Abstract
Barrier mechanisms in the brain are important for its normal functioning and development. Stability of the brain’s internal environment, particularly with respect to its ionic composition, is a prerequisite for the fundamental basis of its function, namely transmission of nerve impulses. In addition, the appropriate and controlled supply of a wide range of nutrients such as glucose, amino acids, monocarboxylates, and vitamins is also essential for normal development and function. These are all cellular functions across the interfaces that separate the brain from the rest of the internal environment of the body. An essential morphological component of all but one of the barriers is the presence of specialized intercellular tight junctions between the cells comprising the interface: endothelial cells in the blood-brain barrier itself, cells of the arachnoid membrane, choroid plexus epithelial cells, and tanycytes (specialized glial cells) in the circumventricular organs. In the ependyma lining the cerebral ventricles in the adult brain, the cells are joined by gap junctions, which are not restrictive for intercellular movement of molecules. But in the developing brain, the forerunners of these cells form the neuroepithelium, which restricts exchange of all but the smallest molecules between cerebrospinal fluid and brain interstitial fluid because of the presence of strap junctions between the cells. The intercellular junctions in all these interfaces are the physical basis for their barrier properties. In the blood-brain barrier proper, this is combined with a paucity of vesicular transport that is a characteristic of other vascular beds. Without such a diffusional restrain, the cellular transport mechanisms in the barrier interfaces would be ineffective. Superimposed on these physical structures are physiological mechanisms as the cells of the interfaces contain various metabolic transporters and efflux pumps, often ATP-binding cassette (ABC) transporters, that provide an important component of the barrier functions by either preventing entry of or expelling numerous molecules including toxins, drugs, and other xenobiotics.
In this review, we summarize these influx and efflux mechanisms in normal developing and adult brain, as well as indicating their likely involvement in a wide range of neuropathologies.
There have been extensive attempts to overcome the barrier mechanisms that prevent the entry of many drugs of therapeutic potential into the brain. We outline those that have been tried and discuss why they may so far have been largely unsuccessful. Currently, a promising approach appears to be focal, reversible disruption of the blood-brain barrier using focused ultrasound, but more work is required to evaluate the method before it can be tried in patients. Overall, our view is that much more fundamental knowledge of barrier mechanisms and development of new experimental methods will be required before drug targeting to the brain is likely to be a successful endeavor. In addition, such studies, if applied to brain pathologies such as stroke, trauma, or multiple sclerosis, will aid in defining the contribution of brain barrier pathology to these conditions, either causative or secondary.
Keywords: Blood-brain barrier, cerebrospinal barrier, CSF-brain barrier, transporters, tight junctions, drug delivery
Introduction
The term blood-brain barrier has a long history. Its current usage describes the structural, physiological, and molecular mechanisms that control the exchange (entry and exit) of molecules between the blood and the brain. The sum of these mechanisms results in the characteristically stable internal environment of the brain, both during development and in the adult. This has been an often confused and misunderstood field of neuroscience.
The main aim of this review is to explain what is known about brain barrier mechanisms and why understanding these mechanisms is fundamental to understanding normal brain development and normal brain function and how disorders of brain barrier mechanisms may contribute to a range of neuropathological conditions. We suggest that the neuroscience community should pay more attention to this topic and we advocate the need for new researchers to move into this intriguing and important field, as major advances in many fields have often come from an influx of new people unfettered by the prevailing dogmas.
The other focus of this review will be to consider the clinically important problem of developing ways to deliver drugs to the brain for treating neurological and psychiatric disorders. This has been a major effort in the blood-brain barrier field for the past 20–30 years but has yielded little of practical value. We list the diverse attempts that have been tried, we analyze some of the possible reasons why they have been unsuccessful, and we suggest some alternative/new approaches to the problem.
What is meant by the term “blood-brain barrier”?
The use of the term “barrier” is in many ways unfortunate 1, as for those outside the field it disguises the multiplicity of mechanisms involved. Perhaps this also explains the almost exclusive focus of people interested in pathological conditions involving the blood-brain barrier on tests of its integrity, largely ignoring until recently the numerous cellular mechanisms at the various blood-brain interfaces that may be disrupted. Almost all of the early work on the blood-brain barrier involved the use of dyes, which could be visualized. This field has recently been reviewed with translations from key oft-cited papers published in their original languages showing that many of the citations were incorrect 2. To set the record straight, it was Lena Stern who was the first to coin the term blood-brain barrier (“barrière hémato-encéphalique” 3) and not, as often cited, Ehrlich 4, Lewandowsky 5, or Goldmann 6. The current understanding of the term “blood-brain barrier” is that it covers a number of morphological entities and a plethora of cellular transport mechanisms both inward and outward, which we next describe briefly.
Morphology of blood-brain barrier interfaces
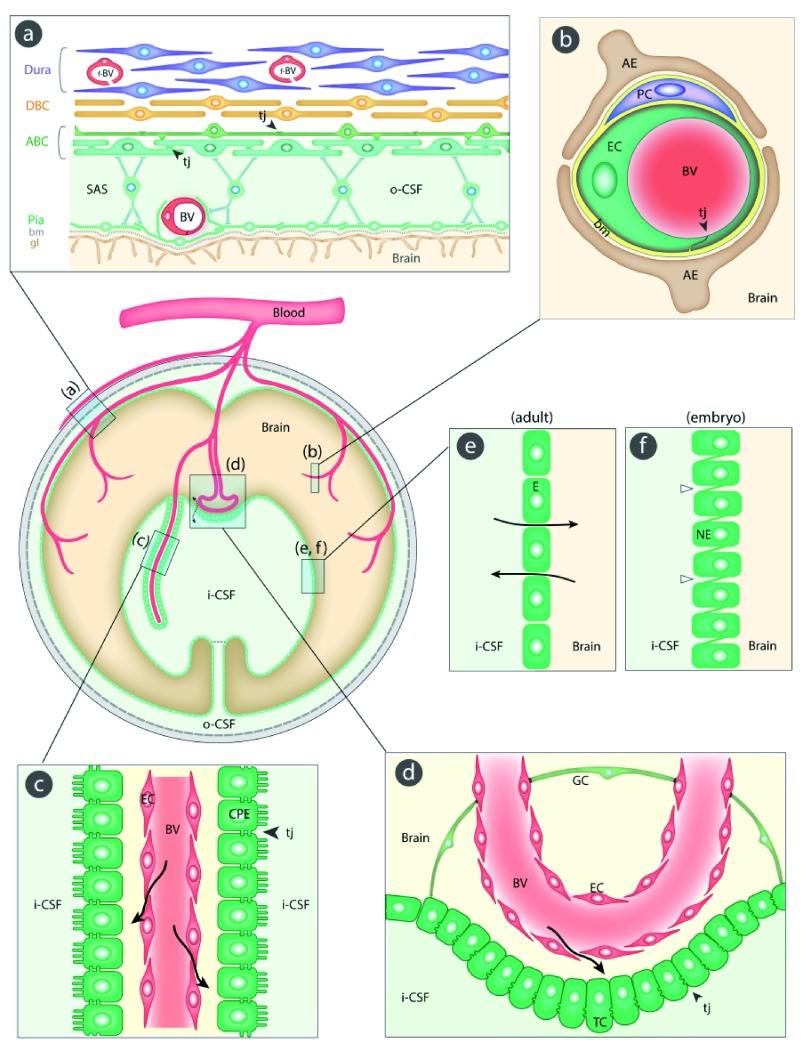
There are six interfaces to be considered. Figure 1 illustrates their sites and main morphological features. An essential component of all interfaces with barrier properties is the presence of specialized junctions between the cells of the interface. In most of the barriers, these junctions are tight junctions; they restrict the movement of molecules between the endothelial and the epithelial cells. As a direct consequence of this restriction, the intercellular junctions have the important functional effect of allowing the numerous transporters within individual cells to operate over the large surface of the barrier interfaces; without this permeability restriction, the inward and outward transporter mechanisms would be ineffective. In recent years, it has become increasingly apparent that there is a much greater complexity involved in the structural organization of the brain barriers; in the case of the blood-brain barrier itself, this includes astrocytes, pericytes, basement membrane, and extracellular matrix ( Figure 1). However, there is much to be learned about the precise role of individual morphological components of the brain barriers and their interactions in normal and pathological brains 7– 10. We shall now consider each barrier interface in turn: (a) the meningeal barrier, shown in Figure 1(a), is structurally the most complex of all the brain barriers and is situated at the meninges (pia, arachnoid, and dura mater). The barrier-forming cells are the outer layer of the arachnoid membrane (the arachnoid barrier cells), which have tight junctions between adjacent cells forming a physical barrier between the outer cerebrospinal fluid (CSF) in the subarachnoid space and more superficial dural layers (dural border cells and the dura mater). The blood vessels in the subarachnoid space have tight junctions with similar barrier characteristics as cerebral blood vessels, although lacking the surrounding pericytes and astrocytic end-feet 11– 13. In contrast, blood vessels within the dura mater are fenestrated; other important components of the barrier are the basement membrane and glia limitans. (b) The blood-brain barrier, shown in Figure 1(b), is situated at the level of cerebral blood vessels between the lumen of the vessel and brain parenchyma. Tight junctions are present between the endothelial cells restricting permeability of the paracellular cleft ( 11 and Text Box). A basement membrane and extracellular matrix 14 surround both the endothelial cells and the pericytes 15, 16. End feet from astroglial cells progressively encircle cerebral blood vessels during development 17. These cellular structures are known collectively as the neurovascular unit 18. (c) The blood-CSF barrier, shown in Figure 1(c), is situated in the choroid plexus within each brain ventricle. In contrast to other cerebral blood vessels, the endothelial cells forming choroid plexus blood vessels are fenestrated and do not form a barrier. The barrier-forming cells are the epithelial cells, which have tight junctions 11 at their apical (CSF) side. Choroid plexus cells have microvilli on their apical side, increasing their exchange surface to the internal CSF. (d) Circumventricular organs, shown in Figure 1(d). These include the median eminence, pineal gland, area postrema, and subfornical organ. The blood vessels have permeability characteristics similar to elsewhere in the body and have the functional property of allowing feedback penetration of peptide hormones controlled by the hypothalamic-pituitary axis. These peptides and other molecules are prevented from entering the CSF by tanycytes, the specialized ependymal cells of these brain areas, connected by tight junctions between their apices; entry into the rest of the brain is prevented by tight junctions between astroglial cells 19, 20. (e) Ependyma in adult brain, shown in Figure 1(e). Apart from areas where there are specialized tanycytes, ependymal cells are linked by gap junctions that do not restrict exchange of even large molecules, such as proteins, between CSF and interstitial space of brain 11, 21. (f) The embryonic CSF-brain barrier, shown in Figure 1(f). In the ventricular zone is a temporary barrier between the CSF and brain parenchyma 21. In early brain development, strap junctions are present between adjacent neuroepithelial cells; these form a physical barrier restricting the movement of larger molecules, such as proteins, but not smaller molecules 22, 23. At later stages of development and in the adult brain, these strap junctions are no longer present when this interface becomes ependyma.
