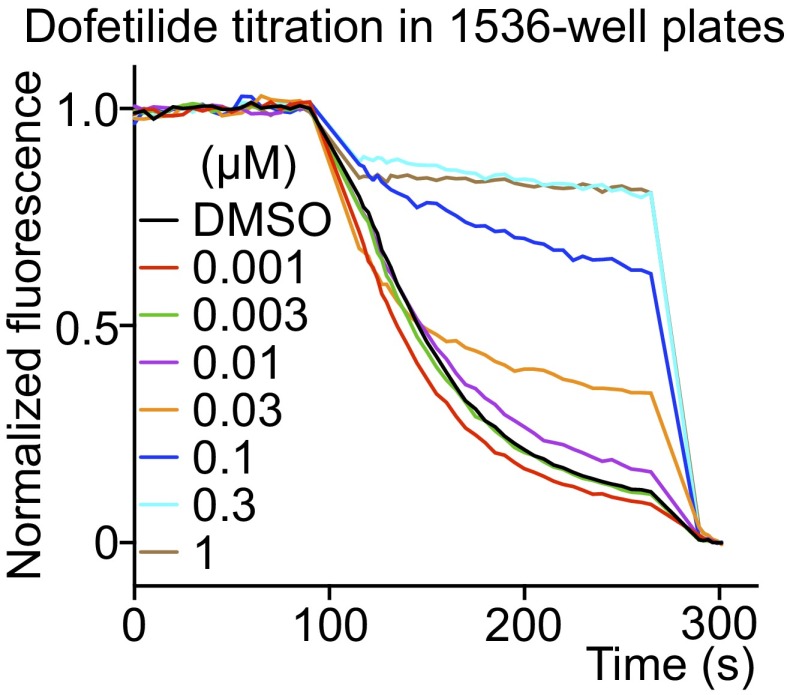
The data shown in this study were carried out using 384-well plates; however, an example using a 1,536-well plate with a similarly high signal demonstrates the ease with which the assay can be scaled (Fig. S8).
Fig. S8.
hERG-mediated efflux in 1,536-well plates. Dofetilide was used as a test positive drug. The flux reaction volume was reduced to a quarter of that used in a 384-well plate maintaining all component ratios. The kinetics and inhibitory activity of dofetilide in the 1536-well plate was similar to values using the 384 wells.
Discussion
The K+ channel family comprises a structurally and functionally diverse class of ion channels. All members share a similar pore but their gating mechanisms vary enormously. That is presumably why we observe close to 80 K+ channels encoded in the human genome, to allow regulation of K+ permeability by a myriad of intracellular ligands, lipids, membrane voltage, and membrane stretch. We still do not understand the biological roles played by many of these channels, mainly because we have not yet developed a sufficient set of pharmacological tools. Gene knockout technology has advanced our understanding to some degree, but such approaches are not good surrogates for acute pharmacology experiments because compensatory expression of a second channel that can fulfill the role of the missing first is all too common, especially given the large number and redundancy of K+ channels (35, 36).
This paper presents an assay called LFA, to identify inhibitors and activators of K+ channels. LFA is made possible by methods that now permit the expression and purification of almost any mammalian K+ channel. We developed this assay to identify pharmacological agents to study the biological roles of many still poorly understood K+ channels. To our knowledge, we identified the first inhibitors of TRAAK and new activators of Slo1. We also discovered that the antiparasitic agent ivermectin activates GIRK2 in an apparently G protein-independent manner. This action could potentially underlie a major side effect of this drug, to induce CNS depression.
These data also suggest that LFA may also be useful for drug development purposes. Fluorescence baseline and initial slope measurements, which are used to quantify flux activity, can be made in parallel on a plate reader in a 2-min interval. Therefore, a 1,536-well plate reader can potentially record about one million measurements per day. Although our laboratory typically runs the assay in a 384-well format, we demonstrated the strong signal measured using 1,536-well plates (Fig. S8) and therefore the assay is easily scalable. Human genetic data point to K+ channels as being likely targets for new therapies for asthma (9, 11, 13), hypertension (15, 37), and perhaps even anticancer therapy (38). LFA can allow rapid screening for identifying new compounds that target K+ channels.
A hERG safety assay based on LFA seems to provide data similar in quality to an electrophysiology assay but at an incomparably higher rate and a tiny fraction of the cost. LFA could easily quantify hERG activity in the vast number of derivative compounds that can be generated through combinatorial synthesis. Such capability would help in efforts to chemically remove hERG activity and realize the potential benefit of a drug.
Methods
Detailed materials and methods are presented in SI Methods.
Protein Purifications.
Mouse GIRK2 was expressed and purified from Pichia pastori yeast cells. Human Gβγ complex was expressed and purified from High Five insect cells. Mouse TRAAK was expressed and purified from P. pastori yeast cells. Human Slo1 was expressed and purified from Sf9 (Spodoptera frugiperda) insect cells. hERG was expressed and purified from HEK293S GnTI− mammalian cells.
Proteoliposome Reconstitution.
Purified proteins were reconstituted into POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine) and POPG [1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol)] vesicles [with a ratio of 3:1 (wt:wt)] by a dialysis method.
High-Throughput LFA Screens.
A 300,000 compound library was constructed from Enamine, Chembridge, and Rockefeller chemical library sources. The primary screen was carried out with a FDSS6000 (Hamamatsu) plate reader with automatic pipetting in 384-well format. The FDSS6000 pipetted and mixed 12 µL compound solution, 6 µL ACMA solution, and 6 µL vesicle solution into an assay plate, and the baseline fluorescence was recorded. Then, 6 µL CCCP was added and mixed to initiate the K+ flux, and the fluorescent signal was monitored every 5 s for 55 cycles. The final concentrations for the components in the LFA reaction were 1 µM compound, 1.3 µM ACMA, and 3.2 µM CCCP.
Hit Confirmation Using LFA.
Once the primary screens were finished and primary hits were selected using offline data analysis, the hits were confirmed using a fluorescent plate reader (Tecan, Infinite M1000 with excitation wavelength 410 nm and emission wavelength 490 nm) with manual pipetting following the same protocol as the primary screen in 384-well plates. Confirmed hits were purchased freshly from the same vendors that had supplied the primary screening compounds and drug titrations were conducted. The flux data were normalized, plotted, and fitted as detailed in SI Methods.
