Abstract
Background
The challenges of identifying acute HIV infection (AHI) have resulted in a lack of critical information on early AHI that constrains the development of therapeutics that are designed to eradicate HIV from the infected host.
Methods
AHI participants were recruited from the Thai Red Cross Anonymous Clinic in Bangkok, Thailand into the RV254/SEARCH010 protocol and categorised according to Fiebig stages as follows: Fiebig I (HIV-RNA+, p24 Ag−, HIV IgM−) and Fiebig II–IV (HIV-RNA+, p24 Ag + or −, HIV IgM− or +, Western blot- or indeterminate). Proviral and viral burden and immune activation levels were compared between Fiebig stage groups at the time of AHI. CD4 and CD4/CD8 ratio were also compared between groups before and up to 96 weeks of ART.
Results
Median age was 27 years and 96% were male. Fiebig I individuals had lower median HIV-DNA in mononuclear cells from blood (3 vs. 190 copies/106 cells) and gut (0 vs. 898 copies/106 cells), and lower HIV-RNA in blood (4.2 vs. 6.2 log10 copies/mL), gut (1.7 vs. 3.1 log10 copies/mg) and cerebrospinal fluid (2.0 vs. 3.8 log10 copies/mL), when compared to Fiebig II–IV individuals (all P<0.01). Median plasma sCD14 level was lower (1.1 vs. 1.6 μg/mL) in Fiebig I individuals as was the frequency of CD8+HLADR+CD38+ T cells in blood (7.6 vs. 14.9%, both P<0.05). The median plasma interleukin 6 levels were similar between stages (0.6 in Fiebig I vs. 0.5 pg/mL in Fiebig II–IV, P>0.05). The frequencies of CD4+HLA-DR+CD38+ T cells were also similar between these stages (2.1 vs. 2.6%, P>0.05). Median CD4 count and CD4/CD8 ratio were higher in Fiebig I: 508 vs. 340 cells/mm3 and 1.1 vs. 0.7, respectively (both P<0.001). After ART, CD4 cell count normalised by week 24 in Fiebig I and week 48 in Fiebig II–IV. However, CD4/CD8 ratio was lower in both groups after 96 weeks of ART compared to healthy Thais (P=0.02).
Conclusions
Compared to later AHI stages, Fiebig I was associated with lower HIV burden in blood and tissue compartments, lower immune activation and higher CD4 and CD4/CD8 ratio. ART in Fiebig I–IV resulted in normalisation of CD4 cell count within the first year, supporting the benefit of early ART. However, the CD4/CD8 ratio was not normalised after 2 years of ART in all AHI stages, suggesting some degree of persistent immunological dysfunction even when ART was instituted as early as Fiebig I.
Keywords: acute HIV infection, Fiebig I, reservoir, immune activation, CD4, CD4/CD8 ratio
Background
Information is limited on the immunological and virological events that occur during the earliest stages of acute HIV infection (AHI) when HIV serology is still non-reactive, due mainly to difficulties in identifying such individuals [1]. The AHI period usually spans the first month of infection and is categorised into four stages by Fiebig and Busch et al. [2]. During the first few hours to days after HIV infection, the virus replicates in tissue but is not yet detected in blood. This so-called ‘eclipse phase’ is immediately followed by the Fiebig I stage in which HIV-RNA in plasma rises to a level of at least 100 copies/mL but the standard HIV diagnostic tests for p24 antigen and HIV antibody are still negative. Documenting immunological and virological parameters during Fiebig I is important to the understanding of HIV pathogenesis [3]. The timing and extent of HIV seeding in blood and tissue cells, CD4 depletion and immune activation are not well understood at the earliest stages of HIV infection [4,5]. Such knowledge could inform therapeutic strategies to mitigate the impact of HIV on the host.
Here we describe a large cohort of individuals with AHI in Thailand and compare immunological and virological data from two Fiebig groups: Fiebig I versus Fiebig II–IV (after the initial detection of p24 antigen and/or HIV antibody). The objective is to identify unique characteristics of Fiebig I individuals with regards to HIV proviral and viral burden, CD4 depletion and immune activation. We also report the CD4 and the CD4/CD8 responses to antiretroviral therapy (ART) in these two groups, since CD4 and CD4/CD8 ratio before and after ART are predictors of long-term morbidity and mortality in HIV [6,7]. Existing data demonstrate that these may not normalise in individuals who start treatment late [8]. However, data comparing these responses in individuals who initiated ART very early have been limited [9,10].
