Abstract
Pancreatic cancer is the fourth leading cause of cancer death in the United States. The microenvironment of pancreatic cancer could be one of the “perfect storms” that support the growth of a cancer. Indeed, pancreatic cancer may be the poster child of a problem with the microenvironment. In this article, we review the rationale and attempts to date on modifying or targeting structural proteins in the microenvironment including hyaluronan (HA) (in primary and metastases), collagen, and SPARC (secreted protein, acidic, and rich in cysteine). Indeed, working in this area has produced a regimen that improves survival for patients with advanced pancreatic cancer (nab-paclitaxel + gemcitabine). In addition, in initial clinical trials, PEGylated hyaluronidase appears promising. We also review a new approach that is different than targeting/destroying the microenvironment and that is orchestrating, reengineering, reprogramming, or normalizing the microenvironment (including normalizing structural proteins, normalizing an immunologically tumor-friendly environment to a less friendly environment, reversing epithelial-to-mesenchymal transition, and so on). We believe this will be most effectively done by agents that have global effects on transcription. There is initial evidence that this can be done by agents such as vitamin D derivatives and other new agents. There is no doubt these opportunities can now be tried in the clinic with hopefully beneficial effects.
Keywords: Extracellular matrix, metastasis, pancreatic cancer, tumor microenvironment
Despite decades of basic and clinical research, effective therapies for the treatment of pancreatic ductal adenocarcinoma (PDAC) remain 1 of the greatest unmet clinical needs in oncology today. Currently, PDAC accounts for approximately 7% of all cancer-related mortality and has the lowest 5-year survival rate among all cancer types in the United States.1 Pancreatic ductal adenocarcinoma is currently the fourth leading cause of death from cancer in the United States with about 46,400 people diagnosed and about 39,500 dying of the disease each year.1 Worldwide it will claim more than 300,000 lives this year.2 It is projected that by 2030, pancreatic cancer will become the second leading cause of cancer-related death in the United States.3 Improved strategies for early detection and for treatment of PDAC are desperately needed.
The poor survival rate we currently observe is the result of multiple contributing factors, including lack of effective preventive or early detection measures; both intrinsic and acquired tumor chemoresistance; high rates of unresectable, metastatic disease in patients at diagnosis; and multiple other factors. In this article, we focus on the tumor microenvironment as a particular challenge in pancreatic cancer. The microenvironment is generally defined in biology as the immediate small-scale environment of an organism, or part of an organism that is a distinct part of a larger environment. While the microenvironment was initially thought to be tumor cells and supportive protein such as collagen, HA, and so on, it is now known to be composed of (a) tumor cell component; (b) a stromal component characterized by a high intratumor pressure that results in relative hypoxia and also acts as a barrier to large molecule penetration; (c) an immunologic environment (with at least myeloid-derived suppressor cells, and regulatory T cells that is “tumor friendly” and conductive to tumor cell survival); (d) an adapted metabolism in which malignant epithelial cells consume protein (in the form of albumin) and lipids as a source of energy4,5; (e) an invasive epithelial-to-mesenchymal transition (EMT) and metastatic phenotype; and (f) an aggressive tumor stem cell compartment that, owing to its unique inherent resistance, becomes enriched after conventional cancer treatment.6
As will be noted in the following sections, the initial attempts on changing the microenvironment have been directed at directing cytotoxics to the microenvironment, and this approach has met some success in improving survival.7 However, additional science is driving different approaches to exploit new findings regarding the microenvironment and how to “orchestrate” the microenvironment. But first we will start at the general level of desmoplasia.
DESMOPLASIA IN TUMOR MICROENVIRONMENT AND THERAPEUTIC APPROACHES
Fibrosis (or desmoplasia) at the primary tumor site is recognized as a common feature of pancreatic cancer and is thought to contribute to chemoresistance and other bad behavior of PDAC.8 Desmoplasia utilizes many of the signaling pathways commonly featured in the normal wound-healing process. In PDAC, however, these pathways are conscripted to promote tumor growth, leading to reduced drug uptake and reduced patient tumor responses.9,10 In patient outcomes, however, tumor metastasis is a primary cause of patient death in many PDAC patients. We need to know the status of desmoplasia in the metastases to effectively target them.11,12 It has been proposed that therapeutics targeting desmoplasia may synergize with chemotherapeutics targeting tumor cells.7,13–16
While primary pancreatic tumors are known to be desmoplastic, whether metastatic lesions are also desmoplastic had not been determined. We recently reported on an analysis of a cohort of PDAC patients in which we saw a negative correlation between PDAC patient survival and the presence of markers of desmoplasia in their tumors.17 We also compared levels of markers of desmoplasia in both primary tumors and metastatic lesions and found both to be elevated.
A key player in the development and maintenance of desmoplasia is the pancreatic stellate cell. In the development of PDAC, the pancreatic stellate cells transform from vitamin A– and lipid-storing cells and begin to secrete transforming growth factor β (TGF-β), as well as platelet-derived growth factor. These key changes result in the induction of transcriptional programs that mediate increased extracellular matrix (ECM) synthesis.18,19 It is thought that these desmoplastic changes, including ECM synthesis, in pancreatic tissue result in vascular and lymphatic dysfunction. Blood vessel compression may mediate altered interstitial fluid pressures resulting from tissue solid stress.15,20 These key features are thought to contribute to the poor drug penetration that has been observed in PDAC. Olive and colleagues21 report significantly reduced uptake of doxorubicin in pancreatic tissues in a genetically engineered mouse (KPC) model for pancreatic cancer. In addition to vascular dysfunction, they reported observing significant hypovascularity in PDAC, resulting in 75% loss of vessel density in PDAC tissues compared with normal pancreas.21 In a prior report, Duda and colleagues22 demonstrated that activated fibroblasts accompanied tumor cell intravasation and promote metastatic dissemination. This report was among the first to demonstrate that metastatic sites may also be desmoplastic. If metastatic lesions are also fibrotic niches that foster tumor growth, as primary tumors certainly are, then one might reasonably expect that the metastatic niche is also innately drug resistant by virtue of this same physiological mechanism. Because of these observations, we ought to understand fully the nature of metastatic desmoplasia if we are to understand how best to address it therapeutically, including components such as HA, collagen, or signaling pathways such as TGF-β. We will now examine some of the various components of desmoplasia.
