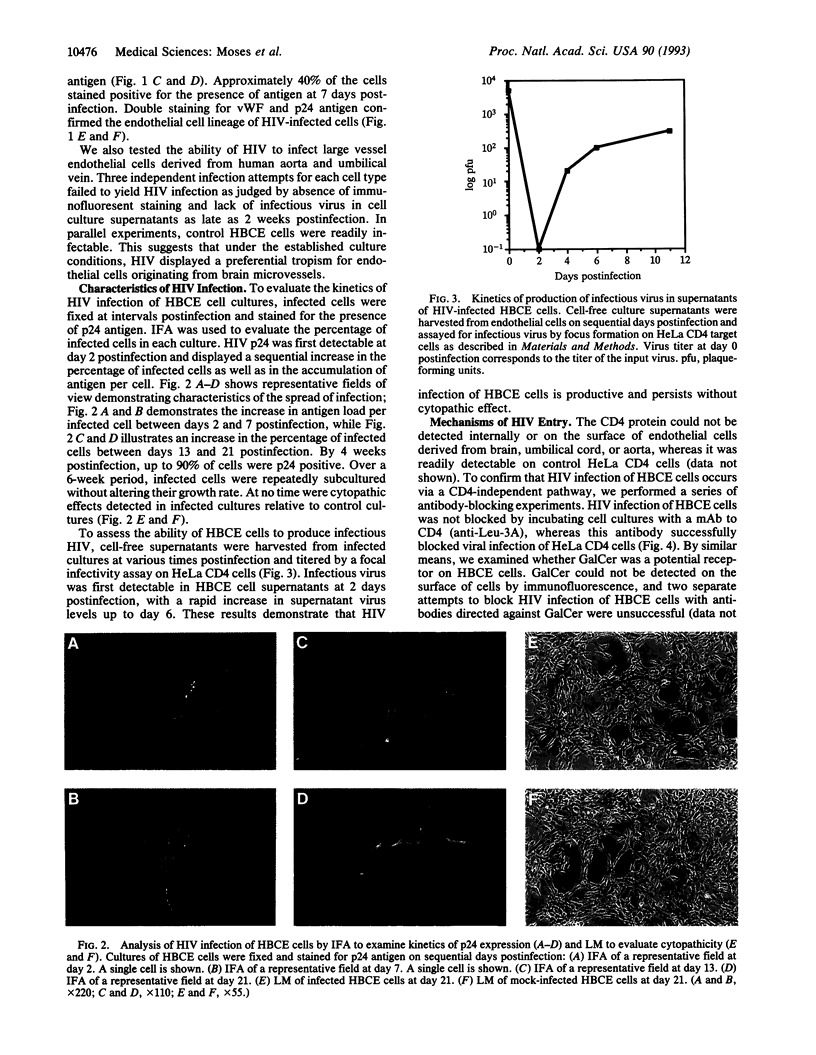
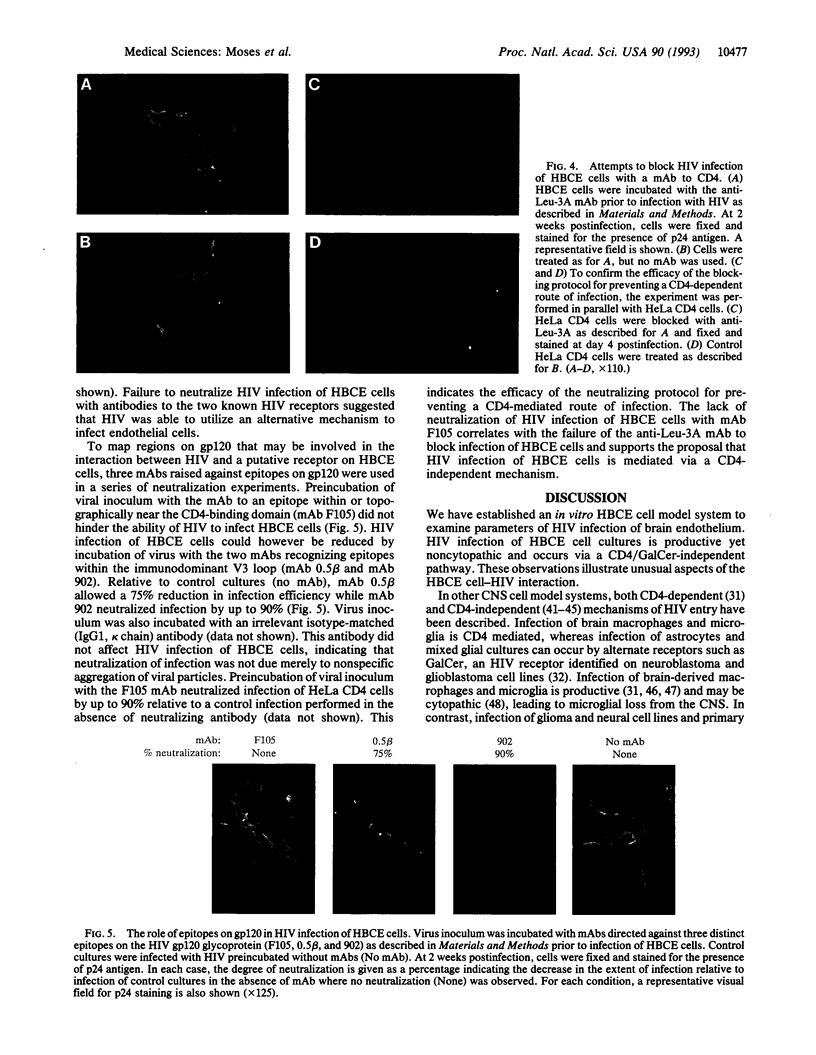
Abstract
Neuropathologic studies of AIDS patients have shown that brain capillary endothelial cells are a cellular target for human immunodeficiency virus (HIV) in vivo. We have established in vitro cultures of primary human brain capillary endothelial (HBCE) cells. Using this model system, we have shown a significant HIV infection of HBCE cells that is productive yet noncytopathic. The infection is mediated by a cellular interaction with gp120 that does not involve CD4 or galactosylceramide. HIV infection of HBCE cells may contribute to AIDS-associated neuropathology by disturbing the physiology of the endothelium and directly or indirectly facilitating dissemination of virus to the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beilke M. A. Vascular endothelium in immunology and infectious disease. Rev Infect Dis. 1989 Mar-Apr;11(2):273–283. doi: 10.1093/clinids/11.2.273. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Brinkmann R., Schwinn A., Narayan O., Zink C., Kreth H. W., Roggendorf W., Dörries R., Schwender S., Imrich H., ter Meulen V. Human immunodeficiency virus infection in microglia: correlation between cells infected in the brain and cells cultured from infectious brain tissue. Ann Neurol. 1992 Apr;31(4):361–365. doi: 10.1002/ana.410310403. [DOI] [PubMed] [Google Scholar]
- Cenacchi G., Re M. C., Preda P., Pasquinelli G., Furlini G., Apkarian R. P., La Placa M., Martinelli G. N. Human immunodeficiency virus type-1 (HIV-1) infection of endothelial cells in vitro: a virological, ultrastructural and immuno-cytochemical approach. J Submicrosc Cytol Pathol. 1992 Apr;24(2):155–161. [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Dreyer E. B., Kaiser P. K., Offermann J. T., Lipton S. A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990 Apr 20;248(4953):364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Sharer L. R., Cho E. S., Myenhofer M., Navia B., Price R. W. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1(6):447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- Gabuzda D. H., Ho D. D., de la Monte S. M., Hirsch M. S., Rota T. R., Sobel R. A. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986 Sep;20(3):289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Geleziunas R., Schipper H. M., Wainberg M. A. Pathogenesis and therapy of HIV-1 infection of the central nervous system. AIDS. 1992 Dec;6(12):1411–1426. doi: 10.1097/00002030-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L. Recent advances in understanding brain capillary function. Ann Neurol. 1983 Oct;14(4):389–395. doi: 10.1002/ana.410140402. [DOI] [PubMed] [Google Scholar]