Methods
The current analysis encompassed data collected between April 2009 and July 2015 from participants enrolled in the RV254/SEARCH010 study (clinicaltrials.gov identification NCT00796146), which is an ongoing prospective study of AHI in Thailand. Briefly, clients of the Thai Red Cross Anonymous HIV testing Clinic in Bangkok were screened in real time for AHI by pooled nucleic acid testing (NAT) or sequential immunoassay as previously described [11] . Participants with a positive NAT and a non-reactive HIV IgG were invited to join the study.
Further testing was performed to categorise them into Fiebig stages as follows: Fiebig I (HIV-RNA+, p24 antigen-, HIV IgM−), Fiebig II (HIV-RNA+, p24 antigen+, HIV IgM−), Fiebig III (HIV IgM+, Western blot-) and Fiebig IV (HIV IgM+, Western blot indeterminate). The corresponding mean cumulative durations from onset of HIV viraemia according to Fiebig et al. are 5 (Fiebig I), 10.3 (Fiebig II), 13.5 (Fiebig III) and 19.1 (Fiebig IV) days [2]. For this study, we reported estimated infection duration from history of HIV exposure within the last 30 days. Flexible sigmoidoscopy and biopsy, and cerebrospinal fluid (CSF) collection were optional procedures. ART was also optional and offered as part of an accompanying protocol (clinicaltrials.gov identification NCT00796263). Treatment included standard doses of a three-drug regimen (tenofovir, lamivudine or emtricitabine, and efavirenz) with some participants receiving a five-drug regimen with the addition of raltegravir and maraviroc during the first 24 weeks. The Thai Chulalongkorn University and relevant US and Canadian institutional review boards approved these studies. All participants provided informed consent.
Laboratory methods were based on assays previously described by our group [12,13]. CD4 cell count was measured by dual-platform flow cytometry (Becton-Dickinson, USA). HIV-RNA in plasma and CSF was performed using the COBAS AMPLICOR HIV-1 Monitor Test v1.5 or Cobas Taqman v2.0 (Roche Molecular Systems, USA). The Siemens Quantiplex HIV-1 3.0 assay was used to measure HIV-RNA (copy/mg of gut tissue). Total HIV-DNA in peripheral blood mononuclear cells (PBMCs) and sigmoid colon were quantified using a modified nested PCR assay for CRF01_AE and B [14]. Immunophenotyping was performed on PBMCs for activated CD4 (CD4+HLA-DR+CD38+) and CD8 (CD8+HLA-DR+CD38+) T cells as previously described [5]. Plasma soluble CD14 (sCD14) and interleukin 6 (IL-6) were measured by ELISA (R&D Systems, Minneapolis, Minnesota, USA). HIV subtyping was performed using the multi-region hybridisation real-time PCR assay for subtypes B, C and CRF01_AE [15].
Statistical analysis
Median (IQR) values were described for each variable. Comparison between Fiebig I and Fiebig II–IV groups was carried out using Mann–Whitney U test or Student t-test for continuous variables; Chi-squared or Fisher's exact test were used for categorical variables. Data from HIV-uninfected Thais were used for comparison when available, and these were either from published data [16] or our concurrent RV304/SEARCH 013 study (clinicaltrials.gov identification NCT01397669). Generalised Estimating Equation model (GEE) was used to estimate mean changes in CD4/CD8 ratio, adjusted by baseline values. Logistic regression model was used to determine predictors for achieving CD4/CD8 ratio ≥1 after 96 weeks of treatment. Statistical tests were two-sided and P values <0.05 were considered statistically significant. Analyses were performed using StataCorp 2013 (StataCorp LP, College Station, TX, USA). Figures were generated using Prism version 6.02 for Windows (GraphPad Software, La Jolla, California, USA).
