Abstract
The use of nonhuman primate (NHP) models to study persistent residual virus and viral eradication strategies in combination antiretroviral therapy (cART)-treated individuals requires regimens that effectively suppress SIV replication to clinically relevant levels in macaques. We developed and evaluated two novel cART regimens in SIVmac239-infected rhesus macaques: (1) a “triple regimen” containing the nucleo(s/t)ide reverse transcriptase inhibitors emtricitabine (FTC) and tenofovir disoproxil fumarate [TDF, prodrug of tenofovir (TFV, PMPA)] with the integrase strand transfer inhibitor dolutegravir (DTG) (n = 3), or (2) a “quad regimen” containing the same three drugs plus the protease inhibitor darunavir (DRV) (n = 3), with each regimen coformulated for convenient administration by a single daily subcutaneous injection. Plasma drug concentrations were consistent across animals within the triple and quad regimen-treated groups, although DTG levels were lower in the quad regimen animals. Time to achieve plasma viral loads stably <30 viral RNA copies/ml ranged from 12 to 20 weeks of treatment between animals, and viral loads <30 viral RNA copies/ml plasma were maintained through 40 weeks of follow-up on cART. Notably, although we show virologic suppression and development of viral resistance in a separate cohort of SIV-infected animals treated with oral DRV monotherapy, the addition of DRV in the quad regimen did not confer an apparent virologic benefit during early treatment, hence the quad regimen-treated animals were switched to the triple regimen after 4 weeks. This coformulated triple cART regimen can be safely, conveniently, and sustainably administered to durably suppress SIV replication to clinically relevant levels in rhesus macaques.
The use of nonhuman primate (NHP) models of AIDS to evaluate virologic and immunologic processes in the setting of suppressive combination antiretroviral therapy (cART) has been limited due, at least in part, to challenges in identifying feasible cART regimens that can sustainably suppress viral replication in macaques infected with pathogenic simian immunodeficiency virus (SIV) to clinically relevant levels such as those seen in cART-suppressed HIV-infected individuals.1 In addition to issues arising from differences in drug potency against SIV vs. HIV and differences in the bioavailability and pharmacokinetics of antiretrovirals in NHPs vs. humans, the feasibility of daily administration of multiple antiretroviral drugs to NHP research animals can pose practical and logistical challenges, especially for drugs given via the oral route more than once daily.
The development of safe and effective cART regimens providing potent antiviral activity against SIV in pathogenic infection models coupled with convenient administration methods that achieve consistent efficacious drug levels would be of great benefit to HIV treatment and cure research and would allow for the in vivo study of residual virus sources that persist in the face of suppressive cART as well as the in vivo evaluation of proposed viral eradication/functional cure strategies. Here, we compared in SIV-infected rhesus macaques the pharmacokinetic and antiviral activity of two cART regimens, one containing three drugs and the other containing four drugs, which were coformulated for administration as a single once daily subcutaneous injection.