Hyaluronan
Hyaluronan is the protein-free polysaccharide found in many tissues throughout the body, with higher concentrations found in the cartilage, skin, eyes, and especially in synovial fluid. Hyaluronan binds multiple water molecules, enhancing volume, rigidity, and viscosity of fluids. In normal tissues, HA is maintained through a balance of HA synthase (HAS) and hyaluronidase activities. Considering its main cellular functions, many had long thought that HA was molecularly inert. With new understanding of the role of the hyaladherins, as well as the HA-binding receptors, CD44, and the receptor for HA-mediated motility (RHAMM), it is now clear that HA plays a much more direct role in regulating many biological processes including cell adhesion, proliferation, and migration, as well as cell invasion and recruitment of neutrophils into the tumor bed.
Hyaluronan levels appear to be elevated in many cancers, including in PDAC, and it often localizes to the stromal compartment of tumor tissues. Hyaluronan has been shown to be involved in the invasion of PDAC cells in experiments demonstrating HA synthesis at the invasive front of expanding tumors.23 Bertrand and colleagues23 report a more than 4-fold increase in HA deposition at the invasive tumor front relative to the adjacent normal tissues. Hyaluronan binding to CD44 promotes tumor migration of colon, breast, and brain cancer cells. In addition, it appears that the level of HA correlates with tumor migration and proliferation indices in breast or ovarian cancer. Induction of expression of the HAS, HAS1, also increases cellular proliferation rates. While studies are ongoing to determine the utility of HAS1 as a biomarker, HA levels may prove to be a suitable biomarker in several cancers including bladder cancer. As HA and HAS1 levels appear altered in multiple disease states, it seems that a balance of HAS and hyaluronidase activities is necessary to maintain the normal tissue homeostasis and function of HA.
Elevated levels of HA have been observed in several malignancies including pancreatic and gastric cancers, as well as in breast and bladder cancers.24–27 In a recent report, we demonstrated that HA negatively correlates with the survival of patient with PDAC.17
Prior studies revealed that HA synthesis can be regulated by corticosteroid administration. For example, cortisol reduced the expression of HA by HAS by 50% in smooth muscle cells. Indeed, Gebhardt and colleagues28 report that HA levels decreased by 50% following topical administration of the corticosteroid dexamethasone. Unfortunately, research of the effects of corticosteroid treatment on HA deposition in solid tumors is limited. Further study may be needed to fully understand its capabilities in solid tumor treatments.
Additional agents that have been reported to inhibit the production of HA by HAS include 4-methylumbelliferone (MU). 4-Methylumbelliferone reportedly synergizes well with gemcitabine in cancer cell lines, with little activity as a single agent. One interesting observation made by Yoshihara and colleagues29 was that MU could decrease the incidence of metastasis in in vivo models for melanoma. 4-Methylumbelliferone also shows activity against squamous cell carcinoma, and prostate and esophageal cancer cell lines, reducing proliferation and migration. 4-Methylumbelliferone (also known as hymecromone) is used clinically as a choleretic and antispasmodic and is sold under multiple commercial names in countries throughout the world (e.g., Adesin C in Japan). It has entered clinical trials in the United States for chronic hepatitis infections (clinicaltrials.gov, NCT00225537), but results from its phase II trial have gone unreported and are unclear. Yet MU is the subject of multiple recent publications and represents an active area of continuing research.30,31
While inhibiting its synthesis has been one approach, promoting breakdown of HA has also been an area of active research too. The hyaluronidases, which include 6 gene isoforms including HYAL1, HYAL2, HYAL3, HYAL4, PHYAL1, and PH20, balance HA levels and overproduction by catabolizing HA to monosaccharides and disaccharides, and smaller HA fragments, acting much as endo-β-acetyl-hexosaminidases. HYAL1 and HYAL2 are the most active, catabolizing as much as 30% of total HA each day in mammals. Hyaluronidase treatment has been proposed as a means to enhance degradation of HA overproduction and accumulation. Administration of hyaluronidase has been shown to synergize well with chemotherapeutics, in multiple tumor types, including pancreas, breast, and brain tumors, as well as in melanoma and sarcoma. The synergy is thought to arise from enhanced penetration of drugs gained following reductions of intratumoral pressures brought on by reduction in HA levels. In some models, the HA degradation may affect the signaling mediated by HA receptors such as CD44.
In preclinical models, hyaluronidase appears to decrease innate chemoresistance in spheroid culture tumor models, enhancing drug penetration and efficacy.32 In xenograft models, additional reports demonstrate enhanced doxorubicin penetration in hyaluronidase-treated tumors.33 These improvements in penetration can exceed 15-fold enhancement in intratumoral concentrations of drugs such as melphalan.34 These results are particularly encouraging as enhancing intratumoral drug concentrations is a continual battle in the effort to maximize both the efficacy and pharmacokinetic properties of drugs. This effort represents an approach, independent of any particular drugs' properties, which may enhance overall efficacy in a variety of conditions in patients.
