Abstract
The blood–brain barrier (BBB) limits the entry of neurotoxic blood-derived products and cells into the brain that is required for normal neuronal functioning and information processing. Pericytes maintain the integrity of the BBB and degenerate in Alzheimer’s disease (AD). The BBB is damaged in AD, particularly in individuals carrying apolipoprotein E4 (APOE4) gene, which is a major genetic risk factor for late-onset AD. The mechanisms underlying the BBB breakdown in AD remain, however, elusive. Here, we show accelerated pericyte degeneration in AD APOE4 carriers >AD APOE3 carriers >non-AD controls, which correlates with the magnitude of BBB breakdown to immunoglobulin G and fibrin. We also show accumulation of the proinflammatory cytokine cyclophilin A (CypA) and matrix metalloproteinase-9 (MMP-9) in pericytes and endothelial cells in AD (APOE4 >APOE3), previously shown to lead to BBB breakdown in transgenic APOE4 mice. The levels of the apoE lipoprotein receptor, low-density lipoprotein receptor-related protein-1 (LRP1), were similarly reduced in AD APOE4 and APOE3 carriers. Our data suggest that APOE4 leads to accelerated pericyte loss and enhanced activation of LRP1-dependent CypA–MMP-9 BBB-degrading pathway in pericytes and endothelial cells, which can mediate a greater BBB damage in AD APOE4 compared with AD APOE3 carriers.
Keywords: Alzheimer’s, blood–brain barrier, neurodegeneration, neurovascular unit, pericytes
Introduction
A neurovascular unit is formed by vascular endothelial cells, pericytes, glial cells (astrocytes, miocroglia), and neurons.1,2 Endothelial cells and perivascular mural cells, pericytes, form the blood–brain barrier (BBB) in the dense microvasculature networks responsible for molecular exchange. The BBB limits entry into the brain of potentially toxic blood-derived products and larger molecules such as peptides and proteins,3,4,5 but allows controlled carrier-mediated bidirectional transendothelial transport of nutrients, such as glucose and amino acids from blood to brain and from brain to blood.1 The cross-talk between different neurovascular unit cell types controls multiple central nervous system functions including regulation of cerebrovascular and BBB integrity and cerebral blood flow. Recent studies in murine transgenic models have shown that pericytes have a critical role in maintaining the BBB integrity, and that loss of pericytes can lead to a long-term BBB breakdown and small vessel disease contributing to neurodegenerative changes.6–9
Pericytes degenerate in Alzheimer’s disease (AD).10–12 Multiple studies have demonstrated loss of cerebrovascular integrity and/or BBB damage in AD12–25 that is accelerated by the apolipoprotein E4 (APOE4) genotype.15,17,22–24 Human apoE has three major apoE isoforms: E2, E3, and E4.26,27 Genetic evidence including recent genome-wide association studies shows that APOE4 is the strongest and most highly replicated genetic risk factor for late-onset AD. Individuals with one copy of APOE4 have a 3.7-fold increase in AD risk and individuals with two copies of APOE4 a 12-fold increase in AD risk relative to APOE3/E3 individuals.26,27 Studies using murine transgenic models have shown that APOE4 increases BBB susceptibility to injury28 and leads to BBB breakdown and microvascular reductions in humanized transgenic APOE4 mice compared with APOE3 mice.29,30 The mechanisms underlying the BBB breakdown in AD, particularly in APOE4 carriers remain, however, elusive.
Recent studies in transgenic APOE2 and APOE3 mice have shown that astrocyte-secreted apoE2 and apoE3 maintain the BBB integrity by suppressing the proinflammatory cyclophilin A (CypA)–metalloproteinase-9 (MMP-9) pathway in pericytes via low-density lipoprotein receptor-related protein-1 (LRP1),29 that is a major apoE receptor.26,27 In contrast, astrocyte-secreted apoE4 fails to effectively suppress CypA–MMP-9 pathway in APOE4 transgenic mice leading to MMP-9-mediated degradation of the BBB tight junction and basement membrane proteins, which causes BBB breakdown.29 Moreover, a recent study in cognitively normal human APOE4 carriers compared with APOE3 carriers has shown an age-dependent increase in CypA and active MMP-9 levels in the cerebrospinal fluid (CSF) suggestive of activation of the CypA–MMP-9 pathway that correlated with increased CSF/plasma albumin ratio indicating BBB breakdown.23 Using postmortem human brain tissue analysis of biomarkers of BBB breakdown, here we show that APOE4 compared with APOE3 accelerates pericyte loss and microvascular reductions in AD, which correlates with the magnitude of BBB breakdown to plasma proteins immunoglobulin G and fibrin. We then show that APOE4 compared with APOE3 leads to a greater accumulation of CypA and MMP-9 in pericytes and endothelial cells in AD suggestive of an enhanced activation of LRP1-dependent CypA–MMP-9 BBB-degrading pathway, which in addition to pericyte loss may contribute to accelerated BBB breakdown in AD APOE4 compared with AD APOE3 carriers.
Materials and Methods
Human Postmortem Tissue Samples
Postmortem paraffin-embedded human frontal cortex tissue samples (Brodmann area 9/10) were obtained from the University of Southern California (USC) Alzheimer’s disease Research Center (ADRC) in accordance with institutional guidelines governed by approved protocols. Informed consent was obtained from all the participants before death, and the tissue collection was approved by the Institutional Review Board (IRB) through the USC ADRC. All the autopsy cases underwent neuropathologic evaluation of AD according to the Consortium to Establish a Registry for Alzheimer’s disease and by the National Institute on Aging and Reagan Institute criteria including assignment of the Braak stage (measure of number and distribution of neurofibrillary tangles). Aged subjects that did not carry the diagnosis of AD or another neurodegenerative disease and showed neuropathologic findings within the normal range for age were used as age-matched controls. Clinical dementia rating (CDR) and Mini-Mental State Examination were available for most but not all individuals. A total of nine controls and 18 AD individuals were used for histopathologic analyses. See Table 1 for detailed demographic data. The postmortem interval (PMI) typically ranged between 3 and 10 hours, but individuals with shorter PMI of 1 and 2 hours (two cases), and somewhat longer PMI of 12 to 13 hours (two cases) were also included in our cohort of n = 27 cases total. The incidence of vascular risk factors (e.g., hypertension, atherosclerosis, etc), the gender ratio, age, and the PMI were comparable between age-matched controls and AD patients. The cause of death in both the groups was either cardiac or respiratory arrest.