Results
The RV254/SEARCH010 study screened 147,563 samples to identify 353 acutely infected individuals. Of these, 292 were enrolled in the study and the first 268 cases were included in this analysis because they were in Fiebig I to IV at time of ART initiation. Twenty-four were excluded either because they progressed to Fiebig V/VI at enrollment (n=21) or they did not initiate ART at enrolment (n=3). The majority of enrollees were young men who have sex with men. The most common HIV clade was CRF01_AE. Fiebig I individuals constituted 16% of participants. The estimated infection duration by history was shorter in Fiebig I (Table 1).
Table 1.
Characteristics of Fiebig I vs. Fiebig II–IV acute HIV-infected participants
| Characteristics | All (n=268) | Fiebig I (n=44) | Fiebig II–IV (n=224) | P value |
|---|---|---|---|---|
| Age (years) | 27(23–32) | 26(23–31) | 27(23–32) | 0.97 |
| Gender male:female, n | 257:11 | 41:3 | 216:8 | 0.40 |
| Infection duration(days), median(IQR) | 19(14–25) | 14(12–21) | 20(15–25) | 0.001 |
| Risk behaviour, n(%) | ||||
| MSM | 249(93) | 39(89) | 210(94) | 0.36 |
| Heterosexual female | 11(4) | 3(7) | 8(4) | |
| Heterosexual male | 8(3) | 2(4) | 6(2) | |
| Fiebig stage, n(%) | ||||
| I(RNA+, p24 antigen-, HIV IgM−) | 44(16) | 44(100) | – | – |
| II(RNA+, p24 antigen+, HIV IgM−) | 80(30) | – | 80(36) | |
| III(HIV IgM+/WB−) | 113(42) | – | 113(50) | |
| IV(HIV IgM+/WB indeterminate) | 31(12) | – | 31(14) |
WB: Western blot
** P<0.001, * P<0.05
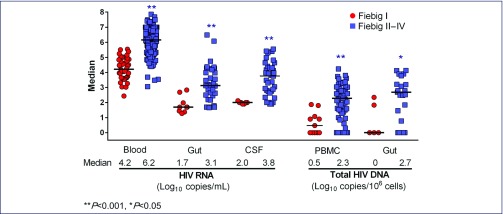
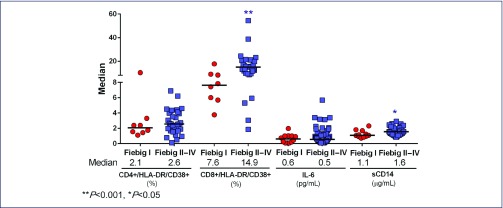
Figure 1a features the viral and proviral burden in different compartments. The HIV-RNA in blood, gut and CSF, and the total HIV-DNA in blood and gut were all significantly lower in Fiebig I versus Fiebig II–IV, P<0.05. The frequency of activated CD8 T cells and sCD14 level were lower in individuals captured in Fiebig I, whereas the frequency of activated CD4 T cells and IL-6 level were not different between Fiebig groups (Figure 1b). Compared to HIV-uninfected Thais, we found that both Fiebig groups had similar IL-6 levels, but higher sCD14 levels (P=0.02 for Fiebig I, P<0.0001 for Fiebig II–IV) and higher frequencies of activated CD8 T cells (P=0.008 for Fiebig I, P<0.0001 for Fiebig II–IV). The median (interquartile ranges, IQR) values from uninfected Thais were 0.5 (0.3–0.8) pg/mL for IL-6 (n=29), 0.8 (0.8–1.0) μg/mL for sCD14 (n=10), and 3.0% (2.8–3.5) for activated CD8 T cells (n=9). The frequency of activated CD4 T cells was similar to nine uninfected Thais (median 1.5%, IQR 1.2–2.1) for Fiebig I (P=0.18), but not for Fiebig II–IV (P=0.03) individuals.
Figure 1a.