Animals.
All animal protocols were approved by the IACUC (Institutional Animal Care and Use Committee) of The Rockefeller University. Newborn C57BL/6J mice (The Jackson Laboratory) were killed for the dissection of hippocampal neurons.
Hippocampal Neuron Cultures.
Hippocampus tissues were dissected from P0 neonatal mice in cold Hank's balanced salt solution without Ca2+ and Mg2+. Dissected tissues were digested by trypsin, gently triturated, and plated on poly-d-lysine– and laminin-coated coverslips. Neurons matured after 1 wk after plating and were patched between 1 and 2 wk.
Plasmid Transfection.
HEK293 or CHO cells were cultured to 80% confluency and transfected with Lipofectamine 2000. Cells were treated with trypsin and plated out on coverslips 12–24 h after transfection and patched 6–48 h after plating.
Electrophysiology.
Different channel constructs were expressed in HEK293 or CHO cells and patched using standard whole-cell or excised configuration.
Data Analysis.
All data are presented as mean ± SEM.
SI Methods
Constructs.
DNA fragments encoding different constructs were amplified by PCR using Phusion DNA polymerase (New England Biolabs) and cloned into expression vectors modified by the MacKinnon laboratory for yeast, insect, and mammalian cells. All constructs were confirmed by sequencing (Genewiz).
Constructs for Protein Purification.
Mouse GIRK2 (mGIRK2) truncation mutant (residues 52–380) was cloned into RML1 vector and was expressed in Pichia pastoris strain SMD1163 (Invitrogen). RML1 vector is modified from pPICZ (Invitrogen) by adding a PreScission protease recognition sequence (LEVLFQ/GP) followed by an EGFP sequence and then a 1D4 tag sequence (TETSQVAPA) to the C terminus of cloned genes.
Human Gβ1 and Gγ2 were individually cloned into pFastbac vector (Invitrogen), and the Gβγ complex was expressed in High Five insect cells (Invitrogen) by virus coinfection.
Mouse TRAAK (mTRAAK) truncation mutant (residues 1–275 with N81Q and N84Q glycosylation mutations) was fused to an N-terminal 27 residue peptide from human TRAAK to increase expression. Chimeric mouse TRAAK construct was cloned into RML2 vector and was expressed in Pichia cells. RML2 vector is modified from pPICZ by adding a PreScission protease recognition sequence followed by an EGFP sequence and then a His10 tag sequence to the C terminus of cloned genes.
Human Slo1 (hSlo1) was cloned into RML11 vector and was expressed in Sf9 (Spodoptera frugiperda) cells (ATCC). RML11 vector is modified from pFastBac vector by adding a PreScission protease recognition sequence followed by an EGFP sequence and then a 1D4 tag sequence to the C terminus of cloned genes.
hERG truncation mutant with internal deletions of unstructured cytoplasmic loops (residues 141–380 and 871–1005) was cloned into RML13 BacMam vector and was expressed in HEK293S GnTI− cells (ATCC). RML13 vector is modified from pEG (39) (a generous gift from Eric Gouaux, The Vollum Institute, Portland, OR), by adding a PreScission protease recognition sequence followed by an EGFP sequence and then a 1D4 tag sequence to the C terminus of cloned genes.
Three of the above constructs (for GIRK2, TRAAK, and hERG) represent channels that have been modified from their full-length form to gain biochemical stability. Although it is possible that disordered loops could form small molecule binding sites, the function of GIRK2 (response to Na+, PIP2, and Gβγ, as well as to toxin inhibitors) and TRAAK (mechanical sensitivity) are indistinguishable from the full-length channels. For the case of the hERG channel, the N-terminal truncation causes increased rates of activation and deactivation. However, the correlation between drug sensitivity between published electrophysiological assays using the full-length channel and the LFA assay using the truncated channel lead us to believe that the truncated channel reports drug sensitivity with good fidelity.
Constructs for Electrophysiology.
mGIRK2 truncation mutant (residues 1–414, for reference the full-length protein is 1–425) was cloned into RML6 vector and was expressed in HEK293 cells (ATCC). RML6 vector is a mammalian cell expression vector adding a thrombin protease recognition sequence (LVPRGS) followed by an EGFP sequence and then a His8 tag sequence to the C terminus of cloned genes.
Rat Kv1.2 was cloned into RML5 vector and was expressed in CHO (ATCC) cells. RML5 vector is a mammalian cell expression vector adding a His8 tag sequence followed by an EGFP sequence then a thrombin protease recognition sequence to the N terminus of cloned genes.
hSlo1 was cloned into RML6 vector and was expressed in CHO cells.
mTRAAK chimeric construct used in protein purification was cloned into RML6 vector and was expressed in CHO cells.
Human ROMK truncation mutant (residues 35–367), mouse Kir2.1 truncation mutant (residues 41–368), and human Kir7.1 truncation mutant (residues 13–360) were cloned into RML6 vector and were expressed in HEK293 cells.
Mouse THIK1, mouse TRESK, rat TWIK2, mouse TALK1, and mouse TASK3 genes were cloned into RML6 vector and were expressed in CHO cells.
Protein Purification.
mGIRK2 purification.
The cloned 1D4-tagged mGIRK2 construct was linearized with Pme1 and transformed into Pichia cells by electroporation (Bio-Rad). Pichia cells were cultured in yeast extract peptone and dextrose (YPD) medium [1% yeast extract, 2% (wt/vol) peptone, 2% (wt/vol) dextrose] in shakers (Infors HT; Multitron) at 220 rpm and 30 °C. Transformants were selected by plating on standard YPD plate with 1 M sorbitol with 1 mg/mL zeocin (Invitrogen). Small-scale expression screens of individual clones were assessed by fluorescence-detection size-exclusion chromatography (40) (FSEC; Shimadzu CBM-20A). Before induction, cells were cultured in a glycerol-based medium (1% NH4SO4, 0.34% yeast nitrogen base, 100 mM K-phoshate buffer, pH 6.0, 1.6 μM biotin, 1% glycerol) at 220 rpm and 30 °C for 24 h. Induction was initiated by switching to a methanol-based medium (1% NH4SO4, 0.34% yeast nitrogen base, 100 mM K-phosphate buffer, pH 6.0, 1.6 μM biotin, 0.5% methanol) at 27 °C and continued for 24 h. The clone with the highest mGIRK2 expression observed by FSEC was chosen for large-scale expression, performed in a fermenter (Infors HT Labfors). Overnight starter cultures of cells grown in the glycerol-based medium with 1 mg/mL zeocin were added to 3 L minimal media (containing 151.2 g glycerol, 2.79 g CaSO4, 54.6 g K2SO4, 21.83 g MgSO4) to an OD600 ∼1 and grown overnight at 29 °C with glycerol feeding and pH maintained at 5.0 by addition of NH4OH. Cells were then starved to deplete glycerol and temperature was reduced to 27 °C. Induction was initiated with slow addition of methanol and continued for ∼24 h. Cells were pelleted, frozen in liquid nitrogen, and stored at −80 °C.
