Abstract
The presence of vector-specific immune responses may hamper the induction of responses to a foreign antigen encoded by the vector. We evaluated the impact of pre-existing immunity to vaccinia virus on the induction of HIV-specific responses after immunization of healthy volunteers with a HIV-1 DNA prime-MVA boost vaccine. Following three priming immunizations with HIV-1 DNA plasmids, the volunteers were boosted with a single injection of recombinant MVA encoding HIV-1 proteins. Pre-existing immunity to vaccinia virus did not reduce the proportion of individuals who responded to HIV-1, but did lower the magnitude of responses. Our results suggest that vaccinia-based vectors can be used to efficiently induce immune responses to vectored HIV-1 antigens, even in individuals with pre-existing immunity to vaccinia virus.
Keywords: HIV-1 vaccine, Modified Vaccinia virus Ankara (MVA), Pre-existing immunity
1. Introduction
Recombinant viral vectors have emerged as a potentially useful modality in vaccine technology. They can carry large foreign genetic load and effectively deliver them intracellularly, resulting in expression of the antigen and induction of long-term cellular and humoral immune responses to the vectored antigen(s) [1–3]. Fowlpox, New York Vaccinia virus (NYVAC) and Modified Vaccinia virus Ankara (MVA) are presently used in prime-boost regimens with plasmid DNA or subunit vaccines [4–7]. MVA is a replication-deficient strain of vaccinia virus that efficiently infects mammalian cells. The strain was developed by serial passage of vaccinia virus Ankara in chicken embryo fibroblasts, resulting in the deletion of genes encoding immunomodulatory functions and host range determinants [2,8–10]. This process resulted in an attenuated virus with a safety profile that is substantially better than that of the original vaccinia virus [9,11]. MVA has an excellent safety record as a smallpox vaccine, for which it was used during the late phase of the smallpox eradication campaign. Moreover, MVA is safe in immunocompromised animals and humans and might therefore be applicable as a vector in HIV-infected persons [12,13].
Immune responses to a vaccine’s vector component may hamper the induction of an effective immune response to the foreign antigen delivered by the vector [14]. Anti-vector antibodies may reduce the actual vaccine dose and thereby prevent efficient delivery of the foreign gene. The negative effect of pre-existing immunity to vaccinia has been studied extensively in the context of neutralizing humoral immunity [15–17]. The impact of anti-vector immunity on the induction of immune responses to an inserted antigen is most likely related to both the efficiency of expression of the vectored gene and the immune status of the host. Prolonged or excessive antigenic stimulation favors the development of effector memory T cells, while a brief period of antigenic stimulation favors the development of the central memory lineage [18–20]. A recent study in monkeys suggests that pre-existing immunity to MVA increases competition between vector-specific and insert-specific T cell responses and thus favors the generation of insert-specific central memory responses relative to effector memory CD4 T cells [21]. As a consequence of smallpox immunizations carried out worldwide until the mid-1970s [22,23], anti-vaccinia virus immunity is present in the older population, but not in younger individuals. The numbers of vaccinia-naïve individuals will therefore continue to increase, enabling wider use of poxviruses as vaccine vectors.
In this study, we have re-analyzed data from an HIV DNA and MVA vaccine trial [24], pertaining to the importance of vector immunity related to insert HIV responses. We demonstrate that pre-existing immunity to vaccinia virus moderately influences the magnitude, but not the frequency, of HIV-1-specific immune responses elicited by an HIV DNA (HIVIS) prime, HIV MVA boost. In addition to strong anti-HIV responses, vaccinia-naïve individuals developed low and transient anti-vaccinia responses.
2. Materials and methods
2.1. Study group
Forty healthy HIV-1 negative Swedish volunteers, 7 women and 33 men, were randomly assigned to four groups to be injected three times with either 1 mg of HIV DNA plasmids intradermally (id) or 2–3.8 mg intramuscularly (im), with or without adjuvant granulocyte macrophage stimulating factor (GM-CSF) [24]. Table 1 shows the outline of the vaccine study and the distribution of the 38 individuals receiving the HIV MVA boost.
Table 1.
Characteristics of 4 treatment groups in an HIV-1 vaccine study that combined DNA priming immunizations and a modified vaccinia virus Ankara (MVA) boosting immunization.
| Treatment group | 3 × HIV DNA priming immunizations |
1 × HIV MVA boosting immunization | |||
|---|---|---|---|---|---|
| A (n=10) | 1 mg | ID | (n = 5) | 108 PFU | IM |
| (n = 5) | 107 PFU | ID | |||
| B (n = 10) | 3.8 mg | IM | (n = 5) | 108 PFU | IM |
| (n = 5) | 107 PFU | ID | |||
| C (n = 10) | 1 mg + GM-CSF | ID | (n = 5) | 108 PFU | IM |
| (n = 4)a | 107 PFU | ID | |||
| D (n = 10) | 2 mg + GM-CSF | IM | (n = 4) | 108 PFU | IM |
| (n = 5) | 107 PFU | ID | |||
DNA priming immunizations were administered at days 0, 30 and 90. The MVA boosting immunization was administered at month 9. GM-CSF, granulocyte macrophage colony stimulating factor; ID, intradermal; IM, intramuscular.