Figure 1. Schematic diagram (center left) of the five main barrier interfaces (a–e) in the brain and an additional one in the embryo (f).
The barrier-forming cellular layers at each interface are colored green. ( a) The meningeal barrier is structurally the most complex of all the brain barriers. Barrier-forming cells are the outer layer of the arachnoid membrane (the arachnoid barrier cells [ABC]); these have tight junctions (arrowheads) between adjacent cells forming a barrier between the outer cerebrospinal fluid (o-CSF) in the subarachnoid space (SAS) and more superficial dural layers (dural border cells [DBC] and the dura mater). Blood vessels (BV) in the SAS have tight junctions with similar barrier characteristics as cerebral blood vessels without surrounding pericytes and astrocytic end-feet 11– 13. Blood vessels within the dura mater are fenestrated (f-BV); bm = basement membrane, gl = glia limitans. ( b) The blood-brain barrier is situated at the level of cerebral blood vessels (BV). Tight junctions (tj, arrowhead) are present between the endothelial cells (EC) restricting the paracellular cleft ( 11 and Text Box); bm = basement membrane, PC = pericytes, AE = end feet from astroglial cells. ( c) The blood-CSF barrier is situated in the choroid plexus within each brain ventricle. Barrier-forming cells are the epithelial cells (CPE), which have tight junctions 11 at their apical side (CSF facing, arrowheads). Blood vessels (BV) are fenestrated and do not form a barrier (arrows); apical microvilli increase exchange surface of epithelial cells to the internal CSF (i-CSF). ( d) Circumventricular organs (including median eminence, pineal gland, area postrema, subfornical organ). Blood vessels have permeability characteristics similar to elsewhere in the body and have the functional property of allowing feedback penetration of peptide hormones controlled by the hypothalamic-pituitary axis. These peptides and other molecules are prevented from entering the CSF by tanycytes (TC), the specialized ependymal cells of these brain areas, connected by tight junctions between their apices (arrowhead); entry into the rest of the brain is prevented by tight junctions between astroglial cells (GC 19, 20). Away from the tanycyte layer, ependymal cells lining the ventricular system are linked by gap junctions that do not hinder free exchange between the CSF and brain interstitial fluid (broken arrow). ( e) Ependyma in adult brain. Apart from areas where there are specialized tanycytes, ependymal cells are linked by gap junctions that do not restrict exchange of even large molecules, such as proteins, between CSF and interstitial space of brain (solid arrows). ( f) The embryonic CSF-brain barrier. In early brain development, strap junctions (open arrowheads) are present between adjacent neuroepithelial cells (NE); these form a barrier restricting the movement of larger molecules, such as proteins, but not smaller molecules.
Transport mechanisms at barrier interfaces
Control of the interstitial ionic environment of the brain
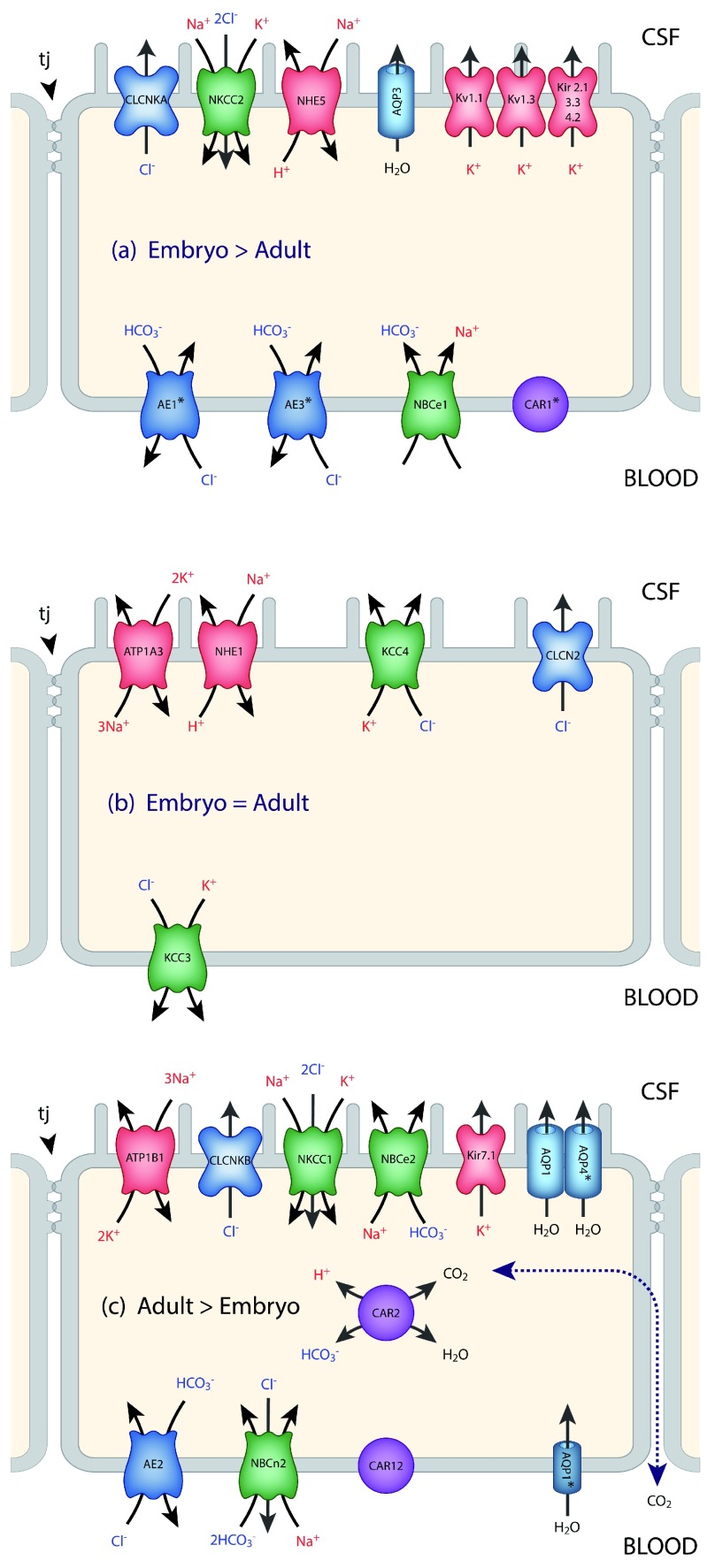
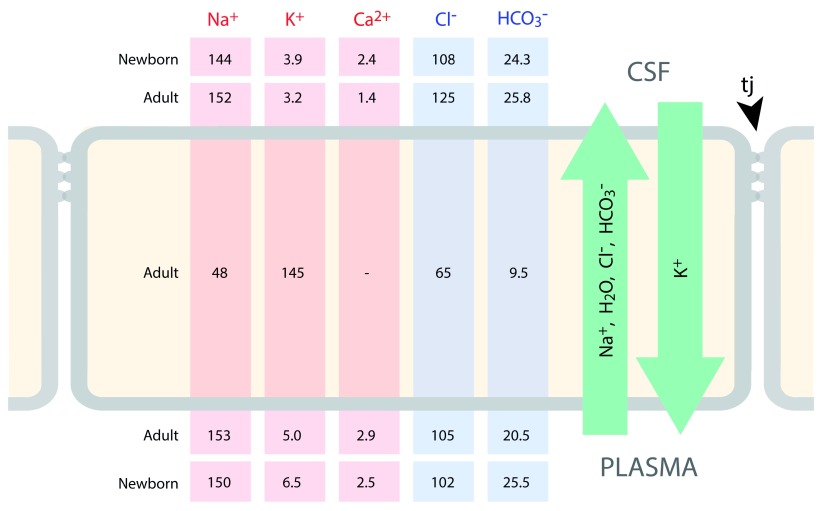
This is absolutely critical for normal function of the brain. As Hugh Davson once put it (paraphrased), without this control our sensory experience would be limited to a series of flashes and bangs. The ionic composition of the interstitial fluid is usually taken to be synonymous with the composition of CSF 24. This, plus the obvious practical point that it is relatively easy to sample CSF, has led to a focus on CSF and the choroid plexus in both the adult 25 and the developing 26 brain; however, CSF composition generally does not reflect blood-brain barrier function (see Text Box). There is a good correlation between expression levels of transporters and ionic concentrations at different ages, at least in the rat ( Figure 2 and Figure 3).