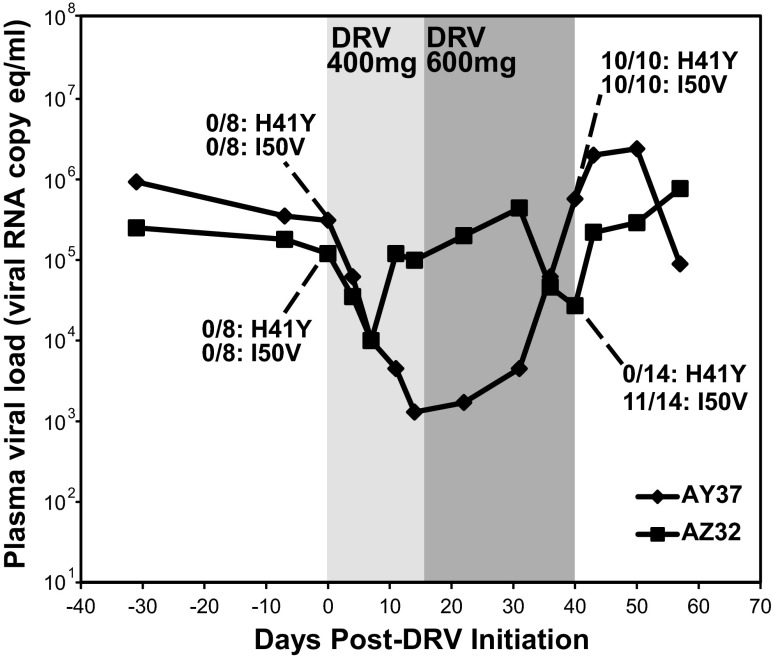
We and others2–5 have previously reported on the use of cART regimens containing the protease inhibitor darunavir (DRV), often with the addition of ritonavir (RTV) as a putative DRV boosting agent based on clinical practice in humans, in SIV-infected rhesus macaques. To specifically evaluate the anti-SIV activity of DRV in vivo in the absence of other antiretroviral drugs, we treated two Indian-origin rhesus macaques chronically infected with SIVmac239 with DRV/RTV “monotherapy” (Fig. 1) and monitored plasma viral RNA levels by quantitative RT-PCR as previously described.5 DRV and RTV (both purchased from the NIH pharmacy) were initially administered via the oral route at fixed doses of 400 mg (equivalent to 36 mg/kg for AZ32 and 54 mg/kg for AY37) and 100 mg (equivalent to 9 mg/kg for AZ32 and 13 mg/kg for AY37), respectively, twice daily. After 2 weeks of treatment, the DRV dose was increased to 600 mg (equivalent to 55 mg/kg for AZ32 and 81 mg/kg for AY37) twice daily while the RTV dose was maintained.
FIG. 1.
Plasma viral load changes with darunavir (DRV)/ritonavir (RTV) “monotherapy.” Plasma viral loads were determined by qRT-PCR assay with a 30-copy/ml quantification limit for two Indian-origin rhesus macaques chronically infected with SIVmac239 and treated with DRV monotherapy at 400 mg (light gray shaded region; body weight adjusted DRV doses of 54 mg/kg for AY37 and 36 mg/kg for AZ32) or 600 mg (dark gray shaded region; body weight adjusted DRV doses of 81 mg/kg for AY37 and 55 mg/kg for AZ32) via the oral route twice daily (animals also received oral RTV at 100 mg twice daily during DRV treatment). The frequency of two viral protease gene mutations (H41Y and I50V) determined by single-genome amplification sequencing is shown for each animal prior to and after DRV treatment.
Prior to treatment, both animals had plasma viral loads >105 viral RNA (vRNA) copies/ml. Within 1 week of treatment initiation, plasma viral loads for both animals declined by >1 log to 104 vRNA copies/ml. Thereafter, viremia in animal AY37 continued to decline by another 1 log over the next 7 days of treatment before rebounding over the subsequent 26 days to pretreatment levels by day 40 despite continued treatment with 600 mg DRV twice daily. After an initial decline, plasma viral loads in animal AZ32 rebounded more quickly, returning to pretreatment levels by day 11 and remaining elevated until again transiently declining by ∼1 log between 2 and 3.5 weeks after switching to the elevated dose of DRV before returning to pretreatment levels by day 40.
To determine if virologic rebound in the setting of DRV treatment was associated with the acquisition of drug resistance mutations, suggestive of SIV selective pressure applied by DRV treatment in infected rhesus macaques, we performed single-genome amplification (SGA)-based viral genome sequencing6 using primers spanning the protease coding region. After 40 total days of DRV/RTV monotherapy, 10 of 10 and 11 of 14 sequences for AY37 and AZ32, respectively, contained the I50V mutation (Fig. 1), previously described as a DRV resistance mutation for HIV-1.7 This mutation was not identified in any of eight viral protease sequences derived from either animal just prior to DRV therapy, suggesting that DRV/RTV monotherapy conferred selection pressure in vivo and that virologic rebound was likely due to the emergence of drug resistance. There was also apparent selection for protease mutation H41Y, a change not previously described with DRV resistance, which was not present in any pretreatment sequences but was present in 10 of 10 posttreatment sequences in one animal, AZ32. The functional importance of the H41Y mutation is unclear, though it may represent a compensatory mutation to restore fitness in the presence of I50V.