Viral and proviral burden in Fiebig I vs. Fiebig II–IV acute HIV infection. The number of participants included for each value is as follows: plasma HIV-RNA (Fiebig I, n=44, Fiebig II–IV, n=224); gut (sigmoid colon) HIV-RNA (Fiebig I, n=9, Fiebig II–IV, n=35); cerebrospinal fluid (CSF) HIV-RNA (Fiebig I, n=7, Fiebig II–IV, n=35); total HIV-DNA in peripheral blood mononuclear cells (PBMCs) (Fiebig I, n=11, Fiebig II–IV, n=60) and total HIV-DNA in gut (sigmoid colon mononuclear cells) (Fiebig I, n=5, Fiebig II–IV, n=21)
Figure 1b.
Immune activation markers in Fiebig I vs. Fiebig II–IV acute HIV infection. The number of participants included for each value is as follows: CD4+/HLA-DR/CD38+ T cells (Fiebig I, n=8, Fiebig II–IV, n=33); CD8+/HLA-DR/CD38+ T cells (Fiebig I, n=8, Fiebig II–IV, n=33); interleukin-6 (IL6) (Fiebig I, n=12, Fiebig II–IV, n=66); soluble CD14 (sCD14) (Fiebig I, n=11, Fiebig II–IV, n=61)
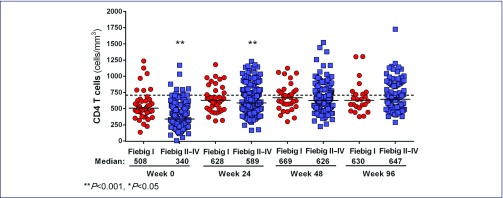
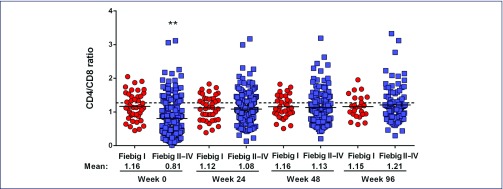
The absolute CD4 T cell count (Figure 2a) and CD4/CD8 ratio (Figure 2b) were compared from baseline and post-ART between the two groups. Before ART, the CD4 cell counts were higher in Fiebig I versus Fiebig II–IV (P<0.001), but both were significantly lower than in 59 controls (mean 730, SD 190), P<0.001 for all. Fiebig I individuals also had higher CD4/CD8 ratio than the Fiebig II–IV individuals (1.1 vs. 0.7, P<0.001), but again both were lower than in uninfected Thai controls (P=0.01 for Fiebig I, P<0.001 for Fiebig II–IV). The mean CD4/CD8 ratio from 216 HIV-uninfected Thais was 1.35 (SD 0.48). The proportions with CD4/CD8 ratio ≥1 were 61% in Fiebig I versus 28% in Fiebig II–IV, P<0.001.
Figure 2a.
CD4+ T cell counts in Fiebig I vs. Fiebig II–IV acute HIV infection before and after antiretroviral therapy. The dotted line represents the mean value in HIV-uninfected Thais. The number of participants included for each time-point is as follows: Week 0 (Fiebig I, n=44, Fiebig II–IV, n=224); Week 24 (Fiebig I, n=41, Fiebig II–IV, n=198); Week 48 (Fiebig I, n=34, Fiebig II–IV, n=162); Week 96 (Fiebig I, n=24, Fiebig II–IV, n=98)
Figure 2b.
CD4/CD8 ratio in Fiebig I vs. Fiebig II–IV acute HIV infection before and after antiretroviral therapy. The dotted line represents the mean value in HIV-uninfected Thais. The number of participants included for each time-point is as follows: Week 0 (Fiebig I, n=44, Fiebig II–IV, n=224); Week 24 (Fiebig I, n=41, Fiebig II–IV, n=197); Week 48 (Fiebig I, n=34, Fiebig II–IV, n=159); Week 96 (Fiebig I, n=24, Fiebig II–IV, n=97)
ART was initiated at a median (IQR) of 0 (0–1) days from study enrollment in both groups. The majority received the three-drug ART regimen (n=187, 70%) and 81 (30%) received the five-drug regimen. After ART, the proportions of participants with plasma HIV-RNA <50 copies/mL were 92% at week 24, 98% at week 48 and 99% at week 96. The CD4 T cell counts and CD4/CD8 ratio were not statistically different between the two Fiebig groups following ART. The CD4 cell count normalised by week 24 in Fiebig I and by week 48 in Fiebig II–IV participants. The CD4/CD8 ratio after treatment did not change from baseline in the Fiebig I group, but in the Fiebig II–IV group, the mean (95% CI) changes by GEE analysis, adjusted for baseline values were 0.27 (0.22–0.32) at week 24, 0.31 (0.26–0.36) at week 48 and 0.36 (0.30–0.42) at week 96 (P<0.001 for all). However, the CD4/CD8 ratio remained persistently low in both groups up to week 96 compared to uninfected controls, P=0.02. By univariate logistic regression analysis, baseline predictors for achieving CD4/CD8 ratio ≥1 after 96 weeks of treatment were CD4>350 cells/mm3 [odds ratio (95% confidence interval, CI) 3.7 (1.7–8.1), P=0.001] and CD4/CD8 ratio higher than the median value of 0.8 [odds ratio (95% CI): 13.0 (5.0–33.5), P<0.001]. Types of ART regimens did not affect HIV-RNA, CD4 and CD4/CD8 ratio.
