Abstract
Collectins are secreted collagen-like lectins that bind, agglutinate, and neutralize influenza A virus (IAV) in vitro. Surfactant proteins A and D (SP-A and SP-D) are collectins expressed in the airway and alveolar epithelium and could have a role in the regulation of IAV infection in vivo. Previous studies have shown that binding of SP-D to IAV is dependent on the glycosylation of specific sites on the HA1 domain of hemagglutinin on the surface of IAV, while the binding of SP-A to the HA1 domain is dependent on the glycosylation of the carbohydrate recognition domain of SP-A. Here, using SP-A and SP-D gene-targeted mice on a common C57BL6 background, we report that viral replication and the host response as measured by weight loss, neutrophil influx into the lung, and local cytokine release are regulated by SP-D but not SP-A when the IAV is glycosylated at a specific site (N165) on the HA1 domain. SP-D does not protect against IAV infection with a strain lacking glycosylation at N165. With the exception of a small difference on day 2 after infection with X-79, we did not find any significant difference in viral load in SP-A−/− mice with either IAV strain, although small differences in the cytokine responses to IAV were detected in SP-A−/− mice. Mice deficient in both SP-A and SP-D responded to IAV similarly to mice deficient in SP-D alone. Since most strains of IAV currently circulating are glycosylated at N165, SP-D may play a role in protection from IAV infection.
The course of a primary respiratory infection with type A influenza virus (IAV) is determined by the rate of viral growth and spread and the development of cell-mediated immunity (9-11). The former is controlled primarily by soluble (6, 8) and cellular (16, 23, 34) components of the immediate innate response. The collectin proteins (collagen-like lectins) are soluble pattern recognition receptors of this multifaceted early host response (24). Two of the members of the collectin family, surfactant protein A (SP-A) and SP-D, are constitutively present in the lining fluids of the respiratory tract, in good position to provide immunological surveillance against both microbes and allergens (7, 35).
The results of in vitro studies suggest that both SP-A and SP-D might contribute to the containment of IAV spread by direct inhibition of IAV infectivity and/or promotion of virus uptake into phagocytic cells (4, 7, 8, 14, 15, 17-19). Although both SP-A and SP-D attach to and neutralize certain strains of IAV, they do so by different mechanisms. SP-D acts like a classic influenza virus β-inhibitor by binding of the collectin carbohydrate-recognition domain (CRD) to high-mannose oligosaccharides present on the coat hemagglutinins (HA) on most strains isolated since 1968 (1, 13, 19). The neutralization of specific strains of IAV by SP-D in vitro correlates with virulence in vivo (32). In the present study we utilize two strains of IAV that vary by only one base change in the HA gene leading to the loss of a single glycosylation site at position 165 in HA (28). Glycosylation of HA N165 is important for the neutralization of IAV by SP-D (32).
In contrast, SP-A neutralizes IAV by directly occupying the HA cell attachment site with the terminal sialic acid on the oligosaccharide located on the CRD of SP-A, presumably blocking this site (3). Unlike the case with SP-D, the attachment of SP-A to influenza virus strains and SP-A-dependent neutralization in vitro are not influenced by the extent of HA glycosylation; in fact, SP-A is slightly more effective at neutralizing IAV strains that lack glycosylation completely (19). These models of SP-A and SP-D attachment suggest that the two collectins bind very similar regions of the HA molecule, albeit by different mechanisms and with different effectiveness. The available data suggest that SP-D binding is of significantly higher avidity and contributes most, if not all, the virus-neutralizing activity in human bronchoalveolar lavage fluid (15).
In addition to neutralizing influenza virus in vitro, SP-A and SP-D agglutinate influenza virus (14), enhance neutrophil uptake, and potentiate influenza virus-induced neutrophil hydrogen peroxide responses (17). SP-A but not SP-D enhances rat alveolar macrophage uptake of IAV in vitro (3). The collectins may also influence the host immune response to IAV without attaching to IAV by interacting in nonopsonic fashion with lung macrophages, neutrophils, dendritic cells, and T cells. At present, there is insufficient information to reliably translate the significance of these many in vitro studies to the physiological or clinical situation. In the present study we use different molecularly defined IAV strains, recombinant forms of SP-A and SP-D, and mouse lines deficient in SP-A, SP-D, and both SP-A and SP-D to dissect the relationship between collectin-IAV neutralization in vitro, viral IAV virulence in vivo, and early host responses.
MATERIALS AND METHODS
Viruses.
Two IAV strains were used in this study. X-79 (H3N2) is a laboratory-derived, high-yielding reassortant of A/PR/8/34 (H1N1) and A/Philippines/82 (H3N2) (36). The HA from A/Philippines/82, present on X-79, is potentially glycosylated at eight sites, including the asparagine at position 165, which predominantly determines β-inhibitor sensitivity (1, 32). Parental X-79 replicates poorly in mice and causes almost no detectable illness (13). We have previously characterized X-79Δ167, a mouse-adapted derivative of X-79 that has a point mutation in codon 167 of the HA1 chain of the HA molecule resulting in an amino acid change from threonine to isoleucine and the loss of the consensus sequence for glycosylation of the critical β-type collectin binding site at position 165 (28). X-79Δ167 is highly virulent in wild-type (WT) mice (28). For mouse inoculations, virus was grown in the allantoic cavity of 10-day embryonated hen's eggs, titered by standard hemagglutination assay, and stored in single-use aliquots at −80°C. The hemagglutination titers of the X-79 and X-79Δ167 stocks were 2,048 and 1,024, respectively. Endotoxin was not detected in the virus stocks by the Limulus Amebocyte Lysate assay (BioWhittaker, Inc., Walkersville, Md.).
Collectins.
Recombinant mouse SP-D and recombinant human SP-A were expressed in Chinese hamster ovary cells and purified by maltose and mannose-agarose affinity chromatography, respectively, as previously described (12). SP-A was also purified from dog bronchoalveolar lavage fluid by sequential butanol and octyl-glucopyranoside extraction as previously described (20).
Virus neutralization assay.
Neutralization of virus infectivity by purified pulmonary collectins was determined by a fluorescent focus reduction assay in MDCK cells cultured in 96-well plates (7 × 104 cells per well). The cells were grown to confluence in RPMI containing 10% fetal calf serum and 1% penicillin and streptomycin. The cells were washed twice in serum-free RPMI and incubated in virus with or without various collectin preparations diluted in RPMI for 1 h at 37°C. The cells were washed twice in serum-free RPMI and incubated in serum-free RPMI for 16 h at 37°C in 5% CO2. The medium was removed, and the cells were washed and then fixed for 1 h in 4% paraformaldehyde in phosphate-buffered saline. The cells were then washed in phosphate-buffered saline containing 0.3% triton X-100 and incubated with monoclonal antibody directed against the IAV nucleoprotein and then with Alexa 488-labeled goat anti-mouse immunoglobulin G. Cell nuclei were counterstained with 4′,6′-diamidino-2-phenylindole before the monolayers were examined by fluorescence microscopy. IAV fluorescent foci were counted directly, and total cell number was determined using NIH Image. Preliminary experiments were conducted to determine a concentration of each virus that resulted in about 400 fluorescent foci per low-power field or roughly 20% of the total cells infected.
