Abstract
Apoptosis is a form of programmed cell death that is critical for basic human development and physiology. One of the more important surprises in cell biology in the last two decades is the extent to which mitochondria represent a physical point of convergence for many apoptosis inducing signals in mammalian cells. Mitochondria not only adjudicate the decision of whether or not to commit to cell death, but also release toxic proteins culminating in widespread proteolysis, nucleolysis, and cell engulfment. Interactions among Bcl-2 family proteins at the mitochondrial outer membrane controls the release of these toxic proteins, and by extension control cellular commitment to apoptosis. This pathway is particularly relevant to cancer treatment, as most cancer chemotherapies trigger mitochondrial mediated apoptosis. In this review, we discuss recent advances in the Bcl-2 family interactions, their control by upstream factors, and how the mitochondria itself alters these interactions. We also highlight recent clinical insights into mitochondrial mediated apoptosis and novel cancer therapies that exploit this pathway.
Introduction
Mitochondria adjudicate the cell death decision in response to many physiological and therapeutic stimuli. The review we highlight seminal and recent advances on how mitochondria and the Bcl-2 family of proteins regulate cell death. In particular we discuss recent advances in the Bcl-2 family interactions, their control by upstream factors, and how the mitochondria itself alters these interactions. We also highlight recent clinical insights into mitochondrial mediated apoptosis and how cancer therapies that exploit this pathway.
Keywords: Apoptosis, Bcl-2, Mitochondria, Cancer
In addition to their long standing role in energetics, mitochondria represent a point of convergence for many cell death signals in mammalian cells. In this review, we summarize seminal and recent advances on how the Bcl-2 family interacts at mitochondria to adjudicate cell death. In addition to the well-studied interactions between the Bcl-2 family, we highlight recent insights into how upstream signals alter the Bcl-2 family, how the mitochondrial environment influences the commitment to death, and the role of mitochondria and the Bcl-2 family in the response to cancer therapeutics. We also discuss the regulation of non-apoptotic death by mitochondria.
Mitochondria regulate apoptosis in vertebrate cells
In mammalian cells, the mitochondrion is a point of convergence for many apoptosis-inducing cues. Interactions at the mitochondrion ultimately determine whether a cell survives or dies in response to many physiologic or therapeutic cell death stimuli. Though early studies reported experimentally controllable cell death (Kerr et al., 1972; Lockshin and Williams, 1965; Lockshin and Zakeri, 2001), it became clear that cell death was a predictable, biologically programmed phenomenon after carefully following the development of single cells in C. elegans (Sulston, 1976). The subsequent discovery of genes regulating cell death in C. elegans demonstrated that cell death could be genetically programmed (Ellis and Horvitz, 1986). Furthermore, homologous genes in mammalian cells suggested the importance of cell death in human physiology and disease (Hengartner and Horvitz, 1994; Yuan et al., 1993) .In particular the caspase family of proteases, which are activated during apoptosis and result in the irreversible destruction of a cell, were found in multiple species (Yuan et al., 1993). In many species, including drosophila, activation of caspases seems not to require mitochondrial participation (White et al., 1996). In contrast, in many mammalian cells the activation of caspases and cell death requires mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome c in response to many cell death stimuli (Liu et al., 1996).
Understanding cellular control of MOMP and release of cytochrome c from mitochondria was enabled by parallel studies into the BCL-2 oncogene (Bakhshi et al., 1985; Cleary and Sklar, 1985; Tsujimoto et al., 1985). These studies indicated that expression of the BCL-2 protein could prevent cell death (Vaux et al., 1988) and promote tumors (McDonnell et al., 1989; Strasser et al., 1990). A family of proteins with homology to BCL-2 (the Bcl-2 family proteins) were found to positively and negatively control the release of cytochrome c and other toxic proteins from the mitochondria (Cory and Adams, 2002; Danial and Korsmeyer, 2004). There are other forms of non-apoptotic programmed cell death (Fuchs and Steller, 2015), but this review will focus on forms of programmed cell death that involve the mitochondrion, with particular attention to the mitochondrial pathway of apoptosis.
Interactions among the Bcl-2 family members regulate commitment to cell death via mitochondrial permeabilization
Perhaps the first clue that the mitochondrion was a critical integrator of apoptotic signaling came with the observation that BCL-2 was localized to the mitochondrion (Hockenbery et al., 1990). The BCL-2 family comprises at least 12 proteins some of which promote and others of which inhibit the onset of apoptosis (Brunelle and Letai, 2009; Chipuk et al., 2010). To a rough approximation, the functional balance between these pro- and anti-apoptotic BCL-2 proteins at the mitochondria determines whether a cell commits to death or not. Both pro-and anti-apoptotic proteins share homology in up to 4 BH (BCL-2 Homology) domains. It should be noted that in addition to their well studies roles in mitochondrial mediated apoptosis, the Bcl-2 family has non apoptotic roles, including in mitochondrial respiration (Perciavalle et al., 2012), and mitochondrial division (Hoppins et al., 2011).
BAX and BAK are referred to as pro-apoptotic effector proteins and are required for mitochondrial mediated apoptosis. Indeed, a double knockout of Bax and Bak is sufficient to prevent mitochondrial mediated apoptosis in response to most insults (Lindsten et al., 2000; Wei et al., 2001). When activated, BAX and BAK oligomerize and form openings in the outer mitochondrial membrane that release cytochrome c (Gross et al., 1998; Wei et al., 2000). Additionally, a third effector protein with homology to BAX and BAK termed BOK appears to govern response to endoplasmic reticulum stress stimuli (Carpio et al., 2015). Loss of cytochrome c from the mitochondria results in the dATP or ATP dependent activation of caspase proteases via the formation of the apoptosome – a seven-fold symmetric complex containing cytochrome c and Apaf-1 (Acehan et al., 2002; Li et al., 1997; Zou et al., 1997). Note that the central role of the mitochondrion in facilitating caspase activation in vertebrates is not shared in drosophila and c. elegans, two important models in cell death studies.