Discussion
The earliest stage of acute infection, Fiebig I, was associated with significantly lower viral and proviral burden, less immune activation and a better CD4 cell count and CD4/CD8 ratio compared to the later stages of Fiebig II–IV; suggesting that intervening with ART in Fiebig I may be more beneficial in mitigating HIV persistence and immune activation, and protecting immune function.
Based on self-reported history of exposure, the duration of HIV infection was shorter in Fiebig I by a median of 6 days with marked lower plasma HIV-RNA by 2 log10 and total HIV-DNA in PBMCs by 1.8 log10 in Fiebig I compared to the other group. These differences may impact long-term outcomes. Plasma HIV-RNA predicts CD4 depletion and subsequent progression to AIDS and death in untreated HIV [17], and both virological failure and poor CD4 recovery are associated with these outcomes in treated HIV [18]. HIV-DNA in PBMCs before ART is correlated with post-treatment HIV-DNA levels, residual viraemia and immune activation [19–22]. Importantly, in the SPARTAC trial, pre-treatment total PBMC HIV-DNA predicted time to viral rebound when ART was removed [23]. Indeed our group has shown that ART initiated at the Fiebig I stage is associated with extremely low frequencies of latently infected cells in PBMCs and in all CD4 subsets [24].
The viral and proviral burden in sigmoid biopsies were also significantly lower in the Fiebig I participants. The gut represents a major HIV reservoir site due partly to the high frequencies of target cells including CCR5+CD4+ and Th17 cells [25,26]. In the Fiebig I participants, the HIV-RNA was 1.4 log10 lower and the total HIV-DNA was 2.7 log10 lower in the gut compared to the Fiebig II–IV group. The central nervous system (CNS) is another important HIV reservoir site where brain microglial cells and astrocytes can harbour HIV, and infected peripheral blood cells traffic to the CNS [27]. CSF HIV-RNA is used as an indirect marker of brain infection, and we show that the CSF HIV-RNA is much lower if infection is identified in Fiebig I.
There was a higher frequency of activated CD8 T cells and a higher level of sCD14 in Fiebig II–IV. In contrast, the frequency of activated CD4 T cells and plasma IL-6 levels were similar in both groups. This is in line with previous reports showing that CD8 T cells are activated early to control viral load set-point in untreated acutely infected persons [28,29]. IL-6 slowly rises following infection; therefore, it was not yet elevated in our participants [30]. sCD14 is a marker of monocyte activation that could be elevated in response to gut endothelial damage and microbial translocation. We have previously shown that gut CD4 depletion occurs after Fiebig I [5], which correlates with the higher sCD14 level observed in later stages of acute infection. Persistent elevation of activated CD8 T cells, sCD14 and IL-6 are associated with AIDS and non-AIDS deaths in chronic HIV infection [31–33]. These data suggest that if HIV is diagnosed early enough, there is an opportunity to intervene and prevent or reverse immune activation. Early ART was associated with normalisation of cellular immune activation, and some, but not all, soluble inflammatory biomarkers [5,34].