Figure 2. Transporters and ion channels in choroid plexus epithelial cells.
Data for the localization of transporters and ion channels are from Damkier et al. 25 and Brown et al. 96. CSF secretion results from coordinated transport of ions and water from basolateral membrane to cytoplasm, then sequentially across apical membrane into ventricles 25. The genes for many of these transporters and ion channels are differentially expressed in the embryo compared to the adult. This is represented in the three panels. It is emphasized that this represents differential expression, not the absence of a gene at one age (details are in 35). On the plasma-facing membrane is parallel Cl -/HCO 3 - exchange (AE2 [ Slc4a2] > in adult and AEI [ Slc4a1], AE3 [ Slc4a3] > in embryo) and Na +/HCO 3 - co-transport (NBC1 [ Slc4a4] > in embryo) with net function bringing Cl - into cells in exchange for HCO 3 - 97. Also basolaterally located is an Na-dependent Cl -/HCO 3 - exchange (NCBE [ Slc4a10] > in adult) that modulates pH and perhaps CSF formation 98. Apical Na + efflux by NHE5 ( Slc9a5 > in embryo) and ATB1 ( Atb1b1 [Na +/K +-ATPase] > in adult) maintains a low cell Na + that sets up a favorable basolateral gradient to drive Na + uptake 99. Na + is extruded into CSF mainly via the Na +/K +-ATPase pump (ATB1 [ Atb1b1]) and, under some conditions, the Na +/K +-Cl - co-transporter NKCC1, Slc12a2 (see 100 for review). Aquaporin (AQP1/3/4) channels on CSF-facing membrane mediate water flux into ventricles 101. Polarized distribution of carbonic anhydrase (CAR) and Na +/K +-ATPases, and aquaporins, enable net ion and water translocation to CSF (see 100 and 102 for reviews). CLCKA (CLCK1) is an inwardly rectifying chloride channel; its gene ( Clcnka) in embryonic choroid plexus is expressed many orders of magnitude higher than in the adult. Clcnkb is expressed at a higher level in the adult. CAR2 has an intracellular distribution and is functionally important for catalyzing the equilibrium that generates H + and HCO 3 -, which is an important part of the mechanism secreting CSF. There are many more channels that show age-related differential expression in choroid plexus, the functions of which are unclear 26.
Figure 3. Ion gradients between CSF and plasma in developing and adult rat brain.
A characteristic of CSF is its stable ionic composition that differs from that of plasma to an extent that cannot be explained by ultrafiltration, as was once thought 24. Data for CSF and plasma (m-equiv/L water) are from 103 and for intracellular ions (mmol/L water) from Figure 8 in 104. The gradients are the consequence of the complex interactions between enzymes (notably carbonic anhydrase) ion transporters and ion channels, as illustrated in Figure 2. The CSF secretion rate in the embryo and newborn is much lower than in the adult 105– 107, which is perhaps explained by the much lower expression of carbonic anhydrase and ATPases in the developing choroid plexus.
Influx mechanisms
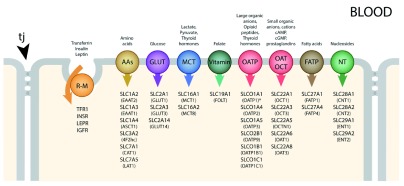
These have been extensively studied only at the blood-brain barrier itself and at the blood-CSF barrier (choroid plexuses). A limitation of early studies with radiolabeled molecules, such as glucose and amino acids, was that it was difficult to distinguish between brain entry and incorporation of labeled molecules into metabolic pathways. This problem was solved by Oldendorf 27 with his short pass technique. In general, essential amino acids were transported into the brain to a greater extent than non-essential amino acids; there was also a high uptake of D-glucose 27. The molecular basis for these inward transport mechanisms has been extensively investigated using gene expression techniques to study the blood-brain barrier itself 28– 30 and also the choroid plexuses 26, 31– 33. These studies have revealed a plethora of genes, particularly those classed as solute linked carriers (SLCs) 34. These are summarized in Figure 4 for transporters identified in both the transcriptome and the proteome in human endothelial cells 29. Some of these carriers will transport only compounds that closely resemble endogenous substrates, as they exhibit high substrate specificity (e.g. GLUT1). Many others (e.g. organic anion transporters [OATs], OAT polypeptides [OATPs], and large amino acid transporter [LAT1]) will accept a broader range of substrates; they provide a potential route of entry into the central nervous system (CNS) for exogenous compounds. Members of the OAT family of solute carriers (SLC) are known to transport a wide range of drugs, such as aspirin, ibuprofen, and various antibiotics, and pesticides (e.g. 2,4-D-dichlorophenoxyacetic acid [2,4-D]). The plant-derived neurotoxin β- N-methylamino-L-alanine (MeAA) and the drug L-DOPA both have amino acid structures that allow entry via the amino acid transporter LAT1. Some environmental toxins are also able to gain entry into the CNS by attaching themselves to an endogenous substrate to be co-transported; for example, methyl-mercury (MeHg) and lead (Pb 2+) attached to cysteine enter via amino acid transporters specific for this amino acid, e.g. SLC1A5 and SLC7A10.
Figure 4. Summary of inward transporter mechanisms in cerebral endothelial cells.
Individual transporters shown are ones identified in human material 29. Tight junctions (tj) between adjacent cells prevent the paracellular passage of hydrophilic compounds. R-M = receptor-mediated, GLUT = glucose transporters, NTs = nucleoside transporters, AAs = amino acid transporters (includes LAT), OATP = organic anion transporting polypeptides, OAT = organic anion transporters, OCT = organic cation transporters, MCT = monocarboxylate transporters, FATP = fatty acid transport protein. Many of these transporters are solute linked carriers (SLCs). Both SLC designations and the original abbreviations are included here. SLC1A2/EAAT2, SLC1A3/EAAT1 high-affinity glutamate, SLC1A4/ASCT1 glutamate/neutral amino acids, SLC2A1/GLUT1, SLC2A3,14/GLUT3 glucose, SLC3A2/4F2hc amino acid transporter heavy chain, SLC6A12/BGT1 neurotransmitter, SLC7A1/CAT1 cationic amino acid, y+ system, SLC7A5/LAT1 amino acid light chain, L system, SLC10A1/NTCP sodium/bile acid cotransporter. SLC16A1/MCT1, SLC16A2/MCT8 monocarboxylates, SLC19A1/RFC folate, SLC22A1/OCT1 organic cations, SLC22A3/OCTN3 organic cations, SLC22A5/OCTN2 organic cation/carnitine, SLC27A1/FATP1 fatty acid, SLC29A1/ENT1 equilibrative nucleosides, SLCO2B1/OATP2B1 organic anions, SLCO1B1/OATP1B1 organic anions. Examples of receptor-mediated transporters are insulin receptor (INSR), transferrin receptor (TFR1), leptin receptor (LEPR), low-density lipoprotein receptor (LDLR), and insulin-like growth factor receptor (IGFR).
Many more Slc genes have been identified in the transcriptome of mouse endothelial cells 28. A large number was also identified in mouse lateral ventricular choroid plexuses 33. For comparison between the two interfaces, see 34. It is striking that the expression of some Slc genes in brain barriers is much higher in the developing brain 28, 35; this correlates with limited information of greater transport of some labeled amino acids and glucose into the developing brain, suggesting that the high expression levels correlate with transporter function (reviewed in 36). Probably several Slcs are responsible for the transport of the same molecules, indicating a significant degree of redundancy.