We next measured the pharmacokinetic steady state plasma concentrations (Css) achieved in the two DRV/RTV-treated animals on day 12 (400 mg) and day 21 (600 mg) of therapy immediately prior to the next scheduled administration for each dose level (Table 1). Oral drug administration, such as that used here for a fixed dose of DRV, requires hiding drugs in food/treats provided ad libidum, which can lead to inconsistencies in dose timing and the amount of dose delivered, and also offers challenges for body weight-normalized drug dosing, which can result in animal-to-animal variability in achieved in vivo drug levels. Plasma DRV levels for both animals at both drug doses exceeded the in vitro protein adjusted (PA) EC95 for DRV (measured in a multiround infection assay on CEMx174 target cells8); however, plasma DRV exposures were highly disparate between the two treated animals, with the measured plasma Css at the 600 mg dose for animal AZ32 more than 10-fold lower than that of animal AY37, consistent with the larger body weight and lower weight-normalized dose in animal AZ32 (Table 1).
Table 1.
Plasma Drug Concentrations
| Animal | Regimen | Sample type/time point | TDF (nM) | FTC (nM) | DTG (nM) | DRV (nM) |
|---|---|---|---|---|---|---|
| Target | 509a | 268a | ||||
| AY37 | DRV (400 mg)/RTV | Css | NA | NA | NA | 2,422 |
| DRV (600 mg)/RTV | Css | NA | NA | NA | 4,236 | |
| AZ32 | DRV (400 mg)/RTV | Css | NA | NA | NA | 1,217 |
| DRV (600 mg)/RTV | Css | NA | NA | NA | 377 | |
| ZI76 | Quad | C2hr | 1,061 | 30,340 | 947 | 5,298 |
| Quad | Css | 268 | 529 | 1,972 | 3,223 | |
| ZI05 | Quad | C2hr | 2,015 | 50,500 | 1,187 | 7,193 |
| Quad | Css | 147 | 243 | 2,021 | 3,556 | |
| ZH34 | Quad | C2hr | 1,789 | 42,016 | 1,030 | 5,441 |
| Quad | Css | 172 | 156 | 1,964 | 5,090 | |
| ZI18 | Triple | C2hr | 1,775 | 35,754 | 3,942 | NA |
| Triple | Css | 255 | 228 | 3,147 | NA | |
| ZI69 | Triple | C2hr | 1,677 | 40,804 | 2,820 | NA |
| Triple | Css | 192 | 287 | 3,852 | NA | |
| ZH37 | Triple | C2hr | 1,907 | 48,076 | 2,686 | NA |
| Triple | Css | 164 | 319 | 2,885 | NA |
Target value represents plasma adjusted 95% effective concentration (PAEC95) determined in multiround infection assays in CEMx174 cells as described.8
TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; DTG, dolutegravir; DRV, darunavir.
In addition, although the measured DRV plasma Css for animal AY37 was ∼1.7-fold higher at the 600 mg dose than at the 400 mg dose, in animal AZ32 the determined Css at 600 mg was more than 3-fold lower than at 400 mg, again highlighting the potential inconsistencies of oral drug administration both within animals and between animals. Consistent with the lower DRV plasma concentrations in animal AZ32, virologic rebound, suggestive of the development of drug resistance, occurred more rapidly following initial plasma viral load decline (Fig. 1).
Recent improvements in the feasibility and effectiveness of cART regimens in SIV-infected macaques have included the development of multidrug cocktails containing two nucleo(t/s)ide RT inhibitors (NRTIs), emtricitabine (FTC) and tenofovir (TFV, PMPA), and one integrase strand transfer inhibitor (INSTI), such as raltegravir (RAL) or dolutegravir (DTG), given with or without oral DRV2,9,10 (Del Prete et al., unpublished results). Coformulating multiple drugs with demonstrated potency against SIV replication in vivo into a single injectable regimen offers several practical advantages, including improved ease and consistency of administration compared with regimens that require multiple daily injections and/or oral drug administration.