Early pilot clinical trials used bovine hyaluronidase in efforts to modulate HA levels in patients. Some of these clinical trials are reviewed in Baumgartner's35 report. One recurring observation was that despite its limitations, hyaluronidase enhanced efficacy in a variety of trial designs. One study demonstrated enhanced activity in the combination of vinblastine and hyaluronidase in Kaposi sarcoma, resulting in reduced recurrence when compared with vinblastine alone.36 Reduced recurrence was also reported by another study looking at carboplatin and etoposide treatment in brain malignancy.37 With a short infusion of hyaluronidase, there was a significant improvement of event-free survival and overall survival. Lastly, this synergism was also seen in patients with bladder cancer where intravesical administration of bovine hyaluronidase reduced recurrence from 32% to 7% with the addition of hyaluronidase (P < 0.05).38
One particular limitation of bovine hyaluronidase has been the development of allergic reactions to this enzyme of bovine origin. As many as 32% of patients were reported to be preimmune to bovine hyaluronidase formulations, resulting in various inflammatory responses, including anaphylaxis.35 Patients who do not already possess cross-reacting antibodies prior to treatment quickly develop antibodies that may preclude any chronic usage of a bovine hyaluronidase-containing regimen.
A recombinant, human hyaluronidase was recently developed (Hylenex) to alleviate some of the major shortcomings of bovine hyaluronidase. Some of the early clinical studies demonstrated that recombinant hyaluronidase did not induce allergic reactions in healthy volunteers following intradermal administration.39 Hylenex is now Food and Drug Administration approved for improved dispersion and delivery of various injectable drugs.39 Recombinant hyaluronidase is currently in clinical trials for multiple indications, including a partially randomized phase I/II trial in patients with newly diagnosed metastatic pancreatic cancer (clinicaltrials.gov, NCT01959139). As HA is synthesized in many locations in the body and used in many normal functions, development of adverse effects such as joint pain has been of particular concern. One report had suggested that inflammation or pain in joints was controlled and alleviated with administration of corticosteroids.39 In the completed clinical trial of single-agent pegylated recombinant hyaluronidase, PEGPH20 (at 50 μg/kg), there was grade 3 muscle/joint pain, whereas doses of 0.5 and 0.75 μg/kg appeared to be well tolerated.40
Because of its elevated levels and its role in desmoplasia in PDAC, HA is currently being pursued as a drug target in clinical trials in patients with advanced PDAC (clinicaltrials.gov, NCT01839487). The initial results of a randomized clinical trial of nanoparticle paclitaxel + gemcitabine, with or without pegylated hyaluronidase PEGPH20, have just been reported, and tumors with high hyaluronic acid levels appear to confer a longer survival after treatment with the triple-drug regimen. The overall results of that important trial are still pending.41
There has been considerable controversy as to whether compression of the tumor vasculature is caused by HA-induced fluid pressure or by solid tissue stress.15,42–44 However, it is fair to say that HA is a reasonable candidate for therapeutic intervention.
Collagen
Collagens I, III, and IV are expressed and deposited at high levels in PDAC. As a major component of desmoplasia, collagens too have been pursued as a potential target in the development of stromal targeting agents (Table 1).45–53 Tumor collagen levels have been shown to correlate negatively with macromolecule penetration.54 In U87 (glioblastoma) and LS174T (colon adenocarcinoma) xenograft tumor model studies, Netti and colleagues10 demonstrated that high collagen levels were associated with significantly decreased diffusion of macromolecules, such as immunoglobulin G antibodies, resulting in 2.1-fold decreases in diffusion coefficients in high versus low collagen content models. Importantly, when collagenases were added to the model system, the decrease in diffusion in high collagen content tumors was abrogated. This and other reports found that collagen content directly impacts diffusion rates and suggested that other stromal macromolecules may also be perturbed in high collagen content tumors.
TABLE 1.
Potential Roles of Collagen in Pancreatic Cancer Biology and Therapeutic Intervention
| Finding | References |
|---|---|
| Collagen I allows pancreatic cancer cells to override checkpoint arrest induced by gemcitabine, i.e., collagen I promotes gemcitabine resistance in pancreatic cancer. | 45 |
| Losartan (an angiotensin II receptor antagonist with antifibrotic activity) induced inhibition of collagen I synthesis (and enhanced the penetration and efficacy of nanotherapeutics [e.g., Doxil] in pancreatic cancer models. | 46 |
| Pancreatic cancer cells grown in 3D collagen gels induced Snail expression, which resulted in matrix metalloproteinase (MMP)–dependent invasion. This work also indicated that the Snail induction accelerates EMT. | 47 |
| Collagen I promotes metastasis in PDAC by increasing motility and EMT. | 48 |
| Relaxin, used to up-regulate matrix-degrading enzymes (MMPs), decreased the length of preexisting collagen fibers and decreased relative diffusive hindrance in the tumor. | 49 |
| Pancreatic cancer cells adhering to ECM proteins including collagen grew better and were more resistant to therapies. | 50 |
| Inhibition of collagen signaling reduces PDAC tumor growth (particularly in SPARC-deficient mice). Collagen can drive EMT. | 51 |
| Collagen I supports the maintenance of ALDH+ pancreatic cancer stem cells. | 52 |
| Treatment with an angiotensin II receptor blocker, candesartan (also known to inhibit TGF-β signaling and collagen synthesis), improves activity of gemcitabine in a genetically engineered mouse model. | 53 |
Collagens can act functionally through interaction with integrins. Integrin subunit expression has been shown to be dysregulated in some cancers. Integrin receptors, such as αvβ3, αvβ6, and α6β4, are thought to be involved in cancer progression.55–57 In addition to signaling via integrin association in cancer, collagens can function in a similar fashion to HA by decreasing penetration of macromolecules into tumor tissues, presumably resulting in poor drug penetration and patient outcomes in PDAC.