Mice.
Mice deficient in SP-A (SP-A−/−) and SP-D (SP-D−/−) and both SP-A and SP-D (SP-AD−/−) were generated from embryonic stem cells targeted with replacement-type vectors as previously described (5, 21, 28). All three strains were backcrossed 10 generations onto a C57/BL6 background. WT C57/BL6 mice were generated from heterozygous matings. Study mice were housed in barrier containment until they were weaned on day 21. Male and female mice were infected with IAV on day 21 and housed separately from controls in isolator cages with free access to food and water. The protocols were reviewed and approved by the Committee for Animal Research of the University of California-San Francisco.
Mouse model.
SP-D−/− and SP-AD−/− mice develop patchy lung inflammation and structural remodeling that becomes evident 3 to 4 weeks after birth (5). To study mice before these changes become prominent and potentially alter host defenses, we inoculated mice with IAV at the time of weaning on day 21. In preliminary experiments, 10 21-day-old mice of each genotype were lightly anesthetized with inhaled methoxyflurane and given a 25-μl intranasal inoculation containing various dilutions of IAV or undiluted allantoic fluid alone. The mice were weighed daily and observed for a total of 14 days. To further define the response to each influenza virus strain, mice were killed by intraperitoneal phenobarbital 2, 4, 6, and 11 days after inoculation using IAV concentrations determined from the preliminary experiments to decrease weight gain but not cause death. The lungs of 10 mice of each genotype on day 2 were lavaged four times with 1-ml aliquots of a solution containing 10 mM Tris, 100 mM NaCl, and 0.2 mM EGTA, pH 7.4, for analysis of total protein, cell count and differential, and SP-A and SP-D levels. The unlavaged lungs of 10 mice of each genotype at each time point were frozen for RNA isolation (right lung) and cytokine measurements (left lung). In rescue experiments, 25 μl of undiluted X-79 virus was incubated for 15 min at 37°C with 10 μg of SP-A, SP-D, or human albumin in a 25-μl volume of 10 mM Tris-100 mM NaCl-2 mM CaCl2 before intranasal inoculation. Additional mice infected with X-79 with or without collectins were sacrificed for histology on day 6.
Cell counts and differentials.
Bronchoalveolar lavage fluid (BAL) was centrifuged at 250 × g for 5 min at 4°C. The pellet was gently resuspended in 200 μl lavage buffer for cell counting. Cytospin slides were stained with Diff-Quik (Dade International, Miami, FL) for cell differential counts. 20 to 25 high-power fields from 10 mice of each genotype on day 2 after inoculation were counted.
SP-A and SP-D levels.
The total protein content of the cell-free BAL was determined using bicinchoninic acid as a substrate. Serial dilutions of cell-free BAL from mice of each genotype on day 2 after inoculation were analyzed for SP-A and SP-D content with a quantitative dot blot assay using monospecific polyclonal antibodies against recombinant mouse SP-A and SP-D, respectively. Standard curves using recombinant mouse SP-A and SP-D expressed in Chinese hamster ovary cells were used to determine the linear range of these assays and calculate absolute SP-A and SP-D BAL pool sizes.
Cytokines.
Interleukin 6 (IL-6) and MIP-2 levels were measured in the presence of protease inhibitors in fresh tissue homogenates of the left lung on days 2, 4, and 6 by sandwich enzyme-linked immunosorbent assay (ELISAs) using standard protocols (Endogen, Woburn, Mass.).
HA real-time quantitative PCR.
RNA was extracted from the right lung 2, 4, 6, and 11 days after IAV inoculation, using the RNeasy reagents according to the manufacturer's recommendations (QIAGEN, Alameda, Calif.). Total RNA (200 ng per sample) was reverse transcribed using RETROscript reagents (Ambion, Austin, Tex.), and real-time quantitative PCR amplification of total cDNA (10 ng per sample) was performed using an ABI PRISM 7700HT sequence detector system with a 384-well block (PE Biosystems, Foster City, Calif.). For HA, the forward primer was 5′-GCTACATGTTCTGTCTGGTTTTCG-3′, the reverse primer was 5′-TTCACTAGCGTTCCGTTTGG-3′, and the probe was 5′6-FAM-AGCACAGCAACGCTGTGCCTGG-3′TAMRA for a product size of 105 bp. Differences in cDNA input were corrected for by normalization to signals obtained using primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). For GAPDH, the forward primer was 5′-TGAGCAAGAGAGGCCCTATCC-3′, the reverse primer was 5′-TAGGCCCCTCCTGTTATTATGG-3′, and the probe was 5′VIC-ACTGAGCATCTCCCTCACAATTTCCATCC −3′TAMRA for a product size of 95 bp. Standard curves for HA and GAPDH were constructed on each plate from serial log dilutions of a stock cDNA pool of several experimental samples (10 pg to 100 ng), and relative quantification in triplicate for each experimental sample was obtained by using the standard curve method. The normalized value obtained on day 11 for SP-D−/− mice was arbitrarily set to 1, and the HA cDNA content of the other experimental samples was expressed as multiples thereof. Control reactions were performed without reverse transcriptase and in the absence of target DNA.
Microscopy.
The lungs from mice 6 days after IAV inoculation were fixed intratracheally at 20 cm H2O with 4% freshly prepared paraformaldehyde in 0.1 M phosphate buffer and then prepared for paraffin sectioning using standard techniques. Mid-sagittal hematoxyln and eosin sections of the right lung were examined for morphological changes.
Mannose receptor PCR.
RNA was extracted from BAL cells of uninfected 21-day-old mice (n = 7 per genotype) using the RNeasy reagents according to the manufacturer's recommendations (QIAGEN). Total RNA (200 ng per sample) was reverse transcribed using RETROscript reagents (Ambion). Real-time quantitative PCR amplification of total cDNA (10 ng per sample, each done in triplicate) was performed using SYBRGold (Molecular Probes, Eugene, Ore.) for detection. The forward primer was 5′-GGTTATGAAAGGCAAGGATGGA-3′, and the reverse primer was 5′-TTGTCTGCACCCTCCGGTACTA-3′, for a product size of 114 bp. Differences in cDNA input were corrected for by normalizing to signals obtained using primers specific for GAPDH.