Table 1.
Demographic data.
| Case ID | Diagnosis | APOE | Age | Gender | PMI (hours) | Vascular risk factors | Braak | CERAD | CDR | MMSE | Disease duration (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 824 | Control | E2/E3 | 87 | F | 7.75 | Hypertension | 0 | Negative | 0 | 30 | NA |
| 870 | Control | E3/E3 | 95 | F | 9 | Hypertension | II | Negative | 1 | NA | NA |
| 903 | Control | E3/E3 | 80 | F | 3.75 | None | I | Negative | 0 | NA | NA |
| 861 | Control | E3/E3 | 74 | F | 10 | None | I | Negative | 0 | 18 | NA |
| 845 | Control | E3/E3 | 90 | F | 5 | Atherosclerosis, Hypertension | I | Negative | 0 | 30 | NA |
| 907 | Control | E3/E3 | 84 | M | 2 | None | I | Negative | 0 | 28 | NA |
| 10473384 | Control | E3/E3 | 84 | M | 5 | Hypertension | III | Negative | NA | 26 | NA |
| 20993308 | Control | E3/E3 | 83 | F | 1 | None | I | Negative | NA | 26 | NA |
| 11072071 | Control | E3/E3 | 88 | M | 8.5 | Hypertension | II | Negative | NA | 27 | NA |
| 427 | AD | E2/E3 | 74 | M | 9 | Atherosclerosis, CAA | V | Frequent | 3 | NA | 15 |
| 787 | AD | E3/E3 | 86 | F | 6 | Atherosclerosis, Hypertension | III | Moderate | NA | NA | Unknown |
| 800 | AD | E3/E3 | 86 | F | 5 | Atherosclerosis, CAA | V | Frequent | 3 | NA | 9 |
| 896 | AD | E3/E3 | 83 | F | 13.25 | Atherosclerosis | V–VI | Moderate | 2 | 0 | 5 |
| 890 | AD | E3/E3 | 71 | M | 5.5 | None | V–VI | Moderate | 2 | 8 | 4 |
| 816 | AD | E3/E3 | 96 | F | 12 | Atherosclerosis, CAA | V | Frequent | NA | NA | Unknown |
| 785 | AD | E3/E4 | 99 | F | 12.25 | Atherosclerosis | III | Moderate | 2 | NA | 7 |
| 902 | AD | E3/E4 | 91 | F | 3.25 | Atherosclerosis | V–VI | Frequent | 1 | 6 | 3 |
| 825 | AD | E3/E4 | 68 | F | 9.75 | Hypertension | III | Moderate | 1 | 15 | 4 |
| 900 | AD | E3/E4 | 87 | M | 5.5 | Atherosclerosis, CAA | V–VI | Frequent | 2 | 19 | Unknown |
| 796 | AD | E3/E4 | 76 | M | 7 | Atherosclerosis, CAA | V | Moderate | 3 | 0 | 2 |
| 867 | AD | E3/E4 | 89 | F | 8.25 | Hypertension | III–IV | Frequent | 2 | 17 | Unknown |
| 407 | AD | E4/E4 | 70 | F | 5 | Atherosclerosis, Hypertension | III | Moderate | NA | NA | Unknown |
| 689 | AD | E4/E4 | 78 | F | 7 | None | V | Moderate | 2 | NA | 8 |
| 719 | AD | E4/E4 | 82 | F | 4.25 | Atherosclerosis, CAA | V–VI | Frequent | 2 | NA | 10 |
| 905 | AD | E4/E4 | 81 | M | 8.5 | Atherosclerosis | III | Moderate | 2 | 11 | 5 |
| 495 | AD | E4/E4 | 75 | M | 9.5 | Hypertension | III–IV | Moderate | NA | NA | Unknown |
| 728 | AD | E4/E4 | 86 | M | 10 | Atherosclerosis, CAA | IV | Moderate | 1 | NA | Unknown |
Abbreviations: AD, Alzheimer’s disease; APOE, apolipoprotein E; CAA, cerebral amyloid angiopathy; CDR, clinical dementia rating; CERAD, Consortium to Establish a Registry for Alzheimer’s disease; F, female; M, male; MMSE, Mini-Mental State Examination; NA, not available; PMI, postmortem interval.
Exclusion Criteria
In this study we excluded any patients with the following conditions: frontotemporal dementia, Lewy body dementia, vascular dementia, Parkinson’s disease dementia, stroke, brain cancer, mental retardation, severe depression, and/or severe weakness leading to invalid mental health assessment.