Efflux mechanisms
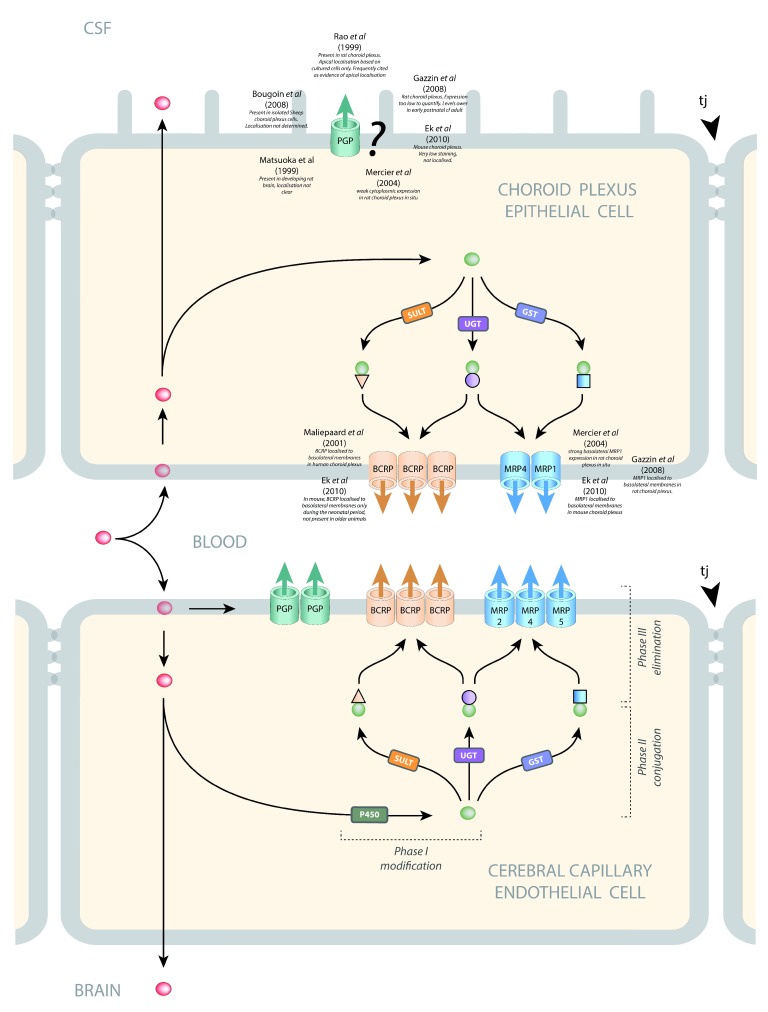
Of particular importance in relation to drug entry into the brain, or rather the failure of most drugs to enter the brain, are the ABC efflux transporters 37, 38. There are 49 members of the ABC protein superfamily ( http://nutrigene.4t.com/humanabc.htm). Many of these are efflux transporters. At the blood-brain barrier interface ( Figure 5), the efflux transporters that have been shown to be expressed and present and appear to be of particular functional importance are ABCB1 (also known as P-glycoprotein [PGP] or MDR1) and ABCG2 (breast cancer resistance protein [BCRP]). ABCC2 (multidrug resistance protein 2 [MRP2]) and ABCC4 (MRP4) have also been demonstrated at this interface 39. At the blood-CSF interface ( Figure 5), ABCC1 (multidrug resistance protein 1 [MRP1]) appears to be the predominant efflux transporter, but ABCC4 (MRP4) and ABCG2 (BCRP) have also been shown to be present 39, 40. In cerebral capillary endothelial cells (blood-brain barrier), PGP 41– 43, BCRP 44, 45, MRP2 46, MRP4 46, and MRP5 47 are localized to the luminal membrane, where they export compounds into the blood. In choroid plexus epithelial cells (blood-CSF barrier), MRP1, MRP4, and BCRP are localized to the basolateral membranes where they export compounds into the stroma of the plexus 40, 48, 49. The subcellular localization of PGP in choroid plexus is not clear. Some studies report staining too low to be able to determine localization 40, 48 or positive staining, but localization was not able to be determined 50, 51. Other studies report cytosolic 52 or subapical localization 42. One study has reported apical membrane localization in cultured choroid plexus epithelial cells 53. A common feature of these outwardly directed efflux transporters is a broad substrate specificity and considerable overlap between transporters (see 54). PGP is unusual in that it intercepts lipid-soluble compounds (red symbols, Figure 5) as they pass through the internal leaflet of the plasma membrane and returns them to the extracellular fluid 55, whereas BCRP and the MRPs bind their substrates from within the cell cytoplasm. Compounds can be exported from the cell by one or more of these efflux pathways. For example, a lipid-soluble compound that manages to avoid interception by PGP as it passes into the cell may then be metabolized by phase I enzymes (e.g. cytochrome P450 oxidases), conjugated by phase II enzymes (sulfotransferases [SULTs], uridine-diphospho-glucuronosyltransferase [UGT], or glutathione S-transferase [GST]), and exported by BCRP and/or MRP.
Figure 5. Efflux pathways in barrier-forming cells.
The main efflux transporters at the blood-brain and blood-CSF interfaces are P-glycoprotein (PGP, MDR1, ABCB1), breast cancer resistance protein (BCRP, ABCG2), and several members of the multidrug resistance protein subfamily (MRP1 ABCC1, MRP2 ABCC2, MRP4 ABCC4, MRP5 ABCC5). In cerebral capillary endothelial cells (blood-brain barrier), PGP 41– 43, BCRP 44, 45, MRP2 46, MRP4 46, and MRP5 47 are localized to the luminal membrane where they export compounds into the blood. In choroid plexus epithelial cells (blood-CSF barrier), MRP1, MRP4, and BCRP are localized to the basolateral membranes where they export compounds into the stroma of the plexus 40, 48, 49. The subcellular localization of PGP in choroid plexus is not clear. Some studies report staining too low to be able to determine localization 40, 48 or positive staining, but localization was not able to be determined 50, 51. Other studies report cytosolic 52 or subapical localization 42. One study has reported apical membrane localization in cultured choroid plexus epithelial cells 53. A common feature of these outwardly directed efflux transporters is a broad substrate specificity and considerable overlap between transporters (see 54). PGP is unusual in that it intercepts lipid-soluble compounds (red symbols) as they pass through the internal leaflet of the plasma membrane and returns them to the extracellular fluid 55, whereas BCRP and the MRPs bind their substrates from within the cell cytoplasm. Compounds can be exported from the cell by one or more of these efflux pathways. For example, a lipid-soluble compound that manages to avoid interception by PGP as it passes into the cell may then be metabolized by phase I enzymes (e.g. cytochrome P450 oxidases), conjugated by phase II enzymes (sulfotransferases [SULTs], uridine-diphospho-glucuronosyltransferase [UGT], or glutathione S-transferase [GST]), and exported by BCRP and/or MRP.
There are probably species differences in the level of expression and function of these various efflux transporters, and it is known that their expression changes with age during brain development at both interfaces 28, 35, 40, 56, 57. No doubt with further studies other members of this large group of transporters will be found to be functional at one or more of the brain barrier interfaces. ABC transporters that have been identified at different brain barriers are shown in Figure 5, together with an indication of differences in mechanisms of their function.