RESULTS
SP-D neutralization of infectivity is IAV strain dependent.
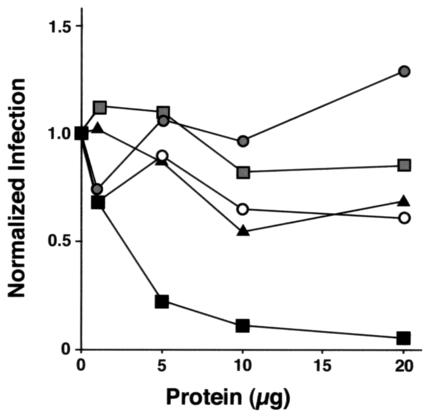
Recombinant mouse SP-D neutralized both X-79 and X-79Δ167 infection of MDCK cells in a dose-dependent manner (Fig. 1), but SP-D was much more effective in neutralizing X-79 than X-79Δ167 (94% neutralization of X-79 compared to 31% neutralization of X-79Δ167). Human recombinant SP-A partially neutralized X-79 infection (39% neutralization) but had no significant effect on the infectivity of X-79Δ167 (Fig. 1). Similar results were found with dog SP-A (not shown).
FIG. 1.
SP-D neutralizes IAV infection of MDCK cells. MDCK cells were grown to confluence in 96-well plates and incubated with a standard dose of IAV in the presence of increasing concentrations of collectins. After 16 h, the cells were stained for IAV and IAV fluorescent foci were counted and expressed as a percentage of total cells assessed by DAPI staining. The data shown are averaged from two separate experiments. Filled squares, recombinant mouse SP-D and X-79; empty circles, recombinant human SP-A and X-79; filled triangles, recombinant mouse SP-D and X-79Δ167; filled circles, recombinant human SP-A and X-79Δ167.
SP-D, but not SP-A, increases after inoculation with X-79.
BAL SP-A levels were unchanged 2 days after X-79 inoculation in WT or SP-D−/− mice. BAL SP-D levels did not change after inoculation of uninfected allantoic fluid, but SP-D increased threefold in BAL from WT and SP-A−/− mice 2 days after X-79 inoculation (P < 0.05; n = 5). We have previously reported similar changes in SP-D levels and stable SP-A levels in older mice after X-79Δ167 inoculation (28).
Weight gain after inoculation with X-79 is blunted in SP-D−/− and SP-AD−/− mice.
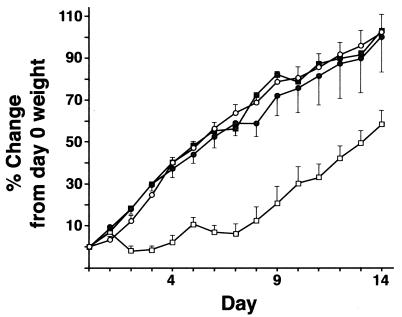
Parental X-79 replicates poorly in WT mice and causes no detectable illness. In pilot studies, 21-day-old mice were inoculated with four serial log dilutions of X-79. All mice survived. WT and SP-A−/− mice inoculated with all dilutions of X-79 gained weight at the same rate as mice given uninfected allantoic fluid or anesthesia only. In contrast, SP-D−/− mice inoculated with the two highest concentrations of X-79 gained weight significantly less rapidly than WT or SP-A−/−-infected mice from days 2 to 7 after inoculation (Fig. 2). The weight curve of infected SP-AD−/− mice was identical to that of infected mice deficient in SP-D alone (data not shown).
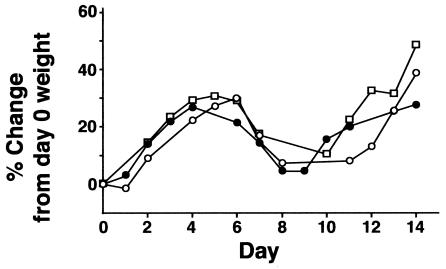
FIG. 2.
Weight change in mice after inoculation with X-79 virus. Mice were weighed daily after intranasal inoculation with X-79 virus or uninfected allantoic fluid. WT mice given X-79 (open circles) and SP-D−/− mice given sterile allantoic fluid (shaded circles) or anesthetic only (shaded squares) gained weight at the same rate as uninfected controls or infected SP-A−/− mice (data not shown). Infected SP-D−/− mice (open squares) failed to gain weight for the first 7 days after inoculation and then recovered. Data are means ± standard errors of the means (n = 10 mice/group). The weights of infected SP-D−/− mice were significantly different from those of infected WT and SP-A−/− mice and uninfected SP-D−/− mice from days 2 to 14 (P < 0.05).
BAL cells after inoculation with X-79.
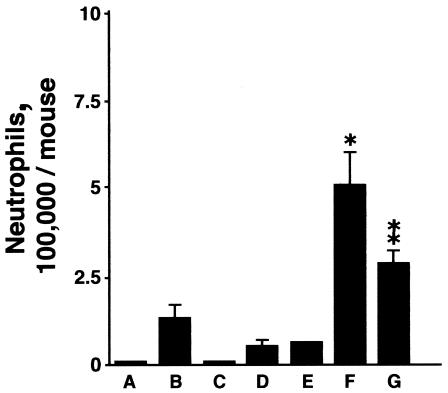
Although there was no overt evidence of inflammation by microscopy in uninfected SP-D−/− mice (see Fig. 9a), the total BAL cell count was significantly increased in control uninfected SP-D−/− mice (2.6 × 105 ± 2.4 × 105) compared to WT (0.8 × 105 ± 0.12 × 105) and SP-A−/− mice (0.7 ± × 105 ± 0.09 × 105) on day 23. No neutrophils were detected in BAL fluid in uninfected mice. In response to uninfected allantoic fluid, SP-D−/− mice but not WT or SP-A−/− mice responded with a small but significant influx of neutrophils on day 2 (Fig. 3). The increase in BAL neutrophils after inoculation with X-79 was significantly greater in SP-D−/− mice than in WT and SP-A−/− mice (Fig. 3). Coinoculation with recombinant mouse SP-D significantly blunted the increase in neutrophils in response to X-79 in SP-D−/− mice (Fig. 3). SP-A or human albumin also had no effect on the neutrophil response in SP-D−/− mice (data not shown).
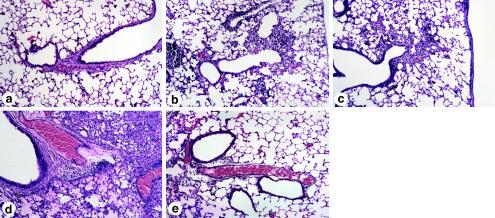
FIG. 9.