In a prior report, Erkan and colleagues58 reported that there existed a positive correlation between patient survival and collagen deposition. The difference in observations made by Erkan et al58 and our report, which found that high collagen levels in patients' tumor were associated with poor patient survival,17 might be explained by their use of the aniline blue dye, which captures all collagens and our use of the collagen- or HA-specific antibodies. It may even suggest that loss of specific collagen subtypes may account for the overall reduction in collagen level Erkan and colleagues found associated with the poor survival cohort. Another explanation for difference in findings might be found in the treatments that the patients received that may have altered the collagen deposition that was observed at the time of resection.
Our more recent findings17 indeed suggest that surrogate markers of desmoplasia are observed in metastatic lesions of PDAC. Considering multiple sites of metastasis, it appears that both primary tumors and metastatic lesions show comparable levels of desmoplasia, including high levels of collagens I, III, and IV. Our data from patient-matched primary and metastasis samples are consistent with the notion that high levels of desmoplasia are observed in both primary tumors and metastatic lesions. Acquisition of pretreatment and posttreatment tissue samples or biopsies would allow an analysis of the effects of treatment on the deposition of ECMs in the course of chemotherapeutic intervention. As we looked only at excised tissue samples, our data reflected most directly the retention of ECMs following treatment. However, our data, taken together with studies demonstrating a negative correlation between ECMs such as collagen and the penetration of macromolecules, suggest that patients with fibrotic primary tumors and metastatic lesions would benefit from therapies targeting the tumor stroma.54 The cellular lineage of the myofibroblasts giving rise to desmoplasia in PDAC is not entirely clear, although it has been suggested that they arise from the primary tumor.22 Certainly, in the liver, where PDAC commonly metastasizes, desmoplasia is also a recognized feature of tumors arising spontaneously. Our data suggest that the noncell autonomous tumor-signaling mechanisms that give rise to desmoplasia appear to be retained postmetastasis. A recent study by Suetsugu et al59 demonstrates that stellate cells could accompany pancreatic cancer cells to distant metastatic sites.
Besides ECM deposition and desmoplasia, collagen has also been shown to play an important role in EMT and metastasis (Table 1). For instance, Shields et al47,60 reported that collagen induces the expression of Snail gene, which in turn accelerates EMT. In addition, Collagen I was reported to support the maintenance of ALDH+ pancreatic cancer stem cells (Table 1).52
There have been a few attempts to decrease collagen production. These have included losartan, relaxin, and candesartan (Table 1).
TGF-β Signaling
Increased TGF-β expression has been observed in multiple cancers, including breast, lung, hepatocellular, and pancreatic cancer. Importantly, TGF-β expression has been correlated with tumor metastasis and progression and poor outcomes in patients.61 In prior reports, it has become clear that TGF-β maintains both tumor suppressing and promoting effects, depending on the model.62 Some have suggested that TGF-β is initially tumor suppressive, but becomes promoting as the stage of disease progresses.61
Transforming growth factor β and its protein family members have been associated with aggressive and invasive tumors, as well as associated with the activation of the stellate cell that leads to pancreatic desmoplasia. Family member proteins are multifunctional and are known to be associated with a variety of different functions, including immune cell regulation, migration, and cell proliferation.63 The TGF-β family of ligands includes TGF-β1, TGF-β2, and TGF-β3. Transforming growth factor β–mediated signaling propagates through 1 of 3 TGF-β receptors (TGF-βRI to TGF-βRIII) and Smad signaling proteins. In many cases, TGF-β binds to a heterodimer of TGF-βRI and TGF-βRII.
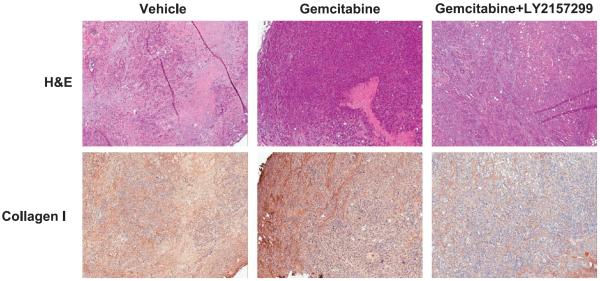
Smad4/DPC4 has been reportedly inactivated in more than 50% of pancreatic tumors. The apparent discrepancy between the correlation of TGF-β and poor outcomes in patients and the finding that Smad4/DPC4 is inactivated in the cancer cells of the majority of PDAC patients suggests that SMAD4-independent pathways play an important role in the progression of some tumors.64 Wild-type Smad4/DPC4 appears to be associated with decreased invasion and improved outcomes in patients.65,66 Some of the Smad-independent TGF-β signaling pathways that have been reported include Ras, PI3K, RhoA, and MAP3K1.67–70 Some of the early findings on the role of TGF-β in cancer came from tetracycline-inducible transgenic MMTV–TGF-β mouse models. In their report, Muraoka-Cook and colleagues71 found a 10-fold enhanced rate of lung metastases following oncogenic transformation by MMTV-PyVT expression and TGF-β induction. Despite the worries that the pleiotropic nature of TGF-β may preclude therapeutic development and intervention, at least in cancer, some initial studies report that might not be the case.72–74 Of note, TGF-β haploinsufficiency itself can inhibit excessive fibrosis and progression of precancerous lesions in preclinical models of pancreatic cancer.75 We recently assessed the effects of the TGF-βRI inhibitor, LY2157299, on tumor stroma using a genetically engineered mouse model for pancreatic cancer. As shown in Figure 1, tumors in mice treated with the combination of LY2157299 and gemcitabine showed a marked reduction in overall stromal content; hematoxylin-eosin staining, top panel) and collagen I deposition (bottom panel) when compared with vehicle-treated mice or gemcitabine-only–treated mice. Our results suggest that TGF-βR inhibition may be an effective means of ameliorating the effects of desmoplasia in pancreatic cancer, which may in turn improve the efficacy of current treatment regimens. With these findings, TGF-β antagonists are currently under investigation as therapeutic agents in a variety of indications (e.g., galunisertib [LY2157299], clinicaltrials.gov, NCT01373164), including hepatocellular carcinoma, pancreatic cancer, or myelodysplastic syndromes.