Tissues Immunofluorescent Staining
For all analyses, formalin-fixed, paraffin-embedded brain tissue samples were used. All tissue was cut to a thickness of 10 µm. Sections were deparrafinized with xylene and rehydrated to distilled water after serial ethanol washes. Subsequently, sections were incubated in 1:10 diluted target antigen retrieval solution, pH 9 (Dako, Carpinteria, CA, USA) for 20 minutes at 95°C. The tissue sections were blocked in 5% donkey serum (Jackson ImmunoResearch, West Grove, PA, USA) containing 0.05% Triton X-100 (Sigma-Aldrich, St Louis, MO, USA) and then incubated with the following primary antibodies overnight at 4°C: goat anti-human PDGFRβ (1:100, R&D Systems, Minneapolis, MN, USA) to detect pericyte marker PDGFRβ,3–6 rabbit anti-human fibrinogen (1:500, Dako), goat anti-human immunoglobulin G (IgG) (1:100, R&D Systems), monoclonal rabbit anti-human LRP-1 (1:100, Abcam, EPR3724, Cambridge, MA, USA), rabbit anti-human CypA (1:500, Abcam, ab42408), rabbit anti-human MMP-9 (1:500, Abcam, ab38898). To visualize brain endothelial vascular profiles, sections were incubated with Dylight 488-conjugated Lycopersicon esculentum lectin (Vector Laboratories, Burlingame, CA, USA; DL-1174; 1:200) for 1 hour at room temperature during the secondary antibody incubation step. Alexa Fluor 568- or 633-conjugated donkey anti-goat secondary antibodies (1:200, Life Technologies, Grand Island, NY, USA) were used to detect goat anti-human PDGFRβ on pericytes.3–6 To visualize fibrin and IgG accumulations, sections were incubated with 568-conjugated donkey anti-rabbit (1:200, Life Technologies) and 568-conjugated donkey anti-goat (1:200, Life Technologies) secondary antibodies, respectively. To visualize LRP-1, CypA, and MMP-9, tissue sections were incubated with Alexa Fluor 568-conjugated donkey anti-rabbit antibody (1:200, Life Technologies). All secondary antibodies were incubated for 1 hour at room temperature. Sudan black-based Autofluorescence Eliminator Reagent (EMD Millipore, Billerica, MA, USA Cat. #2160) was applied to the samples following the manufacturer’s protocol to reduce the tissue autofluoresence before imaging. Tissue sections were coverslipped using fluorescent mounting media (Dako).
Confocal Microscopic Analysis
All coverslipped fluorescently mounted tissue sections were scanned using a custom-built Zeiss 510 meta-confocal laster scanning microscope with a Zeiss Apochromat 259/0.8 NA water immersion objective (Carl Zeiss MicroImaging, Thornwood, NY, USA). A 488-nm argon laser was used to excite Alexa Fluor 488 and DyLight 488 and the emission was collected through a 500- to 550-nm band pass filter. A 543-nm HeNe laser was used to excite Alexa Fluor 568 and the emission was collected through a 560- to 615-nm band pass filter. A 633-nm HeNe laser was used to excite DyLight 649 and the emission was collected through a 650- to 700-nm band pass filter.
Quantitative Image Analysis
All the images were analyzed using NIH-developed ImageJ software (Bethesda, MD, USA). A field size of 420 µm × 420 µm and a maximum z-projection of 8 µm were utilized for all the images. For each analysis described below, five randomly selected fields per section from three non-adjacent tissue sections 100 µm apart per specimen were analyzed.
Pericyte coverage and numbers
The quantification analysis of pericyte coverage and numbers was restricted to PDGFRβ-positive mural cells that were associated with brain capillaries defined as microvessels <6 µm in diameter, as previously described.8,12,29–32 The endothelial-specific L. esculentum lectin was used to visualize endothelial vascular profiles, as previously reported.8,12,29,31 The PDGFRβ coverage of brain capillaries was determined as PDGFRβ-positive area (percentage) occupying lectin-positive endothelial capillary profiles, as we and others have previously described.8,8,29,31,33 Pericyte numbers were determined by counting the number of PDGFRβ-positive cell bodies on the abluminal side of the endothelial membrane (lectin) that colocalized with DAPI (4′,6-diamidino-2-phenylindole)-positive nuclei using the ImageJ Cell Counter plug-in, as we and others previously described.6,31–33 The number of pericytes was expressed per mm2 of tissue.
Microvascular capillary length
Microvascular capillary length was quantified as the length of lectin-positive endothelial profiles (<6 µm in diameter) determined using the ImageJ plug-in length analysis tool from 15 images per specimen derived from five randomly selected fields in the cortex (420 µm × 420 µm) per section from three non-adjacent (∼100 µm apart) sections per specimen, as we described.8,29,32 The length was expressed in millimeter of lectin-positive vascular profiles per mm3 of brain tissue.
Extravascular leakages
The levels of extravascular blood-derived fibrin and IgG indicating BBB breakdown were measured as previously described.8,12,29,31 Briefly, fibrin-positive and/or IgG-positive extravascular signal was obtained by subtracting fibrin and/or IgG signal colocalized with the lectin-positive signal from total fibrin and/or IgG-positive area, yielding a value that reflects only extravascular accumulation of each plasma-derived protein.
LRP1, CypA, and MMP-9 colocalization with PDGFRβ-positive pericytes and lectin-positive endothelial profiles
To quantify the area positive for LRP1, CypA, and MMP-9 in pericytes and brain endothelial cells, the LRP1-positive, CypA-positive, and MMP-9-positive signal (percentage) occupying PDGFRβ-positive pericyte area and/or lectin-positive brain endothelial profiles were determined. The presence of LRP1, CypA, and MMP-9 immunoreactivity in PDGFRβ-positive pericytes and lectin-positive endothelium was determined in single confocal planes. Only single-positive areas were used for the quantification. We utilized a plug-in in NIH ImageJ software, which quantifies and summates colocalized immunofluorescent signal in each individual image—instead of the projection—which is included in a z-stack. This maximizes the specificity allotted to us by confocal microscopy and only includes colocalized signal in a single plane. This approach limits the potential confounding effect of colocalization analysis on maximum projections of confocal stacks that would fail to unambiguously determine the cellular origin as immunofluorescent staining from above and/or below the cell of interest would artifactually appear to colocalize.
Post-image thresholding
To account for background fluorescence and nonspecific staining, we have meticulously optimized blocking and staining conditions, including but not limited to tissue preparation, standard operating procedures for use of primary and secondary antibodies, and antigen retrieval, to maximize specific tissue staining while decreasing background signal. All slides are stained and processed in batch with identical primary and secondary antibodies dilutions, lot number, incubation times, and laser settings as explained above. To minimize the background autofluoresence, we used an additional incubation step with Sudan black reagent that was applied to all control and AD tissue specimens as described above. To account for nonspecific background staining, tissue sections from control and AD specimens were incubated with different sets of the respective secondary antibodies for 1 hour at room temperature as described above, but no primary antibodies. The nonspecific staining is then measured using NIH ImageJ area analysis tool, and then subtracted from the raw signal from the respective immunofluorescent channel. Thus, the thresholded images were generated by subtracting the background staining from the raw images. Importantly, the thresholded images for all studied molecules or cell-biomarkers did not differ in any appreciable manner from the raw images as the background staining for various secondary antibodies ranged between 3 and 5% of the total signal on the raw images.
