Abstract
Purpose
The microRNA-183/96/182 cluster (miR-183/96/182) plays important roles in sensory organs. Because the cornea is replete with sensory innervation, we hypothesized that miR-183/96/182 modulates the corneal response to bacterial infection through regulation of neuroimmune interactions.
Methods
Eight-week-old miR-183/96/182 knockout (ko) mice and their wild-type littermates (wt) were used. The central cornea of anesthetized mice was scarred and infected with Pseudomonas aeruginosa (PA), strain 19660. Corneal disease was graded at 1, 3, and 5 days postinfection (dpi). Corneal RNA was harvested for quantitative RT-PCR. Polymorphonuclear neutrophils (PMN) were enumerated by myeloperoxidase assays; the number of viable bacteria was determined by plate counts, and ELISA assays were performed to determine cytokine protein levels. A macrophage (Mϕ) cell line and elicited peritoneal PMN were used for in vitro functional assays.
Results
MicroRNA-183/96/182 is expressed in the cornea, and in Mϕ and PMN of both mice and humans. Inactivation of miR-183/96/182 resulted in decreased corneal nerve density compared with wt mice. Overexpression of miR-183/96/182 in Mϕ decreased, whereas knockdown or inactivation of miR-183/96/182 in Mϕ and PMN increased their capacity for phagocytosis and intracellular killing of PA. In PA-infected corneas, ko mice showed decreased proinflammatory neuropeptides such as substance P and chemoattractant molecules, MIP-2, MCP1, and ICAM1; decreased number of PMN at 1 and 5 dpi; increased viable bacterial load at 1 dpi, but decreased at 5 dpi; and markedly decreased corneal disease.
Conclusions
MicroRNA-183/96/182 modulates the corneal response to bacterial infection through its regulation of corneal innervation and innate immunity.
Keywords: microRNA, Pseudomonas aeruginosa-induced keratitis, miR-183; 96; 182, innate immunity, sensory innervations
Neuroimmune cross-talk/interactions play important roles in health and diseases.1–12 MicroRNAs (miRNAs) are small, noncoding, regulatory RNAs,13–16 and are proven to be an important mechanism of gene-expression regulation at posttranscriptional levels.13–16 However, the roles of miRNAs in neuroimmune interaction1–12 are still largely unknown. Previously, we identified a conserved miRNA cluster, the miR-183/96/182 cluster (referred to as miR-183/96/182 from here on), including miR-183, miR-96, and miR-182, which are clustered within 4 kb on mouse Chr6qA3 with conservation of synteny to human Chr7q32.2.17,18 It was initially identified as a sensory organ-specific miRNA cluster.17,19,20 Consistently, point mutations in miR-96 resulted in nonsyndromic hearing loss,21,22 suggesting a major role for miR-96 in inner ear function. To uncover in vivo functions of miR-183/96/182, we created a knockout (ko) mouse model19 using a gene-trap (GT) embryonic stem cell (ESC) clone.23–25 The GT construct carries a promoterless reporter, the β-geo cassette (a fusion of β-galactosidase [β-gal] and neomycin resistance genes),23–25 and was inserted in intron 1 of the miR-183/96/182 gene.19 This insertion places the β-geo cassette under the control of native regulatory elements of the gene to faithfully report endogenous expression of miR-183/96/182, providing a powerful tool to study expression patterns of miR-183/96/182 in all organ systems.19 However, the β-geo cassette “hijacks” the transcription of miR-183/96/182, resulting in loss of expression from the miR-183CGT allele; miR-183/96/182 is completely inactivated in miR-183CGT/GT19. Functionally, consistent with its high-level expression in sensory organs, we showed that inactivation of miR-183/96/182 resulted in congenital multisensory defects, including retinal dysfunction,19 hearing loss (unpublished data), and balancing defects.19
In addition to sensory organs, miR-183/96/182 is also reported to be expressed in hematopoietic and immune cells26–29 and thus plays a role in immune system function. Stittrich et al.29 showed that miR-182 and miR-183 were expressed in naïve T cells and were upregulated during T-helper (Th) cell activation. Interleukin-2–induced miR-182 was required for clonal expansion of activated Th cells.29 Kelada et al.30 reported that miR-182 and miR-183 were upregulated in Foxp3+ regulatory T cells (Treg) in a Th2 immune environment, which was required for the suppression of the proliferation of Th2 and naïve T cells, and Th2-driven airway inflammation in a double adoptive transfer system.30 Pucella et al.31 showed that miR-182 was also expressed in B cells and was the most upregulated miRNA during B-cell activation. However, an in vivo study using an miR-182 ko mouse model32 showed that inactivation of miR-182 alone had minimal impact on B- and T-cell development, maturation, and activation, and T-cell–dependent immune response to experimental Listeria monocytogenes infection.31 Pucella et al.31 concluded that miR-182 is largely disposable for adaptive immunity. Consistent with this conclusion, our unpublished data suggest that miR-183/96/182 ko mice have no apparent defects in follicular T-helper cell (TFH) generation (Xiao C, Xu S, written communication, 2016), supporting that miR-183/96/182 does not have a significant role in adaptive immunity in vivo. Recently, miR-183 was shown to mediate TGFβ-induced decreased tumor cytolysis by natural killer cells,33 suggesting that miR-183/96/182 plays a role in the innate immune system.
Microbial keratitis is a potentially blinding disease, costing an estimated $175 million in direct health care expenditures in the United States. Pseudomonas aeruginosa (PA) is a gram-negative opportunistic pathogen capable of inducing keratitis and causes one of the most rapidly developing and destructive diseases of the cornea; it remains the most commonly recovered causative organism in contact lens–related disease.34,35 Currently, bacterial keratitis is mainly treated by topical administration of antibiotics; however, frequent emergence of antibiotic-resistant bacteria poses serious challenges for effective management.36 Development of alternative treatment depends on discoveries of new molecular mechanisms and therapeutic targets. However, the roles of miRNAs in PA-induced keratitis are still largely unknown. Recently, Yang et al.37 reported that miR-155 suppresses macrophage (Mϕ)-mediated bacterial phagocytosis and intracellular killing; inactivation of miR-155 in Mϕ mice increased their resistance to PA-induced keratitis,37 supporting that miRNA plays a role in bacterial keratitis through its modulation of innate immune functions.
The cornea is one of the most densely innervated tissues, receiving input from sensory neurons (SN) of the trigeminal ganglion (TG).38–40 Interactions between SN and resident and infiltrating immune cells play critical roles in corneal health, nerve regeneration, and in the response of this tissue to microbial infection by releasing inflammatory neuropeptides.6,34,38–46 Therefore, we hypothesize that miR-183/96/182 modulates the corneal response to bacterial infection through its regulation of sensory innervation of the cornea and neuroimmune interactions.1–12
More importantly, both adaptive and innate immune systems are actively involved in bacterial clearance and resolution of PA-induced keratitis. A Th1-dominant response is associated with genetic susceptibility, severe corneal disease, and perforation; whereas a Th2-dominant response confers resistance to PA infection and a milder course of disease without corneal perforation.47–50 The Th17 cells also infiltrate the cornea with increased representation in late-stage PA infection, playing a role in sustained inflammatory responses, polymorphonuclear neutrophils (PMN) infiltration and severe corneal disease.51,52 The innate immune system plays a major role in corneal responses to PA infection.34,53–58 Polymorphonuclear neutrophils are the predominant infiltrating cells. Polymorphonuclear neutrophils and Mϕ are recruited to engulf bacteria, and produce large amounts of reactive oxygen species and reactive nitrogen species to kill engulfed bacteria, contributing to bacterial clearance.34,53–57 However, persistence of PMN is associated with increased tissue damage and perforation.53–58 Macrophages restrict PA growth and modulate immune responses by regulating PMN influx and apoptosis, balance of pro- and anti-inflammatory cytokines, and T-cell response.58,59 Therefore, we hypothesize that miR-183/96/182 also modulates the corneal response to PA infection through its regulation of the immune system. Using the miR-183CGT/GT mouse model in combination with in vitro functional assays, here we provide evidence that miR-183/96/182 regulates not only corneal innervation, but also the immune cells integral to the disease.
Materials and Methods
Mice
All animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The miR-183CGT/GT mice are on a 129S2/BL6-mixed background19 and were originally derived from a gene-trap ESC clone.19,24,25 Male and female, young adult (8-week-old) miR-183CGT/GT mice, their heterozygous (miR-183CGT/+) and wild-type (wt) littermates (miR-183C+/+) were used in this study.