FIGURE 1.
The effects of TGF-βRI inhibition on tumor stroma in tumor-bearing LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mice. Mice bearing pancreatic tumors with volumes of at least 100 mm3 were randomized into the 1 of the following treatment groups: vehicle, gemcitabine (80 mg/kg, every 3 days× 4, i.p.) or gemcitabine + LY2157299 (75 mg/kg, twice a day, orally). Upon completion of treatment, mice were killed, and tissues were harvested, formalin fixed, and embedded into paraffin. Collagen I was assessed in the tumor tissues with standard immunohistochemistry techniques. The hematoxylin-eosin staining (top panel) shows decreased stromal content in the tumor tissues treated with the combination compared with the gemcitabine-only– or vehicle-treated tumor tissues. Collagen I staining (bottom panel) was also shown significantly decreased in the combination treatment compared with the gemcitabine only or vehicle treatment.
Epithelial-To-Mesenchymal Transition
Additional hypotheses to address problems associated with desmoplasia include targeting EMT that is induced by TGF-β expression and secretion by cancer-associated fibroblasts (CAFs). It is generally understood that mesenchymal cells are generally more resistant to drug treatment than epithelial cells. Indeed, erlotinib resistance in head and neck squamous cell carcinoma is correlated to increased Zeb-1 expression and decreased E-cadherin expression. Increased Zeb-1 and decreased E-cadherin are classic markers of EMT, and these changes follow TGF-β ligand binding and signal transduction.76,77 Targeting EMT may prove to be a viable approach as multiple studies have shown improved antitumor efficacy, such as with the mucin-reactive PAM4 antibody or with the secreted clusterin–reactive antibody AB-16B5 (clinicaltrials. gov, NCT02412462).78–80
Secreted Protein, Acidic, And Rich In Cysteine
SPARC (secreted protein, acidic, and rich in cysteine) is an albumin-binding protein that is overexpressed in multiple types of cancer.81–83 SPARC expression noted in the stroma in pancreatic cancer but not in tumor cells has been associated with poor survival.84–86 It has been noted that nanoparticle albumin (nab) paclitaxel had shown clinical antitumor activity in several cancer types that overexpressed SPARC.87,88 In a 66-patient pilot trial combining nab-paclitaxel with gemcitabine, an overall response rate (complete plus partial response) of 45% was noted with a median survival of 11.2 months and a 1-year survival of 48%.16 In that trial, the expression of SPARC in the stroma but not in the tumor was correlated with improved survival.
In a multicenter, multiple-country, 861-patient randomized trial of nab-paclitaxel + gemcitabine versus gemcitabine as a single agent, the 2-drug regimen gave a response rate of 23% versus 7% (with the single-agent gemcitabine), median survival of 8.5 months versus 6.7 months (hazard ratio, 0.72, P < 0.001), and 35% survival at 1 year versus 22%, 9% versus 4% at 2 years, and 4% long term (>3 years) for that combination versus 0% on the gemcitabine-alone control arm.7,89 Hidalgo and colleagues90 utilized a different antibody treatment to determine if the presence of SPARC by immunohistochemistry predicted for survival with the nab-paclitaxel + gemcitabine regimen. Despite confirming the results of the pilot trial,16 Hidalgo and colleagues90 could not find an association between SPARC levels and efficacy in patients with metastatic pancreatic cancer enrolled in this definitive 861-patient study (although only 256 patients had enough specimen to have stromal SPARC be evaluated).
In summary, although finding SPARC in pancreatic cancer led to evaluation of nab-paclitaxel because it was believed that SPARC in the tumor stroma would cause accumulation of nab-paclitaxel in the tumor, a very large study did not confirm that to be the case. We are grateful, however, that the nab-paclitaxel + gemcitabine has now become one of the standard of care regimens for patients with advanced pancreatic cancer.
Of special interest is a follow-up trial by Alvarez and colleagues.91 In that trial using endoscopic ultrasound elastography on patients with advanced pancreatic cancer treated with nab-paclitaxel + gemcitabine, they noted a decrease in stiffness of the tumor and on biopsies noted decreases in density of CAFs as well as decreased and disorganized collagen. Their conclusion was that nab-paclitaxel + gemcitabine regimen caused a remarkable alteration of cancer stroma that resulted in “tumor softening.”91
AN ENTIRELY DIFFERENT APPROACH TO THE TUMOR MICROENVIRONMENT
As noted earlier, the tumor microenvironment has many components. It is very tough to know how one is going to be able to “control” the various components of the microenvironment including (at least) (a) tumor cells, (b) stroma, (c) a tumor-friendly immune environment, (d) a special adapted metabolism, (e) the invasive EMT phenotype, and (f) an aggressive tumor stem cell compartment.
In a recent study, Ozdemir and colleagues92 demonstrated that depletion of CAFs in a genetically engineered mouse model for PDAC actually induced immunosuppression, accelerated tumor progression, and reduced survival. In a second study, Rhim et al93 showed that although deletion of sonic hedgehog (Shh) in the KPC mouse model resulted in reduced stromal content, it also induced more proliferative and aggressive tumors. These findings suggest that complete destruction or early inhibition of some components of the tumor microenvironment can potentially promote tumor growth.