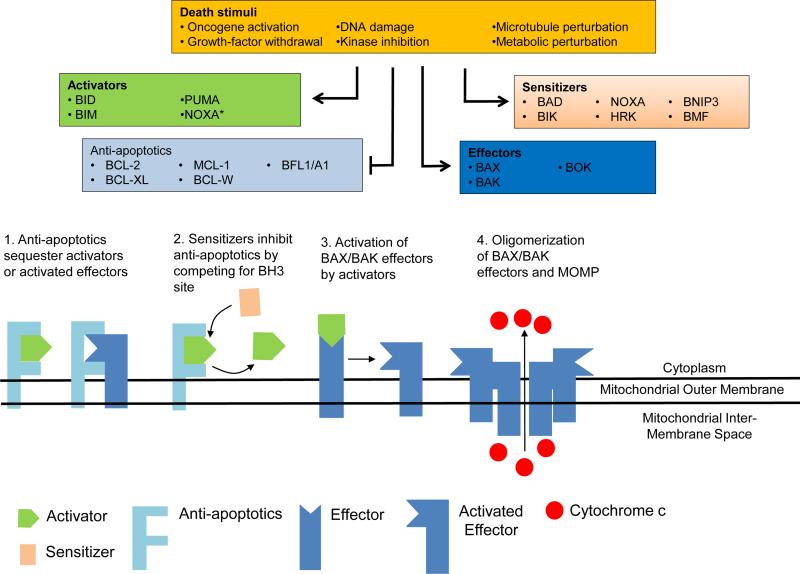
Several pro-apoptotic and anti-apoptotic Bcl-2 family proteins are responsible for the regulation of BAX and BAK activation. BIM (O'Connor et al., 1998) and Bid (Wang et al., 1996) are activator proteins that can directly activate BAX or BAK (Cheng et al., 2001; Kuwana et al., 2005; Letai et al., 2002). In the past decade it has emerged that PUMA (Nakano and Vousden, 2001; Yu et al., 2001) and more recently NOXA (Oda et al., 2000) are also direct activators (Chen et al., 2015; Dai et al., 2011; Kim et al., 2006). Direct activator proteins can be bound and sequestered by the anti-apoptotic proteins BCL-2, BCL-XL (Boise et al., 1993), MCL-1 (Kozopas et al., 1993), and BCL-W (Gibson et al., 1996) at the mitochondria, preventing the homo- and heterodimeric interactions among pro-apoptotic proteins that are necessary for MOMP (Cheng et al., 1996; Cheng et al., 2001). Finally, a subset of pro-apoptotic sensitizer proteins including BAD (Yang et al., 1995), NOXA (Oda et al., 2000), and HRK (Inohara et al., 1997) directly inhibit the anti-apoptotic proteins, but cannot potently directly activate BAX or BAK to induce apoptosis (Letai et al., 2002). The interactions between the effectors, activators, sensitizers, and anti-apoptotic proteins are described in Figure 1, and are described in greater detail below. It should be noted that the segregation of the Bcl-2 family into different categories is done for convenience. Particularly in the case of distinctions between activators and sensitizers these classifications are not absolute. For instance, while BIM and BID appear to have the most potent activating properties, other proteins, notably PUMA and NOXA, also appear to have activating properties. Thus there is likely a spectrum of activator potency among the BH3-only proteins, with BIM and BID likely the most potent, followed by PUMA, NOXA, and perhaps even others whose fainter activator properties have yet to be observed.
Figure 1.
Interactions between the Bcl-2 family of proteins at the mitochondria determines commitment to apoptotic cell death. Physiological or therapeutic apoptotic stimuli alter different classes of Bcl-2 proteins. Activation of effector proteins result in cytochrome c release from mitochondria, and degradation of the cell. (*) Recent evidence indicates that NOXA can also act as an activator BH3 protein.
Bax and Bak are directly activated by activator BH3-only proteins
To commit to the mitochondrial pathway of apoptosis, the sum of all Bcl-2 family interactions must result in the activation of BAX or BAK (Wei et al., 2001). Precisely how BAX and BAK are activated by other Bcl-2 family proteins has been a source of intense research since their discovery. Many proposed models of the how the Bcl-2 family interact have been previously reviewed (Shamas-Din et al., 2013). Several in vitro studies using purified lipid membranes or mitochondria indicate that the activation of BAX and BAK is a result of direct, albeit transient, binding and activation by the BH3-only proteins BID, BIM, and PUMA (Kim et al., 2009; Letai et al., 2002). More recent data indicates that the BH3-only protein NOXA can also directly activate BAX or BAK – though likely more weakly than BIM or BID (Chen et al., 2015; Du et al., 2011). The generation of mice and mouse fibroblasts lacking all of BIM, BID, PUMA and NOXA results in the protection from cell death in response to many apoptosis inducing agents. However, these quadruple knockout cells still undergo apoptosis in response to the DNA damage drug etoposide and in response to UV irradiation, though with reduced kinetics (Chen et al., 2015). This study suggests that while apoptosis progresses in many cases following activation of BAX or BAK by known activator proteins, other modes of activation apparently exist. These modes of activation are not mutually exclusive, and include: that BAX and BAK can auto-activate (Willis et al., 2007); that they can be activated by alternative activators (Follis et al., 2015), perhaps even non-proteins (Pagliari et al., 2005); that an alternative effector like BOK that does not require activation is responsible for apoptosis (Llambi et al., 2016).