Human Cornea, PMN, and Peripheral Blood Mononuclear Cells (PBMCs)
Human corneas were obtained from the Body Bequest Program, Department of Anatomy and Cell Biology, School of Medicine, Wayne State University. The entire human cornea (anterior to the limbus) was collected from cadaveric eyes within 12 hours postmortem and dissected into V-shaped slices from the periphery to the center of the cornea for RNA preparation. The donors were 79 (male), 41 (male), and 54 (female) years old without prior corneal or other eye diseases. Human PMNs and PBMCs were purchased from Zen-Bio, Inc. (Research Triangle Park, NC, USA).
Nerve Staining
Corneas were excised posterior to the limbal region and flattened by four evenly spaced cuts from the periphery toward the center. Corneas were fixed in 2% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB), pH 7.4 overnight (O/N) at 4°C; then, permeabilized with 0.3% Triton X-100 in PBS for 1 hour at room temperature (RT) and blocked with PBS with 0.3% Triton X-100, 2.5% BSA, and 5% donkey serum (Covance, Battle Creek, MI, USA) for 2 hours. Then, corneas were incubated with rabbit anti-βIII-tubulin antibody (Covance) for 2 days at 4°C. After four rinses with PBS plus 0.3% Triton X-100 and 0.05% Tween-20, corneas were incubated with Alexafluor-488–conjugated donkey anti-rabbit secondary antibody (ThermoFisher, Grand Island, NY, USA) O/N at 4°C. Excess antibody was removed with rinses in PBS. Then, the corneas were flattened onto poly-L-lysine-coated slides and coverslipped using Vectashield mounting media (Vector Labs, Burlingame, CA, USA). Controls were treated similarly with omission of primary antibody or addition of nonspecific IgG.
Corneal Infection and Grading
P. aeruginosa infection of the cornea was performed as described before.58 Briefly, mice were anesthetized with ethyl ether in a well-ventilated hood. The cornea of the left eye was wounded; 5.0 × 106 colony forming units (CFU) of PA (strain 19660; ATCC, Manassas, VA, USA) in a 5 μL volume was topically delivered. Corneal disease was graded at 1, 3, and 5 dpi using an established scale58: 0, clear or slight opacity, partially or fully covering the pupil; +1, slight opacity, covering the anterior segment; +2, dense opacity, partially or fully covering the pupil; +3, dense opacity, covering the entire anterior segment; and +4, corneal perforation. Photography with a slit lamp was used to illustrate disease.
RNA Preparation and Real-Time RT-PCR
Total RNA was prepared using the miRVana miRNA isolation kit (Life Technologies, Foster City, CA, USA) for miRNA studies, or the RNeasy (Qiagen, Frederick, MD, USA) and RNA STAT-60 kit (Tel-Test, Friendswood, TX, USA) for mRNA studies as described previously.17,52,60 Quantitative (q)RT-PCR for miRNAs was performed using Taqman miRNA primers and RT-PCR kit (Life Technologies) on a CFX Connect Real-time System (Bio-Rad, Hercules, CA, USA) with snRNA U6 as an endogenous control as described before.17,60 Quantitative RT-PCR assays of protein-coding genes were performed using QuantiFast SYBR Green RT-PCR kit and QuantiTect primers (Qiagen) with 18s rRNA and β-actin as endogenous controls.17,19,60
X-Gal Staining
Staining with X-gal was performed as described before.19,61,62 Briefly, corneas of young adult wt and heterozygous mice (∼8 weeks old) were excised posterior to the limbus. Corneas were fixed in 4% PFA in 0.1M PB, pH 7.4, for 30 minutes on ice, then rinsed in Buffer A (0.1 M PB, pH 7.4, 2 mM MgSO4, 5 mM EGTA) at RT for 5 minutes and a second rinse for 25 minutes, and then soaked in Buffer B (0.1 M PB, pH 7.4, 2 mM MgSO4, 0.01% sodium deoxycholate, 0.02% NP-40) at RT twice for 5 minutes. Corneas then were stained in Buffer C (0.1 M PB, pH 7.4, 2 mM MgSO4, 0.01% sodium deoxycholate, 0.02% NP-40, 5 mM K3Fe[CN]6, 5 mM K4Fe[CN]6, 1 mg X-gal/mL) at 37°C O/N. After washing with PBS, corneas were observed and photographed under a dissection scope (Accu-scope, Inc., Comack, NY, USA). Then, for whole-mount microscopy, the cornea was flattened by four evenly spaced cuts from the periphery toward the center corneas and mounted on Superfrost plus slides (ThermoFisher); for cross-sections, the cornea was soaked in 20% sucrose in 0.1M PB pH 7.4 O/N and mounted in optimum cutting temperature (OCT; Fisher Healthcare, Houston, TX, USA), 12-μm cryoprotected cross-sections were made and examined using light microscopy (Leica DM4000B; Leica Microsystem, Inc., Buffalo Grove, IL, USA).
For TG X-staining, the whole head of a heterozygous mouse was fixed in 4% PFA in 0.1 M PB, pH 7.4 for 2 hours after the skull was opened. Then the head was sequentially soaked in Buffers A and B, stained in Buffer C and postfixed with 4% PFA as described above. Then, the stained head was carefully dissected under a dissection scope to expose the TG.
Enzyme-Linked Immunosorbent Assay
Protein levels for IL-1β and MIP-2 were tested using ELISA kits (R&D Systems, Minneapolis, MN, USA) as described before.53,63 Briefly, corneas were homogenized in 1 mL PBS with 0.1% Tween-20 and protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). An aliquot of each supernatant was assayed in duplicate per the manufacturer's instructions. Sensitivities of the ELISA assays were less than 2.31 pg/mL for IL-1β, and less than 1.5 pg/mL for MIP-2.
Quantitation of Corneal PMN
A myeloperoxidase (MPO) assay was performed as described before.64–67 Briefly, corneas were harvested, homogenized in 1 mL potassium phosphate buffer (50 mM, pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich Corp., St. Louis, MO, USA) using glass microtissue grinders. The samples were freeze-thawed four times and centrifuged at 21,000g for 10 minutes. To measure MPO, a 100-μL supernatant was added to fresh 2.9 mL o-dianisidine dihydrochloride substrate buffer (16.7 mg/mL; Sigma-Aldrich Corp.) with 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was read every 30 seconds for 5 minutes on a Heλios α spectrophotometer (ThermoFisher). Afterward, units of MPO per cornea were calculated: 1 unit of MPO activity is equivalent to approximately 2 × 105 PMN.68
Quantitation of Viable Bacteria
Bacteria were quantitated as described before.64–67 Briefly, each cornea was homogenized in 1.0 mL sterile saline containing 0.25% BSA; 0.1 mL corneal homogenate was serially diluted 1:10 in the same solution and selected dilutions were plated in triplicate on Pseudomonas isolation agar (Becton Dickinson, Sparks, MD, USA). Plates were incubated O/N at 37°C and the number of viable bacteria counted. Results are reported as log10 number of CFU/cornea ± SEM.
In Vitro Transfection and Phagocytosis and Intracellular Killing Assays
A total of 4 × 105 RAW264.7 Mϕ (a cell line derived from the mouse; ATCC) were plated in a 48-well plate and transfected with anti-miR-183/anti-miR-96/anti-miR-182 (10 nM for each) or negative control anti-miR oligonucleotides with scrambled sequences (30 nM; Exiqon, Vedbaek, Denmark) and miRNA mimics of miR-183, miR-96, and miR-182 (10 nM for each) or negative control mimics (30 nM; ThermoFisher) using RNAiMax Lipofectamine (ThermoFisher) as described before.69 All experiments were conducted with four replicate wells. Forty-eight hours after transfection, 1 × 107 CFU PA (strain 19660)/well was added. One hour after coculture at 37°C, gentamicin (300 μg/mL) was added to kill extracellular bacteria. Thirty minutes after gentamicin treatment, cells were washed with 250 μL Hanks' balanced salt solution (HBSS) twice to remove gentamicin and dead extracellular bacteria. For the phagocytosis assay, cells were lysed in 250 μL ice-cold sterile water. The cell lysate was plated on PA isolation agar plates (Becton Dickinson) and incubated at 37°C for 24 hours as described before.64 Colonies were manually counted; CFU/mL of cell lysate was calculated based on dilution factors. For the intracellular killing assay, fresh media was added to the wells after washing with HBSS to remove gentamicin and dead extracellular bacteria, and then cells were incubated for an additional hour before being washed, lysed, and plated. Killing efficiency was calculated as ([CFU/mL at 1 hour] − [CFU/mL at 2 hour])/(CFU/mL at 1 hour).