One way our team and others94,95 are beginning to think about targeting the microenvironment is that instead of using a “brute force” destruction of the microenvironment, the microenvironment needs to be returned to normalcy, or reengineered or “orchestrated” to a normal-like state (immunologically and otherwise).
Figure 2 details how we conceptualize this approach to reach normalcy—which is very much like “tuning” on an amplifier.
FIGURE 2.
Conceptualization of utilizing therapeutics to orchestrate or reengineer the microenvironment to a state of normalcy.
Some of the potential therapeutics being considered to restore normalcy (of the entire microenvironment as well as specific aspects of the microenvironment) are outlined in Table 294,96–103 As outlined in the table, there are some agents such as the vitamin D analog described by Sherman et al, which clearly is a step toward 1 agent restoring the entire pancreatic cancer microenvironment to a state of normalcy. For example, a vitamin D derivative can decrease interleukin 6, decrease CXCL12, decrease collagen, reverse EMT, profoundly increase tumor infiltration of CD8+ cells, and sensitize to the agent gemcitabine.94 As also noted in Table 2, the bromodomain inhibitors are likely to be able to restore that of normalcy. Our working hypothesis is that many of the agents will work by manipulating global transcriptional control (through interactions between superenhancers and transcription factors).
TABLE 2.
List of Potential Therapeutic Agents That Could Restore (Reengineer or Orchestrate the Pancreatic Cancer Microenvironment to a State of Normalcy)
| Areas of Microenvironment to Reset | Agents | Mechanism of Action | References |
|---|---|---|---|
| Multiple | Paricalcitol | Global transcriptional control | 94 |
| Multiple | 5-Azacytidine | Global hypomethylation | 96 |
| Multiple | Istodax | Pan-HDAC inhibitor | 97 |
| Multiple | Bromodomain inhibitors | Global transcription control | 98 |
| Multiple | Minnelide | Global transcription control | 99 |
| Immunologic | Idelalisib | PI3K p110delta | 100 |
| Immune suppression to affect immunity | Anti-CXCR4 | Inactivation of regulatory T cells | 101 |
| Immunologic | Down-regulate CXCR4 | 102 | |
| Decrease myeloid-derived suppressor cells and regulatory T cells | 103 | ||
| Reversal of EMT | TP0903 | AXL inhibitor | In development |
In summary, we believe many of the approaches outlined in Table 2 have an opportunity to have multiple beneficial effects on the tumor microenvironment (hence drive it to normalcy).
CONCLUSION
To try to improve survival for patients with pancreatic cancer, multiple investigators are trying to target the microenvironment. It appears that the first attempt to do that with the use of the nabpaclitaxel (originally thought to target SPARC to deliver paclitaxel to the microenvironment—and now we are less sure of that) did improve survival. It does appear that the nab-paclitaxel + gemcitabine does decrease the presence of CAFs as well as “soften the tumor” as measured by magnetic resonance imaging elastography in patients. The tumor is also less fibrotic on histopathology,
There are additional methods being developed to try to decrease HA and collagen in the microenvironment, hoping for greater delivery of therapeutics. The pegylated hyaluronidase is beginning to show some promise in the area.
However, the use of compounds such has vitamin D derivatives, bromodomain inhibitors, and so on appears to be the future direction for trying to “orchestrate” the microenvironment into a more normal state (rather than just trying to destroy it). These agents can give global transcriptional control (probably via interactions with superenhancers), which gives a whole new approach to the microenvironment. There is promise that one can turn the tumor microenvironment from a tumor supportive one to a nonsupportive one. Ongoing clinical trials are carefully testing that hypothesis.
Acknowledgments
This work was supported by a Stand Up to Cancer Translational Research Grant, a Program of the Entertainment Industry Foundation administered by the American Association for Cancer Research (SUC2-AACR-DT0509). This work was also supported in part by R01 (CA169281) and U01 (CA128454) grants from the national Institutes of Health/National Cancer Institute, by philanthropy from the Katz Family Foundation, and by a grant from the National Foundation for Cancer Research.