Frozen Pichia cells expressing mGIRK2 protein were disrupted by milling (Retsch; model MM301) for five cycles of 3 min at 25 Hz. Cells were kept frozen between cycles by cooling in liquid nitrogen. All subsequent steps were performed at 4 °C. Cell powder was added to lysis buffer [50 mM Hepes-KOH, pH 8.0, 150 mM KCl, 1 mM EDTA, 0.1 mg/mL DNase, a protease inhibitor mixture containing 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin, 100 μg/mL soy trypsin inhibitor, 1 mM benzamidine, 100 μg/mL 4-(2-aminoethyl) benzenesulfonyl fluoride and 1 mM phenylmethysulfonyl fluoride] at a ratio of 1 g cell pellet/4 mL lysis buffer. After 1-h lysis with stirring, the pH of the lysis buffer was readjusted to 8.0 using 1M KOH. Extraction was initiated by adding 60 mM n-Decyl-β-d-Maltopyranoside (DM, Anatrace) and continued for 1 h with stirring. Extracts were collected by centrifugation at 35,000 × g (Beckman, Miami, Avanti J-26XP centrifuge, JA-17 rotor) for 30 min. Washed 1D4 resin was added to the supernatant (1 mL resin/8 g cell) and binding continued for 1 h with rotation. After binding, resin was collected at 245 × g (Beckman, Miami, Avanti J-26XP centrifuge, JS-5.3 rotor) for 5 min. Resin was loaded onto a column and washed with 20 column volumes (cv) of wash buffer (50 mM Hepes-KOH, pH 7.4, 150 mM KCl, 1 mM EDTA, 6 mM DM). PreScission protease [∼1:20 (wt:wt)] was subsequently added to washed resin and on-column cleavage continued for 1.5 h with gentle rotation. Cleaved protein was eluted in wash buffer, and 20 mM DTT was added. Protein was concentrated [100-kDa molecular weight cutoff (MWCO), Amicon Ultra Centrifugal Filter] and applied to a Superdex 200 column (GE Healthcare, 10/300 GL) equilibrated in size exclusion chromatography (SEC) buffer (20 mM Hepes-KOH, pH 7.4, 150 mM KCl, 1 mM EDTA, 20 mM DTT, 4 mM DM). Peak fractions containing mGIRK2 protein were pooled and concentrated (100 kDa MWCO) to 1 mg/mL for reconstitution.
Human Gβγ purification.
Individual baculoviruses expressing untagged human Gβ1 and N-terminal His-tagged (followed by a PreScission Protease cleavable linker) human Gγ2 were produced using the Bac-to-Bac system following manufacturer’s instruction (Invitrogen). High Five cells were cultured in Express Five medium (Life Technologies) supplemented with 18 mM l-glutamine and 1% penicillin/streptomycin in shakers (Infors HT, Multitron) at 120 rpm and 27 °C. Cells were coinfected with Gβ1 and Gγ2 viruses (with a ratio Gβ1: Gγ2 = 2:1) at a density of 2 × 106 cells/mL; 40 h after infection, cells were harvested by centrifugation, frozen with liquid nitrogen, and stored at −80 °C.
All steps were performed at 4 °C. Frozen High Five cells expressing Gβγ protein were lysed by sonication (Branson, Sonifier 450) for three cycles (maximum output, 15 s) on ice in lysis buffer (50 mM Hepes-NaOH, pH 8.0, 100 mM NaCl, 1 mM EDTA, 3 mM 2-mercaptoethanol, a protease inhibitor mixture containing 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin, 100 μg/mL soy trypsin inhibitor, 1 mM benzamidine). Cells were kept cold between cycles by cooling on ice. Cell lysates were centrifuged at 257,000 × g (Beckman, Miami, Optima XL-100K Ultracentrifuge, type 70 i rotor) for 40 min to collect membranes. Membrane pellets were resuspended in resuspension buffer (50 mM Hepes-NaOH, pH 8.0, 100 mM NaCl, 3 mM 2-mercaptoethanol, a protease inhibitor mixture the same as in lysis buffer) and homogenized in 40 mL Dounce Tissue Grinder (Wheaton). Extraction was initiated by adding 2% (wt/vol) Na-cholate (Sigma) and continued for 1 h with stirring. Extracts were collected by centrifugation at 35,000 × g for 30 min. Washed TALON resin (Clontech) was added to the supernatant (5 mL resin/1 L cell) and binding continued for 1 h with rotation. After binding, resin was collected at 245 × g for 5 min and washed briefly with wash buffer (50 mM Hepes-NaOH, pH 8.0, 100 mM NaCl, 3 mM 2-mercaptoethanol, 1% Na-cholate). Resin was loaded to a column and sequentially washed and eluted in wash buffer containing 0, 10, and 200 mM imidazole. PreScission protease [∼1:20 (wt:wt)] was subsequently added to eluted protein and cleavage continued for 1 h with gentle rotation. Cleaved protein was concentrated (10 kDa MWCO, Amicon Ultra Centrifugal Filter) and applied to a Superdex 200 column equilibrated in SEC buffer (20 mM Hepes-KOH, pH7.4, 150 mM KCl, 1 mM EDTA, 20 mM DTT, 4 mM DM). Peak fractions containing Gβγ complex were pooled and concentrated (100 kDa MWCO) to 30 mg/mL and frozen in liquid nitrogen at −80 °C for mGIRK2 reconstitution and LFA.
mTRAAK purification.
For mTRAAK purification, yeast cell transformation, clone selection, and protein expression all followed the protocol of mGIRK2 purification, except for that the methanol induction time was 48 h in the fermenter culture.