One individual were excluded from the analyses due high background in the IFN-γ ELISpot assay.
The HIV DNA plasmids encode envelope gp160 of HIV-1 subtypes A, B and C, rev B, Gag A and B and reverse transcriptase (RT) B, and have been described elsewhere [1,25]. The plasmids were given at 0, 1 and 3 months, using the needle-free device Biojector 2000 (Bioject Medical Technologies Inc., OR, USA). The volunteers were block re-randomized at month 9 to receive a single boost of either 107 plaque-forming units (PFU) id or 108 PFU im of MVA-Chiang Mai double Recombinant (MVA-CMDR) (HIV MVA), which expresses subtype E gp150 Env and Gag/Pol from Thai isolates CM235 and CM240 [1,24] (Earl et al., manuscript in preparation). Blood samples were collected prior to the HIV MVA immunization as well as 2 weeks and 3 months afterwards. Two individuals terminated the trial before the HIV MVA immunization, and one were excluded from the analysis of accumulated ELIspot responses due to high background responses in the immunoassays; hence, the analyses were conducted on 37 vaccinees.
2.2. Neutralization of vaccinia virus
Sera were inactivated at 56 °C for 30 min and subsequently diluted in four fold steps. Serum dilutions and live vaccinia virus (strain Elstree, Bernabiotech, Bern, Switzerland) at a final concentration of 167 PFU/ml were mixed in Eagle’s minimum essential medium with 2% fetal calf serum (FCS, Sigma–Aldrich, Stockholm, Sweden) and incubated for 90 min at 37 °C. Virus and serum mixtures were added in triplicate to fully confluent Green Monkey Kidney (GMK) cells in 24-well tissue culture plates. After 1 h of incubation at 37 °C, the supernatants were replaced with 0.5 ml fresh Eagle’s minimum essential medium with 2% FCS. Cells were subsequently kept at 37 °C with 5% CO2 in air. Forty hours later, the cells were stained and fixed by addition of 50 µl crystal violet in 12% paraformaldehyde. After 30 min the medium was removed and the cells were allowed to dry. The number of plaques formed in each well was quantified in a light microscope. The neutralizing antibody titer was defined as the lowest serum dilution at which the number of foci was reduced by 50%; a titer above 10 was considered a positive response. A purified vaccinia immunoglobulin (Swedish Institute for Infectious Disease Control, Solna, Sweden) was used as a positive control for inter-assay variation.
2.3. Enzyme-linked immunoassay for HIV
ELISA was carried out as previously described [26,27]. In brief, ELISA plates (Nunc, Maxisorp, Odense, Denmark) were coated with recombinant proteins of either HIV subtype B gp160 (Protein Sciences Corporation, Meriden, CT) or recombinant HIV subtype B p55gag, a kind gift from Dr. Susan Barnett, Novartis, MD. Plates were blocked with 5% fat-free milk in phosphate-buffered saline, and serum dilutions were added. Reactive antibodies were detected with goat anti-human immunoglobulin G antibodies conjugated to horseradish peroxidase (BioRad, Stockholm, Sweden) diluted 1/3000. Plates were then developed for 10 min by adding O-phenylene diamine buffer (Sigma–Aldrich, Stockholm, Sweden). The color reaction was stopped with 2.5 mol/L H2SO4, and the optical density was read at 490 nm.
2.4. IFN-γ ELISpot for vaccinia virus and HIV-1
The IFN-γ ELISpot assay was performed as described earlier [24]. In brief, freshly isolated PBMCs were cultured for 20 h in triplicate wells on pre-coated IFN-γ ELISpot plates (Mabtech, Nacka, Sweden), final concentration 200,000 cells/well, with or without heat inactivated vaccinia virus (strain Elstree), HIV antigens or control antigens. Final concentrations of 5 × 104 PFU/well (vaccinia virus), 2.5 µg/ml of HIV-1 peptide pools (Table 2) and 5 µg/ml of CMV, EBV and Influenza virus (CEF) peptides or phytohemagglutinin (PHA) were used. ELIspot responses were considered positive if the number of spot-forming cells was >4 times the background and >55 SFC/106 PBMCs. Here, the magnitude of responses was determined as the accumulated responses to Gag and Env peptide pools less the medium background responses of each sample. Accumulated Gag responses were obtained by summing the responses to Gag I and Gag II peptide pools. Similarly, accumulated Env responses were obtained by summing the responses to Env I, Env II and Env III peptide pools.
Table 2.
HIV-1-specific peptide pools used in the ELISpot assay.
| Peptide pool ID | Protein | Subtype | The peptide sequence corresponds to |
|---|---|---|---|
| Gag WR | p6,p7,p17,p24 | A | HIV MVA boost |
| Gag I | p17 | B | DNA prime |
| Gag II | p24 | A | DNA prime |
| Env I | gp120, including V1 and V2 | A/B | DNA prime |
| Env II | gp120, including V3-V5 | A | DNA prime |
| Env III | gp41 | B | DNA prime |
| RT I | pol | B | DNA prime |
| RT II | pol | B | DNA prime |
All peptides were 15-mers with 10 amino acid (aa) overlap except the Gag WR pool which had peptides with 11 aa overlap.