All the images were analyzed by a masked investigator.
Statistical Analysis
Sample sizes were calculated using nQUERY assuming a two-sided alpha level of 0.05, 80% power, and homogenous variances for the two samples to be compared. Using the means and common standard deviation for different parameters predicted from our published data8,12,29,31,32 and pilot studies, the sample sizes to detect the differences between AD and non-AD groups ⩾25% varied between 5 and 8. Our actual sample sizes for different cellular and molecular biomarkers varied between 6 and 12, thus satisfying a reliable measurement of the predicted effect as defined above. The F-test was conducted to verify that the samples are normally distributed and have homogenous variances. The variances of the respective samples compared between the groups were statistically similar. All data represent the average±s.e.m. For multiple comparisons, a one-way analysis of variance followed by Tukey’s post hoc test was used. A P value <0.05 was considered statistically significant. Simple linear regression and Pearson correlation coefficient were used to determine the correlation between variables using GraphPad Prism 3.0 software.
Results
In all the studies, we compared the biomarkers of BBB breakdown in the cortical tissue (Brodmann area 9/10) derived from nine age-matched non-AD controls, six AD APOE3/APOE3 carriers (APOE3 group), six AD APOE3/APOE4 carriers and six AD APOE4/APOE4 carriers (see Table 1). For the statistical analysis, the AD cases with one and two APOE4 alleles were pooled together in a single APOE4 group (n = 12), as we did not find a significant effect between the two versus one APOE4 allele for the vast majority of the studied BBB biomarkers using the present relatively small cohort of APOE4 carriers.
Accelerated Pericyte Degeneration and Microvascular Reductions in Alzheimer’s Disease Apolipoprotein E4 Carriers
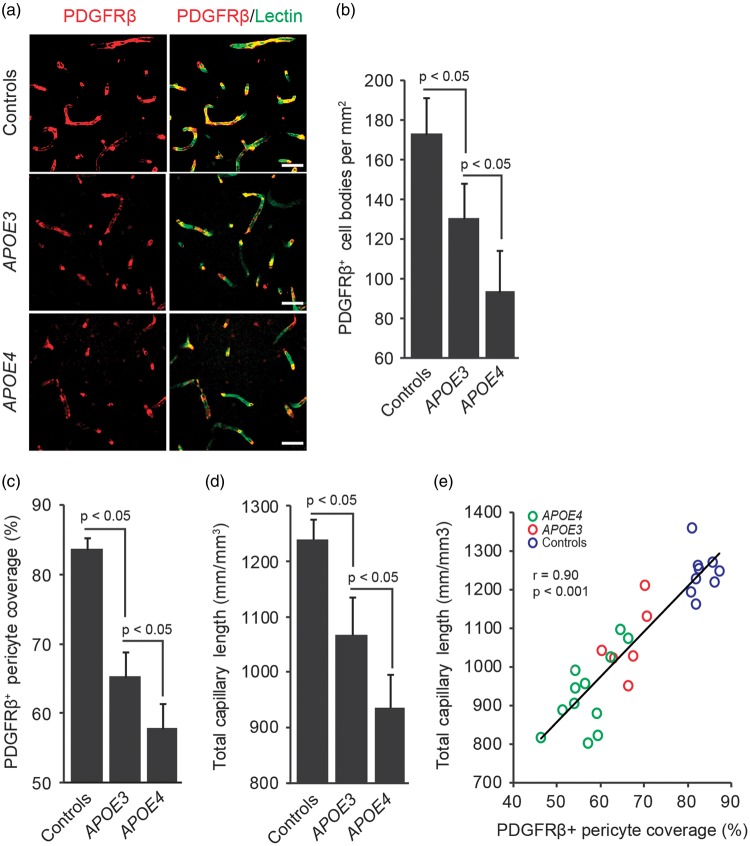
Figures 1A to 1C illustrate that the numbers of PDGFRβ-positive pericytes and pericyte coverage of the capillary wall are reduced in AD APOE3 carriers compared with non-AD controls by 30% and 23%, respectively, and in AD APOE4 carriers compared with AD APOE3 carriers by 31% and 15%, respectively. Supplementary Figure 1 illustrates PDGFRβ-positive pericyte cell bodies that were used for calculations of pericyte numbers shown in Figure 1B. Confocal microscopic analysis of lectin-positive brain endothelial profiles detected significant reductions in the total capillary length in AD APOE3 carriers compared with non-AD controls by 14%, and in AD APOE4 carriers compared with AD APOE3 carriers by an additional 20% (Figure 1D).
Figure 1.
Accelerated pericyte degeneration and microvascular reductions in APOE4 carriers with Alzheimer’s disease. (A) Representative confocal microscopic analysis of PDGFRβ-positive mural cells (red) and lectin-positive endothelial profiles (green) demonstrating mural cell coverage of brain capillaries (yellow-merged) in the frontal cortex of an age-matched neurologically intact control (top), an AD APOE3/APOE3 patient (middle), and an AD APOE4/APOE3 patient (bottom). Scale bars, 25 µm. (B) Quantification of PDGFRβ-positive cell bodies in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group expressed per mm2 of tissue section. See Supplementary Figure 1 for representative images of PDGFRβ+/DAPI+ pericyte cell bodies. (C) Quantification of PDGFRβ-positive pericyte coverage of lectin-positive vascular endothelial profiles in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group. (D) Quantification of total length of lectin-positive brain endothelial profiles in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group. (E) Positive correlation between total length of lectin-positive endothelial vascular profiles and PDGFRβ-positive pericyte coverage of brain capillaries in the frontal cortex of neurologically intact controls (blue), AD APOE3 patients (red), and AD APOE4 patients (green; n = 27). Single data points are from all studied controls and AD patients (n = 27). Mean±s.e.m., n = 9 for non-AD control; n = 6 for AD APOE3 group; and n = 12 for AD APOE4 group. r = Pearson’s coefficient. AD, Alzheimer’s disease; APOE, apolipoprotein E; DAPI, 4′,6-diamidino-2-phenylindole.