Frozen Pichia cells expressing mTRAAK protein were disrupted by milling the same as mGIRK2 purification. All subsequent steps were performed at 4 °C. Cell powder was added to lysis buffer [50 mM Tris, pH 8.0, 150 mM KCl, 0.1 mg/mL DNase, a protease inhibitor mixture the same as used in mGRIK2 purification, 60 mM n-dodecyl-β-d-maltopyranoside (DDM; Anatrace)] at a ratio of 1 g cell pellet/4 mL lysis buffer. Membranes were extracted by gentle stirring for 3 h and collected by centrifugation at 35,000 × g for 45 min. Washed TALON resin was added to the supernatant (1 mL resin/5 g cell pellet) and binding continued for 3 h with gentle stirring. Resin was loaded onto a column and sequentially washed and eluted in wash buffer (50 mM Tris, pH 8.0, 150 mM KCl, 6 mM DDM) containing 10, 30, and 300 mM imidazole. EDTA (1 mM final) and PreScission protease [1:50 (wt:wt)] were added to the elution, and cleavage continued overnight with gentle rotation. Cleaved protein was concentrated (50 kDa MWCO, Amicon Ultra Centrifugal Filter) and applied to a Superdex 200 column equilibrated in SEC buffer (20 mM Tris, pH 8.0, 150 mM KCl, 1 mM EDTA, 1 mM DDM). Peak fractions containing mTRAAK protein were pooled and concentrated (50 kDa MWCO) to 1 mg/mL for reconstitution.
hSLo1 purification.
hSlo1 virus production followed the Bac-to-Bac manufacturer’s instruction. Sf9 cells were cultured in Grace medium (Life Technologies) supplemented with 10% (vol/vol) FBS, 1% pluronic F-68, and 1% penicillin/streptomycin in shakers (Infors HT, Multitron) at 120 rpm (Infors HT, Multitron) and 27 °C. Cells were infected with hSlo1 virus at a density of 2 × 106 cells/mL 60 h after infection, harvested by centrifugation, frozen with liquid nitrogen, and stored at −80 °C.
All steps were performed at 4 °C. Frozen Sf9 cells expressing hSlo1 protein were lysed and extracted in lysis buffer (50 mM Hepes-KOH, pH 8.0, 150 mM KCl, 1 mM EDTA, a protease inhibitor mixture the same as used in GIRK2 purification, 60 mM DM) for 2 h with stirring. Extracts were collected by centrifugation at 257,000 × g for 40 min. Washed 1D4 resin was added to the supernatant (2 mL resin/1 L cell) and binding continued for 1 h with rotation. After binding, resin was collected at 245 × g for 5 min and washed briefly with wash buffer (50 mM Hepes-KOH, pH 7.4, 150 mM KCl, 1 mM EDTA, 6 mM DM). Resin was loaded to a column and further washed with 20 cv wash buffer. PreScission protease [∼1:30 (wt:wt)] was subsequently added to washed resin and on-column cleavage continued for 1.5 h with gentle rotation. Cleaved protein was eluted in wash buffer and 20 mM DTT was added. Protein was concentrated (100 kDa MWCO) and applied to a Superose 6 column (GE Healthcare; 10/300 GL) equilibrated in SEC buffer (20 mM Hepes-KOH, pH7.4, 150 mM KCl, 1 mM EDTA, 20 mM DTT, 4 mM DM). Peak fractions containing hSLO1 protein were pooled and concentrated (100 kDa MWCO) to 1 mg/mL for reconstitution.
hERG purification.
hERG virus production followed the BacMam virus protocol (39). HEK293S GnTI− cells were cultured in FreeStyle 293 Expression Medium (Life Technologies) supplemented with 2% (vol/vol) FBS and 1% penicillin/streptomycin in incubators (Thermo; model 3950) equipped with tabletop orbital shaker at 130 rpm (Thermo, MaxQ HP) and 37 °C with moisture and 8% CO2. Cells were infected with hERG virus at a density of 3 × 106 cells/mL 24 h after infection, and 10 mM sodium butyrate (Sigma) was added to induce protein expression; 36 h after induction, cells were harvested by centrifugation, frozen with liquid nitrogen, and stored at −80 °C.
All steps were performed at 4 °C. Frozen HEK293S GnTI− cells expressing hERG protein were lysed in hypo-osmotic lysis solution (10 mM Hepes-NaOH, pH 7.4, 30 mM KCl, 5 mM DTT, 20 μg/mL DNase, a protease inhibitor mixture the same as used in GIRK2 purification) by stirring to homogeny. Cell lysates were centrifuged at 35,000 × g for 45 min to collect membranes. Membrane pellets were resuspended in extraction buffer [20 mM Hepes-NaOH, pH 7.4, 300 mM KCl, 20 μg/mL DNase, a protease inhibitor mixture the same as used in GIRK2 purification, 1% DDM, 0.2% cholesteryl hemisuccinate (CHS; Anatrace)], and extraction continued for 1 h. Extracts were collected by centrifugation at 20,000 × g for 15 min. Washed GFP-nanobody resin [CNBr-activated Sepharose 4B (GE Healthcare) conjugated with a GFP-binding nanobody (41, 42)] was added to the supernatant (5 mL resin/1 L cell), and binding continued for 1 h with rotation. After binding, resin was collected at 245 × g for 5 min and washed briefly with wash buffer [20 mM Hepes-NaOH, pH 7.4, 300 mM KCl, 0.1% DDM, 0.02% CHS, 0.1 mg/mL lipids [POPE: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC): 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (POPA) = 5:5:1]. Resin was loaded onto a column and further washed with 20 cv wash buffer. PreScission protease [∼1:40 (wt:wt)] was added to washed resin and on-column cleavage continued for 1.5 h with gentle rotation. Cleaved protein was eluted in wash buffer, concentrated (100 kDa MWCO), and applied to a Superose 6 column equilibrated in SEC buffer [20 mM Hepes-NaOH, pH7.4, 300 mM KCl, 0.1% DDM, 0.02% CHS, 0.1 mg/mL lipids (POPE:POPC:POPA = 5:5:1), 0.5 mM Tris(2-carboxyethyl)phosphine (TCEP), 10 mM DTT]. Peak fractions containing hERG protein were pooled for reconstitution.
Proteoliposome Reconstitution.