Mouse Peritoneal PMN Isolation and Ex Vivo Functional Assay
To harvest peritoneal PMN, mice were injected intraperitoneally with 1 mL 9% casein solution 27 and 3 hours before harvesting; a standard peritoneal PMN isolation protocol was followed as described previously.64,67–70 A total of 4 × 105 PMNs were plated on a 48-well plate and infected with 1 × 107 CFU PA (strain 19660). Two hours after infection, gentamicin (300 μg/mL) was added to kill extracellular bacteria. For the phagocytosis assay, 30 minutes after gentamicin treatment, cells were washed, lysed, and plated on PA isolation agar plates as described above. For the intracellular killing assay, cells were incubated for an additional 2 hours after washing; then, cells were lysed and plated. Killing efficiency was calculated as ([CFU/mL at 2 hour] − [CFU/mL at 4 hour])/(CFU/mL at 2 hour).
Statistical Analysis
The difference in clinical score between groups was tested by the Mann-Whitney U test. One-way ANOVA with Bonferroni test (GraphPad Prism; La Jolla, CA, USA) was used to analyze qRT-PCR data of proinflammatory neuropeptides and cytokines. An unpaired, two-tailed Student's t-test was used to determine the significance of qRT-PCR of miRNAs, ELISA, MPO, and plate count data17,19,60; P < 0.05 was considered significant. Each experiment was repeated at least twice to ensure reproducibility and data from a representative experiment are shown. Quantitative data are expressed as the mean ± SEM.
Results
MicroRNA-183/96/182 is Expressed in the Cornea and TG and Is Inactivated in miR-183CGT/GT Mice
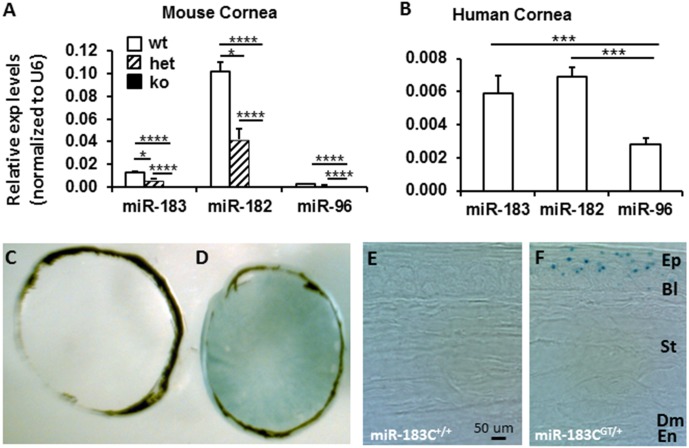
Quantitative RT-PCR analysis showed that all three members of miR-183/96/182 are expressed in the cornea of wt mice (Fig. 1A). Among them, miR-182 and miR-183 showed higher expression than miR-96 (∼32- and 4-fold higher, respectively) (Fig. 1A), suggesting that miR-182 and miR-183 play major roles in the cornea. Consistently, miR-183/96/182 is also expressed in human cornea with a similar pattern as in the mouse (Fig. 1B).
Figure 1.
MicroRNA-183/96/182 is expressed in the cornea in both mice and humans and is inactivated in the ko mice. (A, B) Quantitative RT-PCR analysis in mouse (A) and human (B) cornea. n = 3 for each group. wt, wild-type (miR-183C+/+); het, heterozygous (miR-183CGT/+); ko, knockout (miR-183CGT/GT). (C–F) X-gal staining of wt (C, E) and heterozygous mouse cornea (D, F). *P < 0.05; ****P < 0.0001. Ep, epithelium; Bl, Bowman's layer; St, stroma; Dm, Descemet's membrane; En, endothelium.
In miR-183CGT/GT mice, miR-183/96/182 is inactivated in the cornea. In heterozygous mice, expression of both miR-182 and miR-183 were significantly decreased to approximately one-half of that in wt mice, suggesting a dosage effect because heterozygous mice carry only one wt allele of the miR-183/96/182 gene (Fig. 1A).
Because the β-gal cassette in the miR-183CGT allele follows the endogenous expression pattern of miR-183/96/182,19 to test its spatial expression pattern, we performed X-gal staining in the cornea of miR-183CGT/+ mice. Our results showed that miR-183/96/182 is mainly expressed in the corneal epithelium (Figs. 1C–F). A punctate staining pattern in the corneal epithelium (Fig. 1F) suggested that the expression of miR-183/96/182 may be due to staining of corneal nerve endings.
Inactivation of miR-183/96/182 Resulted in Decreased Nerve Density and Decreased Expression of Proinflammatory Neuropeptides in the Cornea
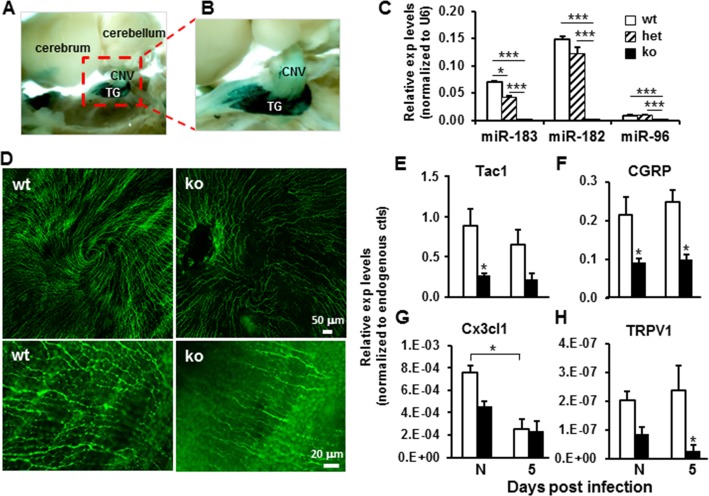
Sensory innervation of the cornea is from the TG.38–40 To test miR-183/96/182 expression in SN of the TG, we performed X-gal staining on miR-183CGT/+ mouse brain, and showed that miR-183/96/182 is highly expressed in the TG (Figs. 2A, 2B). Quantitative RT-PCR further confirmed its expression in TG (Fig. 2C). Similar to the cornea, miR-182 and miR-183 are predominately expressed in the TG, compared with miR-96 (∼16- and 8-fold higher, respectively) (Fig. 2C). MicroRNA-183/96/182 is inactivated in the TG of miR-183CGT/GT mice. In the TG of heterozygous miR-183CGT/+ mice, miR-183 is significantly decreased to approximately one-half of that in wt mice; miR-182 expression is also reduced, although did not reach significance (Fig. 2C). Similarly as in the cornea, these data suggest that miR-183/96/182 expression in TG also has a dosage effect based on numbers of wt alleles (Fig. 2C).
Figure 2.
(A–C) MicroRNA-183/96/182 is expressed in the trigeminal ganglion. (A, B) X-gal staining in the brain of a 6-week-old miR-183CGT/+ mouse. CNV, cranial nerve V; TG, trigeminal ganglia. (C) Quantitative RT-PCR analysis. (D–H) Inactivation of miR-183/96/182 had significant impact on subbasal plexus of corneal nerves and neuropeptide expression in the cornea. (D) Immunofluorescence of flat-mount cornea with anti-β-III-tubulin antibody showing decreased corneal nerve density in the subbasal plexus. Images in the bottom row are under higher magnification of the same corneas as the top row. (E–H) Quantitative RT-PCR analysis on Tac1, CGRP, Cx3cl1, and TRPV1 in the cornea of noninfected (N) and PA-infected (5) wild-type (open bars) and miR-183/96/182 ko animals (filled bars) at 5 dpi. *P < 0.05.
To test whether inactivation of miR-183/96/182 has significant impact on corneal innervation, we performed immunofluorescence analysis using an anti-β-III tubulin antibody, a marker for neurons. Our results showed that, in ko animals, corneal nerve density is drastically decreased; the normal whorl-like pattern of the subbasal nerve plexus39,71–77 is disrupted (Fig. 2D). Local axon enlargements or “beads,” which contain high densities of mitochondria and share similarities to nociceptor terminals,38,39 are decreased in ko compared with wt cornea (Fig. 2D), suggesting that inactivation of miR-183/96/182 has significant consequences on corneal sensory innervation. Consistent with this hypothesis, qRT-PCR using corneal RNA of ko and wt mice showed decreased expression of nociceptor transient receptor potential vanilloid 1 (TRPV1) and SN-released proinflammatory neuropeptides Tac1, the precursor gene for substance P (sP), calcitonin gene-related peptide (CGRP), and chemokine (C-X3-C motif) ligand 1 (Cx3cl1) (Figs. 2E–H).