Our data showed that the CD4 T cell count and CD4/CD8 ratio were better in Fiebig I participants. Over half of Fiebig I but only one-third of Fiebig II–IV individuals had CD4/CD8 ratio ≥1, a ‘normal’ threshold used in several studies [6,7,35]. Both groups, however, had CD4 cell count and CD4/CD8 ratio that were significantly lower than those in uninfected Thai controls [16]. This illustrates the rapidity of immune damage caused by HIV. Low CD4 and CD4/CD8 ratio correlate with poorer HIV disease outcomes [6,7,36]. The CD4/CD8 ratio has been proposed as a better marker for immune dysfunction in HIV than CD4 cell count because it reflects both the CD4 depletion and the activation and proliferation of CD8 T cells in HIV infection [35].
Studies in chronically HIV-infected individuals showed that CD4 and CD4/CD8 ratio recovery were slow even after years of suppressive ART [8,9]. In a large study of adults with chronic HIV infection with a median CD4/CD8 ratio of 0.39, the probability of achieving CD4/CD8 ratio ≥1 was 11.5% at 2 years after ART [8]. Whereas, adults treated within the first 6 months of infection had superior CD4 and CD4/CD8 ratio recovery [9,10]. Here we show that if ART is initiated in Fiebig I–IV AHI, CD4 cell counts recover to normal levels within the first year, with a faster recovery in the Fiebig I individuals. Intriguingly, the CD4/CD8 ratio in Fiebig I remained stable post-ART, whereas in the Fiebig II–IV group, there was a rapid rise in CD4/CD8 ratio within the first 24 weeks of ART. After 96 weeks of ART, both groups had a median CD4/CD8 ratio above 1, but the ratio was lower than in uninfected Thais, and suggests that some persistent immune dysfunction exists even when ART is initiated as early as Fiebig I.
Our rationale for combining the Fiebig II–IV as a group is as follows. First, our intention here is to focus on the Fiebig I group, for whom published data are most lacking. Second, the virological and immunological profiles are largely similar amongst the Fiebig II–IV individuals, particularly for proviral DNA in blood and tissue, and immune activation markers (data not shown). Our data has limitations. The available data from healthy Thais varied, and for some markers, there were as few as nine controls (e.g. activated T cells). This limits the interpretation of the data from our acutely infected participants. Our analysis lacked longitudinal comparisons for most markers for which studies are ongoing. Finally, identifying Fiebig I individuals is not easy. Here we screened large numbers of samples from clients who sought HIV testing at a single centre to enrol 44 Fiebig I participants. Diagnosing AHI requires increased awareness for early testing by the at-risk persons and the healthcare providers. Nucleic acid testing is needed to diagnose Fiebig I acute infection. However, new developments using sample-to-cut-off ratio from standard fourth-generation antigen–antibody combo assays are being validated for early acute infection diagnosis, which could significantly improve the identification of Fiebig I and later stages of AHI [37] .
In summary, our study contributes data on events during the very early AHI period. We demonstrate stark differences in the virological and immunological profiles between Fiebig I and Fiebig II–IV acute infection, highlighting the dynamic processes during AHI. The Fiebig I participants had limited viral and proviral burden, immune activation and CD4 depletion. Initiating ART in Fiebig I may afford the opportunity to markedly mitigate further HIV reservoir seeding and immune damage. The rapid recovery of CD4 cell counts during ART supports initiation of therapy in acute infection. However, the low CD4/CD8 ratio even after very early ART in Fiebig I suggests persistent immune dysfunction and raises the potential need for additional immune therapies that could enhance immune recovery and dampen immune activation.
Acknowledgements
We thank our study participants and staff from the Thai Red Cross AIDS Research Centre, Chulalongkorn University and AFRIMS for their valuable contributions to this study. We thank Ms Piraporn June Ohata for her help in preparing this manuscript.