Lipids in organic solvent were handled with glass pipettes and vials; 10 mg/mL POPE and POPG [1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol)] from Avanti in chloroform were mixed [with a ratio of 3:1 (wt:wt)] and aliquoted in 4 mL per glass vial (Kimble Chase; 16 × 125 mm); 1% brain PIP2 lipid (Avanti) was added for the mGIRK2 inhibitor screen and the mGIRK2 PIP2-based activator screen. Lipid aliquots were air-dried under argon, washed with pentane, and dried again under argon in a laboratory chemical hood. Lipids were protected from light by wrapping with aluminum foil and protected from oxidation by topping with argon in all subsequent steps. Lipids were further dried overnight in a vacuum chamber with loose caps on the vials. Each dried aliquot was rehydrated in 2 mL reconstitution buffer (20 mM Hepes-KOH, pH7.4, 1 mM EDTA, 150 mM KCl, except for hERG 300 mM KCl were used). Lipid solutions were sonicated until translucent in a bath sonicator (Laboratory Supplies; model G112SP1T) with multiple cycles of 1 min on and 1 min off to prevent overheating. Sonicated lipid solutions were mixed with an equal volume of solubilization buffer (reconstitution buffer with 20 mM DM (for mGIRK2, hERG) or 160 mM n-octyl-β-d-maltopyranoside [OM; Anatrace; for the other proteins) and 20 mM DTT (no DTT for mTRAAK)] and incubated for 30 min by rotating. Solubilized lipid-detergent solution was mixed with purified protein at desired ratios and incubated for 1 h at room temperature by rotation. After incubation, protein-lipid-detergent solution was immediately transferred to 50 kDa MWCO dialysis bags (Spectrum Labs) in cold reconstitution buffer with fresh 3 mM DTT (no DTT for mTRAAK) and dialyzed at 4 °C (4 L buffer/20 mL protein-lipid-detergent solution). Reconstitution buffer was changed every 12 h for six cycles. After visible vesicle formation, bags were massaged manually each day to break up large lipid aggregates. Prewetted Bio-Beads SM2 (Bio-Rad, first wetted in methanol, subsequently washed five times with ddH2O and one time with reconstitution buffer) were added to reconstitution buffer (5 mL/4 L buffer) for the last three cycles to remove residual detergents; 50-μL aliquots of the proteoliposomes were flash-frozen in liquid nitrogen and stored at −80 °C.
High-Throughput LFA Screens.
Compound library collections.
Three hundred thousand compounds were collected for screening, of which 100,000 were from Enamine (a preplated diverse subset of their library), 100,000 were from ChemBridge (DIVERSet-EXP and DIVERSet-CL), and 100,000 were from the library available at The Rockefeller University High-Throughput and Spectroscopy Resource Center (RU-HTSRC). The compounds were selected to maximize diversity. These compounds were stored frozen in DMSO at −20 °C in 384-well stock plates at the concentration of 5–10 mM in aliquots. On the day of screening, drug DMSO plates were thawed and small volume of drugs were pipetted out and diluted into screening drug plates filled with drug buffer by robot. The same screening drug plate served multiple K+ channel assays, which reduces the cost of the screens.
The primary screen.
The primary screen was carried out with a FDSS6000 (Hamamatsu) plate reader with automatic pipetting in 384-well format. Before screening, flat-bottomed 384-well plates (Greiner Bio-One; cat. 781076) were filled with stock solutions of different components including drugs, ACMA, and CCCP. Vesicles were added to v-bottomed 384-well plates (Greiner Bio-One; cat. 784201). Drug stock plates were filled with 2.5 µM screening compounds in drug buffer (675 mM NaCl, 20 mM Hepes-NaOH, pH 7.4). Columns 23 and 24 were positive and negative control drugs, respectively. ACMA stock plates were filled with 6.5 µM ACMA in ACMA buffer (20 mM Hepes-NaOH, pH 7.4). CCCP stock plates were filled with 16 µM CCCP in a buffer (5 mM EDTA, 20 mM Hepes-NaOH, pH 7.4). Vesicles containing different channel proteins were thawed in a 37 °C water bath for 30 min and briefly mixed to homogeny by gentle pipetting. Thawing at 37 °C is very critical in obtaining robust LFA signals. Homogeneous vesicles were left at room temperature until immediately before the screening. Vesicles were diluted into a buffer containing no K+ (150 mM NaCl, 20 mM Hepes-NaOH) before their addition to the stock plate to create a stronger K+ gradient across the vesicle membrane, enhancing the flux signal. Vesicles were stable in high K+ solution for at least a day but gradually lost flux activity in low K+ high Na+ buffers, we therefore waited until right before the screen to dilute the vesicles. The vesicle dilutions used for screening are 1–40 for the mGIRK2 inhibitor screen, 1–30 for mGIRK2 PIP2-based activator screen, 1–60 for the mTRAAK inhibitor screen, and 1–30 for the hSlo1 activator screen. Vesicles in stock plates maintained their robust flux signals for at least 20 consecutive plates screened. Empty 384-well clear-bottom plates (Greiner Bio-One; cat. 781096) were used as assay plates to mix flux components and to read fluorescence.
Drug stock plates were loaded to the back port of FDSS6000, and empty assay plates were loaded to the front port. ACMA stock plates, vesicle stock plates, and CCCP stock plates were placed on the rotating stage automatically controlled by FDSS6000. Pipettes loaded on the pipette header of FDSS6000 were automatically washed before and after each pipetting. All screens were conducted at room temperature except for the hSlo1 activator screen, which was performed at 37 °C.
The FDSS6000 pipetted and mixed 12 µL drug solution, 6 µL ACMA solution, and 6 µL vesicle solution into an assay plate, and the baseline fluorescence was recorded (using 380-nm excitation and 510-nm emission). Then 6 µL CCCP was added and mixed to initiate the K+ flux, and the fluorescent signal was monitored every 5 s for 55 cycles. No valinomycin was used during the primary screen because it adhered to the pipette tips and contaminated subsequent plates. The final concentrations for the components in the LFA reaction were 1 µM drug, 1.3 µM ACMA, and 3.2 µM CCCP.