2.5. Lymphocyte proliferation assay with HIV-1 antigens
The lymphocyte proliferation assay was performed as described earlier [24]. In brief, freshly isolated PBMCs were cultured in triplicate wells with or without HIV antigens for 6 days or with PHA for 2 days. The antigens, aldrithiol-2 (AT-2) treated HIV-1 strain MN and control cellular extracts SUPT1, kindly provided by Dr. J Lifson, SAIC Frederick Inc., Frederick, USA, were used at a final concentration of 2.5 µg/ml. The incorporation of thymidine was measured after incubation for 6 h with 3H-thymidine. Stimulation indices (SI) were calculated by dividing the mean 3H-thymidine incorporation of antigen-stimulated cells by the incorporation of cells incubated with medium or the cell control SUPT. A lymphocyte proliferation result was defined as positive if the stimulation index was above eight.
2.6. Statistical analysis
Statistical analyses were performed using the Statistica software version 8 (Statsoft Inc., Tulsa USA). Comparison between medians of two groups of observations was performed by the nonparametric Mann–Whitney U test and intra-group variations were assessed using Wilcoxon signed rank tests. The criterion for statistical significance was p ≤ 0.05. Correlation was determined by the Spearman-rank method. The Bonferroni method was used for adjusting p values for multiple testing. Median values are shown in figures as bars.
2.7. Ethics
The protocols and products were approved by the Karolinska Institute and the Regional Ethics Committee and the Swedish Medical Products Agency. Informed consent was obtained from all participants.
3. Results
Initially, 40 healthy Swedish HIV-1 negative volunteers were randomized into four groups to be injected three times with HIV DNA plasmids intradermally or intramuscularly. GM-CSF, which was only given together with the HIV DNA, decreased cellular reactivity to HIV antigens [24]. Six months after the third DNA immunization, the same volunteers were block re-randomized to receive a single boost of either 107 PFU id or 108 PFU im of recombinant HIV MVA carrying HIV-1 genes. We then evaluated the extent to which pre-existing immunity to vaccinia influenced the induction of HIV-specific responses. This was done by measuring vaccinia neutralizing antibodies and cell-mediated vaccinia immune responses and comparing them to the HIV-specific immune responses.
3.1. Vaccinia-specific humoral immune responses induced by the HIV MVA are dose-dependent
In Sweden, vaccinia vaccination in children was mandatory until 1976 and ceased in 1979. Neutralizing antibody titers to vaccinia were analyzed prior to the HIV MVA boost immunization, as well as 2 weeks and 12 weeks after the immunization (Fig. 1). Nineteen vaccinees had previously received vaccinia immunization, as evidenced by vaccinia scars, vaccinia neutralizing antibody titer and/or their medical history. Prior to immunization with the HIV MVA, 12 of 37 individuals had detectable neutralizing antibodies against vaccinia (Fig. 1C). All 12 had a previous history of vaccinia immunization. Thus, seven individuals with a history of vaccinia vaccination had no detectable anti-vaccinia antibodies (Fig. 1C).
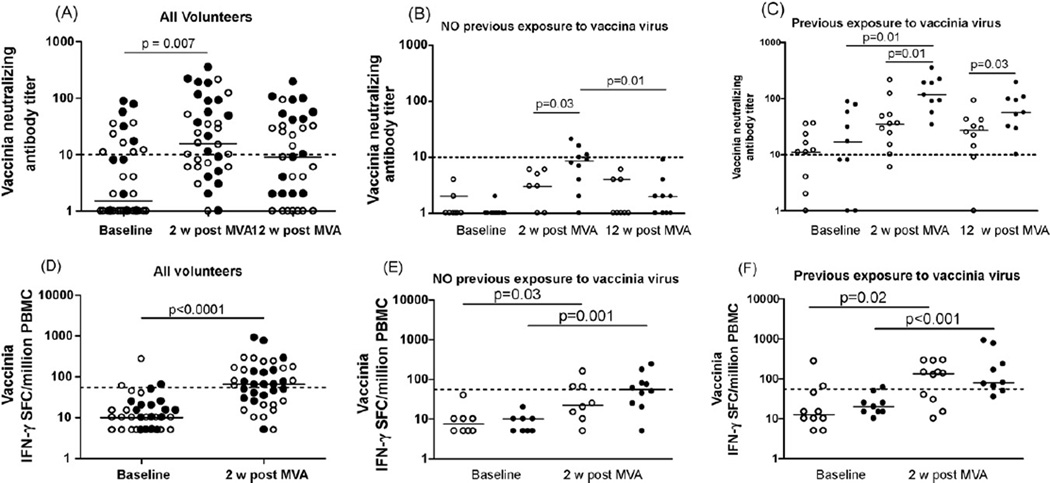
Fig. 1.