Although both BAX and BAK can be activated by both BIM and BID, the efficiency of the possible interactions of these proteins are not identical. Specifically, BIM can preferentially activate BAX, while BID preferentially activates BAK. This has therapeutic consequences as cells that lack BAK are less sensitive to chemotherapeutic agents that require activation of BID (Sarosiek et al., 2013). These results highlight that in response to specific apoptotic stimuli, the cellular context and relative levels of BCL-2 family proteins are critical for how a cell survives or commits to death.
A molecular and structural understanding of how BAX and BAK are activated may facilitate chemical strategies to either accelerate or inhibit apoptosis. Several dynamic insights have been gained into the sequence of molecular and structural events by which BAX and BAK permeabilize the outer mitochondrial membrane. While BAK is constitutively localized to mitochondria, BAX must translocate from the cytosol to the mitochondria to enable apoptosis (Hsu et al., 1997). Elegant dynamic fluorescent studies indicate that the activation of BAX by BID is initiated when caspase truncated BID localizes to the mitochondria where it activates a proportion of soluble BAX enabling insertion of BAX into the outer mitochondrial membrane (Lovell et al., 2008). BAX does not remain permanently associated with the membrane in viable cells, and can cycle between the mitochondria and the cytosol (Edlich et al., 2011). Structural studies have provided insights into the conformational changes that occur to activated BAX and BAK when activated by BH3-only proteins as reviewed in (Czabotar et al., 2014). Specifically, BH3-only activator proteins bind to a hydrophobic groove formed by BH1-3 on BAX or BAK (Czabotar et al., 2013; Leshchiner et al., 2013; Moldoveanu et al., 2013). This results in unfolding of these proteins into two domains termed a “core” and “latch” domain (Brouwer et al., 2014; Czabotar et al., 2013). However, alternative “trigger sites” on BAX for the activating interaction with BH3 domains have also been indentified that are distal from the canonical BH3 binding cleft (Gavathiotis et al., 2008), perhaps reflecting an additional step of BAX activation that is required for mitochondrial targeting.
A comprehensive structure of the BAX and BAK pore in the outer mitochondrial membrane has been long desired, but difficult to obtain (Borner and Andrews, 2014). A low weight oligomer of BAX or BAK results in outer mitochondrial membrane pore that is proposed to be part protein and part lipid, and as more activated BAX is recruited to mitochondria, this opening becomes larger (Gillies et al., 2015; Kuwana et al., 2002). Indeed, a pore structure has been difficult to pin down due to the membrane environment of the BAX/BAK oligomer, and that this oligomer does not have reproducible symmetric features amenable to high resolution structural techniques for membrane embedded proteins. Nonetheless, in addition to the aforementioned high resolution intermediate structures of soluble fragments of BAX and BAK bound to BH3 proteins (Czabotar et al., 2013; Gavathiotis et al., 2008; Moldoveanu et al., 2013), the application of fluorescence and spectroscopic technologies have provided critical clues about how BAX and BAK oligomerize in the outer membrane (Annis et al., 2005; Lovell et al., 2008). In particular a recent study using DEER spectroscopy indicates that BAX dimerizes, spans both sides of the outer mitochondrial membrane and that these BAX dimers further oligomerize to form a protein and lipid based pore (Bleicken et al., 2014). This unique insight highlights the value of technologies that enable the study of these full length proteins in their native environment. Note that the outer membrane pore in apoptosis may contain proteins outside of the Bcl-2 family. In particular, there have been observations that VDAC2 can promote or inhibit apoptosis depending on context. (Cheng et al., 2003; Roy et al., 2009). Notably cells deficient for VDAC1-3 could still undergo apoptosis (Baines et al., 2007), suggesting that VDACs are not effectors of MOMP.
BH3 domains and their binding by anti-apoptotic proteins
The fundamental interaction that defines heterodimerizaton among most Bcl-2 family proteins is the binding of the BH3 domain from a pro-apoptotic protein into a BH3-binding site in an anti-apoptotic protein. Anti-apoptotic proteins (including (BCL-XL, BCL-2, MCL-1, BFL-1 and BCL-W) prevent apoptosis by preventing the oligomerizaton of BAX and BAK. They do this by either binding activators and preventing them from activating BAX or BAK (Cheng et al., 2001), or by binding monomeric BAX or BAK after they have been activated to expose their BH3 domains, preventing their oligomerization (Oltvai et al., 1993; Willis et al., 2005). Anti-apoptotic proteins are predominantly, though not exclusively, localized to mitochondria. In contrast to a static view of anti-apoptotic inhibition of pro-apoptotic proteins at the mitochondria, it has been observed that BCL-XL can retro-translocate BAX that is peripherally associated with mitochondria from the mitochondria outer membrane to the cytoplasm (Edlich et al., 2011). Anti-apoptotic proteins possess all four BCL-2 homology domains (BH1-4). The domains BH1-3 form a hydrophobic cleft that binds and sequesters pro-apoptotic BH3 domains (Muchmore et al., 1996). The hydrophobic cleft on these anti-apoptotic proteins have different affinities for pro-apoptotic proteins resulting in a specific signature of interaction among pro- and anti-apoptotic proteins (Certo et al., 2006; Chen et al., 2005; Kuwana et al., 2005; Opferman et al., 2003). Note that if an empty BCL-2 binds an activator like BIM, this does impair the ability of BIM to activate BAX and induce apoptosis. However, at the same time, it impairs the ability of BCL-XL to bind subsequent pro-apoptotic BH3 domains. In fact it even can leave the cell “primed” for apoptosis, and subsequent sensitizers could displace BIM from BCL-2 to provoke MOMP (Certo et al., 2006).