The RV254/SEARCH 010 Study Group includes: from SEARCH/TRCARC/HIV-NAT: Praphan Phanuphak, Nipat Teeratakulpisarn, James Fletcher, Eugene Kroon, Donn Colby, Duanghathai Sutthichom, Somprartthana Rattanamanee, Peeriya Prueksakaew, Sasiwimol Ubolyam, Pacharin Eamyoung, Suwanna Puttamaswin, Somporn Tipsuk and Putthachard Karnsomlap; from Chulalongkorn University: Rungsun Rerknimitr, Wiriyaporn Ridtitid, Sukalya Lerdlum and Mantana Pothisri; from AFRIMS: Robert J O'Connell, Siriwat Akapirat, Yuwadee Phuang-Ngern, Suchada Sukhumvittaya, Chayada Sajjaweerawan, Surat Jongrakthaitae, Putita Saetun, Nipattra Tragonlugsana, Bessara Nuntapinit, Nantana Tantibul and Hathairat Savadsuk; from MHRP: Michael Eller, Silvia Ratto-Kim, Bonnie Slike, Gustavo Kijak, Trevor Crowell, Sharleen Traynor, Madelaine Ouellette, Oratai Butterworth, Mark Milazzo, Leigh Ann Eller, Ellen Turk, Melissa Walsh; from UCSF: Derek Ochi, Joanna Hellmuth, Collin Adams; from Yale: Leah Le; from VGTI-Florida: Claire Vandergeeten, Remi Fromentin, Wendy Beckman, Lydie Trautmann, Rafick Sekaly; from Monogram Biosciences: Laura Napolitano, Molly Martell, Yolanda Lie and the R&D and PDO groups from Monogram Biosciences (for technical assistance).
Conflicts of interest
Jintanat Ananworanich and Victor Valcour have served on advisory panels for ViiV Healthcare; Jintanat Ananworanich has also served on advisory panels for Merck and Tetralogic. Nicolas Chomont has served on the scientific advisory board of Theravectys. The remaining authors report no relevant conflicts of interest.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense or the Department of Health and Human Services or the Thai Red Cross AIDS Research Center.
Source of funding
This work was supported by cooperative agreements (W81XWH-07-2-0067, W81XWH-11-2-0174) between The Henry M Jackson Foundation for the Advancement of Military Medicine and the US Department of the Army and by an intramural grant from the Thai Red Cross AIDS Research Center. The US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702-5014, is the awarding and administering acquisition office for the cooperative agreement. These funds are supplemented by the National Institute of Mental Health to support the focus on central nervous system studies. This research was supported in part by the Intramural Research Program of NIAID/NIH, the Delaney AIDS Research Enterprise to find a cure (DARE, 1U19AI096109), and R01NS084911-01 (Spudich and Ananworanich). It has also been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The Government Pharmaceutical Organization (GPO) of Thailand, Gilead, Merck and ViiV Healthcare provided support for antiretroviral medications.
References
- 1. Rutstein SE, Sellers CJ, Ananworanich J, Cohen MS. The HIV treatment cascade in acutely infected people: informing global guidelines. Curr Opin HIV AIDS 2015; 10: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fiebig EW, Wright DJ, Rawal BD et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17: 1871–1879. [DOI] [PubMed] [Google Scholar]
- 3. Martin AR, Siliciano RF. Progress toward HIV eradication: case reports, current efforts, and the challenges associated with cure. Ann Rev Med 2015. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg NE, Pilcher CD, Busch MP, Cohen MS. How can we better identify early HIV infections? Curr Opin HIV AIDS 2015; 10: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuetz A, Deleage C, Sereti I et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014; 10: e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buggert M, Frederiksen J, Noyan K et al. Multiparametric bioinformatics distinguish the CD4/CD8 ratio as a suitable laboratory predictor of combined T cell pathogenesis in HIV infection. J Immunol 2014; 192: 2099–2108. [DOI] [PubMed] [Google Scholar]
- 7. Young J, Psichogiou M, Meyer L et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 2012; 9: e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mussini C, Lorenzini P, Cozzi-Lepri A et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2: e98–e106. [DOI] [PubMed] [Google Scholar]
- 9. Cao W, Mehraj V, Trottier B et al. Early initiation rather than prolonged duration of antiretroviral therapy in HIV infection contributes to the normalization of CD8 T-cell counts. Clin Infect Dis 2015; 62: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thornhill J, Inshaw J, Oomeer S et al. Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc 2014; 17: 19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Souza MS, Phanuphak N, Pinyakorn S et al. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS 2015; 29: 793–800. [DOI] [PubMed] [Google Scholar]