Humoral and cellular immune responses to vaccinia virus at baseline and after HIV MVA immunization. Open circles indicate 107 PFU HIV MVA delivered id, closed black circles indicate 108 PFU HIV MVA delivered im. Short horizontal bars indicate medians. The dotted lines indicate the cut off for each assay. (A) Vaccinia virus neutralizing antibody titers in all volunteers. (B) Vaccinia virus neutralizing antibody titers in individuals with no previous exposure to vaccinia virus (vaccinia-naïve individuals). (C) Vaccinia virus neutralizing antibody titers in individuals with previous exposure to vaccinia virus (vaccinia pre-immune individuals). (D) IFN-γ ELISpot responses to vaccinia virus in all volunteers. (E) IFN-γ ELISpot responses to vaccinia virus in individuals with no previous exposure to vaccinia virus. (F) IFN-γ ELISpot responses to vaccinia virus in individuals with previous exposure to vaccinia virus.
Immunization with HIV MVA induced a significant increase in neutralizing antibody titers to vaccinia in 34 of the 37 individuals (p = 0.007, Fig. 1A). In volunteers with no pre-existing immunity to vaccinia the levels of vaccinia antibodies, measured 2 weeks after HIV MVA immunization, were dependent on the dose of HIV MVA (Fig. 1B). In vaccinia-naïve individuals, immunization with 108 PFU of HIV MVA induced higher anti-vaccinia titers than the lower dose of 107 PFU (p = 0.03). At 3 months after immunization, this dose effect could not longer be observed, as the neutralizing titers in all volunteers without pre-existing immunity to vaccinia decreased to a median below 10 (Fig. 1B).
Of the individuals with detectable vaccinia titers prior to the MVA immunization, all except two had increased antivaccinia titers after the HIV MVA (Fig. 1C). In this group, the titers of neutralizing antibodies post-MVA were likewise dependent on the dose of HIV MVA. The higher dose of HIV MVA induced vaccinia 2 two weeks after immunization that were significantly higher than the baseline values (p = 0.01, Fig. 1C).
3.2. HIV MVA immunization induces low cellular immune responses to vaccinia
The vaccinia-specific cellular immune response was measured by stimulation of PBMC with inactivated vaccinia virus at baseline and at 2weeks post-HIV MVA vaccination (Fig. 1D–F). The 12-week samples were not available due to a lack of fresh lymphocytes at that time. Prior to the HIV MVA immunization against vaccinia, only two of the analyzed individuals showed an IFN-γ response above the cut off (55 SFC/million PBMC) (Fig. 1D). The HIV MVA immunization elicited vaccinia-specific IFN-γ responses in 20 of 37 tested individuals (p < 0.0001, Fig. 1D). The magnitude of the vaccinia-specific cellular immune responses in the previously vaccinia-naïve individuals (median 22.5 and 55 SFC/million PBMC respectively for the id and the im group) was below that in the vaccinia pre-immune individuals (median 135 and 80 SFC/million PBMC respectively for the id and the im group). However, the difference between the two groups was not significant.
3.3. Pre-existing immunity to vaccinia lowers the magnitude of the HIV-specific cellular responses
We investigated whether the magnitude of HIV-1 immune responses was related to the pre-existing responses to vaccinia. All vaccinia-naïve individuals mounted an HIV-specific immune response as measured by either IFN-γ ELISpot or lymphoproliferation at 2 weeks after the HIV MVA boost (Fig. 2A and D p = 0.03 p = 0.01). All but one of the individuals, with pre-existing immune responses to vaccinia virus, mounted an HIV-1-specific cellular response. The accumulated HIV-1-specific cellular response was moderately lower in individuals with pre-existing immunity to vaccinia.
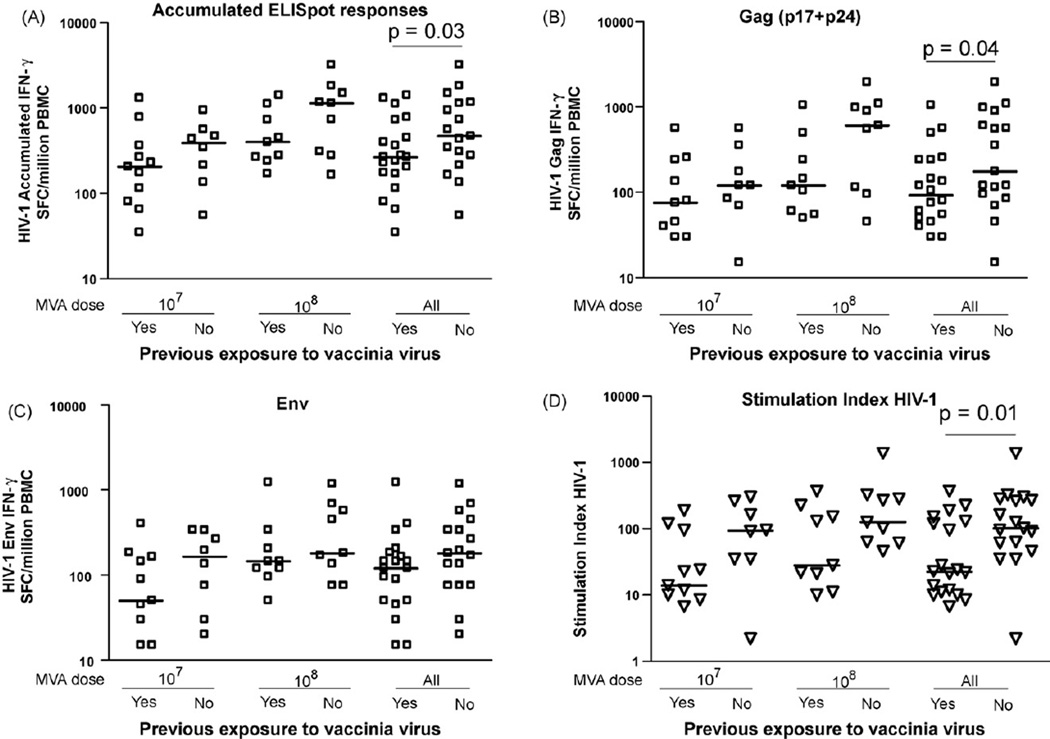
Fig. 2.