- Green D. F., Resnick L., Bourgoignie J. J. HIV infects glomerular endothelial and mesangial but not epithelial cells in vitro. Kidney Int. 1992 Apr;41(4):956–960. doi: 10.1038/ki.1992.146. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Gyorkey P. Human immunodeficiency virus in brain biopsies of patients with AIDS and progressive encephalopathy. J Infect Dis. 1987 May;155(5):870–876. doi: 10.1093/infdis/155.5.870. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Bhat S., Spitalnik S. L., Laughlin M., Stefano K., Silberberg D. H., Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991 Jul 19;253(5017):320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Hollander H., Levy J. A. Neurologic abnormalities and recovery of human immunodeficiency virus from cerebrospinal fluid. Ann Intern Med. 1987 May;106(5):692–695. doi: 10.7326/0003-4819-106-5-692. [DOI] [PubMed] [Google Scholar]
- Jordan C. A., Watkins B. A., Kufta C., Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991 Feb;65(2):736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Lackner A. A., Smith M. O., Munn R. J., Martfeld D. J., Gardner M. B., Marx P. A., Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991 Sep;139(3):609–621. [PMC free article] [PubMed] [Google Scholar]
- Lafon M. E., Steffan A. M., Gendrault J. L., Klein-Soyer C., Gloeckler-Tondre L., Royer C., Kirn A. Interaction of human immunodeficiency virus with human macrovascular endothelial cells in vitro. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1567–1570. doi: 10.1089/aid.1992.8.1567. [DOI] [PubMed] [Google Scholar]
- Lathey J. L., Wiley C. A., Verity M. A., Nelson J. A. Cultured human brain capillary endothelial cells are permissive for infection by human cytomegalovirus. Virology. 1990 May;176(1):266–273. doi: 10.1016/0042-6822(90)90252-m. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., Hollander H., Mills J., Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985 Sep 14;2(8455):586–588. [PubMed] [Google Scholar]
- Li X. L., Moudgil T., Vinters H. V., Ho D. D. CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J Virol. 1990 Mar;64(3):1383–1387. doi: 10.1128/jvi.64.3.1383-1387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Chen I. S. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991 Jul;5(10):2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Gimbrone M. A., Jr Plasmalemmal proteins of cultured vascular endothelial cells exhibit apical-basal polarity: analysis by surface-selective iodination. J Cell Biol. 1986 Dec;103(6 Pt 1):2389–2402. doi: 10.1083/jcb.103.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. H., Wehrly K., Chesebro B. Treatment of HIV tissue culture infection with monoclonal antibody-ricin A chain conjugates. J Immunol. 1989 May 1;142(9):3070–3075. [PubMed] [Google Scholar]