Footnotes
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CACancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamphorst JJ, Cross JR, Fan J, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 9.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 10.Netti PA, Berk DA, Swartz MA, et al. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 11.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houten L, Reilley AA. An investigation of the cause of death from cancer. J Surg Oncol. 1980;13:111–116. doi: 10.1002/jso.2930130205. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neesse A, Frese KK, Bapiro TE, et al. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci U S A. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nabpaclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faouzi S, Le Bail B, Neaud V, et al. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30:275–284. doi: 10.1016/s0168-8278(99)80074-9. [DOI] [PubMed] [Google Scholar]
- 19.Yen TW, Aardal NP, Bronner MP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–134. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- 20.Stylianopoulos T, Martin JD, Chauhan VP, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand P, Girard N, Delpech B, et al. Hyaluronan (hyaluronic acid) and hyaluronectin in the extracellular matrix of human breast carcinomas: comparison between invasive and non-invasive areas. Int J Cancer. 1992;52:1–6. doi: 10.1002/ijc.2910520102. [DOI] [PubMed] [Google Scholar]
- 24.Anttila MA, Tammi RH, Tammi MI, et al. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–155. [PubMed] [Google Scholar]
- 25.Auvinen P, Tammi R, Parkkinen J, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156:529–536. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setala LP, Tammi MI, Tammi RH, et al. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br J Cancer. 1999;79:1133–1138. doi: 10.1038/sj.bjc.6690180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theocharis AD, Tsara ME, Papageorgacopoulou N, et al. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta. 2000;1502:201–206. doi: 10.1016/s0925-4439(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 28.Gebhardt C, Averbeck M, Diedenhofen N, et al. Dermal hyaluronan is rapidly reduced by topical treatment with glucocorticoids. J Invest Dermatol. 2010;130:141–149. doi: 10.1038/jid.2009.210. [DOI] [PubMed] [Google Scholar]
- 29.Yoshihara S, Kon A, Kudo D, et al. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005;579:2722–2726. doi: 10.1016/j.febslet.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 30.Nagy N, Kuipers HF, Frymoyer AR, et al. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol. 2015;6:123. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yates TJ, Lopez LE, Lokeshwar SD, et al. Dietary supplement 4-methylumbelliferone: an effective chemopreventive and therapeutic agent for prostate cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohno N, Ohnuma T, Truog P. Effects of hyaluronidase on doxorubicin penetration into squamous carcinoma multicellular tumor spheroids and its cell lethality. J Cancer Res Clin Oncol. 1994;120:293–297. doi: 10.1007/BF01236386. [DOI] [PubMed] [Google Scholar]
- 33.Beckenlehner K, Bannke S, Spruss T, et al. Hyaluronidase enhances the activity of adriamycin in breast cancer models in vitro and in vivo. J Cancer Res Clin Oncol. 1992;118:591–596. doi: 10.1007/BF01211802. [DOI] [PubMed] [Google Scholar]
- 34.Muckenschnabel I, Bernhardt G, Spruss T, et al. Hyaluronidase pretreatment produces selective melphalan enrichment in malignant melanoma implanted in nude mice. Cancer Chemother Pharmacol. 1996;38:88–94. doi: 10.1007/s002800050452. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner G. The impact of extracellular matrix on chemoresistance of solid tumors—experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131:1–2. [PubMed] [Google Scholar]
- 36.Smith KJ, Skelton HG, Turiansky G, et al. Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of Kaposi's sarcoma. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR) J Am Acad Dermatol. 1997;36:239–242. doi: 10.1016/s0190-9622(97)70288-3. [DOI] [PubMed] [Google Scholar]
- 37.Pillwein K, Fuiko R, Slavc I, et al. Hyaluronidase additional to standard chemotherapy improves outcome for children with malignant brain tumors. Cancer Lett. 1998;131:101–108. doi: 10.1016/s0304-3835(98)00205-5. [DOI] [PubMed] [Google Scholar]
- 38.Maier U, Baumgartner G. Metaphylactic effect of mitomycin C with and without hyaluronidase after transurethral resection of bladder cancer: randomized trial. J Urol. 1989;141:529–530. doi: 10.1016/s0022-5347(17)40881-0. [DOI] [PubMed] [Google Scholar]
- 39.Yocum RC, Kennard D, Heiner LS. Assessment and implication of the allergic sensitivity to a single dose of recombinant human hyaluronidase injection: a double-blind, placebo-controlled clinical trial. J Infus Nurs. 2007;30:293–299. doi: 10.1097/01.NAN.0000292572.70387.17. [DOI] [PubMed] [Google Scholar]
- 40.Shepard HM, Frost GI, Rybak ME, et al. Targeting hyaluronan (HA) in tumor stroma: translational evaluation of pegylated hyaluronidase (PEGPH20, P) in animal models and patients (PTS) with advanced solid tumors. Presented at the 2010 ASCO-NCI-EORTC Annual Meeting on Molecular Markers in Cancer; Hollywood, Florida. October 18–20, 2010; abstract 114. [Google Scholar]
- 41.Hingorani SR, Harris WP, Hendifar AE, et al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: interim results of a randomized phase II study. J Clin Oncol. 2015;33(suppl) abstr 4006. [Google Scholar]
- 42.Chauhan VP, Boucher Y, Ferrone CR, et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell. 2014;26:14–15. doi: 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DelGiorno KE, Carlson MA, Osgood R, et al. Response to Chauhan et al.: interstitial pressure and vascular collapse in pancreas cancer—fluids and solids, measurement and meaning. Cancer Cell. 2014;26:16–17. doi: 10.1016/j.ccr.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dangi-Garimella S, Krantz SB, Barron MR, et al. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP–mediated expression of HMGA2. Cancer Res. 2011;71:1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diop-Frimpong B, Chauhan VP, Krane S, et al. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shields MA, Dangi-Garimella S, Krantz SB, et al. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase–dependent collagen invasion. J Biol Chem. 2011;286:10495–10504. doi: 10.1074/jbc.M110.195628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shintani Y, Hollingsworth MA, Wheelock MJ, et al. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–11753. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- 49.Brown E, McKee T, diTomaso E, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto H, Murakami T, Tsuchida K, et al. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Rivera LB, Carbon JG, Aguilera K, et al. Strategies to combat hypoxia-driven PDAC invasion after antiangiogenic therapy. Presented at the AACR Pancreatic Cancer Special Conference; 2012. abstract A24. [Google Scholar]