Histology of the lung 6 days after inoculation with X-79 virus. Mid-sagittal sections of the right lung were prepared 6 days after inoculation with X-79 virus. (a) Twenty-seven-day-old control SP-D−/− mouse lung; (b) WT mouse lung with X-79; (c) SP-A−/− mouse lung with X-79; (d) SP-D−/− mouse lung after X-79 inoculation; (e) SP-D−/− after X-79 inoculation and cotreatment with recombinant mouse SP-D.
FIG. 3.
BAL neutrophil counts 2 days after X-79 inoculation. BAL neutrophil counts were significantly elevated after inoculation of X-79 compared to results with allantoic fluid only for all three genotypes (P < 0.05; n = 5 mice/group). The neutrophil count was significantly greater for infected SP-D−/− mice than for infected WT and infected SP-A−/− mice (*, P < 0.002; n = 5 mice/group). Cotreatment of SP-D−/− mice with X-79 and recombinant mouse SP-D significantly reduced the neutrophil response to IAV (**, P < 0.05; n = 5 mice/group). (A) WT with allantoic fluid; (B) WT with X-79; (C) SP-A−/− with allantoic fluid; (D) SP-A−/− with X-79; (E) SP-D−/− with allantoic fluid; (F) SP-D−/− with X-79; (G) SP-D−/− with X-79 and recombinant mouse SP-D. Data are means ± standard errors of the means; n = 5 mice/group).
HA mRNA levels after inoculation with X-79.
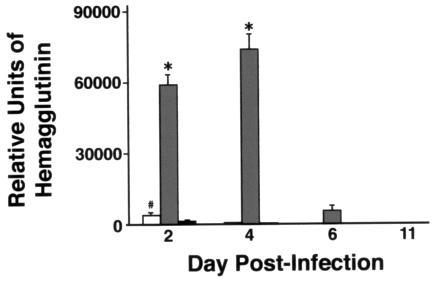
As assessed by real-time quantitative PCR, viral load was increased 40-fold on day 2 and 150-fold on day 4 in SP-D−/− mice compared to results with WT mice (P < 0.005 at both time points). Viral load fell rapidly in SP-D−/− mice between days 4 and 6 after inoculation. The viral load was also significantly increased in SP-A−/− mice compared to results for WT mice on day 2 (threefold; P < 0.05) but to a much less significant extent than with SP-D−/− mice (150-fold). There was no significant difference in viral load between SP-A−/− mice and WT mice on days 4 and 6. No viral RNA was detected on day 11 for any genotype (Fig. 4). Treatment of SP-D−/− mice with recombinant mouse SP-D reduced the viral load by threefold (P < 0.05; n = 5) compared to results with SP-D−/− mice given X-79 alone (data not shown). Treatment with human albumin did not significantly alter the viral load. In the cotreated SP-D−/− mice, the BAL levels of SP-D were 3 μg/mouse after recombinant mouse SP-D administration. These levels compare to BAL levels of 10 μg/mouse and almost 30 μg/mouse in WT mice before and 2 days after X-79 inoculation.
FIG. 4.
Viral load assessed by HA mRNA is increased in SP-D−/− mice during infection with X-79. Three-week-old mice were inoculated intranasally with X-79 virus and sacrificed 2, 4, 6, and 11 days later. HA mRNA levels in lungs were determined by quantitative real-time RT-PCR. Open bars, SP-A−/− mice; shaded bars, SP-D−/− mice; filled bars, WT mice. Data are means ± standard errors of the means (n = 10 mice/group). *, P < 0.005 compared with WT mouse results. #, P < 0.05 compared to WT mouse results.
To assess whether SP-A expression provides SP-D−/− mice with any residual protection against IAV replication, we performed a separate series of experiments with WT, SP-D−/−, and SP-AD−/− mice. The viral load was significantly higher in SP-AD−/− mice than WT mice on days 2, 4, and 6 after inoculation. There was, however, no significant increase in viral load in SP-AD−/− mice over that in SP-D−/− mice at any time point (Fig. 5). These results are most consistent with a primary role for SP-D in the protection of mice from X-79 infection, with minimal if any neutralizing activity of SP-A in vivo.
FIG. 5.
Viral load assessed by HA mRNA is similar in SP-D−/− and SP-AD−/− mice during X-79 infection. Three-week old mice were inoculated intranasally with X-79 virus and sacrificed 2, 4, or 6 days later. Lung HA mRNA levels were determined by quantitative real-time RT-PCR. Open bars, SP-AD−/− mice; shaded bars, SP-D−/− mice; filled bars, WT mice. Data are means ± standard errors of the means (n = 5 mice/group). The viral load was significantly greater in SP-D−/− and SP-AD−/− mice than in WT mice at all time points. The viral load was significantly greater in SP-D−/− mice than in SP-AD−/− mice on day 2, but there were no differences on days 4 and 6.
Cytokine responses to inoculation with X-79.
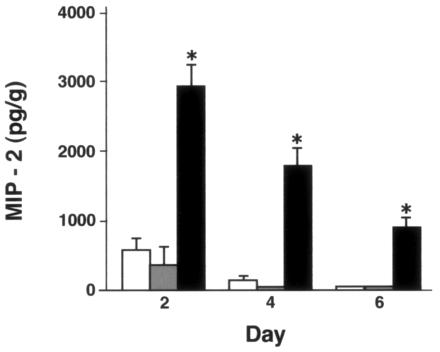
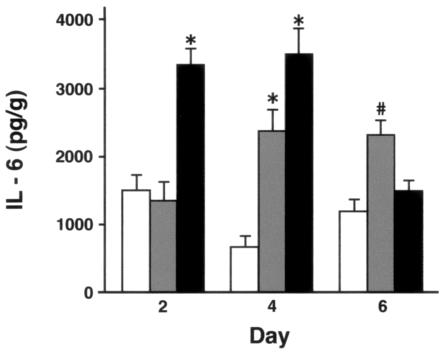
There were no significant differences between genotypes in cytokine levels in lungs of uninfected mice or mice given uninfected allantoic fluid (not shown). On day 2 after X-79 inoculation, MIP-2 levels were significantly greater in SP-D−/− mice than in WT and SP-A−/− mice. MIP-2 levels declined in all three genotypes on days 4 and 6 after inoculation, but the levels in SP-D−/− mice remained significantly elevated above those in WT and SP-A−/− mice at all time points. No significant difference between WT and SP-A−/− mice was detected at any time point (Fig. 6). IL-6 levels were significantly higher than those for WT mice in SP-D−/− mice on day 2 and in both SP-A−/− and SP-D−/− mice on day 4 and were higher in SP-A−/− mice than for the other two genotypes on day 6 (Fig. 7).