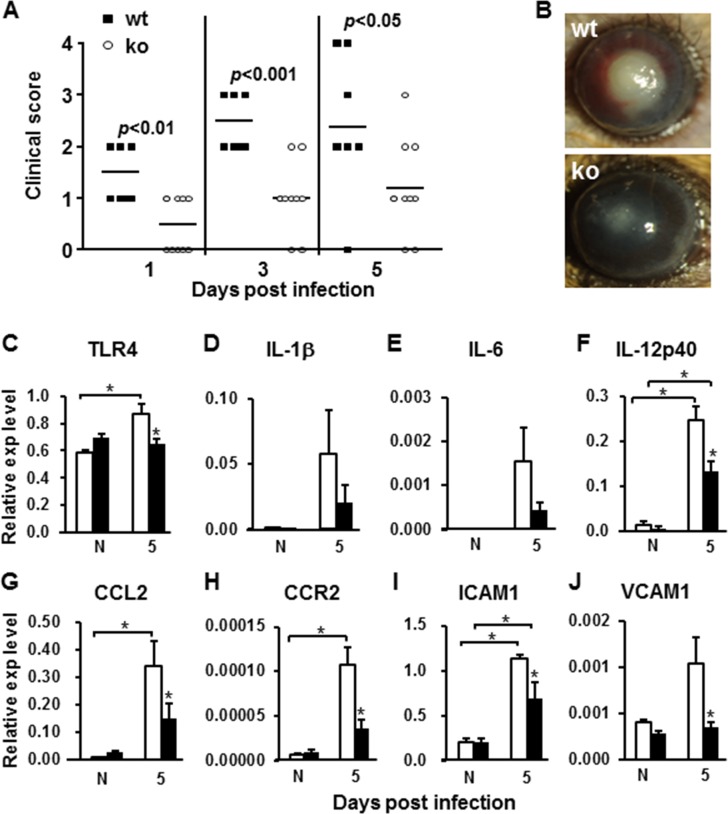
Inactivation of miR-183/96/182 Resulted in Significant Decrease of Corneal Inflammatory Response to PA Infection and Severity of PA-Induced Keratitis
Because inactivation of miR-183/96/182 resulted in decreased nerve density and proinflammatory neuropeptides, we hypothesized that this disrupts neuroimmune cross-talk and modulates immune/inflammatory responses in the cornea. To test this hypothesis, the cornea of ko mice and their wt littermates were infected with PA. Our results showed that miR-183/96/182 ko mice had significantly decreased keratitis at 1, 3, and 5 dpi (Fig. 3A). Most of the ko mice had only slight corneal opacity; none of the corneas perforated (Figs. 3A, 3B). Quantitative RT-PCR analysis of the cornea at 5 dpi showed that many proinflammatory factors and their receptors, which have been shown to play important roles in PA-induced keratitis, were decreased in the infected cornea of ko mice (Figs. 3C–J). In wt animals, pattern-recognition receptor Toll-like receptor 4 (TLR4), IL-1β, IL-6, IL-12p40, chemokine (C-C motif) ligand 2 (CCL2) and its receptor CCR2, and intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) were increased in the infected eyes compared with noninfected contralateral eyes (Figs. 3C–J); whereas in ko mice, increased expression of these proinflammatory factors in infected eyes was mitigated (Figs. 3C–J). Expression levels of most of these inflammatory factors were decreased in the infected eyes of ko mice when compared with their wt littermates (Figs. 3C–J). Enzyme-linked immunosorbent assays demonstrated that, at the protein level, IL-1β was significantly decreased at 1 dpi, whereas macrophage inflammatory protein-2 (MIP-2) was decreased at both 1 and 5 dpi (Figs. 4A, 4B). These results suggest that inactivation of miR-183/96/182 resulted in a decreased inflammatory response in infected corneas and contributed to decreased severity of PA-induced keratitis.
Figure 3.
Inactivation of miR-183/96/182 resulted in decreased expression of proinflammatory cytokines and PA-induced keratitis. (A) Clinical scores at 1, 3, and 5 dpi. n = 8 for wt; n = 10 for ko mice. Horizontal bars represent the mean values. (B) Examples of slit-lamp photography of eyes of wt and ko animals at 5 dpi. (C–J) Inactivation of miR-183/96/182 resulted in decreased expression of proinflammatory cytokines and related receptors in corneas of noninfected (N) and PA-infected (5) wt (open bars) and ko animals (filled bars) at 5 dpi. n = 5 for both genotypes. *P < 0.05.
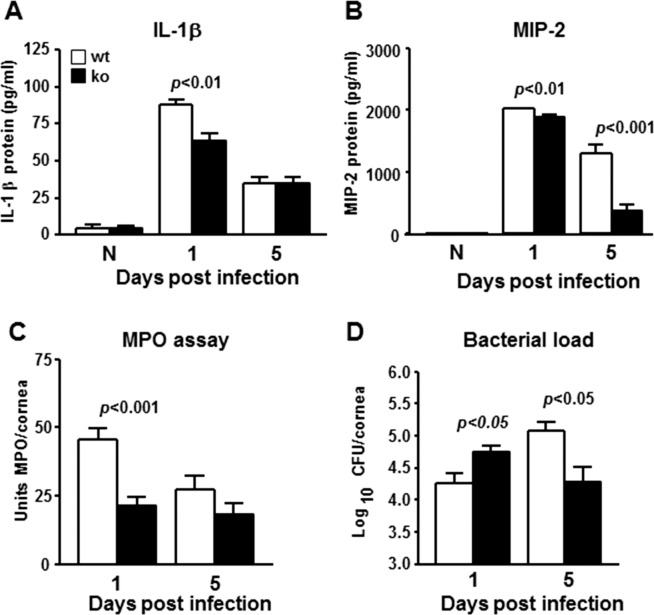
Figure 4.
(A, B) Inactivation of miR-183/96/182 decreased inflammatory cytokines, IL-1β (A) and MIP-2 (B) at protein levels in the infected cornea by ELISA. (C) Myeloperoxidase levels were decreased in the cornea of ko mice. (D) Bacterial load was significantly increased in the cornea of ko mice at 1 dpi, but decreased at 5 dpi.
Polymorphonuclear neutrophils are the predominant infiltrating immune cells in bacterial keratitis and play important roles in bacterial clearance.53–57 To begin to understand the pathological processes involved, we first performed MPO assays to quantify PMN in the cornea. Our results showed that PMN in the infected cornea of ko animals were significantly decreased at 1 dpi and slightly more decreased at 5 dpi. This result is consistent with the observation of decreased expression of chemoattractant neuropeptides (e.g., sP) (Fig. 2E) and cytokines (e.g., CCL2 and MIP-2), and leukocyte adhesion molecules, ICAM1 and VCAM1 (Figs. 3G, 3I, 3J, 4B).
To gain further insight into the progression of pathological processes, we quantified viable bacteria in the infected cornea. Intriguingly, our results showed that bacterial load was increased in the infected cornea of ko compared with wt mice at 1 dpi. This observation is consistent with decreased PMN infiltration in ko animals at this time. However, at 5 dpi, bacterial load in the corneas of wt controls was further increased when compared with 1 dpi; whereas the bacterial load in ko animals was decreased at 5 dpi compared with 1 dpi, and was significantly lower than wt controls. These results suggest that, although they had a higher bacterial burden at 1 dpi, bacteria continued to be cleared more efficiently in ko mice than in wt controls.