Some dogmas and controversies
|
D
ogmas A
nd C
ontroversies I
n B
rain B
arriers B
iology
FACT CHECK |
| (i) The blood-brain barrier in the embryo and newborn is absent or “leaky”. Incorrect. Widely believed and stated, usually without experimental basis and in spite of much evidence to the contrary 2. |
| (ii) Induction of tight junctions in early brain development depends on astrocytes. Incorrect. Functionally effective tight junctions are present well before the differentiation of astrocytes 15, 117. |
| (iii) The paracellular pathway (intercellular space) in blood-brain barrier and choroid plexuses is the route for water, small molecules, and ion exchange between blood, brain, and cerebrospinal fluid (CSF). Most likely not correct. It is based on transepithelial resistance measurements 118 with no direct evidence, as water, ions (e.g. Na +), and small lipid-insoluble molecules (e.g. sucrose) cannot yet be visualized with sufficient resolution. Recent methods using visualizable, similar small molecules have shown transfer through epithelial cells of choroid plexus and not via paracellular pathway 119. Other limitations of Frömter & Diamond 118 are discussed in Ek et al. 119. |
| (iv) Increased penetration of molecules into brain parenchyma in pathological conditions is due to the breakdown of tight junctions at the barrier interfaces. Probably not correct in many cases. Most studies do not use electron microscopy (EM) required to define state of tight junctions. Evidence is emerging that in e.g. stroke, tight junctions are intact and transfer may be intracellular across cells forming the barrier (e.g. Krueger et al. 120); some studies have shown ultrastructural changes in both cellular constituents and tight junctions 121. In addition, regulation of various transporters may change in some pathologies 122, 123. |
| (v) Evans blue binds tightly and specifically to plasma albumin and can be used to measure penetration of albumin into brain in barrier dysfunction. Incorrect. Evans blue binds to multiple proteins in plasma and to cells and tissues in reversible equilibrium 124. |
| (vi) Lack of lymphatics in brain. Incorrect. Evidence going back many years suggesting drainage pathway via cervical lymphatics is now supported by evidence from 2-photon microscopical studies 125. |
| (vii) Brain extracellular space (ECS) volume. Controversial. Decades-long discrepancy between EM measurements (negligible ECS) and physiological measurements (approx. 15%) has been resolved by cryo-fixation-EM 126. Important for interpretation of blood-brain barrier transfer studies. |
| (vii) Concentration of markers in the CSF is a measure of blood-brain barrier permeability. Untrue. Commonly used, especially in human studies, but is misleading because concentration of any molecule in the CSF does not necessarily reflect transfer across the blood-brain barrier 127 and is influenced by its transfer through blood/CSF barrier and only indirectly and variably via the blood-brain barrier 128; also CSF drainage, uptake into the brain across ependyma 24, and possibly lymphatic drainage 125 all influence levels in the CSF. |
| (viii) The brain is a site of immune privilege. Recent findings of a functional and classical lymphatic system suggest this concept needs further study 129. |
Drug targeting to the central nervous system
This has been a major field of endeavor over the past 20–30 years. It has been largely unsuccessful in that few of the proposed methods appear to have been independently replicated, and we are not aware of any neuropharmaceutical drugs that have been identified using these methods. There are several comprehensive reviews of the methods that have been developed 58– 60. Here, we provide only a list of these methods ( Table 1), with some observations on their limitations. We deal in more detail with methods designed to allow entry of drugs into the brain by disruption of the blood-brain barrier. This is the only method of drug delivery that has translated to clinical practice, albeit on a limited basis. Newer, more focal methods hold promise of significant advances using this approach.
The following drug delivery approaches have been tried:
(i) In vitro blood-brain or blood-CSF barrier models (see reviews in 61– 64)
The hallmarks of success of such systems are generally held to be a high transendothelial resistance (TEER) and limited permeability to barrier integrity markers such as 14C-sucrose 64. The only in vivo TEER values that have been measured are for pial blood vessels 65. It is unclear whether these reflect the properties of vessels within the brain. The type of endothelial cells isolated in preparation of the cultures is often not clear 66. But perhaps the biggest limitation of these systems is that few attempts have been made to characterize at the molecular level the barrier and transport properties in vitro compared to those in vivo. This would seem to be particularly important given the propensity for cells to transform in culture. Where attempts have been made, the extent to which the in vivo properties are retained is limited 67.
(ii) Receptor-mediated and adsorptive-mediated transcytosis (see Table 1)
Lajoie and Shusta 60 review a number of more recent developments using alternative targets on cerebral endothelial cells, but it is too soon to tell whether these will be more successful than earlier developed methods.
(iii) Influx transporters
As indicated above, there are numerous influx transporters in brain endothelial cells. In the case of only a few, it has been possible to use these to achieve penetration of a therapeutic compound into the brain. The best known, and one of the earliest to be described, is L-DOPA for the treatment of Parkinson’s disease, e.g. 68. Its introduction transformed the treatment of this condition, but, after prolonged experience, it is clear that it has serious clinical limitations.
(iv) Inhibition of efflux transporters (see above, 69, and Table 1)
The number of ABC transporters that have been shown to be functionally effective at the blood-brain barrier is only a small proportion of the known total of 49. A huge number of drugs and other xenobiotics are excluded from the brain 70, which explains the lack of specificity of ABC transporters. Unless some way could be found to limit the effect of the inhibitor to cerebral endothelial cells, and preferably only those in the neurological target area of the brain, this is unlikely to be a viable method of promoting drug entry to the brain.
(v) Modulation of integrity of the blood-brain barrier
Three methods have been tried − osmotic opening, ultrasound, and electrical stimulation ( Table 2).
Reversible osmotic opening of the blood-brain barrier was first demonstrated in animals by Rapoport et al. 71 using a variety of hypertonic electrolyte and non-electrolyte solutions. Brightman et al. 72 showed that barrier opening to horseradish peroxidase was due to opening of cerebral vessel tight junctions. Since 1979, Neuwelt has pioneered the use of osmotic opening of the blood-brain barrier as a means of delivering chemotherapeutic agents to treat brain tumors 73. He has built up an impressive array of animal and patient imaging techniques, which allowed careful evaluation of the use of hypertonic solutions to open the barrier under well-controlled, carefully monitored conditions and to develop methods for mitigating some of the potentially devastating side effects 74, 75. Reversible osmotic opening of the blood-brain barrier is the only technique for improving drug delivery to the brain that has successfully translated to the clinic. It is not widely used, probably because it requires repeated hospital admissions and general anesthesia, as well as being associated with increased risk of stroke and epileptic seizures 76 and other surgical and neurological problems 75.
Focused ultrasound disruption of the blood-brain barrier in laboratory animals was first investigated in the 1950s 77. In that study and in subsequent ones, it was necessary to perform a craniectomy in order to achieve sufficient ultrasound energy to produce effects in the brain (e.g. 78). A major advance was to combine intravenous injection of gas bubbles, previously developed as a contrast agent for ultrasound imaging, with focused ultrasound 79; this reduced the ultrasound power required to disrupt the blood-brain barrier and was shown to be effective through the intact skull in rabbits. Subsequent studies have evaluated the safety of the procedure 80, effects of different anesthetic agents 80, and feasibility in large animals 81. The mechanism of the interaction between the micro-bubbles and the focused ultrasound beam is unclear. Several possibilities are discussed by Burgess and Hynynen 82 and by Timbie et al. 83. The advantages of this approach compared to osmotic disruption of the blood-brain barrier are that (a) it is non-invasive (does not require craniotomy), (b) it can be targeted to a specific lesion, e.g. tumor, or region of neurological disorder such as the basal ganglia in Parkinson’s disease, (c) it is transient, although the estimates of duration barrier opening have varied from 6 to 24 hours in different studies, and (d) under well-defined conditions of ultrasound parameters, there appears to be no evidence of ischemia, apoptosis, or cognitive dysfunction (tested in primates 82). Investigations so far have concentrated on the mechanical disruptive effects of the method, but studies are needed to investigate possible effects on cellular transport across cerebral endothelial cells 84. Also, it needs to be considered whether the effects might be different in pathological brains.
Table 1. Drug targeting to the central nervous system.
| Method & Key
References |
Rationale | Limitations |
|---|---|---|
| In vitro barrier systems 61– 64 | Potential for high-throughput
screening |
In vitro transformation of cell properties. Only
limited gene expression of in vivo characteristics |
| Receptor- or adsorptive-
mediated transcytosis 60, 108 |
Uses known receptors (e.g. Tf,
insulin) & cellular mechanisms |
Not restricted to brain; only about 15% reaches
brain. Limited capacity |
| Influx transporters | ||
| SLC transporters 109 | Naturally occurring transporters
that also transport wide range of drugs |
Many of the transporters are ubiquitously
expressed, widespread effects likely. Limited transport capacity. Drugs may also be substrates for ABC efflux transporters |
| Efflux transporters | ||
| Inhibition of efflux
transporters 110 |
ABC transporters are major
reason for drugs not reaching brain |
Not restricted to brain, widespread side effects
likely from both drug entry into other organs and entry of other xenobiotics that may be present |
| Modulation of integrity of the blood-brain barrier | ||
| See Table 2 | ||
| Bypassing the barriers | ||
| Convection-enhanced
delivery 111 |
Localized delivery to site of
pathology |
Invasive. Potential damaging effect not yet fully
evaluated |
| Injection into CSF 112 | Bypasses barriers | Invasive, requires repeated administration or
infusion, not targeted to sites of pathology |
Table 2. Modulation of integrity of blood-brain barrier as method of drug delivery to the brain.
| Method and Key References | Level of Preclinical
Evaluation |
Invasive | In Clinical Use |
|---|---|---|---|
| Reversible osmotic opening 73– 76 | Substantial | Yes | Yes, in small
number of centers |
| Focused ultrasound + micro-bubbles 79, 81, 83, 84 | Substantial | No | No |
| Electrical stimulation | |||
| Non-thermal electroporation 76 | Limited | Yes | No |
| Pulsed electromagnetic stimulation 113 | |||
| Sphenopalatine ganglion stimulation 114, 115 | Limited | Yes | No |
| Electric field application 116 | Limited | No | No |
| Convection-enhanced delivery 111 | Limited | Yes | No |
What next?
The concentration in the past 25 years on developing in vitro systems for testing barrier permeability to drugs and the various drug delivery methods outlined above has been at the expense of fundamental research aimed at better understanding of brain barrier mechanisms. We list here some major questions, the answers to which might aid the development of more effective drug delivery strategies as well as our understanding of the involvement of brain barrier mechanisms in a wide range of neuropsychiatric conditions.