For mGIRK2 inhibitor screen, the positive control was mGIRK2 vesicles reconstituted with PIP2 only, the negative control was mGIRK2 reconstituted with both Gβγ and PIP2, and the Z-factor was 0.84. For mGIRK2 activator screen, the positive control was DM-solubilized Gβγ, the negative control was DM only, and the Z-factor was 0.81. For mTRAAK inhibitor screen, the positive control was a nonspecific pore blocker in the beginning and RU-TRAAK-1 for the rest of the screen, the negative control was DMSO only, and the Z-factor was 0.91. For hSlo1 activator screen, the positive control was Ca2+ in the beginning and RU-Slo1-2 for the rest of the screen, the negative control was DMSO only, and the Z-factor was 0.82.
Hit confirmation using LFA.
Once the primary screens were finished and primary hits were selected using offline data analysis, the hits were confirmed using a fluorescent plate reader (Tecan, Infinite M1000 with excitation wavelength 410 nm and emission wavelength 490 nm) with manual pipetting following the same protocol as the primary screen in 384-well plates. After monitoring the flux signal for 55 cycles, 1 µL 8 µM valinomycin (in DMSO) was added to allow K+ ions to pass through the membrane and reach equilibrium. The final fluorescence value was then recorded. Valinomycin addition causes little change in ACMA fluorescent signal under conditions where the channels are active, but it causes a sharp drop in signal under conditions where the channels are blocked or inactive. The use of valinomycin is an important control because fluorescent compounds may be identified as inhibitors in the primary screen, but these are likely false positives if the fluorescent signal does not decrease after valinomycin addition. Similarly, compounds that lyse the vesicles may show up as false positives in the primary screen, and can be discarded after an anomalous postvalinomycin result.
Once the hits were confirmed, fresh compounds were purchased from the same vendors that had supplied the primary screening compounds and drug titrations were conducted. Each compound titration was performed in a single column of a 384-well plate to ensure the simultaneous addition of CCCP using a multichannel pipette in a Tecan Infinite M1000 plate reader. The flux data were normalized, plotted, and fitted as detailed later.
Explanation of the IC50 Offset in the hERG Assay.
In the LFA, efflux of K+ is coupled to ionophore-mediated influx of H+. Through analysis of efflux rates as a function of protein-to-lipid ratio with various K+ channels, we know that under many circumstances (specific channels such as hERG and at high protein-to-lipid ratios), when K+ channels are not blocked, the influx of H+ limits the efflux of K+. Consequently, reduced K+ efflux is observed only when K+ channels are sufficiently blocked to allow K+ efflux to become rate limiting. This effect causes an offset in the estimate of the IC50 of a K+ channel blocker. To demonstrate with a simple model how this occurs, we model the processes of K+ efflux and H+ influx as conductors in series: and . Therefore, recorded conductance is
| [S1] |
If a blocker at concentration x inhibits K+ conductance according to
| [S2] |
where is the K+ conductance in the absence of blocker, then
| [S3] |
where Kd is the equilibrium dissociation constant for the blocker binding to its inhibitory site on the channel. Fig. 5G shows that a titration curve is shifted ∼10-fold when = 10 . By setting
| [S4] |
we find that the IC50 for the LFA curve is
| [S5] |
This model explains in simple terms why LFA can contain an offset in the estimated affinity of a blocker. As shown by the absence of false-negative and false-positive determinations in Tables S1 and S2, respectively, the offset does not render the assay inaccurate in its ability to identify channel blocking agents.
Animals.
All animal protocols were approved by IACUC of The Rockefeller University. Newborn C57BL/6J mice (The Jackson Laboratory) were killed for the dissection of hippocampal neurons.
Hippocampal Neuron Cultures.
Twelve-millimeter round poly-d-lysine–coated glass coverslips (neuVitro) were further coated with 10 μg/mL laminin (Invitrogen) in Dulbecco's PBS buffer (DPBS; Life Technologies) at 37 °C for 3 h in 24-well plates (Falcon). Before dissection, coated coverslips were washed four times with DPBS and incubated with plating medium (Neurobasal-A medium; Life Technologies) supplemented with 1× B27 supplement, 2% (vol/vol) horse serum, 2 mM GlutaMAX supplement, and 25 μM glutamate at 37 °C with moisture and 5% CO2. Hippocampus tissues from P0 mice were quickly dissected out in cold Hank's balanced salt solution (HBSS; Life Technologies) without Ca2+ and Mg2+. Hippocampus tissues were digested with 0.25% trypsin (Sigma; 1:250 tryptic activity) at 37 °C for 15 min. Digested tissues were washed four times with warm HBSS without Ca2+ and Mg2+ and gently triturated by a P200 pipette. Viable cells were counted with Trypan Blue (Lonza) and plated on coated coverslips in plating medium at a density of 0.5–1 × 105 cells per well. Four days after plating, plating medium was gradually changed to culturing medium (plating medium without glutamate). Culturing medium was renewed every 3 d. Neurons matured after 1 wk and were patched between 1 and 2 wk after plating.
Cell Line Cultures.
HEK293 cells were cultured in DMEM (Life Technologies) supplemented with 10% (vol/vol) FBS, 2 mM l-glutamine, and 1% penicillin/streptomycin in an incubator (Thermo Forma; Series II 3110) at 37 °C with moisture and 5% CO2. CHO-K1 cells were cultured in DMEM: Nutrient Mixture F-12 (DMEM/F12; Life Technologies) supplemented with 10% (vol/vol) FBS, 2 mM l-glutamine, and 1% penicillin/streptomycin in an incubator at 37 °C with moisture and 5% CO2. Cells were cultured to 80% confluency and transfected with Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol and passaged 12–24 h after transfection onto 12-mm poly-d-lysine– and laminin-coated glass coverslips (BioCoat) at low density. Patch-clamp recordings were performed 6–48 h after plating.
Electrophysiology.
All recordings were performed at room temperature. Pipettes of borosilicate glass (Sutter Instruments; BF150-86-10) were pulled to 2- to 4-MΩ resistance (except the pipettes used to patch BK were 1–2 MΩ) with a micropipette puller (Sutter Instruments; P-97) and polished with a microforge (Narishige; MF-83). Recordings were made with an Axopatch 200B amplifier (Molecular Devices) using standard whole-cell or excised patch-clamp techniques. Current-clamp experiments were performed on the I-fast mode. Recordings were acquired at 10 kHz and filtered at 1 kHz (Molecular Devices; 1440A). Pressure stimulation in inside-out patches for TRAAK was performed with a high-speed pressure clamp (ALA Scientific; HSPC) controlled through the Clampex 10 software (Molecular Devices). Pressure application velocity was set to the maximum rate of 8.3 mmHg/ms.