MicroRNA-183/96/182 is Expressed in Mϕ and PMN and Plays an Important Role in Modulation of Innate Immune Cell Function
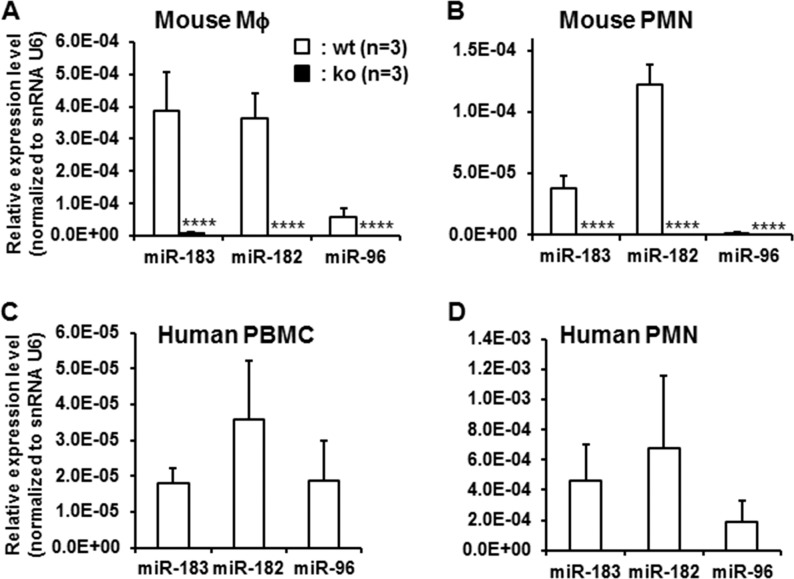
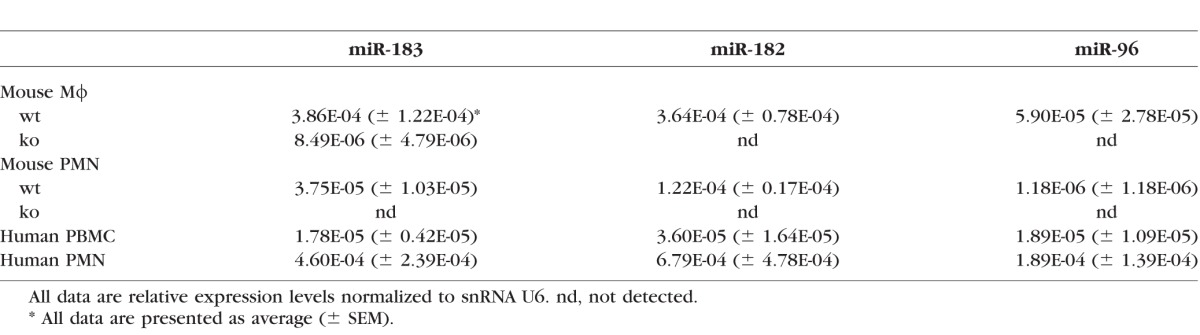
Quantitative RT-PCR results showed that miR-183/96/182 is expressed in both PMNs and Mϕ of wt mice, while inactivated in miR-183CGT/GT animals (Figs. 5A, 5B; Table). In Mϕ, miR-183 and miR-182 are predominantly expressed, whereas miR-96 is expressed at a significantly lower level, approximately one-sixth of the level of miR-183 and miR-182 (Fig. 5A). In PMN, only miR-182 and miR-183 are expressed, with miR-182 predominant (∼3.3-fold over miR-183); whereas miR-96 is barely detectable (Fig. 5B). Similarly, miR-183/96/182 is also expressed in human PMNs and PBMCs (Figs. 5C, 5D; Table), suggesting that miR-183/96/182 modulates the functions of PMNs and Mϕ in both mice and humans.
Figure 5.
MicroRNA-183/96/182 is expressed in mouse Mϕ and PMNs (A, B) and in human PBMCs and PMNs (C, D). It is inactivated in the miR-183/96/182 ko mice (A, B). n = 3 for each group. ****P < 0.0001.
Table.
MicroRNA-183/96/182 Expression in Mouse Mϕ and PMNs
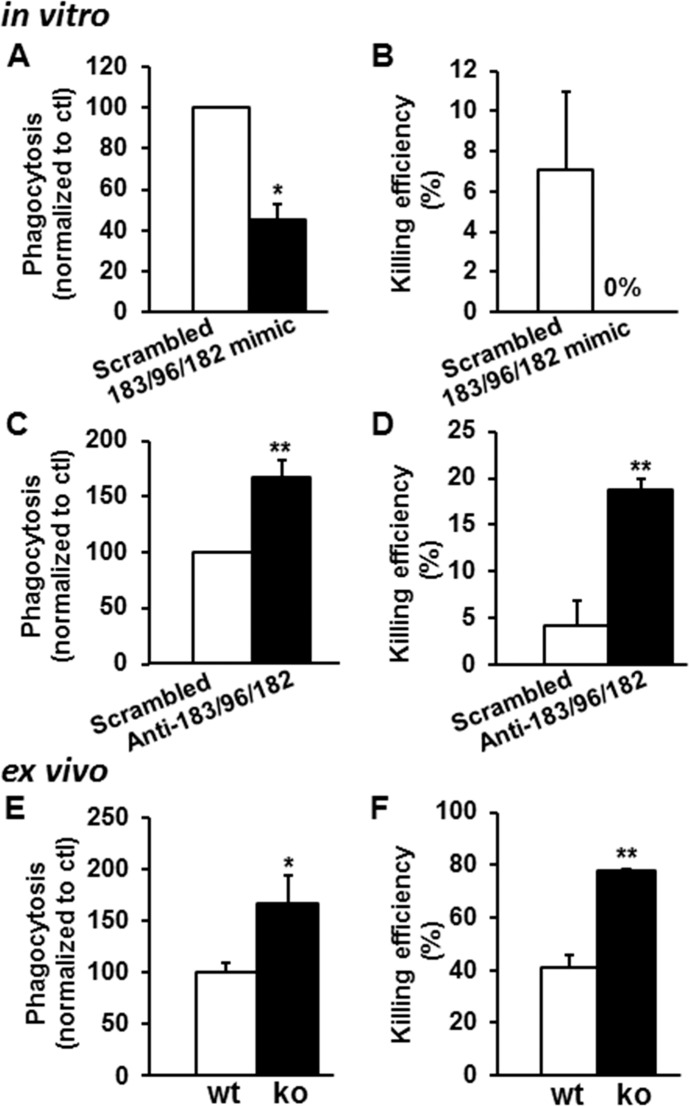
In vitro phagocytosis and intracellular killing assay using a mouse Mϕ cell line, RAW264.7, showed that simultaneous overexpression of miR-182, -183, and -96 in Mϕ significantly decreased their phagocytosis of PA by approximately 55.1% (Fig. 6A) and abolished their killing capacity (Fig. 6B); whereas knockdown of miR-182, -183, and -96 resulted in a significant increase of phagocytosis (by ∼67.3%) (Fig. 6C) and intracellular killing efficiency (4.2%–18.8%) (Fig. 6D). To test the role of miR-183/96/182 in PMNs, we isolated peritoneal PMNs from ko and wt mice for phagocytosis and intracellular killing assays. Our results showed that, consistent with the knockdown experiment in Mϕ, inactivation of miR-183/96/182 in PMNs resulted in a significant increased phagocytosis capacity (∼66.5%) (Fig. 6E) and intracellular killing efficiency (∼37%; range, 41.0%–78.0%) (Fig. 6F).
Figure 6.
MicroRNA-183/96/182 modulates phagocytosis and intracellular killing capacities of Mϕ and PMNs. (A–D) In vitro overexpression (A, B) and knockdown (C, D) of miR-183/96/182 in Mϕ cell line, Raw264.7. (E, F) Ex vivo phagocytosis and intracellular killing assays in PMN from miR-183/96/182 ko and wt mice. n = 4 for each group. *P < 0.05; **P < 0.01.
Discussion
Bacterial keratitis is a potentially blinding corneal infectious disease and PA is the most commonly recovered causative organism in contact lens–related cases. PA-induced keratitis is also one of the most rapidly developing and destructive diseases of the cornea.34,35 However, the roles of miRNAs in bacterial keratitis are still largely unknown. In this regard, Yang et al.37 recently provided evidence that miR-155 modulates the corneal response to PA infection through its regulation of Mϕ. Our study is the second report describing the involvement of miRNAs in PA-induced keratitis. In the current report, we demonstrated that miR-183/96/182 modulates the corneal response to PA infection through its regulation of sensory innervation of the cornea and innate immune cell functions (both PMN and Mϕ). Inactivation of miR-183/96/182 in mice resulted in a decreased inflammatory response and less severe keratitis, providing evidence that miR-183/96/182 is involved in the pathogenesis of PA-induced keratitis.
By X-gal staining and qRT-PCR analyses, we demonstrated that miR-183/96/182 is highly expressed in mouse cornea, with miR-182 and miR-183 predominately expressed, suggesting their major roles in the cornea (Fig. 1). Similar to mouse cornea, miR-183/96/182 is also expressed in human cornea, suggesting clinical relevance to the findings in mice. In the cornea, its expression is mostly in the epithelium with a unique punctate pattern, suggesting that its expression in the cornea is likely in the nerve endings (Figs. 1C–E). Consistently, miR-183/96/182 is highly expressed in the TG, where the SN to the cornea reside, with similar ratios among the three miRNAs in the cornea (Figs. 2A–C). Inactivation of miR-183/96/182 resulted in a decrease of corneal nerve density and beaded nociceptor terminals (Fig. 2D) and decreased expression of TRPV1 and proinflammatory neuropeptides, including sP, CGRP, and Cx3cl1 (Figs. 2E–H). It has been shown that sP- and CGRP-expressing SN coexpress TRPV140,78; activation of TRPV1 in sensory nerve endings by capsaicin results in calcium influx-dependent neuropeptide release, including sP and CGRP.79,80 Therefore, our data suggest that decreased sensory innervation contributes to the decreased release of proinflammatory neuropeptides in the cornea and inflammatory responses. However, we do not exclude the possibility that decreased production of these neuropeptides from other sources (e.g., the corneal epithelium and tears)38 also contributed to the overall decreased expression of proinflammatory neuropeptides.