IFN-γ ELISpot responses to HIV-1 peptide pools after HIV MVA boost, related to vaccinia vaccination status and dose. Short horizontal bars indicate medians. (A) Distribution of accumulated HIV-1 IFN-γ ELISpot responses in vaccinia-naïve and pre-immune individuals. (B) Distribution of Gag (p17 + p24) IFN-γ ELISpot responses in vaccinia-naïve and pre-immune individuals. (C) Distribution of Env IFN-γ ELISpot responses in vaccinia-naïve and pre-immune individuals. (D) Distribution of HIV-1 antigen-specific T cell proliferative responses in vaccinia-naïve and pre-immune individuals.
The higher dose of MVA, 108 PFU, induced HIV-1-specific responses that were significantly stronger than those observed after injection of the lower dose of 107 PFU of MVA. In the higher dose group, the median of the accumulated HIV-specific IFN-γ responses in vaccinia-naïves was 990 SFC/million PBMC as compared to 400 SFC/million PBMC in vaccinia pre-immune individuals (Fig. 2A). In the lower dose group, the median of the accumulated HIV-specific IFN-γ responses in vaccinia-naïves was 390 SFC/million PBMC as compared to 205 SFC/million PBMC in vaccinia pre-immune individuals (Fig. 2A).
Cellular responses to HIV Gag and HIV Env followed similar trends, with higher insert-specific cellular responses in vaccinia-naïve persons than in persons with pre-existing immunity (Fig. 2B and C). However, the cellular immune responses to Env elicited by the higher dose of HIV MVA seemed to be less influenced by preexisting anti-vaccinia antibody titers than the responses elicited by the lower dose (Fig. 2C). Pre-existing titers to vaccinia had a negative influence on the magnitude of HIV cellular responses, but the inverse relationship between pre-existing anti-vaccinia neutralizing antibody titers and an HIV-specific cellular immune response did not reach significance (not shown).
Similar to our findings in the ELISpot assays, the T cell proliferative responses were clearly higher in vaccinia-naïve individuals than in individuals with pre-existing vaccinia-immunity (p = 0.01, Fig. 2D). This observation was independent of the injected dose of HIV MVA. There was a tendency for the magnitude of HIV-specific proliferation to be lower in the individuals with pre-existing vaccinia antibody titers (p = 0.04, r = 0.4 data not shown).
3.4. Pre-existing vaccinia immunity lowers the HIV-1-specific humoral responses
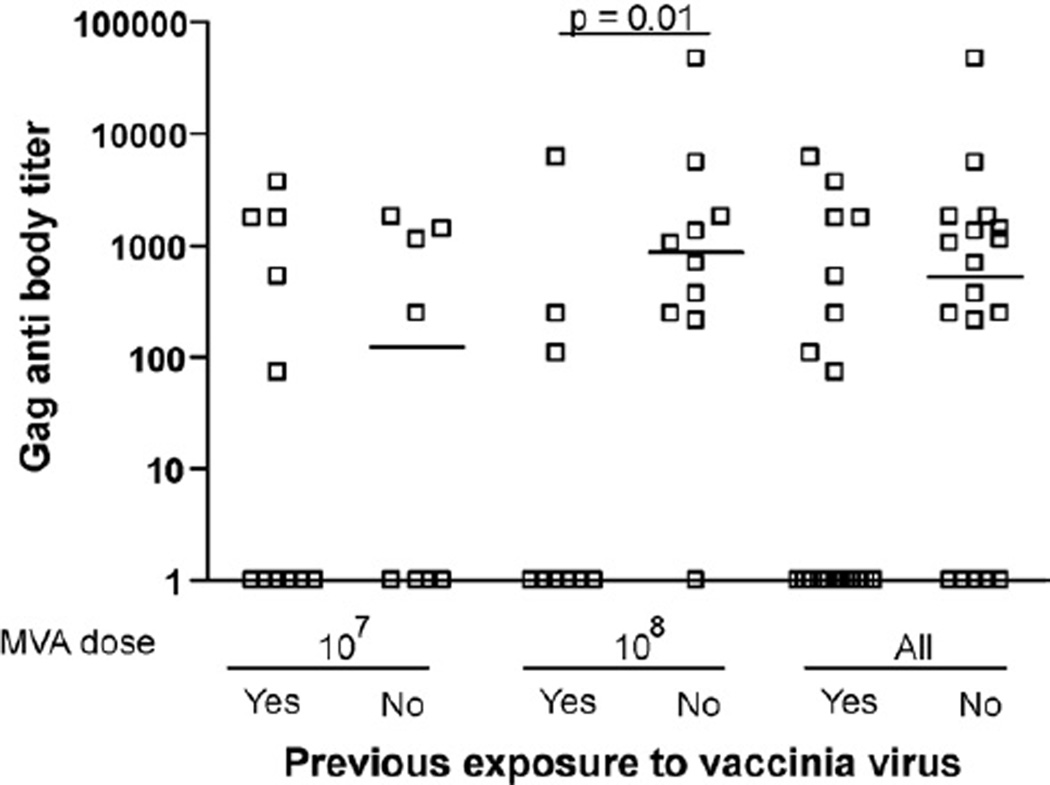