Although DRV has apparent anti-SIV activity in vivo (Fig. 1) it is unclear whether it might enhance the antiviral activity of the TFV/FTC/DTG cART regimen, particularly if better DRV dose consistency and in vivo drug levels can be achieved. Additionally, although TFV has become a core component of most cART regimens used in SIV-infected rhesus macaques, occasional renal toxicity has been noted in association with acute high dose plasma exposure of TFV coupled with chronic administration; chronic administration is often required for studies of persistent residual virus in the setting of suppressive cART and for evaluations of viral eradication/functional cure strategies.5,11,12 We therefore sought to develop and evaluate two new coformulated injectable drug regimens in SIVmac239-infected Indian-origin rhesus macaques.
The first regimen, hereafter referred to as the “triple regimen,” contained FTC, DTG, and TDF (TFV prodrug; tenofovir disoproxil fumarate) coformulated as a once daily subcutaneous injectable; TDF can achieve in vivo antiviral potency similar to TFV but with lower plasma exposure.13,14 Although TFV is typically administered to SIV-infected macaques at doses ranging from 15 to 30 mg/kg,1 in the triple regimen TDF was administered at 5.1 mg/kg, with FTC and DTG administered at 50 mg/kg and 2.5 mg/kg, respectively. The second regimen, hereafter termed the “quad regimen,” contained the same compounds and dosages as the triple regimen with the addition of DRV at 40 mg/kg, with all four compounds again coformulated to enable administration via a single once daily subcutaneous injection.
For compounds used in the coformulated triple and quad regimens, TDF and FTC were manufactured by Gilead Sciences, Inc., DTG sodium salt and free base were purchased from Shanghai Medicilon, Inc., and DRV ethanolate was purchased from Toronto Research Chemical Inc. To make 1 liter of the triple regimen, first ∼1 liter of a 15% (w/w) Kleptose solution was prepared in sterile water using Kleptose HPB parenteral grade (Roquette). Next 2.5 g of DTG free base equivalent was placed in a 1-liter volumetric flask and filled to 80% with the 15% Kleptose solution and then stirred and sonicated until all material was dissolved, in about 30 min. Next 5.1 g of TDF was added and again the solution was stirred and sonicated until all material was dissolved, in about 5 min. Then, 50 g of FTC was added and the solution was again stirred and sonicated until all material was dissolved, in about 3 min. Finally, the total volume was brought up to 1 liter with the 15% Kleptose solution, pH confirmed/adjusted (final pH ∼4.2), filtered through a 0.22-μm filter, aliquoted (100-ml bottles), and stored frozen at −20°C.
To make 100 ml of the quad regimen, first a DRV/DTG solution was prepared in Polyethylene glycol 400 (PEG400; Spectrum Chemical) by adding 4.34 g of DRV-ethanolate and 0.25 g of DTG free base to a 50-ml volumetric flask and bringing up to 90% full with PEG400 followed by stirring for 1 h and then sonication for 20 min. The total volume was then brought up to 50 ml with PEG400, followed by stirring for an additional hour and sonicating for 10 min. Next, a TDF/FTC solution was prepared in 30% Kleptose; 0.51 g of TDF, 5.0 g of FTC, and 15 g of Kleptose were added to a 50-ml volumetric flask, then sterile water was added to bring to ∼90% full and the solution was stirred for 1 h. The total volume was then brought up to 50 ml with water and the solution stirred for an additional 3 h. Finally the 50 ml of DRV/DTG/PEG400 solution and 50 ml of TDF/FTC/Kleptose solution were combined in a 100-ml bottle, stirred, sterile filtered, and stored frozen at −20°C.
All cART formulations were made fresh on a monthly basis and stored frozen at −20°C until the day of administration, upon which individual bottles were thawed to room temperature prior to subcutaneous administration at 1 ml/kg animal body weight.