As reported previously,8,12,31 reductions in the pericyte coverage correlated positively with the reductions in total capillary length in the studied AD patients and controls as shown by individual single point data regression analysis (Figure 1E).
Accelerated Blood–Brain Barrier Breakdown in Alzheimer’s Disease Apolipoprotein E4 Carriers Correlates with Pericyte Degeneration
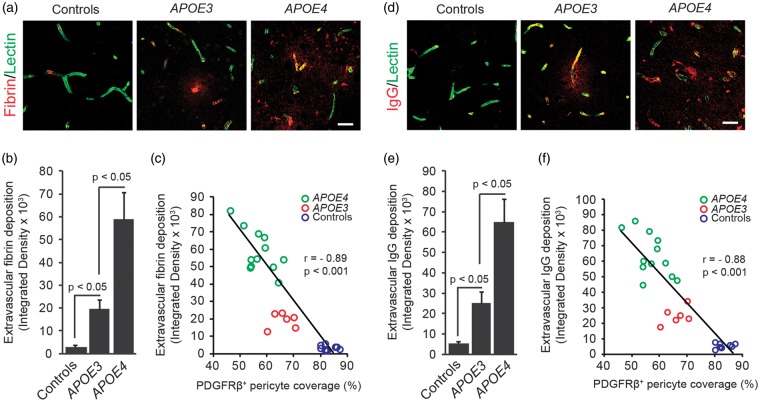
To determine the degree of BBB breakdown, we next determined the levels of fibrin and IgG extravascular accumulations depicting vascular capillary leakages. Figures 2A and 2B, show a 6.9-fold increase in fibrin perivascular deposits in AD APOE3 carriers compared with non-AD controls, and a 3.1-fold increase in AD APOE4 carriers compared with AD APOE3 carriers. There was a significant (P < 0.001) correlation between the loss of pericyte coverage and the magnitude of fibrin deposits in AD APOE3 and AD APOE4 carriers and non-AD controls (Figure 2C). We also found increases in the IgG extravascular accumulation by 5.3-fold and 2.6-fold in AD APOE3 carriers compared with non-AD controls, and in AD APOE4 compared with AD APOE3 carriers, respectively (Figures 2D and 2E). As for fibrin, there was a significant (P < 0.001) correlation between the loss of pericyte coverage and the magnitude of IgG extravascular deposits (Figure 2F). In addition, we found a negative correlation between loss of pericyte coverage and Braak stage or CDR, i.e., the greater the loss of pericyte coverage, the greater the Braak stage and the CDR, and a positive correlation between IgG extravascular deposits and Braak stage and CDR, i.e., the greater the IgG leakage, the greater the Braak stage and the CDR, as shown in Supplementary Figures 2A to 2D.
Figure 2.
Increased fibrin and immunoglobulin G (IgG) extravascular deposits in APOE4 carriers with Alzheimer’s disease correlates with pericyte degeneration. (A) Representative confocal microscopic analysis of fibrin extravascular deposits (red) and lectin-positive endothelial profiles (green) in the frontal cortex of an age-matched neurologically intact control (left), an AD APOE3/APOE3 patient (middle), and an AD APOE4/APOE3 patient (right). Scale bar, 25 µm. (B) Quantification of fibrin extravascular fibrin in the frontal cortex in neurologically intact controls, AD APOE3 group, and AD APOE4 group. (C) Negative correlation between fibrin extravascular deposits and PDGFRβ-positive pericyte coverage of brain capillaries in the frontal cortex of neurologically intact controls (blue), AD APOE3 patients (red), and AD APOE4 patients (green). Single data points are from all controls and AD patients (n = 27). (D) Representative confocal microscopic analysis of IgG extravascular deposits (red) and lectin-positive endothelial profiles (green) in the frontal cortex of an age-matched neurologically intact control (left), an AD APOE3/APOE3 patient (middle), and an AD APOE4/APOE3 patient (right). Scale bar, 25 µm. (E) Quantification of IgG extravascular deposits in the frontal cortex in neurologically intact controls, AD APOE3 group, and AD APOE4 group. (F) Negative correlation between IgG extravascular deposits and PDGFRβ-positive pericyte coverage of brain capillaries in the frontal cortex of neurologically intact controls (blue), AD APOE3 patients (red), and AD APOE4 patients (green). Single data points are from all controls and AD patients (n = 27). Mean±s.e.m., n = 9 for non-AD control; n = 6 for AD APOE3 group; and n = 12 for AD APOE4 group. r = Pearson’s coefficient. AD, Alzheimer’s disease; APOE, apolipoprotein E.