- Pincus S. H., Wehrly K., Chesebro B. Use of a focal infectivity assay for testing susceptibility of HIV to antiviral agents. Biotechniques. 1991 Mar;10(3):336–342. [PubMed] [Google Scholar]
- Posner M. R., Hideshima T., Cannon T., Mukherjee M., Mayer K. H., Byrn R. A. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol. 1991 Jun 15;146(12):4325–4332. [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumarola-Sune T., Navia B. A., Cordon-Cardo C., Cho E. S., Price R. W. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987 May;21(5):490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Sabatier J. M., Vives E., Mabrouk K., Benjouad A., Rochat H., Duval A., Hue B., Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991 Feb;65(2):961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Kaaden O., Copeland T. D., Oroszlan S., Hunsmann G. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol. 1986 Nov;67(Pt 11):2533–2538. doi: 10.1099/0022-1317-67-11-2533. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Steffan A. M., Lafon M. E., Gendrault J. L., Schweitzer C., Royer C., Jaeck D., Arnaud J. P., Schmitt M. P., Aubertin A. M., Kirn A. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1582–1586. doi: 10.1073/pnas.89.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler M. H., Eskin T. A., Benn S., Angerer R. C., Angerer L. M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986 Nov 7;256(17):2360–2364. [PubMed] [Google Scholar]
- Vazeux R., Brousse N., Jarry A., Henin D., Marche C., Vedrenne C., Mikol J., Wolff M., Michon C., Rozenbaum W. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987 Mar;126(3):403–410. [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., O'Leary T. J., Baskin G. B., Benveniste R., Harris C. A., Nara P. L., Rhodes R. H. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987 May;127(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Watkins B. A., Dorn H. H., Kelly W. B., Armstrong R. C., Potts B. J., Michaels F., Kufta C. V., Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990 Aug 3;249(4968):549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Weber J., Clapham P., McKeating J., Stratton M., Robey E., Weiss R. Infection of brain cells by diverse human immunodeficiency virus isolates: role of CD4 as receptor. J Gen Virol. 1989 Oct;70(Pt 10):2653–2660. doi: 10.1099/0022-1317-70-10-2653. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]