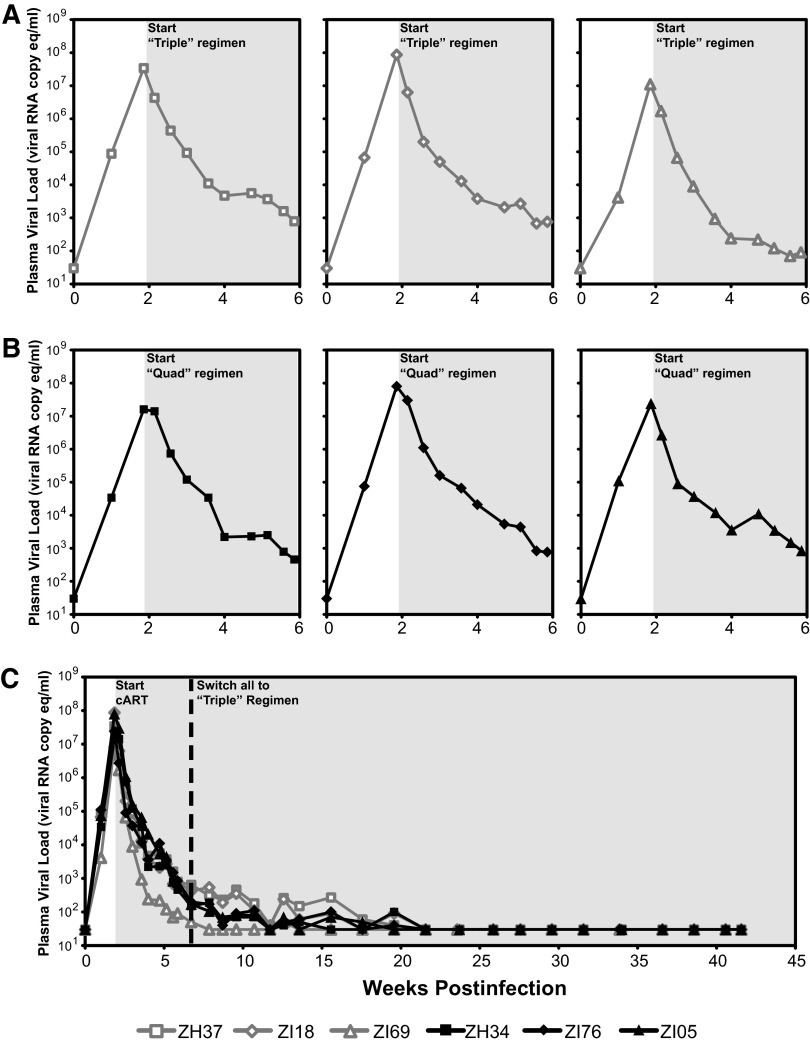
Six Indian-origin rhesus macaques were intravenously inoculated with 300 IU (based on infectious titer determined on TZM-bl reporter cells15) of SIVmac239X16 (Fig. 2). On day 13 postinfection (dpi), three animals began treatment with the triple regimen while the remaining three animals began treatment with the quad regimen. Pretreatment viral loads on day 13 ranged from 8.6 × 106 to 7.0 × 107 vRNA copies/ml plasma, with mean pretreatment plasma viral loads of 4.2 × 107 vRNA copies/ml and 3.1 × 107 vRNA copies/ml for the triple and quad regimen treatment groups, respectively. The addition of DRV in the quad regimen conferred no apparent increase in the kinetics of first phase viral load decline over the first 12 days of treatment when compared with the triple regimen (p = 0.26), and plasma viral loads declined by a similar amount, 4.5–5 logs, after 4 weeks on cART for both treatment groups (Fig. 2). After 4 weeks of treatment the quad treatment group was switched to the triple regimen and all animals continued to receive the triple regimen for an additional 36 weeks of treatment with plasma viral load monitoring. Durable suppression of plasma viral loads to below the limit of quantification (<30 vRNA copies/ml) was achieved in all six animals after 12–20 weeks of treatment and was maintained through 40 weeks of follow-up on cART. The animal that achieved suppression to <30 vRNA copies/ml plasma most rapidly (animal ZI69 in the triple regimen group) notably had the lowest pretreatment viral load of 8.6 × 106 vRNA copies/ml plasma. Complete blood counts (CBC) and serum chemistries were monitored throughout treatment. No systemic reactions or blood abnormalities were noted during cART treatment, and both regimens were well tolerated.