- 52.Rasheed ZA, Jung C, Huang A, et al. The extracellular matrix regulates pancreatic cancer stem cell functionvia focal adhesion kinase signaling. Presented at the AACR Pancreatic Cancer Special Conference; 2012. abstract A70. [Google Scholar]
- 53.Tada M, Omata M, Moses HL, et al. The exploration of novel strategy for treatment of pancreatic ductal adenocarcinoma targeting tumor microenvironment. Presented at the AACR Pancreatic Cancer Special Conference; 2012. abstract A85. [Google Scholar]
- 54.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22:276–284. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 55.Grzesiak JJ, Ho JC, Moossa AR, et al. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas. 2007;35:293–301. doi: 10.1097/mpa.0b013e31811f4526. [DOI] [PubMed] [Google Scholar]
- 56.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 57.Linder S, Castanos-Velez E, von Rosen A, et al. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1321–1327. [PubMed] [Google Scholar]
- 58.Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Suetsugu A, Snyder CS, Moriwaki H, et al. Imaging the interaction of pancreatic cancer and stellate cells in the tumor microenvironment during metastasis. Anticancer Res. 2015;35:2545–2551. [PubMed] [Google Scholar]
- 60.Shields MA, Ebine K, Sahai V, et al. Snail cooperates with KrasG12D to promote pancreatic fibrosis. Mol Cancer Res. 2013;11:1078–1087. doi: 10.1158/1541-7786.MCR-12-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 62.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 63.Naber HP, ten Dijke P, Pardali E. Role of TGF-beta in the tumor stroma. Curr Cancer Drug Targets. 2008;8:466–472. doi: 10.2174/156800908785699342. [DOI] [PubMed] [Google Scholar]
- 64.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 65.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhowmick NA, Ghiassi M, Bakin A, et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulder KM, Morris SL. Activation of p21ras by transforming growth factor beta in epithelial cells. J Biol Chem. 1992;267:5029–5031. [PubMed] [Google Scholar]
- 69.Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 70.Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10870–10876. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- 71.Muraoka-Cook RS, Kurokawa H, Koh Y, et al. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–9011. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- 72.Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang YA, Dukhanina O, Tang B, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zion O, Genin O, Kawada N, et al. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas. 2009;38:427–435. doi: 10.1097/MPA.0b013e3181967670. [DOI] [PubMed] [Google Scholar]
- 75.Adrian K, Strouch MJ, Zeng Q, et al. Tgfbr1 haploinsufficiency inhibits the development of murine mutant Kras-induced pancreatic precancer. Cancer Res. 2009;69:9169–9174. doi: 10.1158/0008-5472.CAN-09-1705. [DOI] [PubMed] [Google Scholar]
- 76.Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res. 2009;15:532–542. doi: 10.1158/1078-0432.CCR-08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Postigo AA. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. EMBO J. 2003;22:2443–2452. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gold DV, Karanjawala Z, Modrak DE, et al. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380–7387. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 79.Gold DV, Modrak DE, Ying Z, et al. New MUC1 serum immunoassay differentiates pancreatic cancer from pancreatitis. J Clin Oncol. 2006;24:252–258. doi: 10.1200/JCO.2005.02.8282. [DOI] [PubMed] [Google Scholar]
- 80.Ocean AJ, Pennington KL, Guarino MJ, et al. Fractionated radio-immunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: a phase 1 trial. Cancer. 2012;118:5497–5506. doi: 10.1002/cncr.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 82.Socinski MA, Manikhas GM, Stroyakovsky DL, et al. A dose finding study of weekly and every-3-week nab-paclitaxel followed by carboplatin as first-line therapy in patients with advanced non–small cell lung cancer. J Thorac Oncol. 2010;5:852–861. doi: 10.1097/jto.0b013e3181d5e39e. [DOI] [PubMed] [Google Scholar]
- 83.Yardley DA, Daniel BR, Inhorn RC, et al. SPARC microenvironment signature (SMS) analysis of a phase II trial of neoadjuvant gemcitabine (G), epirubicin (E), and nab-paclitaxel (nab-P) in locally advanced breast cancer (LABC) J Clin Oncol. 2010;28(15s) 2010; (suppl; abstr 10574) 28, 15s (suppl; abstr 10574) [Google Scholar]
- 84.Guweidhi A, Kleeff J, Adwan H, et al. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg. 2005;242:224–234. doi: 10.1097/01.sla.0000171866.45848.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 86.Watkins G, Douglas-Jones A, Bryce R, et al. Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids. 2005;72:267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 87.Koukourakis MI, Giatromanolaki A, Brekken RA, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non–small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 88.Massi D, Franchi A, Borgognoni L, et al. Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol. 1999;30:339–344. doi: 10.1016/s0046-8177(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 89.Goldstein D, Maraghi RHE, Hammel P, et al. Updated survival from a randomized phase III trial (MPACT) of nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients (pts) with metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2014;32(suppl 3):2014. abstr 178^) 32, abstr 178. [Google Scholar]
- 90.Hidalgo M, Plaza C, Illei P, et al. SPARC analysis in the phase III MPACT trial of nab-paclitaxel (nab-p) plus gemcitabine (gem) vs gem alone for patients with metastatic pancreatic cancer. Ann Oncol. 2014;25(suppl_2):ii105–ii117. [Google Scholar]
- 91.Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor–mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63:1769–1781. doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Carvalho DD, You JS, Jones PA. DNA methylation and cellular reprogramming. Trends Cell Biol. 2010;20:609–617. doi: 10.1016/j.tcb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ischenko I, Petrenko O, Hayman MJ. Analysis of the tumor-initiating and metastatic capacity of PDX1-positive cells from the adult pancreas. Proc Natl Acad Sci U S A. 2014;111:3466–3471. doi: 10.1073/pnas.1319911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sahai V, Kumar K, Knab LM, et al. BET bromodomain inhibitors block growth of pancreatic cancer cells in three-dimensional collagen. Mol Cancer Ther. 2014;13:1907–1917. doi: 10.1158/1535-7163.MCT-13-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chugh R, Sangwan V, Patil SP, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Greeno E, Borazanci E, Gockerman J, et al. Phase I dose escalation and pharmokinetic study of 14-O-phosphonooxymethyltriptolide. Presented at the AACR 2015 Annual Meeting; Philadelphia, PA. 2015. abstract CT207. [Google Scholar]
- 102.Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell–mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]