Accumulation of Cyclophilin A and Matrix Metalloproteinase-9 in Pericytes and Endothelium in Alzheimer’s Disease Apolipoprotein E4 Carriers
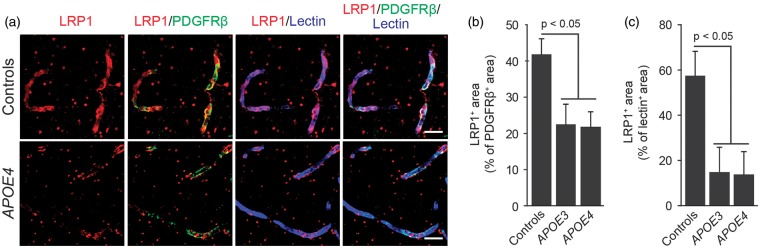
To better understand possible molecular mechanisms contributing to accelerated BBB breakdown in APOE4 carriers, we next studied the relative levels of proteins involved in the LRP1–CypA–MMP-9 pathway previously shown to control the BBB integrity in transgenic APOE3 and APOE4 mice.29 Our data show that LRP1 levels in pericytes and brain endothelial cells of the BBB are similarly reduced irrespectively of the APOE genotype in AD APOE3 and AD APOE4 patients compared with controls by approximately 55% in pericytes and 74% in endothelial cells, as shown by triple immunostaining analysis for LRP1, pericyte marker PDGFRβ, and endothelial-specific lectin, respectively (Figures 3A to 3C). A substantial reduction in LRP1 endothelial levels found in this study is consistent with a previous report of 75% reduction in LRP1 brain endothelial level in AD patients compared with non-AD controls determined with Von Willebrand Factor as an endothelial-specific marker.34
Figure 3.
Lipoprotein receptor-related protein 1 (LRP1) levels are reduced in pericytes and brain endothelial cells in Alzheimer’s disease irrespective of APOE genotype. (A) Representative confocal microscopic analysis of LRP-1 immunodetection (red), and colocalization of LRP-1 (red) with PDGFRβ-positive pericytes (green, yellow-merged) and lectin-positive endothelial profiles (blue, magenta-merged) in the frontal cortex of an age-matched neurologically intact control (top) and an AD APOE4/APOE3 patient (bottom). Scale bar, 20 µm. (B) Quantification of LRP-1-positive signal colocalizing with PDGFRβ-positive pericytes in the frontal cortex of neurologically intact controls, AD APOE3 group and AD APOE4 group. (C) Quantification of LRP-1-positive signal colocalizing with lectin-positive brain endothelial cells in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group. Mean±s.e.m., n = 9 for non-AD control; n = 6 for AD APOE3 group; and n = 12 for AD APOE4 group. AD, Alzheimer’s disease; APOE, apolipoprotein E.
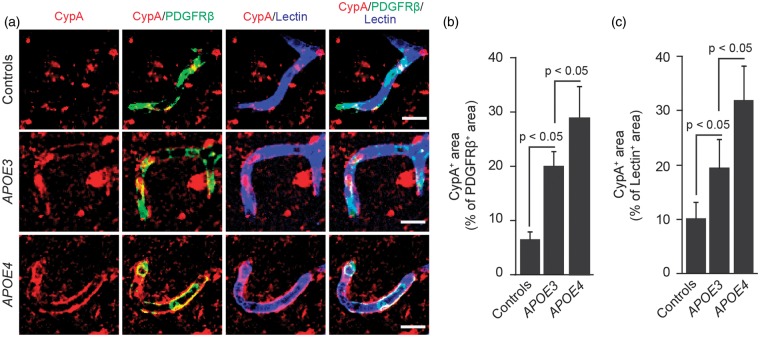
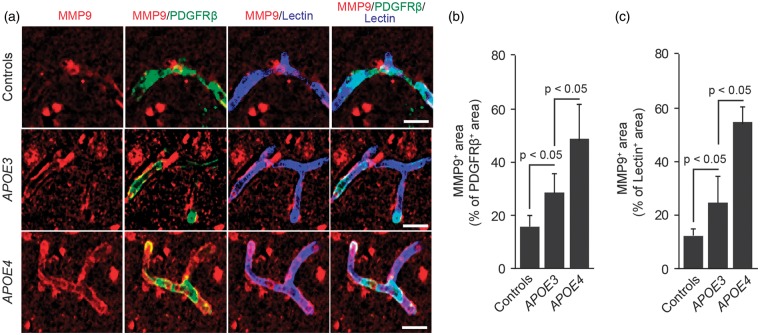
Figures 4A to 4C illustrates accumulation of CypA in pericytes and endothelial cells in AD APOE3 carriers compared with non-AD controls by 3.4-fold and 1.4-fold, respectively, and in AD APOE4 carriers compared with AD APOE3 carriers by approximately 46% and 70%, respectively. We also found an increase in MMP-9 levels in pericytes and endothelial cells in AD APOE3 carriers compared with non-AD controls by 32% and 79%, respectively, and in AD APOE4 carriers compared with AD APOE3 carriers by approximately 82% and 132%, respectively (Figures 5A to 5C). In addition, we found that increased levels of CypA in pericytes correlate positively with Braak stage and/or CDR, i.e., the greater the CypA levels, the greater the Braak stage and the CDR, as shown in Supplementary Figures 3A to 3D. However, there was no statistically significant correlation between MMP-9 levels in vascular cells and Braak stage or CDR (not shown).
Figure 4.
Cyclophilin A levels are elevated in pericytes and brain endothelial cells in APOE4 carriers with Alzheimer’s disease. (A) Representative confocal microscopic analysis of cyclophilin A (CypA) immunodetection (red) and colocalization of CypA (red) with PDGFRβ-positive pericytes (green, yellow-merged) and lectin-positive endothelial profiles (blue, magenta-merged) in the frontal cortex of an age-matched neurologically intact control (top), an AD APOE3/APOE3 patient (middle), and an AD APOE4/APOE3 patient (below). Scale bar, 15 µm. (B) Quantification of CypA-positive signal colocalizing with PDGFRβ-positive pericytes in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group. (C) Quantification of CypA-positive signal colocalizing with lectin-positive brain endothelial cells in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group. Mean ± s.e.m., n = 9 for non-AD control; n = 6 for AD APOE3 group; and n = 12 for AD APOE4 group. AD, Alzheimer’s disease; APOE, apolipoprotein E.
Figure 5.