FIG. 6.
Lung MIP-2 levels are significantly higher in SP-D−/− mice after X-79 inoculation. MIP-2 levels in lung homogenates were measured by ELISA 2, 4, and 6 days after intranasal inoculation with X-79 virus. MIP-2 levels in uninfected mice or after inoculation with sterile allantoic fluid ranged from undetectable to 200 pg/g. The control values did not differ significantly between genotypes (data not shown). After X-79 inoculation, MIP-2 levels were significantly higher in SP-D−/− mice (filled bars) than in WT (open bars) and SP-A−/− (shaded bars) mice. There were no significant differences between WT and SP-A−/− mice. Data are means ± standard errors of the means (n = 5 mice/group). *, P < 0.0001 compared with WT results.
FIG. 7.
Lung IL-6 levels are significantly higher in SP-D−/− mice after inoculation with X-79. IL-6 levels in lung homogenates were measured by ELISA 2, 4, and 6 days after intranasal inoculation with X-79 virus. IL-6 was detectable in uninfected mice but did not differ between genotypes and did not change after inoculation with sterile allantoic fluid (not shown). After X-79 inoculation, IL-6 levels were significantly higher (*, P < 0.001) in SP-D−/− mice (filled bars) than in WT (open bars) and SP-A−/− (shaded bars) mice on day 2. There were no significant differences between WT and SP-A−/− mice on day 2. On day 4, IL-6 levels were higher in both SP-A−/− (P < 0.002) and SP-D−/− (*, P < 0.001) mice compared to WT results. The levels in SP-D−/− mice were significantly higher than those in SP-A−/− mice on day 4, but on day 6, IL-6 levels were higher in SP-A−/− mice than in both WT and SP-D−/− mice (#, P < 0.01). Data are means ± standard errors of the means (n = 4 to 7 mice/group).
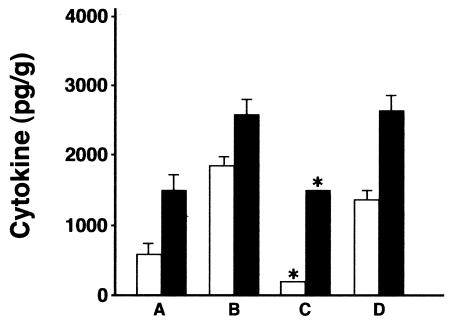
Treatment of SP-D−/− mice with SP-D completely prevented the increase in MIP-2 and IL-6, with cytokine levels at or below those seen in WT infected mice. Treatment of SP-D−/− mice with human albumin also partially blunted the increase in MIP-2 (P < 0.05), but human albumin had no significant effect on the increase in IL-6 levels (Fig. 8).
FIG. 8.
SP-D cotreatment reduces the cytokine response to X-79 virus in SP-D−/− mice. MIP-2 (open bars) and IL-6 (black bars) were measured by ELISA 2 days after inoculation of SP-D−/− mice with X-79 ± 10 μg of recombinant mouse SP-D or human albumin. (A) WT infected mice; (B) SP-D−/− infected mice; (C) SP-D−/− infected mice with 10 μg of recombinant mouse SP-D; (D) SP-D−/− infected mice plus 10 μg of human albumin. Cotreatment with recombinant mouse SP-D (C) significantly reduced the MIP-2 and IL-6 to levels similar to those for infected WT mice. Human albumin had a small but significant effect on MIP-2 levels but no effect on IL-6 levels. Data are means ± standard errors of the means (n = 5 mice/group). *, P < 0.05 compared to results for SP-D−/− mice with X-79 without cotreatment.
Microscopy after inoculation with X-79.
The inflammation, surfactant accumulation, and airspace enlargement that develops spontaneously in SP-D−/− mice with age was not apparent on day 27 in uninfected mice, equivalent to day 6 after inoculation (Fig. 9a). Mid-sagittal sections of the right lung showed peribronchial inflammation and edema in all mice inoculated with X-79 6 days previously. The extent of peribronchial and alveolar inflammation and edema was qualitatively much greater in SP-D−/− mice (Fig. 9d) than in WT (Fig. 9b) or SP-A−/− mice (Fig. 9c). Coinoculation of SP-D substantially reduced the inflammation and edema caused by X-79 infection (Fig. 9e).
Mannose receptor mRNA.
The macrophage mannose receptor may enhance IAV clearance by alveolar macrophages (30). We therefore measured mannose receptor mRNA in BAL cells from all genotypes to exclude the possibility that the increased viral load in SP-D−/− mice was secondary to a decrease in mannose receptor mRNA. There was no significant difference in the amounts of mannose receptor mRNA detected in BAL cells from WT mice and SP-D−/− mice, but the BAL cells from SP-A−/− mice contained significantly more mannose receptor mRNA than those from either WT mice (fourfold; P < 0.01; n = 7) or SP-D−/− mice (threefold; P < 0.05; n = 7).
Responses to inoculation with X-79Δ167.
In contrast to X-79, X-79Δ167 is a highly virulent virus in mice. The 50% lethal dose (LD50) for our X-79Δ167 stock in mature WT mice is a 10−4 dilution with death occurring on days 6 to 8 after intranasal inoculation (28). In order to study viral clearance, mice in this study were inoculated with a sublethal 10−5 dilution of X-79Δ167 in endotoxin-free saline. The pattern of weight loss from days 5 through 9 followed by weight gain from days 9 through 14 was similar for all three genotypes after inoculation with X-79167 (Fig. 10). On day 2 after inoculation with a 10−5 dilution of X-79Δ167, the BAL neutrophil counts were not different between genotypes (per mouse, WT, 0.5 × 104 ± 0.2 × 104; SP-A−/−, 1.0 × 104 ± 0.2 × 104; SP-D−/−, 1.5 × 104 ± 0.4 × 104).
FIG. 10.
Weight change in mice after inoculation with X-79Δ167 virus. Mice were weighed daily after intranasal inoculation with X-79Δ167 virus (10−1 LD50). WT mice (squares), SP-D−/− mice (filled circles), and SP-A−/− mice (open circles) all had similar patterns of initial weight gain similar to the control pattern (see controls in Fig. 3) followed by weight loss from days 5 to 10 and then recovery. No differences between genotypes were observed (n = 10 per genotype).
HA mRNA levels after inoculation with X-79Δ167.