The BH3 domain is a roughly 20 amino-acid amphipathic alpha-helix possessed by all Bcl-2 family proteins (Zha et al., 1996). This domain is necessary for the pro-death function of all pro-death proteins, including effectors, activators, and sensitizers. The amino acid sequence is poorly conserved across Bcl-2 proteins, with the exception of the LXXXXD sequence that appears consistently near the C-terminus (Aouacheria et al., 2013; DeBartolo et al., 2014; Sattler et al., 1997). The BH3 domain by itself is sufficient to replicate the pro-death function of intact BH3-only proteins, both effectors and sensitizer (Letai et al., 2002). While the sensitizer BH3-only proteins lack the ability to directly activate BAX or BAK, they nonetheless exert a pro-death function by their ability to inhibit anti-apoptotic proteins. This means that they compete for the BH3 binding site in anti-apoptotic proteins, displacing or preventing the binding of activators or effectors. The BH3 domains of the most potent activators, like BID, BIM and PUMA, interact promiscuously with all anti-apoptotic proteins. In contrast, among the sensitizers, including NOXA, BAD, BMF, HRK, and BIK, there is important selectivity in interaction with anti-apoptotic proteins (Certo et al., 2006; Chen et al., 2005; Kuwana et al., 2005; Opferman et al., 2003). For example, BAD selectively binds and inhibits BCL-2, BCL-XL, and BCL-W, while HRK selectively binds only BCL-XL. NOXA selectively binds MCL-1, and with less affinity, BFL-1. Proteins such as PUMA, and BMF are more promiscuous, and can interact with and inhibit most or all anti-apoptotic proteins. Thus, the commitment to cell death depends on whether the right combination of pro-apoptotic proteins exists in a cell to evade the specific anti-apoptotic proteins present in a cell.
Modulation of the Bcl-2 proteins by upstream events in the cell
During a countless variety of basic physiologic processes (including cell cycle, metabolism, DNA damage response, oncogenesis), and in response to therapeutics too numerous to list (including DNA damaging agents, microtubule perturbagens, kinase inhibitors, and many other selective pathway inhibitors) the Bcl-2 family are modified by upstream factors (Kutuk and Letai, 2008). Several types of modifications of Bcl-2 family proteins have been studied including control of transcript levels and degradation, and also control over protein phosphorylation and protein localization. Several examples of protein transcript control include the upgregulation of PUMA and NOXA by p53 (Nakano and Vousden, 2001; Oda et al., 2000), and miRNA control of BCL-XL and BCL-2 levels (Cimmino et al., 2005; Wang et al., 2015). Control over protein degradation has been observed for several pro and anti-apoptotic proteins. For example, MCL-1 is degraded by the ubiquitin ligases MULE and FBW7 (Inuzuka et al., 2011; Wertz et al., 2011). Bcl-2 family proteins can also be regulated by miRNA(Cimmino et al., 2005). The widespread use of technologies like RNASeq and RPPA will enable a better understanding of how Bcl-2 family transcripts and proteins are modified in different cellular conditions and in response to different therapeutic cues.
In addition to modifying protein levels, activities of Bcl-2 proteins can be modified by protein phosphorylation. Examples include BCL-XL phosphorylation in cardiomyocytes at Ser14 which antagonizes BCL-XL binding of BAX (Del Re et al., 2014), and NOXA phosphorylation at Ser13 which prevents its pro-apoptotic activity (Lowman et al., 2010). BIM can be extensively phosphorylated at some sites that promote apoptosis and at other sites that prevent apoptosis (Hubner et al., 2008). BAX and BAK are subject to phosphorylation events that may inhibit their pro-apoptotic effect (Fox et al., 2010; Gardai et al., 2004; Xin and Deng, 2005). Though many modifications may be specific to cell types and apoptotic inducing cues, understanding these modifications potentially have therapeutic outcomes such as the inhibition of BCL2 phosphorylation , which increases sensitivity of cells to ABT-737 (Konopleva et al., 2006). Finally, the localization of Bcl-2 proteins can be modified by upstream factors – for example in human embryonic stem cells, BAX is constrained at the Golgi until released by DNA damage signals (Dumitru et al., 2012), and BAX can be constrained in the cytoplasm by Bcl-XL (Edlich et al., 2011). These phosphorylation and localization modes of Bcl-2 family regulation indicate the challenge of understanding regulation of cell death based on total levels of Bcl-2 family proteins.