HIV Gag-specific antibodies were detected in 21 of 37 individuals after HIV MVA immunization. Responses ranged from titers of 50 to 10,200, with a median anti-Gag titer of 900 in vaccinia-naïve individuals (Fig. 3). The influence of pre-existing immunity to vaccinia was most evident in the high-dose HIV MVA group, where the HIV Gag-specific antibodies were significantly higher in vaccinia-naïve individuals compared to vaccinia pre-immune individuals (Fig. 3, p = 0.01). This trend was also observed in the group that received a lower dose of vaccinia, but the differences did not reach significance. The immunization regimen induced anti-gp160 antibodies in only 1 of 37 vaccinees; thus, no correlation could be assessed between pre-existing immunity to vaccinia and gp160 antibodies (data not shown).
Fig. 3.
HIV-1 humoral responses (to Gag) after HIV MVA boost, in vaccinia-naïve and pre-immune individuals, related to dose.
3.5. Relationship between age and cellular responses to HIV-1 after immunization
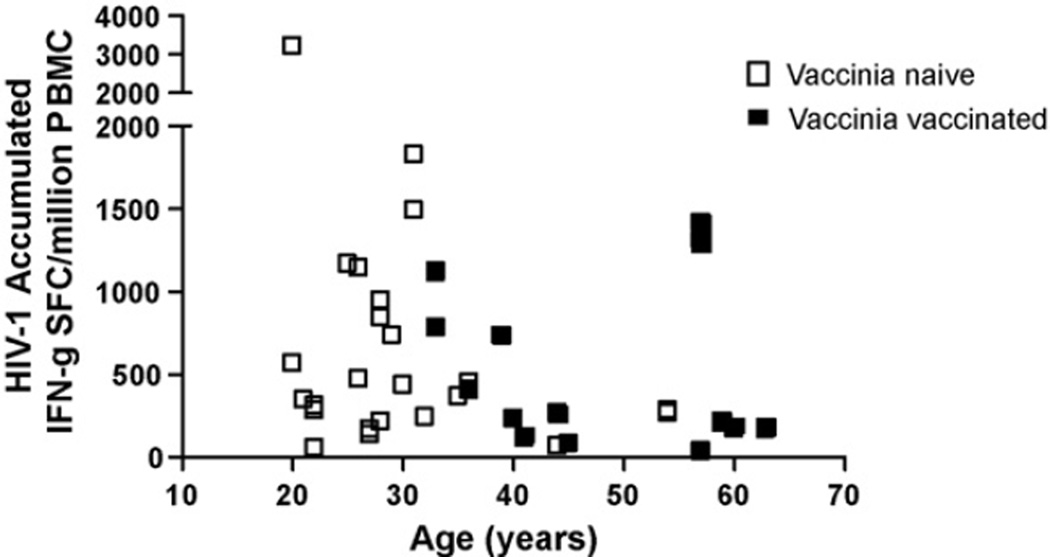
We have previously reported that the HIV-1 Gag-specific cellular responses, measured with the HIV peptides corresponding to the recombinant HIV genes in the HIV MVA construct, were lower in older individuals [24]. Therefore, we investigated whether age also influenced the total HIV-1-specific cellular responses. While the influence of age on the accumulated HIV-1-specific cellular responses (Gag and Env) did not reach significance (Fig. 4), there is a clear trend towards lower HIV cellular responses in the older individuals. Hence, age could be an additional cause of the lowered HIV-specific cellular and humoral immune responses in vaccinia pre-immune individuals.
Fig. 4.
Relationship of accumulated HIV-1 IFN-γ ELISpot responses and age in all immunized individuals. Open white boxes indicate vaccinia-naïve individuals; closed black boxes indicate vaccinia pre-immune individuals.
4. Discussion
The smallpox vaccine is one of the most efficient vaccines ever used, and in humans is capable of inducing long-term protective immunity to Variola, Vaccinia and Monkey Pox [28]. In the present study, we analyzed the influence of immune response to vaccinia virus on the induction of HIV-specific immune responses in individuals primed with HIV DNA and boosted with a recombinant vaccinia vector carrying HIV-1 genes. The mechanism by which pre-existing anti-vector immunity limits responses to delivered antigens involves not only direct humoral neutralization of the vaccinia virus, but most likely also anti-vector cellular responses that can eradicate antigen-expressing cells after infection. Experience from both human trials and animal experiments has demonstrated that strong immune responses to a viral vector can hamper the induction of an effective immune response to the vectored antigen [23,29,30].