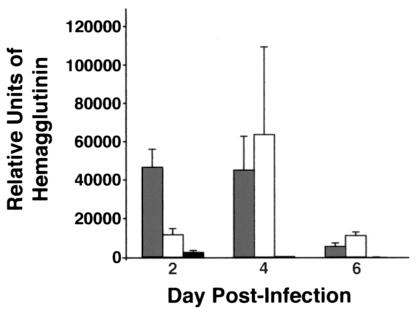
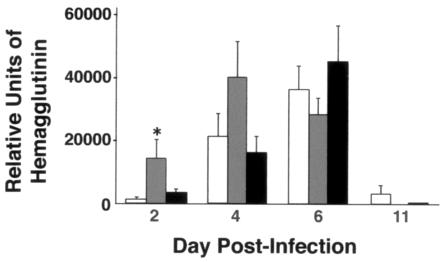
As assessed by real-time quantitative PCR, the viral load of X-79Δ167 was not significantly different between genotypes on days 4, 6, and 11. By day 11, viral load was very low for all mice. On day 2, SP-D−/− mice had a small but significantly higher viral load than either WT or SP-A−/− mice (Fig. 11).
FIG. 11.
Viral load assessed by HA mRNA is similar for all genotypes during infection with X-79Δ167. Three-week-old mice were inoculated intranasally with X-79Δ167 virus and sacrificed 2, 4, 6, and 11 days later. Lung HA mRNA levels were determined by quantitative real-time RT-PCR. Open bars, WT mice; grey bars, SP-D−/− mice; black bars, SP-A−/− mice. Data are means ± standard errors of the means (n = 10 mice/group). *, P < 0.05 compared with WT results.
Cytokines responses to inoculation with X-79Δ167.
Cytokine levels in lungs were measured on days 2, 4, 6, and 11 after inoculation with X-79Δ167. Both IL-6 and MIP-2 were significantly increased above uninfected levels for all three genotypes at each time point except for the MIP-2 levels on day 11 in SP-D−/− mice. In contrast to infection with X-79, neither IL-6 nor MIP-2 levels were significantly increased in SP-D−/− mice compared to WT mice at any time point. Levels of MIP-2 but not IL-6 were higher on days 4 and 6 in SP-A−/− mice than levels in both WT and SP-D−/− mice (Table 1). The cytokine levels after X-79Δ167 inoculation peaked on day 6, coincident with rapid weight loss, for all three genotypes and were declining towards baseline on day 11 as mice of all genotypes reestablished weight gain.
TABLE 1.
Cytokine levels after X-79Δ167 inoculationa
| Cytokine | Day | Level for genotype
|
||
|---|---|---|---|---|
| WT | SP-A−/− | SP-D−/− | ||
| MIP-2 | Preinoculation | Not detected | 164 ± 59 | 309 ± 84 |
| 2 | 1,038 ± 155 | 155 ± 37* | 989 ± 254 | |
| 4 | 396 ± 181 | 1,546 ± 392* | 533 ± 514 | |
| 6 | 1,385 ± 336 | 2,360 ± 190* | 1,267 ± 162 | |
| 11 | 1,016 ± 139 | 745 ± 111 | 337 ± 51* | |
| IL-6 | Preinoculation | 550 ± 79 | 1,054 ± 139 | 605 ± 92 |
| 2 | 3,423 ± 218 | 3,688 ± 366 | 3,331 ± 272 | |
| 4 | 4,009 ± 341 | 3,055 ± 877 | 4,232 ± 1,247 | |
| 6 | 6,934 ± 488 | 6,256 ± 711 | 4,807 ± 374* | |
| 11 | 1,252 ± 152 | 2,197 ± 216* | 1,135 ± 151 | |
Data are means ± standard errors of the means (pg/g of lung). *, P < 0.05 compared to WT (n = 5 to 10 per group).
DISCUSSION
IAV is a major human pathogen causing significant morbidity and more than 20,000 deaths per year in the United States. While the eventual recovery from IAV infection depends on the development of cell-mediated immunity (10), local mediators of innate immunity are important in limiting early viral growth and spread (23). In this study, we show that SP-D but not SP-A, both members of the collectin protein family, has a role in controlling viral replication and spread and reducing the early host response to IAV infection. This protective role of SP-D is strongly related to the state of glycosylation of HA, specifically at position 165 (N165), close to the IAV cell attachment site. Our studies add to the current literature by providing data on single- and double-knockout mice on the same genetic background and by more precisely localizing the key site on IAV for the neutralizing role of SP-D in vivo. In vitro studies have previously defined N165 as important for SP-D, conglutinin, and mannose-binding lectin (Mbl) neutralizing activity in vitro (1). SP-D attachment presumably sterically blocks the virus-cell receptor interaction needed for infection. Poorly glycosylated influenza virus strains or mouse- and bovine-adapted strains with a mutation that prevents N165 glycosylation lack SP-D attachment sites and are not neutralized effectively by SP-D (32). Additional glycosylation at positions 126 and 144 on the HA1 head domain may also contribute to SP-D and Mbl sensitivity (32). These sites were preserved in the X-79Δ167 strain and were possibly responsible for the weak residual neutralizing activity of SP-D against this strain in vitro and on day 2 in vivo. Glycosylation around the cell attachment site on the HA1 domain decreases binding of neutralizing antibodies, suggesting that the continual changes in amino acids around the sialic acid binding cleft resulting in antigenic drift result in a dynamic balance between SP-D and neutralizing antibody inhibition.
The collectins are a protein family characterized by extended collagen-like domains and calcium-dependent carbohydrate recognition domains homologous to the large C-type lectin superfamily (7). In the mouse and human, three collectins, SP-A, SP-D, and Mbl, have been identified. Each has IAV-neutralizing activity in vitro, with relative potency being dependent on the specific IAV strain tested (1, 19). Gene-targeted mice on a C57/BL6 background were used in this study to extend these studies. The phenotypes of SP-A- and SP-D-deficient mice in several different backgrounds have been reported previously. While uninfected SP-A−/− mice are largely indistinguishable from WT mice except for the absence of a surfactant fraction called tubular myelin (25), SP-D−/− mice develop patchy pulmonary inflammation, airspace remodeling, and surfactant accumulation (5, 33). These apparently noninfectious changes start early in life but are pronounced by 6 weeks of age (33). Mice in this study were inoculated at 3 weeks of age. The BAL cell counts prior to inoculation were already elevated due to increased numbers of macrophages, but baseline cytokine levels and the histology of the SP-D−/− lung were similar to those of the WT at this age. SP-A levels did not change in SP-D−/− mice in response to IAV infection with either strain of virus. The viral load, neutrophil counts, and cytokine levels were all significantly higher in SP-D−/− mice inoculated with X-79 than in WT or SP-A−/− mice. In contrast, there were no sustained differences in viral load between genotypes when the mice were inoculated with X-79Δ167, a strain resistant to neutralization by SP-D. These results support a role for SP-D in limiting early viral growth and spread, provided specific sites on HA are glycosylated. Similar results have recently been reported with mature Swiss Black mice (27).