- 12. Ananworanich J, Schuetz A, Vandergeeten C et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7: e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ananworanich J, Chomont N, Fletcher JLK et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J Virus Erad 2015; 1: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vandergeeten C, Fromentin R, Merlini E et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol 2014; 88: 12385–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kijak GH, Tovanabutra S, Sanders-Buell E et al. Distinguishing molecular forms of HIV-1 in Asia with a high-throughput, fluorescent genotyping assay, MHAbce v.2. Virology 2007; 358: 178–191. [DOI] [PubMed] [Google Scholar]
- 16. Webster HK, Pattanapanyasat K, Phanupak P et al. Lymphocyte immunophenotype reference ranges in healthy Thai adults: implications for management of HIV/AIDS in Thailand. Southeast Asian J Trop Med Public Health 1996; 27: 418–429. [PubMed] [Google Scholar]
- 17. Mellors JW, Munoz A, Giorgi JV et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126: 946–954. [DOI] [PubMed] [Google Scholar]
- 18. Molina JM, Cahn P, Grinsztejn B et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 2011; 378: 238–246. [DOI] [PubMed] [Google Scholar]
- 19. Groves KC, Bibby DF, Clark DA et al. Disease Progression in HIV-1-Infected Viremic Controllers. J Acquir Immune Defic Syndr 2012; 61: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laanani M, Ghosn J, Essat A et al. Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin Infect Dis 2015; 60: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 21. Malatinkova E, De Spiegelaere W, Bonczkowski P et al. Impact of a decade of successful antiretroviral therapy initiated at HIV-1 seroconversion on blood and rectal reservoirs. Elife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruggiero A, De Spiegelaere W, Cozzi-Lepri A et al. During stably suppressive antiretroviral therapy integrated HIV-1 DNA load in peripheral blood is associated with the frequency of CD8 cells expressing HLA-DR/DP/DQ. EBioMedicine 2015; 2: 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams JP, Hurst J, Stohr W et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 2014; 3: e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ananworanich J, Vandergeeten C, Chomchey N et al. Early ART intervention restricts the seeding of the HIV reservoir in long lived central memory CD4 T cells. Conference on Retroviruses and Opportunistic Infections. March 2013. Atlanta, GA, USA. Abstract 47.
- 25. Kim CJ, McKinnon LR, Kovacs C et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol 2013; 191: 2164–2173. [DOI] [PubMed] [Google Scholar]
- 26. Yukl SA, Shergill AK, Ho T et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis 2013; 208: 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valcour V, Chalermchai T, Sailasuta N et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker B, McMichael A. The T-cell response to HIV. Cold Spring Harb Perspect Med 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ndhlovu ZM, Kamya P, Mewalal N et al. Magnitude and kinetics of CD8(+) T cell activation during hyperacute HIV infection impact viral set point. Immunity 2015; 43: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stacey AR, Norris PJ, Qin L et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009; 83: 3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duffau P, Wittkop L, Lazaro E et al. Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS 2015; 29: 2099–2108. [DOI] [PubMed] [Google Scholar]
- 32. Hamlyn E, Fidler S, Stohr W et al. Interleukin-6 and D-dimer levels at seroconversion as predictors of HIV-1 disease progression. AIDS 2014; 28: 869–874. [DOI] [PubMed] [Google Scholar]
- 33. Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203: 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Utay N, Ananworanich J, Suteeraporn P et al. Inflammation persists despite early initiation of ART in acute HIV infection. Conference on Retroviruses and Opportunistic Infections. 2015. Seattle, WA, USA. Abstract 47.
- 35. Tinago W, Coghlan E, Macken A et al. Clinical, immunological and treatment-related factors associated with normalised CD4+/CD8+ T-cell ratio: effect of naive and memory T-cell subsets. PLoS One 2014; 9: e97011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Serrano-Villar S, Sainz T, Lee SA et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10: e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramos EM, Ortega J, Daza G et al. Use of the sample-to-cutoff ratio (S/CO) to identify recency of HIV-1 infection. Conference on Retroviruses and Opportunistic Infection. February 2015. Seattle, WA, USA. Abstract 627.