For whole-cell recordings of the mGIRK2 channel for inhibitor characterizations, the pipette solution was 10 mM Hepes-KOH, 20 mM NaCl, 130 mM KCl, 5 mM EGTA, and 2 mM MgCl2, pH 7.4 (adjusted with KOH), and the bath solution was 10 mM Hepes-KOH, 150 mM KCl, 5 mM EGTA, and 2 mM MgCl2, pH 7.4 (adjusted with KOH). For whole-cell recordings of the mGIRK2 channel with ivermectin, symmetrical pipette and bath solutions were 10 mM Hepes-KOH, 150 mM KCl, 5 mM EGTA, and 2 mM MgCl2, pH 7.4 (adjusted with KOH). For whole-cell recordings of the Kv1.2, hKir1.1, mKir2.1, hKir7.1, or K2P channels, the pipette solution was 10 mM Hepes-KOH, 150 mM KCl, 5 mM EGTA, and 2 mM MgCl2, pH 7.4 (adjusted with KOH), and the bath solution was 10mM Hepe-NaOH, 15 mM KCl, 140 mM NaCl, 2 mM MgCl2, and 1 mM CaCl2, pH 7.4 (adjusted with NaOH). For inside-out recordings of the mTRAAK channel, the pipette solution was 10 mM Hepe-NaOH, 15 mM KCl, 140 mM NaCl, 2 mM MgCl2, and 1 mM CaCl2, pH 7.4 (adjusted with NaOH), and the bath solution was 10 mM Hepes-KOH, 150 mM KCl, 5 mM EGTA, and 2 mM MgCl2, pH 7.4 (adjusted with KOH). For whole-cell and inside-out recordings of the hSlo1 channel, symmetrical pipette and bath solutions were 10 mM Hepes-KOH, 150 mM KCl, 5 mM EGTA, and 2 mM MgCl2, pH 7.4 (adjusted with KOH). For whole-cell hippocampal neuron recordings, the pipette solution was 10 mM Hepes-KOH, 150 mM K-gluconate, 5 mM NaCl, 5 mM EGTA, 2 mM MgCl2, 4 mM Mg-ATP, 0.4 mM Na-GTP, and 10 mM phosphocreatine (Tris salt), pH 7.4 (adjusted with KOH), and the bath solution was 10 mM Hepes-NaOH, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, and 10 mM glucose, pH 7.4 (adjusted with NaOH).
All drug perfusions were performed using home-made bath perfusion chambers except for the hippocampal neuron recordings, which used a fast pressurized microperfusion system (ALA Scientific; ALAVC3X8PP).
Data Analysis.
All data are presented as mean ± SEM.
Flux titration data were first normalized to eliminate baseline fluorescence fluctuations (due to ACMA pipetting variance and intrinsic fluorescence of testing compounds) using the following equation:
| [S6] |
where Fnormalized is the normalized fluorescence plotted in the flux titration figures, F is measured fluorescence in arbitrary units, Fstart is the average of measured fluorescence before addition of CCCP, and Fend is the measured end point fluorescence after addition of valinomycin. Normalizations were performed with Excel (Microsoft). Plots were made using Prism software (GraphPad).
The initial slopes of flux after CCCP addition (the average slopes of first 10 s after CCCP addition) were extracted from normalized flux titration data. The slope values were normalized to the maximum slope to obtain normalized flux activity plotted in the dose–response curves of flux titrations. Of note, in an inhibitor titration, the maximum slope is the slope of DMSO control whereas in an activator titration the maximum slope is the slope at highest activator concentration tested. The dose–response curves of flux titrations were fitted with the Hill equation with OriginPro software (OriginLab)
| [S7] |
where y is the normalized flux activity (the normalized initial slope), START is the normalized flux activity of DMSO control, END is the normalized flux activity at the highest drug concentration tested, x is drug concentration, and n is the Hill coefficient. The Hill coefficient ranged between 0.8 and 3.2.
Current-voltage curves of Slo1 recordings (Fig. 4D and Fig. S5) were fitted with the Boltzmann equation in Prism
| [S8] |
where I is the measured current, Ibottom is the measure current at the lowest voltage, Itop is the measure current at the highest voltage, V is the command depolarization voltage to open the channels, Vmid is the command voltage at which the channels have reached halfway between Ibottom and Itop, F is the Faraday constant, R is the gas constant, T is the absolute temperature, and Z is the apparent valence of the voltage dependence.
The scatter plot of hERG positive control drugs (Fig. 5F) was fitted with linear regression in OriginPro (OriginLab).
Acknowledgments
We thank J. Fraser Glickman, Antonio Luz, and Jeanne Chiaravalli-Giganti at the High-Throughput and Spectroscopy Resource Center of The Rockefeller University for assistance with the screens; Maria L. Garcia and Gregory J. Kaczorowski for advice with data analyses; Stephen G. Brohawn for assistance with K2P constructs and TRAAK purification; Yi Chun Hsiung for assistance with insect and mammalian cell cultures; and members of the R.M. laboratory for helpful discussions. R.M. is an investigator in the Howard Hughes Medical Institute. Other funding support includes National Institutes of Health Grant GM43949, American Asthma Foundation extension award, The Robertson Therapeutic Development Fund, and The Bridge Fund (to R.M.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 5472.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602815113/-/DCSupplemental.