The potential mechanism of SP-D protection remains to be determined. The dependence of SP-D neutralizing activity on IAV glycosylation is consistent with a key role for the CRD domain. Although the CRD domain alone is sufficient to protect mice against aspergillosis (29, 30) and respiratory syncytial virus (22), an SP-D mutant lacking the ability to assemble beyond a single trimeric subunit only partially protects mice from IAV infection (37). This finding supports the importance of the full oligomeric quaternary structure of SP-D in preventing IAV replication and associated inflammation. Other CRD-containing lectins in the lung could also conceivably contribute to strain-dependent IAV clearance. Specifically, the macrophage mannose receptor may have a role in macrophage uptake and clearance of glycosylated IAV strains (31). Although decreased expression of the mannose receptor could potentially contribute to IAV load, we found no significant difference in mannose receptor mRNA levels in macrophages from 3-week-old WT and SP-D−/− mice. Interestingly, we found a significant increase in macrophage mannose receptor RNA in macrophages from SP-A−/− mice, in apparent contrast to the reduced surface expression of mannose receptor on alveolar macrophages from SP-A−/− mice (2). Although surface expression of the mannose receptor was not assessed in our study, the significant reduction of IAV load in SP-D−/− mice by recombinant mouse SP-D suggests that the susceptibility of these mice to IAV infection is due directly to SP-D deficiency and not to a secondary change in another immune mediator.
Although SP-A has previously been shown to bind and neutralize nonglycosylated strains of IAV (4, 19), we did not detect significant neutralizing activity of either recombinant human SP-A or dog SP-A towards either X-79 or X-79Δ167 in our in vitro assay. Consistent with these results, both strains of IAV replicated similarly in WT and SP-A−/− mice. We also saw no difference in viral replication in WT and SP-A−/− mice in a prior study using outbred SP-A−/− mice and a dose of X-79Δ167 10 times the LD50 (28). In that study, the SP-A−/− mice responded to the virus with increased MIP-2 levels, and neutrophil counts compared to those for the WT (28). In this study, 3-week-old SP-A−/− mice inoculated with 1/100 the dose of X-79Δ167 also had significantly higher MIP-2 levels in the lung on days 4 and 6 after inoculation than WT mice. Taken together, our results suggest that SP-A might alter the host response to viral infection without directly influencing viral growth and spread, at least when the virus is resistant to neutralization by SP-D. SP-A−/− mice inoculated with X-79 responded similarly to WT mice. This result is consistent with the observation that SP-D neutralizes X-79 and that SP-D levels increase threefold within 2 days of inoculation in both WT and SP-A−/− mice. The response to X-79 in mice deficient in both SP-A and SP-D was similar to that in mice deficient in SP-D alone, consistent with a primary role for SP-D and a minimal, if any, role for SP-A in protecting mice from IAV infection. These results and conclusions are somewhat different from those recently reported by LeVine and colleagues using mature 129 strain SP-A−/− mice and H3N2 A/Philadelphia/82 IAV, a strain glycosylated at N165 and neutralized by SP-D (26). Despite finding a significant increase in SP-D levels in response to Phil/82, titers of virus and inflammatory responses were higher in SP-A−/− mice in these experiments (26). The reasons for the differences between studies remain to be resolved.
In summary, in young mice SP-D helps control IAV growth and spread, thereby minimizing the host inflammatory response provided that key sites on the viral surface are glycosylated. Since most IAV strains currently circulating in the human population are glycosylated at N165, SP-D may play a role in limiting the ability of IAV to spread to the lower respiratory tract in most individuals. Low SP-D levels in premature infants, chronic smokers, and patients with cystic fibrosis may contribute to the increase morbidity from IAV seen in these populations.
Acknowledgments
This work was supported by grants HL-58047 and HL-24075 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Viruses and the anti-IAV nucleoprotein monoclonal antibody were kindly provided by David Schnurr of the Division of Communicable Disease Control, Department of Health Services, Richmond, CA 94804.
REFERENCES
- 1.Anders, E. M., C. A. Hartley, and D. C. Jackson. 1990. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc. Natl. Acad. Sci. USA 87:4485-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beharka, A. A., C. D. Gaynor, B. K. Kang, D. R. Voelker, F. X. McCormack, and L. S. Schlesinger. 2002. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J. Immunol. 169:3565-3573. [DOI] [PubMed] [Google Scholar]
- 3.Benne, C. A., B. Benaissa-Trouw, J. A. van Strijp, C. A. Kraaijeveld, and J. F. van Iwaarden. 1997. Surfactant protein A, but not surfactant protein D, is an opsonin for influenza A virus phagocytosis by rat alveolar macrophages. Eur. J. Immunol. 27:886-890. [DOI] [PubMed] [Google Scholar]
- 4.Benne, C. A., C. A. Kraaijeveld, J. A. van Strijp, E. Brouwer, M. Harmsen, J. Verhoef, L. M. van Golde, and J. F. van Iwaarden. 1995. Interactions of surfactant protein A with influenza A viruses: binding and neutralization. J. Infect. Dis. 171:335-341. [DOI] [PubMed] [Google Scholar]
- 5.Botas, C., F. Poulain, J. Akiyama, C. Brown, L. Allen, J. Goerke, J. Clements, E. Carlson, A. M. Gillespie, C. Epstein, and S. Hawgood. 1998. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc. Natl. Acad. Sci. USA 95:11869-11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conn, C. A., J. L. McClellan, H. F. Maassab, C. W. Smitka, J. A. Majde, and M. J. Kluger. 1995. Cytokines and the acute phase response to influenza virus in mice. Am. J. Physiol. 268:R78-R84. [DOI] [PubMed] [Google Scholar]
- 7.Crouch, E., K. Hartshorn, and I. Ofek. 2000. Collectins and pulmonary innate immunity. Immunol. Rev. 173:52-65. [DOI] [PubMed] [Google Scholar]
- 8.Dawson, T. C., M. A. Beck, W. A. Kuziel, F. Henderson, and N. Maeda. 2000. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 156:1951-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty, P. C., D. J. Topham, and R. A. Tripp. 1996. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol. Rev. 150:23-44. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 11.Gerhard, W., K. Mozdzanowska, M. Furchner, G. Washko, and K. Maiese. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159:95-103. [DOI] [PubMed] [Google Scholar]