Matrix metalloproteinase-9 levels are elevated in pericytes and brain endothelial cells in APOE4 carriers with Alzheimer’s disease. (A) Representative confocal microscopic analysis of matrix metalloproteinase-9 (MMP-9) immunodetection (red) and colocalization of MMP-9 (red) with PDGFRβ-positive pericytes (green, yellow-merged) and lectin-positive brain endothelial cells (blue, magenta-merged) in the frontal cortex of an age-matched neurologically intact control (top), an AD APOE3/APOE3 patient (middle), and an AD APOE4/APOE3 patient (below). Scale bar, 15 µm. (B) Quantification of MMP-9-positive signal colocalizing with PDGFRβ-positive pericytes in the frontal cortex of neurologically intact controls, AD APOE3 group, and AD APOE4 group. (C) Quantification of MMP-9-positive signal colocalizing with lectin-positive brain endothelial cells in the frontal cortex of neurologically intact controls, AD APOE3 group and AD APOE4 group. Mean ± s.e.m., n = 9 for non-AD control; n = 6 for AD APOE3 group; and n = 12 for AD APOE4 group.
Interestingly, we found positive correlation between loss of pericyte coverage and loss of LRP1, as well as negative correlations between loss of pericyte coverage and levels of CypA and MMP-9 in pericytes, i.e., the greater the loss of pericyte coverage, the greater the levels of CypA and MMP-9 (Supplementary Figure 4). These findings suggest that loss of pericytes could be related to activation of CypA–MMP-9 pathway, although the nature of this relationship cannot be revealed by the postmortem tissue analysis and would require additional experimental studies in transgenic APOE4 mice.
Discussion
In this study we show that AD APOE4 carriers develop accelerated pericyte degeneration and BBB breakdown compared with AD APOE3 carriers, whereas AD APOE3 carriers show a significantly greater loss of pericytes compared with non-AD controls, which correlates with the magnitude of BBB breakdown to the studied plasma proteins fibrin and IgG. These findings support previous studies demonstrating BBB damage in AD12–25 and suggest that APOE genotype influences the degree of BBB damage.15,17,22–24 The present study extends, however, previous findings by showing that a greater BBB disruption in AD APOE4 compared with AD APOE3 carriers could be related at least, in part, to a greater loss of pericyte population that has been shown to lead to a small vessel disease and a chronic BBB breakdown in murine transgenic models6–9 and is associated with BBB breakdown in human neurodegenerative disorders such as AD10–12 and amyotrophic lateral sclerosis.31
At the molecular level, we show increased accumulation of CypA and MMP-9 in pericytes and endothelial cells in AD APOE4 carriers compared with AD APOE3 carriers, which, in turn, develop greater levels of CypA and MMP-9 in the BBB vascular cells compared with non-AD controls. In APOE4 transgenic mice, activation of CypA–MMP-9 pathway in pericytes has been shown to mediate BBB breakdown by degrading the BBB tight junction proteins ZO-1, occludin, and claudin-5 and the basement membrane protein of the capillary wall, collagen IV, that are all substrates for MMP-9.29 ApoE3, but not apoE4, effectively inhibits CypA–MMP-9 pathway in pericytes in vitro and in transgenic APOE3 mice in vivo acting through LRP1, which leads to LRP1-dependent transcriptional suppression of CypA and a subsequent transcriptional MMP-9 inhibition, as reported.29 Compared with apoE3, apoE4 interacts weakly with LRP1 on vascular cells,29,35 which leads to a loss of inhibition of CypA–MMP-9 pathway and its activation over time causing a progressive age-dependent BBB breakdown as shown in transgenic APOE4 mice.29
LRP1 levels are reduced in brain capillaries in AD35,36 that is not influenced by APOE genotype as we show in the present study by demonstrating a comparable loss of LRP1 from brain capillaries in AD APOE3 and AD APOE4 carriers. On the basis of previous findings in transgenic APOE3 and APOE4 mice and cultured vascular cells,29 one would expect to see that diminished brain capillary LRP1 levels in AD APOE3 patients compared with non-AD APOE3 controls lessens the ability of apoE3 to inhibit CypA–MMP-9 pathway, which, in turn, results in elevated CypA and MMP-9 levels in vascular cells, as shown in this study. This likely contributes to BBB breakdown in AD APOE3 patients similar to that reported in transgenic APOE3 mice after LRP1 silencing.29 The degree of BBB breakdown in AD APOE3 carriers was, however, less pronounced than in AD APOE4 carriers in spite of comparable LRP1 reductions. As a much weaker ligand for LRP1 in vascular cells, apoE4 compared with apoE329 is likely much less effective in inhibiting LRP1-dependent CypA–MMP-9 pathway in human AD vasculature with diminished LRP1 further elevating CypA and MMP-9 causing a greater BBB breakdown, as found in the present study, and reported in transgenic APOE4 mice.29 Thus, diminished LRP1 levels at the BBB probably generates a double hit in AD APOE4 patients with weak ligand–receptor affinity and receptor downregulation, whereas AD APOE3 patients have a single hit and only the receptor downregulation.