We observed that even when 30 years had passed since immunization, several volunteers still had clearly detectable levels of vaccinia neutralizing antibodies in their serum. A previous study of the persistence of immunity following smallpox vaccination showed that vaccinia-specific antibodies may persist up to 75 years after vaccination, whereas T cell responses slowly decline, with a half-life of 8–15 years [22]. In this study, the B cell memory response appeared to be more dependent on the dose of HIV MVA than the cellular responses. A higher dose of HIV MVA delivered im induced significantly higher serological vaccinia-specific responses than did a lower dose delivered id. After the HIV MVA boost, the anti-vaccinia antibodies persisted at high levels for several months in individuals displaying pre-existing vaccinia responses prior to the immunization. In vaccinia-naïve individuals, on the other hand, the primary anti-vaccinia antibody responses induced by HIV MVA were transient; after 12 weeks, the titers of neutralizing antibodies had dropped to baseline levels in a majority of the vaccinees. Ex-vivo vaccinia-specific cellular immune responses were below the level of detection prior to HIV MVA immunization, but as evidenced by the clear boost of the vaccinia-specific IFN-γ response, the smallpox vaccine, in addition to the humoral responses, induced a long-lived T cell memory response (see Fig. 1E and F).
Cells of the innate and adaptive immune system undergo profound age-related changes; altered frequencies and functional defects of innate and adaptive immune cells can lead to weaker and more quickly declining humoral and cellular responses to vaccination in the elderly [31]. We have observed that the presence of pre-existing neutralizing antibody titers to vaccinia decreased the magnitude of both cellular and humoral HIV-specific responses elicited by HIV-1 DNA and HIV MVA immunization. However, preexisting antibodies to vaccinia are perhaps not the sole explanation for the lower magnitude of HIV-specific cellular responses in the vaccinia pre-immune group, as the individuals in this group were significantly older than the vaccinia-naïve. Using an HIV-1 Gag peptide pool representing the priming HIV DNA, we showed that age had a moderate negative effect on the accumulated HIV-1-specific responses (Fig. 4). Since all older volunteers had previously received smallpox immunizations, the reduced responses in these individuals can most probably be explained by a combination of age and pre-existing vaccinia immunity.
As a consequence of the worldwide smallpox immunization that was carried out until the mid-1970s [22,23], anti-vaccinia virus immunity is present in the older population, but not in younger individuals, enabling the future use of poxvirus-based vaccines. However, the increasing interest in poxvirus as vectors for a variety of pathogens, such as malaria and tuberculosis, might in the long run restrict the use of these vectors, due to the potential drawbacks of vector-specific immunity. Heterologous prime-boost regimens, defined as a sequential immunization using different vaccine modalities, are one way to avoid the potential impact of pre-existing immunity against the vector [4–7,14]. By reducing the risk of neutralization of the vaccine vectors, the use of heterologous prime-boost promotes efficient delivery of the inserted gene and favors expansion of the insert-specific memory population over the vector-specific responses [32,33].
We have shown that pre-existing immunity to vaccinia did not affect the proportion of vaccinees that had a positive cellular response to HIV peptide pools after three HIV DNA priming vaccinations and a HIV MVA boosting vaccination. However, preexisting humoral immunity to vaccinia decreased the magnitude of the cellular HIV-specific responses. Furthermore, we found that one immunization with HIV MVA can boost both humoral and cellular responses to vaccinia, even though almost 30 years have passed since smallpox vaccinations ceased. However, 3 months after immunization with HIV MVA, anti-vaccinia antibody responses in vaccinia-naïve individuals had declined to the levels detected before immunization. It may therefore be beneficial to lengthen the interval between sequentially delivered doses of a vaccine vector to elicit the best conceivable HIV-specific immune responses.
In summary, our results suggest that recombinant MVA can efficiently be used to induce immune responses to vectored HIV- 1 antigens in humans, despite pre-existing immunity to vaccinia virus.
Acknowledgments
This study was supported by the European Union (INCO-DEV A4 ICFP501A4PR03, AVIP 503487); Swedish International Development Cooperation Agency (Sida); Department of Research Cooperation (SAREC) (SWE-2004-120, HIV2004-000809, 2004:813); Swedish Research Council (Vetenskapsrådet, K2004-16x-07743-19); Läkare mot AIDS Forskningsfond (04-050301 and 01-051101). Construction of the HIV-1 modified vaccinia virus Ankara was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and the US Military HIV Research Program. The production costs were funded by the US Military HIV Research Program, Walter Reed Army Institute of Research.