(i) Different approaches to drug development
Given the overwhelming importance of efflux transporters in excluding drugs from the brain, we need better understanding of the molecular nature of their mechanism(s) of action, as this would allow the development of drugs that evade these mechanisms. However, there would still be a need to develop ways of targeting the drugs not just to the cerebral vasculature but also to specific brain regions. This might come from better knowledge of the molecular characteristics of cerebral endothelial and peripheral endothelial cells as well as identifying such differences in different brain regions, as suggested by Pachter’s work 66, 85.
(ii) Rapid high-throughput screening of drugs with potential for neurotherapeutic treatments
In the past, most such drugs have been developed in vitro but failed as useful agents in vivo because of inability to cross the blood-brain barrier 86. Two plausible approaches, on which a start has been made, are (a) to use in initial screens organisms that are easily available in large numbers, e.g. flies 87, 88 and zebrafish 89, 90. These organisms utilize ABC efflux transporters as in mammals, although the morphological sites at which they function are different in invertebrates 87 and the actual ABC transporters that are functionally important may be different in different species. Also, (b) we require a better understanding of the ABC transporters in human brain barriers (adult and developing) and their level of function. It should then be possible to devise appropriate cell-based screens using fluorophore-tagged drugs and competitors 91.
(iii) What is the risk to the developing brain of drugs administered to pregnant women?
As indicated above, several ABC transporters are expressed at high levels in embryonic brain both in blood vessels and in the choroid plexuses 28, 35. However, it is uncertain if expression levels can be equated to functional exclusion of drugs from the brain. If this can be shown, then coupled with similar transporter activity in the placenta, this suggests that the developing brain may be much better protected than is implied by the discredited but still current dogma that the blood-brain barrier in the embryo is unformed or “leaky” (see Text Box). Nevertheless, the loss of the placental protection in prematurely born infants may mean that they are more vulnerable to the ill effects of drugs than their full-term counterparts.
(iv) Involvement of barrier mechanism in brain disorders
The literature on possible involvement of blood-brain barrier mechanisms is too extensive to cover in this review. Recent papers describing different aspects of the pathobiology of brain barrier mechanisms are 92, which is particularly comprehensive, and 69, 93– 95. For decades, the focus has been on dysfunction defined by supposed disruption of the blood-brain barrier often defined with unsuitable markers (see Text Box). Only comparatively recently has attention turned to the possibility that transporter dysfunction may be involved. It is usually unclear whether barrier dysfunction is a cause or a consequence of a particular neurological disorder. This is an area in which further research with modern technology is likely to be fruitful.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Patrizia Ferretti, Stem Cells and Regenerative Medicine Section, UCL Institute of Child Health, London, UK
Daniela Virgintino, Department of Basic Medical Sciences, Neuroscience, and Sensory Organs, University of Bari School of Medicine, Bari, Italy
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Krogh A: The active and passive exchanges of inorganic ions through the surfaces of living cells and through living membranes generally. Proc R Soc Lond B. 1946;133(871):140–200. 10.1098/rspb.1946.0008 [DOI] [PubMed] [Google Scholar]
- 2. Saunders NR, Dreifuss JJ, Dziegielewska KM, et al. : The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci. 2014;8:404. 10.3389/fnins.2014.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stern L, Gautier R: Recherches sur le liquide céphalo-rachidien. 1. Les rapports entre le liquide céphalo-rachidien et la circulation sanguine. Arch Int Physiol. 1921;17(2):138–192. 10.3109/13813452109146211 [DOI] [Google Scholar]
- 4. Ehrlich P: Das Sauerstoffbedürfnis des Organismus. Eine Farbenanalytische Studie.Berlin: Hirschwald;1885. Reference Source [Google Scholar]
- 5. Lewandowsky M: Zur Lehre von der Cerebrospinalflüssgkeit. Z Clin Med. 1900;40:480–494. [Google Scholar]
- 6. Goldmann EE: Die äussere und innere Sekretion des gesunden und kranken Organismus im Lichte der ‘vitalen Färbung.1909. [Google Scholar]
- 7. Mäe M, Armulik A, Betsholtz C: Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17(26):2750–2754. 10.2174/138161211797440113 [DOI] [PubMed] [Google Scholar]
- 8. Sá-Pereira I, Brites D, Brito MA: Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45(2):327–347. 10.1007/s12035-012-8244-2 [DOI] [PubMed] [Google Scholar]
- 9. Alvarez JI, Saint-Laurent O, Godschalk A, et al. : Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis. 2015;74:14–24. 10.1016/j.nbd.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 10. Liddelow S, Barres B: SnapShot: Astrocytes in Health and Disease. Cell. 2015;162(5):1170–1170.e1. 10.1016/j.cell.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 11. Brightman MW, Reese TS: Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40(3):648–677. 10.1083/jcb.40.3.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nabeshima S, Reese TS, Landis DM, et al. : Junctions in the meninges and marginal glia. J Comp Neurol. 1975;164(2):127–169. 10.1002/cne.901640202 [DOI] [PubMed] [Google Scholar]
- 13. Brøchner CB, Holst CB, Møllgård K: Outer brain barriers in rat and human development. Front Neurosci. 2015;9:75. 10.3389/fnins.2015.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engelhardt B, Sorokin L: The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. 10.1007/s00281-009-0177-0 [DOI] [PubMed] [Google Scholar]
- 15. Daneman R, Zhou L, Kebede AA, et al. : Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Errede M, Girolamo F, Rizzi M, et al. : The contribution of CXCL12-expressing radial glia cells to neuro-vascular patterning during human cerebral cortex development. Front Neurosci. 2014;8:324. 10.3389/fnins.2014.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caley DW, Maxwell DS: Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970;138(1):31–47. 10.1002/cne.901380104 [DOI] [PubMed] [Google Scholar]
- 18. Neuwelt EA: Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54(1):131–40– discussion 141–2. 10.1227/01.NEU.0000097715.11966.8E [DOI] [PubMed] [Google Scholar]
- 19. Krish B, Leonhardt H: The functional and structural border of the neurohemal region of the median eminence. Cell Tissue Res. 1978;192(2):327–339. 10.1007/BF00220750 [DOI] [PubMed] [Google Scholar]
- 20. Price CJ, Hoyda TD, Ferguson AV: The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist. 2008;14(2):182–194. 10.1177/1073858407311100 [DOI] [PubMed] [Google Scholar]
- 21. Møllgård K, Balslev Y, Lauritzen B, et al. : Cell junctions and membrane specializations in the ventricular zone (germinal matrix) of the developing sheep brain: a CSF-brain barrier. J Neurocytol. 1987;16(4):433–444. 10.1007/BF01668498 [DOI] [PubMed] [Google Scholar]
- 22. Fossan G, Cavanagh ME, Evans CA, et al. : CSF-brain permeability in the immature sheep fetus: a CSF-brain barrier. Brain Res. 1985;350(1–2):113–124. 10.1016/0165-3806(85)90255-X [DOI] [PubMed] [Google Scholar]
- 23. Whish S, Dziegielewska KM, Møllgård K, et al. : The inner CSF-brain barrier: developmentally controlled access to the brain via intercellular junctions. Front Neurosci. 2015;9:16. 10.3389/fnins.2015.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davson H, Segal MB: Physiology of the CSF and Blood-Brain Barriers.Boca Raton: CRC Press;1996. Reference Source [Google Scholar]
- 25. Damkier HH, Brown PD, Praetorius J: Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93(4):1847–1892. 10.1152/physrev.00004.2013 [DOI] [PubMed] [Google Scholar]
- 26. Liddelow SA, Dziegielewska KM, Ek CJ, et al. : Mechanisms that determine the internal environment of the developing brain: a transcriptomic, functional and ultrastructural approach. PLoS One. 2013;8(7):e65629. 10.1371/journal.pone.0065629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oldendorf WH: Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221(6):1629–1639. [DOI] [PubMed] [Google Scholar]
- 28. Daneman R, Zhou L, Agalliu D, et al. : The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5(10):e13741. 10.1371/journal.pone.0013741 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Shawahna R, Uchida Y, Declèves X, et al. : Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8(4):1332–1341. 10.1021/mp200129p [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Geier EG, Chen EC, Webb A, et al. : Profiling solute carrier transporters in the human blood-brain barrier. Clin Pharmacol Ther. 2013;94(6):636–639. 10.1038/clpt.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Marques F, Sousa JC, Coppola G, et al. : Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS. 2011;8(1):10. 10.1186/2045-8118-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Ho HT, Dahlin A, Wang J: Expression Profiling of Solute Carrier Gene Families at the Blood-CSF Barrier. Front Pharmacol. 2012;3:154. 10.3389/fphar.2012.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liddelow SA, Temple S, Møllgård K, et al. : Molecular characterisation of transport mechanisms at the developing mouse blood-CSF interface: a transcriptome approach. PLoS One. 2012;7(3):e33554. 10.1371/journal.pone.0033554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saunders NR, Daneman R, Dziegielewska KM, et al. : Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med. 2013;34(2–3):742–752. 10.1016/j.mam.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 35. Kratzer I, Liddelow SA, Saunders NR, et al. : Developmental changes in the transcriptome of the rat choroid plexus in relation to neuroprotection. Fluids Barriers CNS. 2013;10(1):25. 10.1186/2045-8118-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saunders NR, Dziegielewska KM, Møllgård K, et al. : Influx mechanisms in the embryonic and adult rat choroid plexus: a transcriptome study. Front Neurosci. 2015;9:123. 10.3389/fnins.2015.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dean M, Rzhetsky A, Allikmets R: The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11(7):1156–1166. [DOI] [PubMed] [Google Scholar]