Interestingly, we also found that loss of pericyte coverage, extravascular accumulations of plasma-derived proteins (i.e., IgG) and increased CypA levels, but not MMP-9, in pericytes correlate with Braak stage and cognitive impairment (i.e., CDR). These data may suggest a possible role of BBB breakdown in the development of neuropathology and dementia in AD. However, human postmortem studies reflect end-stage disease and therefore cannot determine the exact time course of the BBB breakdown, CypA and MMP-9 changes in vascular cells, and/or APOE genotype, and whether these changes precede and/or contribute to neurodegeneration. Future longitudinal imaging studies in the living human brain in APOE4 carriers and non-carriers are needed to address these important questions. Ideally, such studies should combine measurements of regional BBB integrity, particularly in the hippocampus, as recently reported,37 with serial CSF biomarkers analyses of vascular/BBB and/or other cell-specific injury, and brain connectivity and structural changes, and neurophsychological testing.38
Elevated CSF CypA and active MMP-9 levels, and an increase in albumin CSF/plasma ratio suggestive of BBB breakdown have been shown in living older cognitively normal APOE4/APOE3 carriers compared with the corresponding APOE3/APOE3 and APOE2/APOE3 carriers with no cognitive impairment,23 suggesting that APOE4 allele compared with APOE3 and/or APOE2 leads to increased BBB permeability during cognitively normal aging. In addition, a recent study on the basis of postmortem brain tissue analysis reported that CypA mRNA levels are reduced in APOE2 carriers further supporting the role of CypA in regulating neurovascular function.39
On a technical note, an earlier study reported decreased absolute values for the capillary length density in the cortex in control subjects.40 A number of factors might have contributed to discrepant results with the present study such as to name a few, use of a different molecular marker to determine capillary length, i.e., endothelial-specific lectin versus the basement membrane protein collagen IV, different thickness of tissue sections, i.e., 10 µm versus 50 µm that can potentially affect differential penetration of primary antibodies into the respective tissue specimens, differences in the Brodman areas studied, different PMI, i.e., 3 to 10 hours versus 14 to 24 hours, and a different quantification method, i.e., 15 images per specimen derived from five fields per section in three adjacent sections 100 µm apart per specimen versus stereological measurements, in the present versus earlier study,40 respectively. The source of this discrepancy and/or the relative contributions of the factors listed above remain unclear. Importantly, the use of lectin-positive endothelial profiles to determine capillary length as in the present study has been validated with another endothelial marker CD31 in murine models8,29 and has been shown to accurately reflect the length of perfused cortical capillaries in vivo determined by a fluorescein-conjugated dextran angiography and multiphoton microscopy in the living murine brain.8
Nonetheless, the present study suggests that BBB breakdown in AD APOE4 carriers could be related to accelerated pericyte degeneration and/or activation of CypA–MMP-9 pathway. Although, the relative contributions of pericyte degeneration and activation of CypA–MMP-9 pathway to BBB breakdown remain elusive at the present time, it is possible that activation of MMP-9 accelerates pericyte loss by at least two ways: (1) by allowing a greater ingress of blood-derived products across the BBB that are taken up and degraded by pericytes, which, over time, has been shown to lead to pericyte loss in animal models;8,32 and (2) by degrading the extracellular matrix and important cell adhesion molecules around brain capillaries that can lead to separation of pericytes from the capillary wall and their cell death.9,25
Future longitudinal CSF and BBB imaging biomarkers studies in living human APOE4 and APOE3 carriers with no cognitive impairment and/or with mild cognitive impairment should continue interrogating the role of BBB breakdown in the pathogenesis of dementia because of AD and other causes, as well as the role of pericyte loss and the studied molecular biomarkers of BBB breakdown in the living human brain.
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institutes of Health grants R37NS34467 (BVZ), R37AG23084 (BVZ), R01AG039452 (BVZ), Zilkha Senior Scholar Support (BVZ), and P50AG05142 (USC ADRC).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
MRH, SVR, QM, ZZ, and EAW performed the experiments and analyzed the data. CAM helped with the neuropathologic analysis. BVZ designed the experiments, analyzed the data and wrote the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 2.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic B. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res 1995; 12: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem 1987; 49: 310–315. [DOI] [PubMed] [Google Scholar]
- 5.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin,d-Alanine2-d-leucine5-enkephalin and their N-terminal amino acid (tyrosine). Brain Res 1985; 336: 125–132. [DOI] [PubMed] [Google Scholar]
- 6.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 7.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol 2001; 64: 575–611. [DOI] [PubMed] [Google Scholar]
- 11.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer's disease: a study in Golgi technique and electron microscopy. J Neurol Sci 2012; 322: 117–121. [DOI] [PubMed] [Google Scholar]
- 12.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol 2013; 23: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol Scand 1990; 81: 323–326. [DOI] [PubMed] [Google Scholar]
- 14.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur J Clin Invest 2002; 32: 360–371. [DOI] [PubMed] [Google Scholar]
- 15.Salloway S, Gur T, Berzin T, Tavares R, Zipser B, Correia S, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer's disease. J Neurol Sci 2002; 203-204: 1837. [DOI] [PubMed] [Google Scholar]
- 16.Cullen KM, Kócsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab 2005; 25: 1656–1667. [DOI] [PubMed] [Google Scholar]
- 17.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging 2007; 28: 977–986. [DOI] [PubMed] [Google Scholar]
- 18.Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition fluid biomarkers and cognition. Stroke 2009; 40: 3455–3460. [DOI] [PubMed] [Google Scholar]
- 19.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med 2009; 13: 2911–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol Aging 2009; 30: 337–352. [DOI] [PubMed] [Google Scholar]
- 21.Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer's disease. Curr Gerontol Geriatr Res 2012; 2012: 184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hultman K, Strickland S, Norris EH. The APOE ɛ4/ɛ4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J Cereb Blood Flow Metab 2013; 33: 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol 2013; 70: 1198–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zonneveld HI, Goos JD, Wattjes MP, Prins ND, Scheltens P, van der Flier WM, et al. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology 2014; 82: 698–704. [DOI] [PubMed] [Google Scholar]
- 25.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol 2014; 24: 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron 2009; 63: 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011; 10: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem 2011; 286: 17536–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 2015; 35: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 2013; 125: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Park L, Zhou J, Zhou P, Pistick R, El Jamal S, Younkin L, et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci USA 2013; 110: 3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 2004; 43: 333–344. [DOI] [PubMed] [Google Scholar]
- 35.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008; 118: 4002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000; 106: 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montagne A, Pa J, Zlokovic BV. Vascular plasticity and cognition during normal aging and dementia. JAMA Neurol 2015. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, et al. APOE2 enhances neuroprotection against Alzheimer's disease through multiple molecular mechanisms. Mol Psychiatry 2014; 19: 1243–1250. [DOI] [PubMed] [Google Scholar]
- 40.Kreczmanski P, Schmidt-Kastner R, Heinsen H, Steinbusch HW, Hof PR, Schmitz C. Stereological studies of capillary length density in the frontal cortex of schizophrenics. Acta Neuropathol 2005; 109: 510–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.