References
- 1.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57(4):387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 2.Shieh C-C, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: Molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev. 2000;52(4):557–594. [PubMed] [Google Scholar]
- 3.Hibino H, et al. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev. 2010;90(1):291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 4.Brohawn SG, del Mármol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335(6067):436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci USA. 2014;111(9):3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516(7529):126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honoré E. The neuronal background K2P channels: Focus on TREK1. Nat Rev Neurosci. 2007;8(4):251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberg JI, et al. hERG K+ channels: Structure, function, and clinical significance. Physiol Rev. 2012;92(3):1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 9.Nardi A, Olesen S-P. BK channel modulators: A comprehensive overview. Curr Med Chem. 2008;15(11):1126–1146. doi: 10.2174/092986708784221412. [DOI] [PubMed] [Google Scholar]
- 10.Ponte CG, et al. Selective, direct activation of high-conductance, calcium-activated potassium channels causes smooth muscle relaxation. Mol Pharmacol. 2012;81(4):567–577. doi: 10.1124/mol.111.075853. [DOI] [PubMed] [Google Scholar]
- 11.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel beta1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L802–L810. doi: 10.1152/ajplung.00104.2006. [DOI] [PubMed] [Google Scholar]
- 12.Bentzen BH, Olesen S-P, Rønn LCB, Grunnet M. BK channel activators and their therapeutic perspectives. Front Physiol. 2014;5:389. doi: 10.3389/fphys.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibold MA, et al. An african-specific functional polymorphism in KCNMB1 shows sex-specific association with asthma severity. Hum Mol Genet. 2008;17(17):2681–2690. doi: 10.1093/hmg/ddn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkov GV, et al. β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537(Pt 2):443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichhorn B, Dobrev D. Vascular large conductance calcium-activated potassium channels: Functional role and therapeutic potential. Naunyn Schmiedebergs Arch Pharmacol. 2007;376(3):145–155. doi: 10.1007/s00210-007-0193-3. [DOI] [PubMed] [Google Scholar]
- 16.Mouhat S, Andreotti N, Jouirou B, Sabatier J-M. Animal toxins acting on voltage-gated potassium channels. Curr Pharm Des. 2008;14(24):2503–2518. doi: 10.2174/138161208785777441. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder K, Neagle B, Trezise DJ, Worley J. Ionworks HT: A new high-throughput electrophysiology measurement platform. J Biomol Screen. 2003;8(1):50–64. doi: 10.1177/1087057102239667. [DOI] [PubMed] [Google Scholar]
- 18.Dunlop J, Bowlby M, Peri R, Vasilyev D, Arias R. High-throughput electrophysiology: An emerging paradigm for ion-channel screening and physiology. Nat Rev Drug Discov. 2008;7(4):358–368. doi: 10.1038/nrd2552. [DOI] [PubMed] [Google Scholar]
- 19.Beacham DW, Blackmer T, O’ Grady M, Hanson GT. Cell-based potassium ion channel screening using the FluxOR assay. J Biomol Screen. 2010;15(4):441–446. doi: 10.1177/1087057109359807. [DOI] [PubMed] [Google Scholar]
- 20.Whiteaker KL, et al. Validation of FLIPR membrane potential dye for high throughput screening of potassium channel modulators. J Biomol Screen. 2001;6(5):305–312. doi: 10.1177/108705710100600504. [DOI] [PubMed] [Google Scholar]
- 21.Bagriantsev SN, et al. A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. ACS Chem Biol. 2013;8(8):1841–1851. doi: 10.1021/cb400289x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priest B, Bell IM, Garcia M. Role of hERG potassium channel assays in drug development. Channels. 2008;2(2):87–93. doi: 10.4161/chan.2.2.6004. [DOI] [PubMed] [Google Scholar]
- 23.Huang X-P, Mangano T, Hufeisen S, Setola V, Roth BL. Identification of human Ether-à-go-go related gene modulators by three screening platforms in an academic drug-discovery setting. Assay Drug Dev Technol. 2010;8(6):727–742. doi: 10.1089/adt.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trailovic SM, Nedeljkovic JT. Central and peripheral neurotoxic effects of ivermectin in rats. J Vet Med Sci. 2011;73(5):591–599. doi: 10.1292/jvms.10-0424. [DOI] [PubMed] [Google Scholar]
- 25.Geyer J, Janko C. Treatment of MDR1 mutant dogs with macrocyclic lactones. Curr Pharm Biotechnol. 2012;13(6):969–986. doi: 10.2174/138920112800399301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adelsberger H, Lepier A, Dudel J. Activation of rat recombinant α1β2γ2S GABAa receptor by the insecticide ivermectin. Eur J Pharmacol. 2000;394(2-3):163–170. doi: 10.1016/s0014-2999(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 27.Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276(16):12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- 28.Lynagh T, Lynch JW. Molecular mechanisms of Cys-loop ion channel receptor modulation by ivermectin. Front Mol Neurosci. 2012;5:60. doi: 10.3389/fnmol.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nausch B, et al. NS19504: A novel BK channel activator with relaxing effect on bladder smooth muscle spontaneous phasic contractions. J Pharmacol Exp Ther. 2014;350(3):520–530. doi: 10.1124/jpet.113.212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca-activated K+ channel. J Gen Physiol. 1997;110(3):257–281. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L, Guthrie H. Automated electrophysiology in the preclinical evaluation of drugs for potential QT prolongation. J Pharmacol Toxicol Methods. 2005;52(1):123–135. doi: 10.1016/j.vascn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Piper DR, et al. Development of the predictor HERG fluorescence polarization assay using a membrane protein enrichment approach. Assay Drug Dev Technol. 2008;6(2):213–223. doi: 10.1089/adt.2008.137. [DOI] [PubMed] [Google Scholar]
- 33.Rampe D, Roy ML, Dennis A, Brown AM. A mechanism for the proarrhythmic effects of cisapride (Propulsid): High affinity blockade of the human cardiac potassium channel HERG. FEBS Lett. 1997;417(1):28–32. doi: 10.1016/s0014-5793(97)01249-0. [DOI] [PubMed] [Google Scholar]
- 34.Wible BA, et al. HERG-Lite: A novel comprehensive high-throughput screen for drug-induced hERG risk. J Pharmacol Toxicol Methods. 2005;52(1):136–145. doi: 10.1016/j.vascn.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Parrish JZ, et al. Krüppel mediates the selective rebalancing of ion channel expression. Neuron. 2014;82(3):537–544. doi: 10.1016/j.neuron.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao J, et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77(5):899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 37.Ji W, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40(5):592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014;206(2):151–162. doi: 10.1083/jcb.201404136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goehring A, et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat Protoc. 2014;9(11):2574–2585. doi: 10.1038/nprot.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14(4):673–681. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Rothbauer U, et al. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Proteomics. 2008;7(2):282–289. doi: 10.1074/mcp.M700342-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Fridy PC, et al. A robust pipeline for rapid production of versatile nanobody repertoires. Nat Methods. 2014;11(12):1253–1260. doi: 10.1038/nmeth.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]