The composition, structure and subcellular location of mitochondria influence sensitivity to apoptosis
While much attention is paid to interactions between Bcl-2 proteins, in recent years data has emerged suggesting that features of the mitochondria itself influence the cellular commitment to apoptosis. These features include the lipid composition, the size of mitochondria, and the location of mitochondria relative to other organelles. The outer mitochondrial membrane lipid environment is the predominant site of interactions between the Bcl-2 family. In fact, this environment is so important that the interaction of small molecules mimicking Bcl-2 proteins can differ when in solution and when in a membrane environment (Aranovich et al., 2012). Moreover the precise lipid composition of mitochondria can also alter the cellular sensitivity for apoptosis. For example, cellular levels of sphingomylenease appears to promote MOMP (Chipuk et al., 2012). Lipid composition of mitochondria can also preferentially facilitate the activity of specific pro-apoptotic proteins. For example, tBID is better recruited and undergoes conformation changes in membranes with cardiolipin (Shamas-Din et al., 2015). Not only does mitochondrial lipid composition influence apoptosis, but mitochondria morphology and size can also determine sensitivity to MOMP (Pyakurel et al., 2015; Renault et al., 2015). Finally, a recent study indicates that mitochondrial interactions with the endoplasmic reticulum can result in mitochondria inner membrane remodeling downstream of BAX and BAK activation and efficient cytochrome c release (Prudent et al., 2015). These studies indicate that future insights into the physical composition mitochondria, and its physical location within the cell will be essential to understanding how cells commit to apoptosis. Note that while there is an appreciation of these particular influences on MOMP, our knowledge is as yet not sufficiently precise to construct clinically useful predictive models based on initial conditions relevant to these properties.
MOMP as the point of no return
The onset of MOMP in a cell is often considered the point of no return in the cellular commitment to apoptosis. Indeed once MOMP occurs, cells initiate caspase activation (Li et al., 1997), and undergo cleavage of genomic DNA (Li et al., 2001), and exposure of phosphadityl serine (PS) (Fadok et al., 2000). In vivo, the exposure of PS and other “eat-me” signals on the surface of the apoptotic cell facilitates rapid engulfment by phagocytes. Recently, the link between caspase activation and PS exposure has been determined. Flippases constantly internalize PS in healthy cells and are inactivated by caspases (Segawa et al., 2014) while scramblases actively promote PS externalization and are activated by caspases (Suzuki et al., 2013; Suzuki et al., 2010). When caspases are activated after MOMP, these flippases are inactivated and scramblases are activated resulting of external exposure of PS. Note that the exposure of pro-phagocytic signals like phosphatidyl serine on the plasma membrane enforce an irreversible aspect to apoptosis in vivo that will not be observed in monoculture in vitro.
Mitochondria within a single cell generally undergo MOMP within a few minutes of each other. At high spatial and temporal imaging resolution, it is clear that some mitochondria in the cell undergo MOMP first, and then MOMP proceeds as a wave across the rest of the cell (Bhola et al., 2009; Lartigue et al., 2008). This suggests a feed forward mechanism to amplify the initial apoptotic signal, or that the sensitivity of individual mitochondria for apoptosis is spatially organized. It has also been observed that the cell can tolerate MOMP occurring in a single or a few mitochondria in the cell without undergoing cell wide MOMP (Ichim et al., 2015). Some studies even suggest that some cells can even survive widespread caspase activation and DNA damage (Tang et al., 2015; Tang et al., 2012). When thinking about the Bcl-2 family in the cell, it is unclear how much mitochondria need to undergo MOMP prior to the full cell wide commitment to death. Remarkably, in contrast to the cell wide commitment to death more typically observed, developing neurons prune specific axons in a BAX and caspase dependent process, leaving the remaining neurons intact and functioning (Cusack et al., 2013; Nikolaev et al., 2009).
Cell death via the mitochondrial permeability transition pore (PTP)
In addition to MOMP by the Bcl-2 family, mitochondria can induce necrotic cell death by opening of a mitochondrial permeability transition pore (mPTP) resulting in swelling and rupture of mitochondria. Genetic studies indicate that cyclophilin D is involved in mPTP mediated death, and that loss of cyclophilin D does not protect cells from agents that induce mitochondrial mediated apoptosis (Baines et al., 2005). This suggests that mPTP contributes to a necrotic death distinct from mitochondrial mediated apoptosis. Surprisingly, loss of BAX and BAK inhibits mitochondrial swelling and rupture following mPTP opening indicating that some of the proteins involved in mitochondrial mediated apoptosis may nonetheless affect the mPTP (Karch et al., 2013; Whelan et al., 2012).
It is important to recognize that many other forms of programmed cell death that do not use the mitochondria have been reported (Fuchs and Steller, 2015). This includes use of the extrinsic pathway of apoptosis which relies on the activity of initiator caspases such as caspase-8 that cleave and activate broadly acting caspases such as caspase-3 (Fulda and Debatin, 2006). Notably, the extrinsic pathway of apoptosis, and the mitochondrial mediated pathway of apoptosis are not truly distinct. Depending on the cellular context, death receptor mediated activation of initiator caspase 8 via the extrinsic apoptotic pathway results in the cleavage of the BID protein, which in turn induces mitochondrial mediated apoptosis (Li et al., 1998; Luo et al., 1998). This mitochondrial amplification loop is utilized for cell death via the extrinsic apoptotic pathway in many contexts. This again highlights the importance of cellular context when assessing apoptotic signaling.
What is the connection to human disease and therapy? Focus on Cancer
Degenerative diseases often result from excessive cell death. However, the links between degenerative disease and the mitochondrial apoptotic pathways are less developed, and there are no current clinical efforts targeting direct inhibition of apoptosis in these diseases. Cancer is the reciprocal case, where there is persistence of malignant cells and the therapeutic goal is to kill them. Oncology has likely been exploiting indirect targeting of apoptosis for decades. In recent years, novel therapeutics have directly targeted Bcl-2 family proteins. We will therefore briefly discuss therapies and diagnostics related to the mitochondrial apoptotic pathway in cancer.