FIG. 2.
Plasma viral load changes in triple regimen-treated and quad regimen-treated animals. Plasma viral loads were determined by qRT-PCR assay with a 30-copy/ml quantification limit for six Indian-origin rhesus macaques infected with SIVmac239X and treated with the triple regimen (open gray symbols) or the quad regimen (black filled symbols) starting at 13 days postinfection (light gray shaded region). (A, B) Plasma viral loads are shown for individual study animals during the acute phase of infection and first 4 weeks of treatment with the triple regimen (A) or quad regimen (B). (C) Plasma viral loads for all six animals through 42 weeks of monitoring are shown. After 4 weeks of treatment, quad regimen-treated animals were switched to the triple regimen (dashed vertical line).
Plasma drug concentrations at 2 h postdose (C2hr) on day 1 (start of cART) and at 24 h post-dose on day 30 (i.e., steady state trough levels, Css) were determined for all regimen drugs in the six treated animals (Table 1). Consistent plasma drug concentrations were achieved for all of the coformulated compounds, with mean Css drug levels that exceeded the target levels for DRV and DTG. Importantly, peak (C2hr) plasma tenofovir levels in both treatment groups (mean = 1,704 nM both groups combined) were substantially lower than peak levels in animals treated with TFV at 20 mg/kg (mean = 36,000 nM; unpublished results) and were similar to maximum tenofovir levels measured in humans following a single oral dose of TDF.17 Because TDF can achieve higher active intracellular drug levels at lower plasma exposures than TFV,18 this lower peak plasma concentration may mitigate the potential renal toxicity associated with high dose plasma exposures of TFV while achieving therapeutic intracellular concentrations of active drug metabolites.
Although, TDF and FTC concentrations were comparable between the triple and quad regimen-treated animals, mean plasma DTG C2hr and Css levels were 3.0-fold (66%) and 1.7-fold (40%) lower, respectively, in the quad regimen-treated animals, likely due to the presence of DRV in the quad regimen as has been noted in human studies when DTG has been coadministered with DRV.19,20 Notably, trough (Css) DRV levels were higher and more consistent between animals in the quad regimen-treated macaques than in the monotherapy-treated macaques (Table 1), despite twice daily administration of DRV at comparable or higher weight-normalized doses in the monotherapy-treated animals. The higher levels and improved consistency achieved with once daily subcutaneous dosing were likely due to improved drug bioavailability and greater control over dose timing and weight-dependent dosing than is afforded by the oral route.
Although we show that DRV is active against SIV replication in infected rhesus macaques, and despite the achievement of higher and more consistent plasma DRV levels with subcutaneous administration, the addition of DRV to the coformulated TDF/FTC/DTG triple cART regimen did not lead to a measurable increase in the rate of plasma viral load decay in treated animals. Although DTG Css levels were lower in quad regimen-treated animals compared with triple regimen-treated animals, raising the possibility that the lower DTG levels may have offset an added antiviral benefit from the addition of DRV, we note that the DTG levels in quad regimen-treated animals were still ∼4-fold greater than the in vitro PAEC95 for DTG against SIV. It thus seems unlikely that the 1.7-fold reduction in DTG plasma levels with the DRV-containing quad regimen resulted in a marked reduction in the overall antiviral activity of the regimen. In either case, for these specific coformulated injectable drug regimens, the addition of DRV to the regimen does not appear to confer any additional virologic benefit.
Overall, our findings show that a two class (NRTI/INSTI), three drug cART regimen formulated for coadministration of all three drugs as a once daily subcutaneous injection can effectively and durably suppress SIVmac239 replication in Indian-origin rhesus macaques to clinically relevant levels (<30 vRNA copies/ml plasma). The feasibility of this dosing regimen, coupled with its pharmacokinetic consistency and potential for improved safety through the use of the TDF prodrug form of TFV, makes it a highly attractive regimen for studies of AIDS virus persistence and latency and for the evaluation of viral eradication strategies in a relevant in vivo model system.