- 38. Hartz AMS, Bauer B: ABC transporters in the CNS - an inventory. Curr Pharm Biotechnol. 2011;12(4):656–673. 10.2174/138920111795164020 [DOI] [PubMed] [Google Scholar]
- 39. Strazielle N, Ghersi-Egea JF: Efflux transporters in blood-brain interfaces of the developing brain. Front Neurosci. 2015;9:21. 10.3389/fnins.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ek CJ, Wong A, Liddelow SA, et al. : Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol Lett. 2010;197(1):51–59. 10.1016/j.toxlet.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 41. Beaulieu E, Demeule M, Ghitescu L, et al. : P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–544. 10.1042/bj3260539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bendayan R, Lee G, Bendayan M: Functional expression and localization of P-glycoprotein at the blood brain barrier. Microsc Res Tech. 2002;57(5):365–380. 10.1002/jemt.10090 [DOI] [PubMed] [Google Scholar]
- 43. Roberts LM, Black DS, Raman C, et al. : Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–438. 10.1016/j.neuroscience.2008.06.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Cooray HC, Blackmore CG, Maskell L, et al. : Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13(16):2059–2063. 10.1097/00001756-200211150-00014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Daood M, Tsai C, Ahdab-Barmada M, et al. : ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics. 2008;39(4):211–218. 10.1055/s-0028-1103272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bauer B, Hartz AM, Lucking JR, et al. : Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28(6):1222–1234. 10.1038/jcbfm.2008.16 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Nies AT, Jedlitschky G, König J, et al. : Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129(2):349–360. 10.1016/j.neuroscience.2004.07.051 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Gazzin S, Strazielle N, Schmitt C, et al. : Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol. 2008;510(5):497–507. 10.1002/cne.21808 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Maliepaard M, Scheffer GL, Faneyte IF, et al. : Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–3464. [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Matsuoka Y, Okazaki M, Kitamura Y, et al. : Developmental expression of P-glycoprotein (multidrug resistance gene product) in the rat brain. J Neurobiol. 1999;39(3):383–392. [DOI] [PubMed] [Google Scholar]
- 51. Bougoin S, Lomet D, Kerboeuf D, et al. : Evidence that the choroids plexus in female sheep express P-glycoprotein. Neuro Endocrinol Lett. 2008;29(4):438–442. [PubMed] [Google Scholar]
- 52. Mercier C, Masseguin C, Roux F, et al. : Expression of P-glycoprotein (ABCB1) and Mrp1 (ABCC1) in adult rat brain: focus on astrocytes. Brain Res. 2004;1021(1):32–40. 10.1016/j.brainres.2004.06.034 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Rao VV, Dahlheimer JL, Bardgett ME, et al. : Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96(7):3900–3905. 10.1073/pnas.96.7.3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Löscher W, Potschka H: Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6(8):591–602. 10.1038/nrn1728 [DOI] [PubMed] [Google Scholar]
- 55. Higgins CF, Gottesman MM: Is the multidrug transporter a flippase? Trends Biochem Sci. 1992;17(1):18–21. 10.1016/0968-0004(92)90419-A [DOI] [PubMed] [Google Scholar]
- 56. Schumacher U, Møllgård K: The multidrug-resistance P-glycoprotein (Pgp, MDR1) is an early marker of blood-brain barrier development in the microvessels of the developing human brain. Histochem Cell Biol. 1997;108(2):179–182. 10.1007/s004180050159 [DOI] [PubMed] [Google Scholar]
- 57. Virgintino D, Errede M, Girolamo F, et al. : Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J Neuropathol Exp Neurol. 2008;67(1):50–61. 10.1097/nen.0b013e31815f65d9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. He Y, Yao Y, Tsirka SE, et al. : Cell-culture models of the blood-brain barrier. Stroke. 2014;45(8):2514–2526. 10.1161/STROKEAHA.114.005427 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Patel MM, Goyal BR, Bhadada SV, et al. : Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23(1):35–58. 10.2165/0023210-200923010-00003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Lajoie JM, Shusta EV: Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2015;55:613–631. 10.1146/annurev-pharmtox-010814-124852 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Palmiotti CA, Prasad S, Naik P, et al. : In vitro cerebrovascular modeling in the 21 st century: current and prospective technologies. Pharm Res. 2014;31(12):3229–3250. 10.1007/s11095-014-1464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilhelm I, Krizbai IA: In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol Pharm. 2014;11(7):1949–1963. 10.1021/mp500046f [DOI] [PubMed] [Google Scholar]
- 63. Stanimirovic DB, Bani-Yaghoub M, Perkins M, et al. : Blood-brain barrier models: in vitro to in vivo translation in preclinical development of CNS-targeting biotherapeutics. Expert Opin Drug Discov. 2015;10(2):141–155. 10.1517/17460441.2015.974545 [DOI] [PubMed] [Google Scholar]
- 64. Wolff A, Antfolk M, Brodin B, et al. : In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J Pharm Sci. 2015;104(9):2727–2746. 10.1002/jps.24329 [DOI] [PubMed] [Google Scholar]
- 65. Revest PA, Jones HC, Abbott NJ: Transendothelial electrical potential across pial vessels in anaesthetised rats: a study of ion permeability and transport at the blood-brain barrier. Brain Res. 1994;652(1):76–82. 10.1016/0006-8993(94)90319-0 [DOI] [PubMed] [Google Scholar]
- 66. Macdonald JA, Murugesan N, Pachter JS: Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature. J Neurosci Res. 2010;88(7):1457–1474. 10.1002/jnr.22316 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Ohtsuki S, Ikeda C, Uchida Y, et al. : Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol Pharm. 2013;10(1):289–296. 10.1021/mp3004308 [DOI] [PubMed] [Google Scholar]
- 68. Cotzias GC, Papavasiliou PS, Gellene R: Experimental treatment of parkinsonism with L-Dopa. Neurology. 1968;18(3):276–277. [PubMed] [Google Scholar]
- 69. Qosa H, Miller DS, Pasinelli P, et al. : Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015;1628(Pt B):298–316. 10.1016/j.brainres.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grandjean P, Landrigan PJ: Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. 10.1016/S0140-6736(06)69665-7 [DOI] [PubMed] [Google Scholar]
- 71. Rapoport SI, Hori M, Klatzo I: Reversible osmotic opening of the blood-brain barrier. Science. 1971;173(4001):1026–1028. 10.1126/science.173.4001.1026 [DOI] [PubMed] [Google Scholar]
- 72. Brightman MW, Hori M, Rapoport SI, et al. : Osmotic opening of tight junctions in cerebral endothelium. J Comp Neurol. 1973;152(4):317–325. 10.1002/cne.901520402 [DOI] [PubMed] [Google Scholar]
- 73. Neuwelt EA, Maravilla KR, Frenkel EP, et al. : Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64(2):684–688. 10.1172/JCI109509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Doolittle ND, Muldoon LL, Culp AY, et al. : Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol. 2014;71:203–243. 10.1016/bs.apha.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Bellavance MA, Blanchette M, Fortin D: Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10(1):166–177. 10.1208/s12248-008-9018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Rodriguez A, Tatter SB, Debinski W: Neurosurgical Techniques for Disruption of the Blood-Brain Barrier for Glioblastoma Treatment. Pharmaceutics. 2015;7(3):175–187. 10.3390/pharmaceutics7030175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bakay L: Development of the blood-brain barrier. In: Bakay L, editor. The blood brain barrier, with special regard to the use of radioactive isotopes Springfield, IL: Charles C Thomas;1956;77–149. [Google Scholar]
- 78. Patrick JT, Nolting MN, Goss SA, et al. : Ultrasound and the blood-brain barrier. Adv Exp Med Biol. 1990;267:369–381. [DOI] [PubMed] [Google Scholar]