Many conventional and novel targeted therapies kill via mitochondrial mediated apoptosis. This includes chemicals that induce DNA damage, microtubule inhibitors, several kinase inhibitors including MEK, EGFR, BRAF inhibitors, and recent direct chemical inhibitors of BCL-2 and BCL-XL (Souers et al., 2013; Tse et al., 2008). The role of mitochondrial mediated apoptosis in these chemically induced cell deaths is in part confirmed by knockout experiments (Chen et al., 2015; Wei et al., 2001), correlations of apoptotic proteins with therapeutic outcomes (Faber et al., 2011; Lindner et al., 2013), and correlations of functional assessments of apoptotic sensitivity in primary human tumors with therapeutic outcomes using BH3 profiling (Davids et al., 2012; Montero et al., 2015; Ni Chonghaile et al., 2011; Vo et al., 2012).
BH3 profiling represents a powerful approach for testing the apoptotic sensitivity in cancer therapeutics (Letai et al., 2002; Ryan et al., 2010). This assay evaluates apoptotic sensitivity of cancer cells by measuring whether synthetic peptides modeled after BH3 domains induce cytochrome c loss in mitochondria from tumor cells. In practice a cell that is highly predisposed to apoptosis (one that is primed for apoptosis) has mitochondria that lose cytochrome c after exposure to low concentrations of promiscuously acting peptide such as those modeled after the BH3 domains of BIM, BID and PUMA. Conversely, a cell that is poorly predisposed for apoptosis (one that is poorly primed for apoptosis) has mitochondria that fail to undergo cytochrome c loss after exposure to high concentrations of promiscuous peptides. BH3 profiling is applicable to cells directly isolated from human samples and with little or no ex vivo culture thereby preserving tumor fidelity to its in vivo environment (Ryan et al., 2010). Moreover, an important advantage of BH3 profiling is that it is a functional measurement of predisposition to apoptotic priming, and avoids the practical obstacle of measuring all of the molecular components in a single cell.
Using BH3 profiling, it has been demonstrated that the apoptotic priming of primary human tumor cells is predictive of response to several conventional chemotherapies. This includes tumor cells from AML, ALL, CLL, MM, and ovarian cancers (Davids et al., 2012; Ni Chonghaile et al., 2011; Vo et al., 2012). The more primed a tumor cells is for apoptosis, the more likely the patient is to respond to therapy. Conversely, normal primary human cells are usually less primed than the cancer cells (Ni Chonghaile et al., 2011). This difference in apoptotic sensitivity explains the therapeutic index of many conventional chemotherapies. BH3 profiling can also be used to predict sensitivity to small molecule BH3 mimetics (Pan et al., 2014). In addition to measuring the baseline apoptotic priming, BH3 profiling can be used to measure the change in apoptotic priming in response to therapeutic chemicals (Montero et al., 2015). This approach, termed dynamic BH3 profiling, is particularly useful for novel therapies that do not act on ubiquitous targets, but act to inhibit specific pathways that are active in some cells but not in others.
Several small molecules that mimic sensitizer BH3 proteins and disrupt the interactions of pro-apoptotic and anti-apoptotic proteins have now entered the clinic. These include navitoclax (ABT-263) an inhibitor of BCL-2 and BCL-XL (Tse et al., 2008), and venetoclax (ABT-199) a selective inhibitor of BCL-2 (Souers et al., 2013). ABT-199 and ABT-263 have been used in clinical trials with impressive single agent clinical outcomes for a few cancers. The on-target BCL-XL dependent toxicity of thrombocytopenia has limited clinical use of ABT-263, but exciting clinical results have been obtained for ABT-199 as a single agent and in combination therapies, particularly in CLL, AML, and select non-Hodgkins lymphomas (Roberts et al., 2016; Roberts et al., 2012; Souers et al., 2013). ABT-263 demonstrated more modest activity when tested as a single agent in solid tumors (Gandhi et al., 2011). This suggests that that while selective inhibition of anti-apoptotic proteins may increase apoptotic sensitivity, it is not always sufficient to induce apoptosis in a clinical setting. This may also suggest that the other non-targeted anti-apoptotic proteins can compensate for the inhibition of BCL-2 and BCL-XL. The clinical success of a selective BCL-2 inhibitor in the clinic, has spurred investigation into selective inhibitors of other anti-apoptotic proteins. Several inhibitors of MCL-1 have been produced as tool compounds (Leverson et al., 2015; Varadarajan et al., 2013), though thus far have not entered clinical trials. Whether a therapeutic index exists for these drugs, or for drugs that mimic promiscuous sensitizer or activator BH3 domains remains to be seen. Note that many compounds with purported selective or even pan-BCL-2 family inhibitory properties may well kill via a mechanism of action distinct from BH3 mimetic action (Vogler et al., 2009).
Different normal and cancerous cells rely on different subsets of pro-apoptotic Bcl-2 proteins to facilitate apoptosis and conversely rely on different anti-apoptotic proteins for survival. For example, in normal T-cell development, early progenitors primarily rely on BCL-XL for survival while more differentiated pre-thymic T-cells rely on BCL-2 (Ryan et al., 2010). Mirroring this pattern, in early T-cell progenitor acute lymphoblastic leukemia (ETP-ALL), cells are mainly BCL-2 dependent, but more differentiated lymphoblasts in T-cell ALL are primarily BCL-XL dependent (Chonghaile et al., 2014). This therapeutically significant difference shows how cellular and physiologic context affects the balance of apoptotic signals. CLL and ALL are cancers where dependence on individual anti-apoptotic proteins is relatively homogeneous. The more common circumstance will likely be that histologically defined cancers will instead be made up of subsets that have differing dependence on anti-apoptotic proteins, as has been shown in AML and multiple myeloma.