Toll-like receptor 4 is a classic member of the pathogen-recognition receptor TLR family and functions as a primary sensor to detect gram-negative bacteria by binding to bacteria-derived lipopolysaccharides (LPS) expressed in bacterial cell walls.81 As previously described,82 PA infection induces TLR4 expression in the cornea of wt mice (Fig. 3D); however, intriguingly, in miR-183/96/182 ko mice, PA infection in the cornea did not result in upregulation of TLR4 (Fig. 3C); the level of TLR4 in the infected cornea of ko mice was significantly decreased compared with wt controls (Fig. 3C). Toll-like receptor 4 is expressed in TRPV1-containing trigeminal SN.83 It has been shown that LPS binding sensitizes TRPV1 and increases neuropeptide release from SN.84 Therefore, decreased expression of TLR4 in the cornea of miR-183/96/182 ko animals may contribute to decreased neuropeptide release.
The Cx3cl1, also known as fractalkine and a ligand for Cx3cr1, is a neuronally derived proinflammatory neuropeptide.85 In the peripheral nervous system, dorsal root ganglion-released Cx3cl1 promotes microglial production of inflammatory factors and contributes to chronic pain.86–90 In cornea, Cx3cl1/Cx3cr1 signaling mediates direct physical association between corneal nerves and resident Mϕ, which is suggested to act as an indicator of malfunctioning neuroimmune communication in diseases.44 Decreased expression of Cx3cl1 (Fig. 2G) may have compromised neuroimmune communication in the cornea and contributed to the changes of the overall corneal response to PA infection (Fig. 3).
Polymorphonuclear neutrophils are the predominant infiltrating cells in PA keratitis and are essential for microbial clearance.53–57 Our data showed that the number of PMNs was significantly decreased (by ∼50%) in the infected cornea of ko mice at 1 dpi (Fig. 4C), resulting in decreased bacterial clearance and increased bacterial load (Fig. 4D). Substance P has been shown to be a potent chemoattractant and to induce leukocyte activation, NF-κB activation, cytokine production, and plasma extravasation.91–95 Decreased expression of sP (Fig. 2E), together with decreased expression of proinflammatory cytokines, including multiple chemoattractant molecules (e.g., CCL2), also known as monocyte chemoattractant protein (MCP)-1 (Fig. 3G), ICAM1 (Fig. 3I), and MIP-2 (Fig. 4B), may have contributed to decreased recruitment of PMNs, and possibly Mϕ, to the infected cornea.53,56,96–98
Intriguingly, at 5 dpi, bacterial load in the cornea of ko mice was significantly decreased compared with wt controls (Fig. 4D), whereas the number of PMNs showed no significant difference between ko and wt cornea (Fig. 4C). This cannot be explained by the decreased corneal nerve density and proinflammatory peptides. An alternative hypothesis is that inactivation of miR-183/96/182 has direct impact on PMN function and other components of the innate immune system. Supporting this hypothesis, our studies confirmed that miR-183/96/182 is expressed in Mϕ and PMNs in both mice and humans (Fig. 5; Table). More importantly, our in vitro and ex vivo data showed that inactivation or knockdown of miR-183/96/182 in Mϕ and PMNs resulted in a significant increase in their capacity for phagocytosis and intracellular killing of PA (Fig. 6). Therefore, in spite of the decreased number of PMNs and Mϕ in the infected cornea, bacteria were efficiently cleared without excessive collateral damage to the cornea, leading to decreased keratitis. Substance P has also been shown to be a potent anti-apoptosis regulator.99,100 During PA infection, sP delays apoptosis of PMNs58; persistence of PMNs is associated with increased corneal damage and perforation.53–58 Therefore, decreased sP in the infected cornea of the ko animals could result in increased apoptosis of PMNs and contribute to decreased severity of keratitis.
In addition to the innate immune system, emerging evidence suggests that miR-183/96/182 is expressed in the adaptive immune system.29–31 Although in vitro and ex vivo data suggest potential roles of miR-183/96/182 in modulating adaptive immune functions, including clonal expansion of activated Th cells29 and Th2-induced Treg,30 other reports31 and our unpublished data (Xiao C, Xu S, written communication, 2016) do not support significant functions of miR-183/96/182 in adaptive immunity in vivo. Further investigation is needed to determine whether inactivation of miR-183/96/182 affects adaptive immunity and whether this impacts the corneal response to PA keratitis.
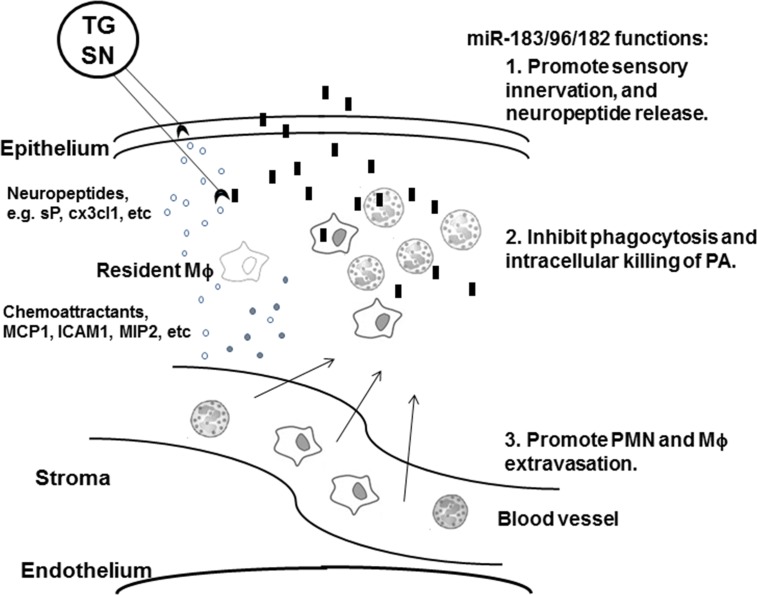
Overall, our data suggest that miR-183/96/182 is important in the corneal response to PA infection through its regulation of both sensory innervation of the cornea and innate immune functions at multiple levels in the pathogenesis of PA-induced keratitis (Fig. 7). The impact of inactivation of miR-183/96/182 on innate immune functions plays a major role in modulating the corneal response to PA infection and development of keratitis. It will be worthwhile to further tease out the effects of the developmental defects leading to a disrupted pattern of corneal innervation from the effects of gene ablation on innate immunity per se.
Figure 7.
Schematic illustration of the roles of miR-183/96/182 in the corneal response to PA infection. In wt animals, miR-183/96/182 promotes the integrity of sensory innervation and proinflammatory neuropeptide release, including sP and Cx3cl1; in innate immune system, miR-183/96/182 modulates extravasation of PMN and Mϕ and their capacity of phagocytosis and intracellular killing of bacteria. When miR-183/96/182 is inactivated, sensory innervation to the cornea is decreased, which leads to decreased release of proinflammatory neuropeptide on PA infection, which leads to decreased activation of resident Mϕ and decreased chemoattractant cytokines, decreased infiltration of PMN, and possibly Mϕ, to the infected cornea; meanwhile, inactivation of miR-183/96/182 potentiates PMNs and Mϕ to increase their capacity of phagocytosis and intracellular killing of bacteria, leading to efficient removal of invading PA with less collateral damage to corneal tissue, and therefore, decreased keratitis.  , sensory nerve ending;
, sensory nerve ending;  , PMN;
, PMN;  , Mϕ;
, Mϕ;  , PA;
, PA;  , neuropeptides and cytokines.
, neuropeptides and cytokines.
To our knowledge, our data provide the first evidence that miR-183/96/182 plays an important role in innate immunity and modulates phagocytosis and bacterial killing capacities of both PMNs and Mϕ. This finding has important implications to a wide range of diseases in which innate immunity has a critical role to disease outcome. It is also the first report that an miRNA cluster has a significant functional role in both sensory and innate immune systems. These results lead us to suggest that miR-183/96/182 represents a new therapeutic target for treatment of bacterial keratitis.