- 79. Hynynen K, McDannold N, Vykhodtseva N, et al. : Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–646. 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Aryal M, Vykhodtseva N, Zhang YZ, et al. : Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: a safety study. J Control Release. 2015;204:60–69. 10.1016/j.jconrel.2015.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Wei KC, Tsai HC, Lu YJ, et al. : Neuronavigation-guided focused ultrasound-induced blood-brain barrier opening: a preliminary study in swine. AJNR Am J Neuroradiol. 2013;34(1):115–120. 10.3174/ajnr.A3150 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Burgess A, Hynynen K: Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem Neurosci. 2013;4(4):519–526. 10.1021/cn300191b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Timbie KF, Mead BP, Price RJ: Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. 10.1016/j.jconrel.2015.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Meairs S: Facilitation of Drug Transport across the Blood-Brain Barrier with Ultrasound and Microbubbles. Pharmaceutics. 2015;7(3):275–293. 10.3390/pharmaceutics7030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Paul D, Cowan AE, Ge S, et al. : Novel 3D analysis of Claudin-5 reveals significant endothelial heterogeneity among CNS microvessels. Microvasc Res. 2013;86:1–10. 10.1016/j.mvr.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Pardridge WM: Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1(2):131–139. 10.1038/nrd725 [DOI] [PubMed] [Google Scholar]
- 87. Hindle SJ, Bainton RJ: Barrier mechanisms in the Drosophila blood-brain barrier. Front Neurosci. 2014;8:414. 10.3389/fnins.2014.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Limmer S, Weiler A, Volkenhoff A, et al. : The Drosophila blood-brain barrier: development and function of a glial endothelium. Front Neurosci. 2014;8:365. 10.3389/fnins.2014.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Umans RA, Taylor MR: Zebrafish as a model to study drug transporters at the blood-brain barrier. Clin Pharmacol Ther. 2012;92(5):567–570. 10.1038/clpt.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fleming A, Diekmann H, Goldsmith P: Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One. 2013;8(10):e77548. 10.1371/journal.pone.0077548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fardel O, Le Vee M, Jouan E, et al. : Nature and uses of fluorescent dyes for drug transporter studies. Expert Opin Drug Metab Toxicol. 2015;11(8):1233–1251. 10.1517/17425255.2015.1053462 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Neuwelt EA, Bauer B, Fahlke C, et al. : Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–182. 10.1038/nrn2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blanchette M, Daneman R: Formation and maintenance of the BBB. Mech Dev. 2015;138(Pt 1):8–16. 10.1016/j.mod.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 94. Marques F, Sousa JC: The choroid plexus is modulated by various peripheral stimuli: implications to diseases of the central nervous system. Front Cell Neurosci. 2015;9:136. 10.3389/fncel.2015.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stolp HB, Dziegielewska KM: Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol. 2009;35(2):132–146. 10.1111/j.1365-2990.2008.01005.x [DOI] [PubMed] [Google Scholar]
- 96. Brown PD, Davies SL, Speake T, et al. : Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129(4):957–970. 10.1016/j.neuroscience.2004.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Murphy VA, Johanson CE: Acidosis, acetazolamide, and amiloride: effects on 22Na transfer across the blood-brain and blood-CSF barriers. J Neurochem. 1989;52(4):1058–1063. 10.1111/j.1471-4159.1989.tb01847.x [DOI] [PubMed] [Google Scholar]
- 98. Damkier HH, Nielsen S, Praetorius J: Molecular expression of SLC4-derived Na +-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R2136–46. 10.1152/ajpregu.00356.2007 [DOI] [PubMed] [Google Scholar]
- 99. Pollay M, Hisey B, Reynolds E, et al. : Choroid plexus Na+/K+-activated adenosine triphosphatase and cerebrospinal fluid formation. Neurosurgery. 1985;17(5):768–772. [DOI] [PubMed] [Google Scholar]
- 100. Johanson CE, Duncan JA, 3rd, Klinge PM, et al. : Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. 10.1186/1743-8454-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Oshio K, Watanabe H, Song Y, et al. : Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19(1):76–78. 10.1096/fj.04-1711fje [DOI] [PubMed] [Google Scholar]
- 102. Owler BK, Pitham T, Wang D: Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res. 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Amtorp O, Sorensen SC: The ontogenetic development of concentration differences for protein and ions between plasma and cerebrospinal fluid in rabbits and rats. J Physiol. 1974;243(2):387–400. 10.1113/jphysiol.1974.sp010759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Johanson CE, Murphy VA: Acetazolamide and insulin alter choroid plexus epithelial cell [Na+], pH, and volume. Am J Physiol. 1990;258(6 Pt 2):F1538–46. [DOI] [PubMed] [Google Scholar]
- 105. Bass NH, Lundborg P: Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-( 14C)inulin after intrathecal infusion. Brain Res. 1973;52:323–332. 10.1016/0006-8993(73)90668-9 [DOI] [PubMed] [Google Scholar]
- 106. Johanson CE, Woodbury DM: Changes in CSF flow and extracellular space in the developing rat. In “ Drugs and the developing brain.” Vernadakis A, Weiner N, editors. New York: Plenum;1974;281–287. 10.1007/978-1-4684-3063-9_15 [DOI] [Google Scholar]
- 107. Johanson CE, Reed DJ, Woodbury DM: Developmental studies of the compartmentalization of water and electrolytes in the choroid plexus of the neonatal rat brain. Brain Res. 1976;116(1):35–48. 10.1016/0006-8993(76)90247-X [DOI] [PubMed] [Google Scholar]
- 108. Pardridge WM: Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. 10.1038/jcbfm.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liang Y, Li S, Chen L: The physiological role of drug transporters. Protein Cell. 2015;6(5):334–350. 10.1007/s13238-015-0148-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bauer B, Hartz AM, Fricker G, et al. : Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med (Maywood). 2005;230(2):118–127. [DOI] [PubMed] [Google Scholar]
- 111. Kantorovich S, Astary GW, King MA, et al. : Influence of neuropathology on convection-enhanced delivery in the rat hippocampus. PLoS One. 2013;8(11):e80606. 10.1371/journal.pone.0080606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Abbott NJ: Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36(3):437–449. 10.1007/s10545-013-9608-0 [DOI] [PubMed] [Google Scholar]
- 113. Gherardini L, Bardi G, Gennaro M, et al. : Novel siRNA delivery strategy: a new “strand” in CNS translational medicine? Cell Mol Life Sci. 2014;71(1):1–20. 10.1007/s00018-013-1310-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yarnitsky D, Gross Y, Lorian A, et al. : Blood-brain barrier opened by stimulation of the parasympathetic sphenopalatine ganglion: a new method for macromolecule delivery to the brain. J Neurosurg. 2004;101(2):303–309. 10.3171/jns.2004.101.2.0303 [DOI] [PubMed] [Google Scholar]
- 115. Yarnitsky D, Gross Y, Lorian A, et al. : Increased BBB permeability by parasympathetic sphenopalatine ganglion stimulation in dogs. Brain Res. 2004;1018(2):236–240. 10.1016/j.brainres.2004.05.103 [DOI] [PubMed] [Google Scholar]
- 116. Dereymaeker A, Gonsette R: An experimental permeabilization of the blood-brain barrier by electric field application. Eur Neurol. 1977;15(6):333–339. 10.1159/000114822 [DOI] [PubMed] [Google Scholar]
- 117. Saunders NR, Liddelow SA, Dziegielewska KM: Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. 10.3389/fphar.2012.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Frömter E, Diamond J: Route of passive ion permeation in epithelia. Nat New Biol. 1972;235(53):9–13. 10.1038/newbio235009a0 [DOI] [PubMed] [Google Scholar]
- 119. Ek CJ, Dziegielewska KM, Stolp H, et al. : Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum ( Monodelphis domestica). J Comp Neurol. 2006;496(1):13–26. 10.1002/cne.20885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Krueger M, Bechmann I, Immig K, et al. : Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35(2):292–303. 10.1038/jcbfm.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Knowland D, Arac A, Sekiguchi KJ, et al. : Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. 10.1016/j.neuron.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Prasad S, Sajja RK, Naik P, et al. : Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J Pharmacovigil. 2014;2(2):125. 10.4172/2329-6887.1000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Willyerd FA , Empey PE , Philbrick A, et al. : Expression of ATP-Binding Cassette Transporters B1 and C1 after Severe Traumatic Brain Injury in Humans. J Neurotrauma. 2016;33(2):226–231. 10.1089/neu.2015.3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Saunders NR, Dziegielewska KM, Møllgård K, et al. : Markers for blood-brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci. 2015;9:385. 10.3389/fnins.2015.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Iliff JJ, Goldman SA, Nedergaard M: Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14(10):977–9. 10.1016/S1474-4422(15)00221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Korogod N, Petersen CC, Knott GW: Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. eLife. 2015;4:e05793. 10.7554/eLife.05793 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 127. Hammarlund-Udenaes M, Fridén M, Syvänen S, et al. : On the rate and extent of drug delivery to the brain. Pharm Res. 2008;25(8):1737–1750. 10.1007/s11095-007-9502-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 128. de Lange EC, Danhof M: Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin Pharmacokinet. 2002;41(10):691–703. 10.2165/00003088-200241100-00001 [DOI] [PubMed] [Google Scholar]
- 129. Louveau A, Smirnov I, Keyes TJ, et al. : Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]