It is important to clarify a common confusion regarding apoptosis and cancer cells. One of the identified hallmarks of cancer is that tumor cells have to evade apoptosis (Hanahan and Weinberg, 2000). Cancer cells often display phenotypes that might trigger apoptosis, such as invasive growth through basement membranes, genomic instability, and growth in the absence of survival factors. Their survival nonetheless does imply that cancer cells have selected for evasion of apoptosis that might have been induced by these mechanisms, or they would have died. However, this does not necessarily mean that cancer cells are resistant to subsequent apoptotic signaling, or that cancer cells are generally required to have blocked or incompetent apoptotic signaling, as is often stated. If this were truly the case, drugs that act by inducing apoptosis in cancer cells would tend to have a negative therapeutic index, not have a role in cancer therapy. In fact the therapeutic index of conventional chemotherapy, observed widely across nearly all cancer histologies, likely largely depends upon cancer cells generally having a greater propensity to apoptosis than non-malignant cells. Using the BH3 profiling assay to measure overall apoptotic priming in primary human tumor cells, it is observed that not only can cancer cells respond to mitochondrial apoptotic signaling, but tumor cells are often more predisposed to apoptosis when compared to normal cells (Ni Chonghaile et al., 2011; Vo et al., 2012).
Despite decades of progress in our understanding of mitochondrial mediated apoptosis and interactions between the Bcl-2 family of proteins, much remains to be discovered. In particular our understanding of how upstream signals alter the Bcl-2 family, and how the mitochondria environment itself contributes to the commitment to cell death remain exciting areas of study. Even with incomplete knowledge, however, agents directly targeting key mitochondrial regulators of cell death have made it to the clinic and demonstrated superbly selective anti-cancer activity (Roberts et al., 2016). Moreover, although the development of orthogonally acting non-apoptotic therapies represents exciting approaches to treatment, sensitizing cells for the mitochondrial pathway of apoptosis and augmenting existing apoptosis-inducing therapies will continue to provide an exciting approach to eradicate tumors, as it has for the past 6 decades.
Acknowledgements
We acknowledge support from NIH grant P01CA066996. A.L. is a consultant for, and receives sponsored research support for AbbVie, Astra-Zeneca, Tetralogic and XrX. P.B. supported by NIH grant 1R21CA188858-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Molecular cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. The EMBO journal. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheria A, Rech de Laval V, Combet C, Hardwick JM. Evolution of Bcl-2 homology motifs: homology versus homoplasy. Trends in cell biology. 2013;23:103–111. doi: 10.1016/j.tcb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranovich A, Liu Q, Collins T, Geng F, Dixit S, Leber B, Andrews DW. Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Molecular cell. 2012;45:754–763. doi: 10.1016/j.molcel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Bhola PD, Mattheyses AL, Simon SM. Spatial and temporal dynamics of mitochondrial membrane permeability waves during apoptosis. Biophys J. 2009;97:2222–2231. doi: 10.1016/j.bpj.2009.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Jeschke G, Stegmueller C, Salvador-Gallego R, Garcia-Saez AJ, Bordignon E. Structural model of active Bax at the membrane. Molecular cell. 2014;56:496–505. doi: 10.1016/j.molcel.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Borner C, Andrews DW. The apoptotic pore on mitochondria: are we breaking through or still stuck? Cell Death Differ. 2014;21:187–191. doi: 10.1038/cdd.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JM, Westphal D, Dewson G, Robin AY, Uren RT, Bartolo R, Thompson GV, Colman PM, Kluck RM, Czabotar PE. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Molecular cell. 2014;55:938–946. doi: 10.1016/j.molcel.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. Journal of cell science. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio MA, Michaud M, Zhou W, Fisher JK, Walensky LD, Katz SG. BCL-2 family member BOK promotes apoptosis in response to endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2015;112:7201–7206. doi: 10.1073/pnas.1421063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen HC, Kanai M, Inoue-Yamauchi A, Tu HC, Huang Y, Ren D, Kim H, Takeda S, Reyna DE, Chan PM, et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nature cell biology. 2015;17:1270–1281. doi: 10.1038/ncb3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Molecular cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molecular cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Molecular cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, Loh ML, Hunger SP, Wood B, DeAngelo DJ, et al. Maturation Stage of T-cell Acute Lymphoblastic Leukemia Determines BCL-2 versus BCL-XL Dependence and Sensitivity to ABT-199. Cancer Discov. 2014;4:1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary ML, Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nature reviews. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nature communications. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. The Journal of cell biology. 2011;194:39–48. doi: 10.1083/jcb.201102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, Wilson WH, Brown JR, Letai A. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120:3501–3509. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBartolo J, Taipale M, Keating AE. Genome-wide prediction and validation of peptides that bind human prosurvival Bcl-2 proteins. PLoS computational biology. 2014;10:e1003693. doi: 10.1371/journal.pcbi.1003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Molecular cell. 2014;54:639–650. doi: 10.1016/j.molcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. The Journal of biological chemistry. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Molecular cell. 2012;46:573–583. doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, Incio J, Digumarthy SR, Pollack SF, Song Y, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer discovery. 2011;1:352–365. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Follis AV, Llambi F, Merritt P, Chipuk JE, Green DR, Kriwacki RW. Pin1-Induced Proline Isomerization in Cytosolic p53 Mediates BAX Activation and Apoptosis. Molecular cell. 2015;59:677–684. doi: 10.1016/j.molcel.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL, Ismail F, Azad A, Ternette N, Leverrier S, Edelmann MJ, Kessler BM, Leigh IM, Jackson S, Storey A. Tyrosine dephosphorylation is required for Bak activation in apoptosis. The EMBO journal. 2010;29:3853–3868. doi: 10.1038/emboj.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nature reviews. Molecular cell biology. 2015;16:329–344. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. The Journal of biological chemistry. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, Sutherland GR, Baker E, Adams JM, Cory S. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996;13:665–675. [PubMed] [Google Scholar]
- Gillies LA, Du H, Peters B, Knudson CM, Newmeyer DD, Kuwana T. Visual and functional demonstration of growing Bax-induced pores in mitochondrial outer membranes. Molecular biology of the cell. 2015;26:339–349. doi: 10.1091/mbc.E13-11-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. The EMBO journal. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Molecular cell. 2011;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Molecular cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Molecular cell. 2015;57:860–872. doi: 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Ding L, Chen S, Nunez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). The EMBO journal. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife. 2013;2:e00772. doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British journal of cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature cell biology. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Molecular cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuk O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr Mol Med. 2008;8:102–118. doi: 10.2174/156652408783769599. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Molecular cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Llambi F, et al. coming out online at Cell on March 3rd - 10.1016/j.cell.2016.02.026.