References
- 1.Brave A, Boberg A, Gudmundsdotter L, Rollman E, Hallermalm K, Ljungberg K, et al. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol Ther. 2007;15(9):1724–1733. doi: 10.1038/sj.mt.6300235. [DOI] [PubMed] [Google Scholar]
- 2.Moss B, Carroll MW, Wyatt LS, Bennink JR, Hirsch VM, Goldstein S, et al. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JM, Amara RR, McClure HM, Patel M, Sharma S, Yi H, et al. Multiprotein HIV type 1 clade BDNA/MVA vaccine: construction, safety, and immunogenicity in Macaques. AIDS Res Hum Retroviruses. 2004;20(6):654–665. doi: 10.1089/0889222041217419. [DOI] [PubMed] [Google Scholar]
- 4.Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, Schmidt C, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25(11):2120–2127. doi: 10.1016/j.vaccine.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205(1):63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson HL, Sharma S, Zhao J, Kannanganat S, Lai L, Chennareddi L, et al. Immunogenicity in macaques of the clinical product for a clade B DNA/MVA HIV vaccine: elicitation of IFN-gamma, IL-2, and TNF-alpha coproducing CD4 and CD8 T cells. AIDS Res Hum Retroviruses. 2007;23(12):1555–1562. doi: 10.1089/aid.2007.0165. [DOI] [PubMed] [Google Scholar]
- 7.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006;74(10):5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter G, Moss B. Novel vaccinia vector derived from the host range restricted and highly attenuated MVA strain of vaccinia virus. Dev Biol Stand. 1995;84:195–200. [PubMed] [Google Scholar]
- 9.Meisinger-Henschel C, Schmidt M, Lukassen S, Linke B, Krause L, Konietzny S, et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J Gen Virol. 2007;88(Pt 12):3249–3259. doi: 10.1099/vir.0.83156-0. [DOI] [PubMed] [Google Scholar]
- 10.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89(22):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stickl H-M. MVA vacciniation against smallpox: clinical tests with an attenuated live vaccinia virus strain. Dtsch Med Wochenschr. 1974;99(47):2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79(Pt 5):1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 13.Cosma A, Buhler S, Nagaraj R, Staib C, Hammarin AL, Wahren B, et al. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin Diagn Lab Immunol. 2004;11(2):406–410. doi: 10.1128/CDLI.11.2.406-410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77(1):799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallo S, Maa JS, Rodriguez JR, Rodriguez D, Esteban M. Humoral immune response elicited by highly attenuated variants of vaccinia virus and by an attenuated recombinant expressing HIV-1 envelope protein. Virology. 1989;173(1):323–329. doi: 10.1016/0042-6822(89)90250-x. [DOI] [PubMed] [Google Scholar]
- 16.Flexner C, Murphy BR, Rooney JF, Wohlenberg C, Yuferov V, Notkins AL, et al. Successful vaccination with a polyvalent live vector despite existing immunity to an expressed antigen. Nature. 1988;335(6187):259–262. doi: 10.1038/335259a0. [DOI] [PubMed] [Google Scholar]
- 17.Perkus ME, Piccini A, Lipinskas BR, Paoletti E. Recombinant vaccinia virus: immunization against multiple pathogens. Science. 1985;229(4717):981–984. doi: 10.1126/science.2992092. [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 19.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8 + T cell lineage commitment. Nat Immunol. 2005;6(8):793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177(2):831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 21.Amara RR. Short talk: preexisting immunity to vaccinia reduces the frequency of SIV-specific CCR-5+ve but not CCR-7+ve CD4 T cells elicited by a DNA/MVA vaccine and enhances the control of SIV251 challenge. Keystone Symposia HIV Vaccine & Pathogenesis. 2008 [Google Scholar]
- 22.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 23.Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, et al. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 24.Sandstrom E, Nilsson C, Hejdeman B, Brave A, Bratt G, Robb M, et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008;198(10):1482–1490. doi: 10.1086/592507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rollman E, Brave A, Boberg A, Gudmundsdotter L, Engstrom G, Isaguliants M, et al. The rationale behind a vaccine based on multiple HIV antigens. Microbes Infect. 2005;7(14):1414–1423. doi: 10.1016/j.micinf.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Lundholm P, Asakura Y, Hinkula J, Lucht E, Wahren B. Induction of mucosal IgA by a novel jet delivery technique for HIV-1 DNA. Vaccine. 1999;17(15–16):2036–2042. doi: 10.1016/s0264-410x(98)00404-6. [DOI] [PubMed] [Google Scholar]
- 27.Hinkula J, Rosen J, Sundqvist VA, Stigbrand T, Wahren B. Epitope mapping of the HIV-1 gag region with monoclonal antibodies. Mol Immunol. 1990;27(5):395–403. doi: 10.1016/0161-5890(90)90163-t. [DOI] [PubMed] [Google Scholar]
- 28.Parrino J, Graham BS. Smallpox vaccines: past, present, and future. J Allergy Clin Immunol. 2006;118(6):1320–1326. doi: 10.1016/j.jaci.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belyakov IM, Moss B, Strober W, Berzofsky JA. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc Natl Acad Sci USA. 1999;96(8):4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney JF, Wohlenberg C, Cremer KJ, Moss B, Notkins AL. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988;62(5):1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46(7):1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82(10):4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moorthy VS, Imoukhuede EB, Keating S, Pinder M, Webster D, Skinner MA, et al. Phase 1 evaluation of 3 highly immunogenic prime-boost regimens, including a 12-month reboosting vaccination, for malaria vaccination in Gambian men. J Infect Dis. 2004;189(12):2213–2219. doi: 10.1086/421118. [DOI] [PubMed] [Google Scholar]