Acknowledgments
We thank Babara Rosso-Norgen, mortuary supervisor of the Body Bequest Program, Department of Anatomy and Cell Biology, School of Medicine, Wayne State University, for coordinating procurement of human corneal samples.
Supported by Grants R01EY016058 (LDH), R01EY023226 (EB), and P30EY004068 (LDH) from the National Eye Institute, National Institutes of Health, and by a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology/Kresge Eye Institute. LDH is the recipient of a 2012 Alcon Research award.
Disclosure: C.K. Muraleedharan, None; S.A. McClellan, None; R.P. Barrett, None; C. Li, None; D. Montenegro, None; T. Carion, None; E. Berger, None; L.D. Hazlett, None; S. Xu, None
References
- 1. Kerschensteiner M,, Meinl E,, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009; 158: 1122–1132. [DOI] [PubMed] [Google Scholar]
- 2. Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004; 5: 575–581. [DOI] [PubMed] [Google Scholar]
- 3. Brogden KA,, Guthmiller JM,, Salzet M,, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005; 6: 558–564. [DOI] [PubMed] [Google Scholar]
- 4. Tsui H,, Razavi R,, Chan Y,, Yantha J,, Dosch HM. ‘Sensing' autoimmunity in type 1 diabetes. Trends Mol Med. 2007; 13: 405–413. [DOI] [PubMed] [Google Scholar]
- 5. Razavi R,, Chan Y,, Afifiyan FN,, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006; 127: 1123–1135. [DOI] [PubMed] [Google Scholar]
- 6. Shechter R,, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if' but ‘how'. J Pathol. 2013; 229: 332–346. [DOI] [PubMed] [Google Scholar]
- 7. Popovich PG,, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008; 9: 481–493. [DOI] [PubMed] [Google Scholar]
- 8. Tsui H,, Winer S,, Chan Y,, et al. Islet glia, neurons, and beta cells. Ann N Y Acad Sci. 2008; 1150: 32–42. [DOI] [PubMed] [Google Scholar]
- 9. Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006; 6: 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson U,, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012; 209: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiu IM,, Heesters BA,, Ghasemlou N,, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013; 501: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinman L. Lessons learned at the intersection of immunology and neuroscience. J Clin Invest. 2012; 122: 1146–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambros V. The functions of animal microRNAs. Nature. 2004; 431: 350–355. [DOI] [PubMed] [Google Scholar]
- 14. Bartel DP. MicroRNAs: genomics biogenesis, mechanism, and function. Cell. 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 15. Wightman B,, Ha I,, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993; 75: 855–862. [DOI] [PubMed] [Google Scholar]
- 16. Lee RC,, Feinbaum RL,, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993; 75: 843–854. [DOI] [PubMed] [Google Scholar]
- 17. Xu S,, Witmer PD,, Lumayag S,, Kovacs B,, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007; 282: 25053–25066. [DOI] [PubMed] [Google Scholar]
- 18. Xu S. microRNA expression in the eyes and their significance in relation to functions. Prog Retin Eye Res. 2009; 28: 87–116. [DOI] [PubMed] [Google Scholar]
- 19. Lumayag S,, Haldin CE,, Corbett NJ,, et al. Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc Natl Acad Sci U S A. 2013; 110: E507–E516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wienholds E,, Kloosterman WP,, Miska E,, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005; 309: 310–311. [DOI] [PubMed] [Google Scholar]
- 21. Lewis MA,, Quint E,, Glazier AM,, et al. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009; 41: 614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mencia A,, Modamio-Hoybjor S,, Redshaw N,, et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009; 41: 609–613. [DOI] [PubMed] [Google Scholar]
- 23. Friedrich G,, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991; 5: 1513–1523. [DOI] [PubMed] [Google Scholar]
- 24. Hansen J,, Floss T,, Van Sloun P,, et al. A large-scale, gene-driven mutagenesis approach for the functional analysis of the mouse genome. Proc Natl Acad Sci U S A. 2003; 100: 9918–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schnutgen F,, De-Zolt S,, Van Sloun P,, et al. Genomewide production of multipurpose alleles for the functional analysis of the mouse genome. Proc Natl Acad Sci U S A. 2005; 102: 7221–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Georgantas RW,, III, Hildreth R,, Morisot S,, et al. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2007; 104: 2750–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pal S,, Baiocchi RA,, Byrd JC,, Grever MR,, Jacob ST,, Sif S. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 2007; 26: 3558–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barad O,, Meiri E,, Avniel A,, et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004; 14: 2486–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stittrich AB,, Haftmann C,, Sgouroudis E,, et al. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nat Immunol. 2010; 11: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 30. Kelada S,, Sethupathy P,, Okoye IS,, et al. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS Pathog. 2013; 9: e1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pucella JN,, Yen WF,, Kim MV,, et al. miR-182 is largely dispensable for adaptive immunity: lack of correlation between expression and function. J Immunol. 2015; 194: 2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin ZB,, Hirokawa G,, Gui L,, et al. Targeted deletion of miR-182, an abundant retinal microRNA. Mol Vis. 2009; 15: 523–533. [PMC free article] [PubMed] [Google Scholar]
- 33. Donatelli SS,, Zhou JM,, Gilvary DL,, et al. TGF-beta-inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci U S A. 2014; 111: 4203–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004; 23: 1–30. [DOI] [PubMed] [Google Scholar]
- 35. Stapleton F,, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond). 2012; 26: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mesaros N,, Nordmann P,, Plesiat P,, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007; 13: 560–578. [DOI] [PubMed] [Google Scholar]
- 37. Yang K,, Wu M,, Li M,, et al. miR-155 suppresses bacterial clearance in Pseudomonas aeruginosa-induced keratitis by targeting Rheb. J Infect Dis. 2014; 210: 89–98. [DOI] [PubMed] [Google Scholar]
- 38. Muller LJ,, Marfurt CF,, Kruse F,, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003; 76: 521–542. [DOI] [PubMed] [Google Scholar]
- 39. Marfurt CF,, Cox J,, Deek S,, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010; 90: 478–492. [DOI] [PubMed] [Google Scholar]
- 40. Murata Y,, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006; 1085: 87–94. [DOI] [PubMed] [Google Scholar]
- 41. Jones MA,, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998; 66: 421–435. [DOI] [PubMed] [Google Scholar]
- 42. Troger J,, Kieselbach G,, Teuchner B,, et al. Peptidergic nerves in the eye, their source and potential pathophysiological relevance. Brain Res Rev. 2007; 53: 39–62. [DOI] [PubMed] [Google Scholar]
- 43. Sarkar J,, Chaudhary S,, Jassim SH,, et al. CD11b+GR1+ myeloid cells secrete NGF and promote trigeminal ganglion neurite growth: implications for corneal nerve regeneration. Invest Ophthalmol Vis Sci. 2013; 54: 5920–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seyed-Razavi Y,, Chinnery HR,, McMenamin PG. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Invest Ophthalmol Vis Sci. 2014; 55: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 45. Shaheen BS,, Bakir M,, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014; 59: 263–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belmonte C,, Acosta MC,, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004; 78: 513–525. [DOI] [PubMed] [Google Scholar]
- 47. Kwon B,, Hazlett LD. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J Immunol. 1997; 159: 6283–6290. [PubMed] [Google Scholar]
- 48. Hazlett LD,, McClellan S,, Kwon B,, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci. 2000; 41: 805–810. [PubMed] [Google Scholar]
- 49. Berk RS,, Leon MA,, Hazlett LD. Genetic control of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1979; 26: 1221–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berk RS,, Beisel K,, Hazlett LD. Genetic studies of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1981; 34: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suryawanshi A,, Cao Z,, Thitiprasert T,, Zaidi TS,, Panjwani N. Galectin-1-mediated suppression of Pseudomonas aeruginosa-induced corneal immunopathology. J Immunol. 2013; 190: 6397–6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li C,, McClellan SA,, Barrett R,, Hazlett LD. Interleukin 17 regulates Mer tyrosine kinase-positive cells in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2014; 55: 6886–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kernacki KA,, Barrett RP,, Hobden JA,, Hazlett LD. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000; 164: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 54. Kernacki KA,, Barrett RP,, McClellan S,, Hazlett LD. MIP-1alpha regulates CD4+ T cell chemotaxis and indirectly enhances PMN persistence in Pseudomonas aeruginosa corneal infection. J Leukoc Biol. 2001; 70: 911–919. [PubMed] [Google Scholar]