- 12.Haagsman, H. P., R. T. White, J. Schilling, K. Lau, B. J. Benson, J. Golden, S. Hawgood, and J. A. Clements. 1989. Studies of the structure of lung surfactant protein SP-A. Am. J. Physiol. 257:L421-L429. [DOI] [PubMed] [Google Scholar]
- 13.Hartley, C. A., P. C. Reading, A. C. Ward, and E. M. Anders. 1997. Changes in the hemagglutinin molecule of influenza type A (H3N2) virus associated with increased virulence for mice. Arch. Virol. 142:75-88. [DOI] [PubMed] [Google Scholar]
- 14.Hartshorn, K., D. Chang, K. Rust, M. White, J. Heuser, and E. Crouch. 1996. Interactions of recombinant human pulmonary surfactant protein D and SP-D multimers with influenza A. Am. J. Physiol. 271:L753-L762. [DOI] [PubMed] [Google Scholar]
- 15.Hartshorn, K. L., E. C. Crouch, M. R. White, P. Eggleton, A. I. Tauber, D. Chang, and K. Sastry. 1994. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Investig. 94:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartshorn, K. L., A. B. Karnad, and A. I. Tauber. 1990. Influenza A virus and the neutrophil: a model of natural immunity. J. Leukoc. Biol. 47:176-186. [DOI] [PubMed] [Google Scholar]
- 17.Hartshorn, K. L., K. B. Reid, M. R. White, J. C. Jensenius, S. M. Morris, A. I. Tauber, and E. Crouch. 1996. Neutrophil deactivation by influenza A viruses: mechanisms of protection after viral opsonization with collectins and hemagglutination-inhibiting antibodies. Blood 87:3450-3461. [PubMed] [Google Scholar]
- 18.Hartshorn, K. L., K. N. Sastry, D. Chang, M. R. White, and E. C. Crouch. 2000. Enhanced anti-influenza activity of a surfactant protein D and serum conglutinin fusion protein. Am. J. Physiol. Lung Cell Mol. Physiol. 278:L90-L98. [DOI] [PubMed] [Google Scholar]
- 19.Hartshorn, K. L., M. R. White, V. Shepherd, K. Reid, J. C. Jensenius, and E. C. Crouch. 1997. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am. J. Physiol. 273:L1156-L1166. [DOI] [PubMed] [Google Scholar]
- 20.Hawgood, S., B. J. Benson, and R. L. Hamilton, Jr. 1985. Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry 24:184-190. [DOI] [PubMed] [Google Scholar]
- 21.Hawgood, S., M. Ochs, A. Jung, J. Akiyama, L. Allen, C. Brown, J. Edmondson, S. Levitt, E. Carlson, A. M. Gillespie, A. Villar, C. J. Epstein, and F. R. Poulain. 2002. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L1002-L1010. [DOI] [PubMed] [Google Scholar]
- 22.Hickling, T. P., H. Bright, K. Wing, D. Gower, S. L. Martin, R. B. Sim, and R. Malhotra. 1999. A recombinant trimeric surfactant protein D carbohydrate recognition domain inhibits respiratory syncytial virus infection in vitro and in vivo. Eur. J. Immunol. 29:3478-3484. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann, P., H. Sprenger, A. Kaufmann, A. Bender, C. Hasse, M. Nain, and D. Gemsa. 1997. Susceptibility of mononuclear phagocytes to influenza A virus infection and possible role in the antiviral response. J. Leukoc. Biol. 61:408-414. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe, H. J., and K. B. Reid. 1994. Collectins—soluble proteins containing collagenous regions and lectin domains—and their roles in innate immunity. Protein Sci. 3:1143-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korfhagen, T. R., M. D. Bruno, G. F. Ross, K. M. Huelsman, M. Ikegami, A. H. Jobe, S. E. Wert, B. R. Stripp, R. E. Morris, S. W. Glasser, C. J. Bachurski, H. S. Iwamoto, and J. A. Whitsett. 1996. Altered surfactant function and structure in SP-A gene targeted mice. Proc. Natl. Acad. Sci. USA 93:9594-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeVine, A. M., K. Hartshorn, J. Elliott, J. Whitsett, and T. Korfhagen. 2002. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am. J. Physiol. Lung Cell Mol. Physiol. 282:L563-L572. [DOI] [PubMed] [Google Scholar]
- 27.LeVine, A. M., J. A. Whitsett, K. L. Hartshorn, E. C. Crouch, and T. R. Korfhagen. 2001. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 167:5868-5873. [DOI] [PubMed] [Google Scholar]
- 28.Li, G., J. Siddiqui, M. Hendry, J. Akiyama, J. Edmondson, C. Brown, L. Allen, S. Levitt, F. Poulain, and S. Hawgood. 2002. Surfactant protein-A-deficient mice display an exaggerated early inflammatory response to a beta-resistant strain of influenza A virus. Am. J. Respir. Cell Mol. Biol. 26:277-282. [DOI] [PubMed] [Google Scholar]
- 29.Madan, T., U. Kishore, M. Singh, P. Strong, H. Clark, E. M. Hussain, K. B. Reid, and P. U. Sarma. 2001. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J. Clin. Investig. 107:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madan, T., U. Kishore, M. Singh, P. Strong, E. M. Hussain, K. B. Reid, and P. U. Sarma. 2001. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect. Immun. 69:2728-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reading, P. C., J. L. Miller, and E. M. Anders. 2000. Involvement of the mannose receptor in infection of macrophages by influenza virus. J. Virol. 74:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reading, P. C., L. S. Morey, E. C. Crouch, and E. M. Anders. 1997. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 71:8204-8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wert, S. E., M. Yoshida, A. M. LeVine, M. Ikegami, T. Jones, G. F. Ross, J. H. Fisher, T. R. Korfhagen, and J. A. Whitsett. 2000. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc. Natl. Acad. Sci. USA 97:5972-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijburg, O. L., S. DiNatale, J. Vadolas, N. van Rooijen, and R. A. Strugnell. 1997. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J. Virol. 71:9450-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright, J. R. 1997. Immunomodulatory functions of surfactant. Physiol. Rev. 77:931-962. [DOI] [PubMed] [Google Scholar]
- 36.Xu, X., E. D. Kilbourne, H. E. Hall, and N. J. Cox. 1994. Nonimmunoselected intrastrain genetic variation detected in pairs of high-yielding influenza A (H3N2) vaccine and parental viruses. J. Infect. Dis. 170:1432-1438. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, L., K. L. Hartshorn, E. C. Crouch, M. Ikegami, and J. A. Whitsett. 2002. Complementation of pulmonary abnormalities in SP-D(−/−) mice with an SP-D/conglutinin fusion protein. J. Biol. Chem. 277:22453-22459. [DOI] [PubMed] [Google Scholar]