- Lartigue L, Medina C, Schembri L, Chabert P, Zanese M, Tomasello F, Dalibart R, Thoraval D, Crouzet M, Ichas F, et al. An intracellular wave of cytochrome c propagates and precedes Bax redistribution during apoptosis. Journal of cell science. 2008;121:3515–3523. doi: 10.1242/jcs.029587. [DOI] [PubMed] [Google Scholar]
- Leshchiner ES, Braun CR, Bird GH, Walensky LD. Direct activation of full-length proapoptotic BAK. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E986–995. doi: 10.1073/pnas.1214313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell death & disease. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lindner AU, Concannon CG, Boukes GJ, Cannon MD, Llambi F, Ryan D, Boland K, Kehoe J, McNamara DA, Murray F, et al. Systems analysis of BCL2 protein family interactions establishes a model to predict responses to chemotherapy. Cancer research. 2013;73:519–528. doi: 10.1158/0008-5472.CAN-12-2269. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W-X, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Lockshin RA, Williams CM. Programmed Cell Death--I. Cytology of Degeneration in the Intersegmental Muscles of the Pernyi Silkmoth. J Insect Physiol. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nature reviews. Molecular cell biology. 2001;2:545–550. doi: 10.1038/35080097. [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Lowman XH, McDonnell MA, Kosloske A, Odumade OA, Jenness C, Karim CB, Jemmerson R, Kelekar A. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Molecular cell. 2010;40:823–833. doi: 10.1016/j.molcel.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K, Kriwacki RW, Green DR. BID-induced structural changes in BAK promote apoptosis. Nature structural & molecular biology. 2013;20:589–597. doi: 10.1038/nsmb.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, Piao H, Horowitz NS, Berkowitz RS, Matulonis U, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160:977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Molecular cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. The EMBO journal. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer discovery. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nature cell biology. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J, Zunino R, Sugiura A, Mattie S, Shore GC, McBride HM. MAPL SUMOylation of Drp1 Stabilizes an ER/Mitochondrial Platform Required for Cell Death. Molecular cell. 2015;59:941–955. doi: 10.1016/j.molcel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Pyakurel A, Savoia C, Hess D, Scorrano L. Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Molecular cell. 2015;58:244–254. doi: 10.1016/j.molcel.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Floros KV, Elkholi R, Corrigan KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla JJ, Buettner C, et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Molecular cell. 2015;57:69–82. doi: 10.1016/j.molcel.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12895–12900. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek KA, Chi X, Bachman JA, Sims JJ, Montero J, Patel L, Flanagan A, Andrews DW, Sorger P, Letai A. BID Preferentially Activates BAK while BIM Preferentially Activates BAX, Affecting Chemotherapy Response. Molecular cell. 2013;51:751–765. doi: 10.1016/j.molcel.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Bindner S, Chi X, Leber B, Andrews DW, Fradin C. Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax. The Biochemical journal. 2015;467:495–505. doi: 10.1042/BJ20141291. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Kale J, Leber B, Andrews DW. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harbor perspectives in biology. 2013;5:a008714. doi: 10.1101/cshperspect.a008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Sulston JE. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341:403–406. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- Tang HL, Tang HM, Fung MC, Hardwick JM. In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity. Sci Rep. 2015;5:9015. doi: 10.1038/srep09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HL, Tang HM, Mak KH, Hu S, Wang SS, Wong KM, Wong CS, Wu HY, Law HT, Liu K, et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Molecular biology of the cell. 2012;23:2240–2252. doi: 10.1091/mbc.E11-11-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer research. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985;229:1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell death and differentiation. 2013;20:1475–1484. doi: 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, Deangelo DJ, Frattini MG, Letai A. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, Cohen GM. Different forms of cell death induced by putative BCL2 inhibitors. Cell death and differentiation. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- Wang JX, Gao J, Ding SL, Wang K, Jiao JQ, Wang Y, Sun T, Zhou LY, Long B, Zhang XJ, et al. Oxidative Modification of miR-184 Enables It to Target Bcl-xL and Bcl-w. Molecular cell. 2015;59:50–61. doi: 10.1016/j.molcel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes & development. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & development. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Tahaoglu E, Steller H. Cell killing by the Drosophila gene reaper. Science. 1996;271:805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes & development. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Molecular cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. The Journal of biological chemistry. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]