- 55. Hazlett LD. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells Langerhans cells, PMN, and cytokines. DNA Cell Biol. 2002; 21: 383–390. [DOI] [PubMed] [Google Scholar]
- 56. Rudner XL,, Kernacki KA,, Barrett RP,, Hazlett LD. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol. 2000; 164: 6576–6582. [DOI] [PubMed] [Google Scholar]
- 57. Kernacki KA,, Barrett RP,, McClellan SA,, Hazlett LD. Aging and PMN response to P. aeruginosa infection. Invest Ophthalmol Vis Sci. 2000; 41: 3019–3025. [PubMed] [Google Scholar]
- 58. Zhou Z,, Barrett RP,, McClellan SA,, et al. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2008; 49: 4458–4467. [DOI] [PubMed] [Google Scholar]
- 59. McClellan SA,, Huang X,, Barrett RP,, van Rooijen N,, Hazlett LD. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J Immunol. 2003; 170: 5219–5227. [DOI] [PubMed] [Google Scholar]
- 60. Kovacs B,, Lumayag S,, Cowan C, Xu S. microRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2011; 52: 4402–4409. [DOI] [PubMed] [Google Scholar]
- 61. Xu S,, Wang Y,, Zhao H,, et al. PHR1, a PH domain-containing protein expressed in primary sensory neurons. Mol Cell Biol. 2004; 24: 9137–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mombaerts P,, Wang F,, Dulac C,, et al. Visualizing an olfactory sensory map. Cell. 1996; 87: 675–686. [DOI] [PubMed] [Google Scholar]
- 63. Thakur A,, Barrett RP,, Hobden JA,, Hazlett LD. Caspase-1 inhibitor reduces severity of Pseudomonas aeruginosa keratitis in mice. Invest Ophthalmol Vis Sci. 2004; 45: 3177–3184. [DOI] [PubMed] [Google Scholar]
- 64. Foldenauer ME,, McClellan SA,, Berger EA,, Hazlett LD. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J Immunol. 2013; 190: 5649–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McClellan SA,, Zhang Y,, Barrett RP,, Hazlett LD. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators downregulated. Invest Ophthalmol Vis Sci. 2008; 49: 1502–1511. [DOI] [PubMed] [Google Scholar]
- 66. Hazlett LD,, McClellan SA,, Barrett RP,, Liu J,, Zhang Y,, Lighvani S. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2007; 48: 797–807. [DOI] [PubMed] [Google Scholar]
- 67. Berger EA,, McClellan SA,, Vistisen KS,, Hazlett LD. HIF-1alpha is essential for effective PMN bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis. PLoS Pathog. 2013; 9: e1003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Williams RN,, Paterson CA,, Eakins KE,, Bhattacherjee P. Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr Eye Res. 1982; 2: 465–470. [DOI] [PubMed] [Google Scholar]
- 69. Cowan C,, Muraleedharan CK,, O'Donnell JJ,, III, et al. microRNA-146 inhibits thrombin-induced NF-kappaB activation and subsequent inflammatory responses in human retinal endothelial cells. Invest Ophthalmol Vis Sci. 2014; 55:4944–4951. [DOI] [PubMed]
- 70. Swamydas M,, Luo Y,, Dorf ME,, Lionakis MS. Isolation of mouse neutrophils. Curr Protoc Immunol. 2015; 110: 3.20.1–3.20.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Al-Aqaba MA,, Fares U,, Suleman H,, Lowe J,, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010; 94: 784–789. [DOI] [PubMed] [Google Scholar]
- 72. Auran JD,, Koester CJ,, Kleiman NJ,, et al. Scanning slit confocal microscopic observation of cell morphology and movement within the normal human anterior cornea. Ophthalmology. 1995; 102: 33–41. [DOI] [PubMed] [Google Scholar]
- 73. Patel DV,, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005; 46: 4485–4488. [DOI] [PubMed] [Google Scholar]
- 74. Ueda S,, del Cerro M,, LoCascio JA,, Aquavella JV. Peptidergic and catecholaminergic fibers in the human corneal epithelium. An immunohistochemical and electron microscopic study. Acta Ophthalmol Suppl. 1989; 192: 80–90. [DOI] [PubMed] [Google Scholar]
- 75. Dvorscak L,, Marfurt CF. Age-related changes in rat corneal epithelial nerve density. Invest Ophthalmol Vis Sci. 2008; 49: 910–916. [DOI] [PubMed] [Google Scholar]
- 76. Leiper LJ,, Ou J,, Walczysko P,, et al. Control of patterns of corneal innervation by Pax6. Invest Ophthalmol Vis Sci. 2009; 50: 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu CQ,, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007; 48: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 78. Caterina MJ,, Schumacher MA,, Tominaga M,, Rosen TA,, Levine JD,, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 79. Flores CM,, Leong AS,, Dussor GO,, Harding-Rose C,, Hargreaves KM,, Kilo S. Capsaicin-evoked CGRP release from rat buccal mucosa: development of a model system for studying trigeminal mechanisms of neurogenic inflammation. Eur J Neurosci. 2001; 14: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Franco-Cereceda A,, Henke H,, Lundberg JM,, Petermann JB,, Hokfelt T,, Fischer JA. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987; 8: 399–410. [DOI] [PubMed] [Google Scholar]
- 81. Takeuchi O,, Hoshino K,, Kawai T,, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999; 11: 443–451. [DOI] [PubMed] [Google Scholar]
- 82. Huang X,, Du W,, McClellan SA,, Barrett RP,, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006; 47: 4910–4916. [DOI] [PubMed] [Google Scholar]
- 83. Wadachi R,, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006; 85: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Diogenes A,, Ferraz CC,, Akopian AN,, Henry MA,, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 85. Ji RR,, Xu ZZ,, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Verge GM,, Milligan ED,, Maier SF,, Watkins LR,, Naeve GS,, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004; 20: 1150–1160. [DOI] [PubMed] [Google Scholar]
- 87. Souza GR,, Talbot J,, Lotufo CM,, Cunha FQ,, Cunha TM,, Ferreira SH. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci U S A. 2013; 110: 11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Clark AK,, Yip PK,, Grist J,, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007; 104: 10655–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Milligan ED,, Zapata V,, Chacur M,, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004; 20: 2294–2302. [DOI] [PubMed] [Google Scholar]
- 90. Dorgham K,, Ghadiri A,, Hermand P,, et al. An engineered CX3CR1 antagonist endowed with anti-inflammatory activity. J Leukoc Biol. 2009; 86: 903–911. [DOI] [PubMed] [Google Scholar]
- 91. Vishwanath R,, Mukherjee R. Substance P promotes lymphocyte-endothelial cell adhesion preferentially via LFA-1/ICAM-1 interactions. J Neuroimmunol. 1996; 71: 163–171. [DOI] [PubMed] [Google Scholar]
- 92. Quinlan KL,, Naik SM,, Cannon G,, et al. Substance P activates coincident NF-AT- and NF-kappa B-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol. 1999; 163: 5656–5665. [PubMed] [Google Scholar]
- 93. Quinlan KL,, Song IS,, Naik SM,, et al. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol. 1999; 162: 1656–1661. [PubMed] [Google Scholar]
- 94. Steinhoff MS,, von Mentzer B,, Geppetti P,, Pothoulakis C,, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014; 94: 265–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. O'Connor TM,, O'Connell J,, O'Brien DI,, Goode T,, Bredin CP,, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004; 201: 167–180. [DOI] [PubMed] [Google Scholar]
- 96. Xue ML,, Thakur A,, Cole N,, et al. A critical role for CCL2 and CCL3 chemokines in the regulation of polymorphonuclear neutrophils recruitment during corneal infection in mice. Immunol Cell Biol. 2007; 85: 525–531. [DOI] [PubMed] [Google Scholar]
- 97. Hobden JA,, Masinick-McClellan S,, Barrett RP,, Bark KS,, Hazlett LD. Pseudomonas aeruginosa keratitis in knockout mice deficient in intercellular adhesion molecule 1. Infect Immun. 1999; 67: 972–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hobden JA,, Masinick SA,, Barrett RP,, Hazlett LD. Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci. 1995; 36: 1107–1114. [PubMed] [Google Scholar]
- 99. Bockmann S,, Seep J,, Jonas L. Delay of neutrophil apoptosis by the neuropeptide substance P: involvement of caspase cascade. Peptides. 2001; 22: 661–670. [DOI] [PubMed] [Google Scholar]
- 100. Kang BN,, Jeong KS,, Park SJ,, et al. Regulation of apoptosis by somatostatin and substance P in peritoneal macrophages. Regul Pept. 2001; 101: 43–49. [DOI] [PubMed] [Google Scholar]