Acknowledgments
The authors thank the members of the Quantitative Molecular Diagnostics Core, the Viral Evolution Core, and the Specimen Support Core of the AIDS and Cancer Virus Program as well as the nonhuman primate care staff in the Laboratory Animal Sciences Program, at Leidos Biomedical Research, Inc. We also thank Rebecca Kiser for assistance with study scheduling.
All rhesus macaques (Macaca mulatta) in this study were treated according to a protocol approved by the IACUC of the National Cancer Institute and housed and cared for in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) standards in AAALAC-accredited facilities.
This work was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Author Disclosure Statement
G.S.J., B.L., J.H., J.Z., J.H., and R.G. are employees of Gilead Sciences and may own Gilead stock and/or stock options.
References
- 1.Del Prete GQ, Lifson JD. Considerations in the development of nonhuman primate models of combination antiretroviral therapy for studies of AIDS virus suppression, residual virus, and curative strategies. Curr Opin HIV AIDS 2013;8(4):262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shytaj IL, Norelli S, Chirullo B, et al. : A highly intensified ART regimen induces long-term viral suppression and restriction of the viral reservoir in a simian AIDS model. PLoS Pathog 2012;8(6):e1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis MG, DaFonseca S, Chomont N, et al. : Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension. AIDS 2011;25(11):1347–1356 [DOI] [PubMed] [Google Scholar]
- 4.Ling B, Piatak M, Jr., Rogers L, et al. : Effects of treatment with suppressive combination antiretroviral drug therapy and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) on SIV-infected Chinese rhesus macaques. PLoS One 2014;9(7):e102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Prete GQ, Shoemaker R, Oswald K, et al. : Effect of suberoylanilide hydroxamic acid (SAHA) administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected Indian rhesus macaques. Antimicrob Agents Chemother 2014;58(11):6790–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keele BF, Li H, Learn GH, et al. : Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 2009;206(5):1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Meyer S, Vangeneugden T, van Baelen B, et al. : Resistance profile of darunavir: Combined 24-week results from the POWER trials. AIDS Res Hum Retroviruses 2008;24(3):379–388 [DOI] [PubMed] [Google Scholar]
- 8.Forthal DN, Landucci G, Cole KS, et al. : Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol 2006;80(18):9217–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitney JB, Hill AL, Sanisetty S, et al. : Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014;512(7512):74–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis MG, Norelli S, Collins M, et al. : Response of a simian immunodeficiency virus (SIVmac251) to raltegravir: A basis for a new treatment for simian AIDS and an animal model for studying lentiviral persistence during antiretroviral therapy. Retrovirology 2010;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. : Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: Summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother 2008;52(9):3144–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders-Beer BE, Spano YY, Golighty D, et al. : Clinical monitoring and correlates of nephropathy in SIV-infected macaques during high-dose antiretroviral therapy. AIDS Res Ther 2011;8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks SG, Barditch-Crovo P, Lietman PS, et al. : Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother 1998;42(9):2380–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barditch-Crovo P, Deeks SG, Collier A, et al. : Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 2001;45(10):2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morcock DR, Thomas JA, Sowder RC 2nd, et al. : HIV-1 inactivation by 4-vinylpyridine is enhanced by dissociating Zn(2+) from nucleocapsid protein. Virology 2008;375(1):148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Prete GQ, Park H, Fennessey CM, et al. : Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J Virol 2014;88(14):8077–8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004;43(9):595–612 [DOI] [PubMed] [Google Scholar]
- 18.Durand-Gasselin L, Van Rompay KK, Vela JE, et al. : Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol Pharm 2009;6(4):1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song I, Borland J, Min S, et al. : Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir. Antimicrob Agents Chemother 2011;55(7):3517–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song I, Min SS, Borland J, et al. : The effect of lopinavir/ritonavir and darunavir/ritonavir on the HIV integrase inhibitor S/GSK1349572 in healthy participants. J Clin Pharmacol 2011;51(2):237–242 [DOI] [PubMed] [Google Scholar]