Summary
In activated B lymphocytes, AID initiates antibody variable (V) exon somatic hypermutation (SHM) for affinity maturation in germinal centers (GCs) and IgH switch (S) region DNA breaks (DSBs) for class-switch recombination (CSR). To resolve long-standing questions, we have developed an in vivo assay to study AID-targeting of passenger sequences replacing a V exon. First, we find AID targets SHM hotspots within V exon and S region passengers at similar frequencies and that the normal SHM process frequently generates deletions, indicating that SHM and CSR employ the same mechanism. Second, AID mutates targets in diverse non-Ig passengers in GC B cells at levels similar to those of V exons, definitively establishing the V exon location as "privileged" for SHM. Finally, Peyer's patch GC B cells generate a reservoir of V exons that are highly mutated before selection for affinity maturation. We discuss implications of these findings for harnessing antibody diversification mechanisms.
INTRODUCTION
Antibodies are the secreted form of B cell antigen receptors (BCRs), the basic subunit of which is a pair of identical immunoglobulin (Ig) heavy (IgH) and light (IgL) chains. N-terminal regions of IgH and IgL chains provide the antigen-binding variable ("V") region of antibodies. Ig V regions are encoded by exons ("V exons") assembled by V(D)J recombination during bone marrow B cell development. V(D)J recombination creates diverse antibody repertoires by assembling multitudes of different germline V, D and J combinations and by diversifying junctions between these segments through nucleotide deletions and insertions (Alt et al., 2013). V exons contain three highly variable domains termed complementarity-determining regions (CDRs), which encode antigen-contact sites and determine binding-specificity (Di Noia and Neuberger, 2007). CDR1 and CDR2 are encoded by germline V segments; whereas CDR3 is encoded by V(D)J junctional regions and, therefore, has the greatest diversity (Hwang et al., 2015). Conserved framework regions (FWRs) between CDRs impart antibody structure. Due to junctional diversity, about 2/3 of V exons are assembled out of frame and do not encode proteins. These "non-productive" V exons are often present in B cells in which the other IgH (and/or IgL) locus is productively rearranged and supports development (Mostoslavsky et al., 2004).
The mouse expresses different antibody classes determined by expressed IgH constant regions exons (CHs). The first developmentally expressed CH (Cμ) generates primary B cells expressing IgM. Newly generated IgM-expressing B cells migrate to peripheral lymphoid organs where, upon antigen activation, they further diversify primary antibody V exon repertoires by somatic hypermutation (SHM) and change expressed CH antibody effector functions via IgH class switch recombination (CSR) (Hwang et al., 2015). SHM occurs in response to antigen-dependent B cell activation in specialized lymphoid structures termed germinal centers (GCs) (Victora and Nussenzweig, 2012). SHM introduces mainly point mutations into V exons (Di Noia and Neuberger, 2007). GC B cells with SHMs that result in increased BCR antigen-binding affinity are positively selected, leading to affinity maturation, and those that decrease BCR affinity or cause loss of BCR expression are negatively selected (Di Noia and Neuberger, 2007; Victora and Nussenzweig, 2012). IgH CSR occurs within or outside GCs and can be activated in cultured IgM-expressing primary B cells (Stavnezer et al., 2008). During CSR, DNA double strand breaks (DSBs) are introduced into long, repetitive switch (S) regions that precede Cμ (Sμ) and each downstream CH. Joining a donor Sμ DSB to a downstream acceptor S region DSB effects CSR to IgG, IgE, or IgA (Hwang et al., 2014).
Both V exon SHM and IgH CSR are initiated by activation-induced cytidine deaminase (AID) (Muramatsu et al., 2000), an enzyme that deaminates cytosines (C) to uridines (U) in single-stranded DNA. AID is targeted transcriptionally to V exons and S regions, where it acts on both DNA strands (Alt et al., 2013). Co-opted base excision repair (BER) or mismatch repair (MMR) factors convert AID-initiated lesions into mutational or DSB outcomes (Di Noia and Neuberger, 2007; Peled et al., 2008). Uridine/guanine (U/G) mismatches resulting from AID activity are converted to transition or transversion mutations at initiating C/G residues by replication over uracils or over an abasic site upon uracil removal by BER (Di Noia and Neuberger, 2007). MMR also generates transition or transversion mutations and extends SHM to flanking adenine/thymidine (A/T) residues by error prone DNA polymerase activity following excision of DNA patches around AID-generated uracils (Peled et al., 2008). DSBs can be generated by BER in the form of adjacent nicks on both DNA strands or by MMR in the form of overlapping gaps (Saribasak and Gearhart, 2012; Chahwan et al., 2012). AID preferentially deaminates cytidines in short RGYW (R=A/G, Y=C/T, W=A/T) or related motifs (Liu and Schatz, 2009; Hackney et al., 2009). Compared to the genome, such motifs are mildly enriched in certain V exons (Hackney et al., 2009). AGCT, a canonical RGYW motif, occurs at high density in the core of long, highly repetitive mammalian S regions, where its palindromic sequence provides AID substrates on both DNA strands (Han et al., 2011; Zarrin et al., 2004). However, AID-targeting patterns on core S regions had not been measured due to the repetitive S region nature.
While transcription targets AID to different S regions (Alt et al., 2013), mechanisms that differentially target AID to sequences within a V exon or between V exons and S regions have remained enigmatic. AID targets both productive and non-productive V exons during SHM at high frequency (10−3 mutations per base pair) (Odegard and Schatz, 2006). Within V exons, SHMs are focused at RGYW motifs in CDRs of both productive and non-productive alleles (Di Noia and Neuberger, 2007; Dorner et al., 1997; Betz et al., 1993a; Betz et al., 1993b; Wei et al., 2015). RGYW motifs do not appear sufficient for targeting, since identical motifs mutate less frequently in FWRs (Dorner et al., 1997; Wei et al., 2015). In this regard, non-Ig sequences driven by Ig promoters and enhancers in transgenes can, in some cases, undergo substantial SHM (Yelamos et al., 1995). However, as such transgene results are variable and could reflect influences of integration site and copy number, they have not generally been considered to represent normal SHM processes (Yang and Schatz, 2007; Maul and Gearhart, 2010a). Thus, the question of what mechanisms enable high AID targeting within V exons and within CDRs, in particular, has remained an enigma.
Another AID targeting enigma comes from findings that B cells or B cell lines activated in culture to undergo robust CSR have not been found to undergo SHM of adjacent, transcribed V exons (Nagaoka et al., 2002; Maul et al., 2014; Liu and Schatz, 2009). Such findings led to the conclusion that differential mechanisms target AID to V exons versus S regions (Maul and Gearhart, 2010b; Storb, 2014). While such mechanisms have been elusive, one postulate is that these activities involve differential activation of CSR versus SHM factors by AID (Muramatsu et al., 2007). V exon SHM is considered to predominantly involve point mutations (Di Noia and Neuberger, 2007) and rarely lead to DSBs (Betz et al., 1993a). Estimates of the frequency of V exon DSBs have been highly variable, likely due to the limited number of sequences analyzed (Goossens et al., 1998, Betz et al., 1993a; Betz et al., 1993b; Bross et al., 2000; Briney et al, 2012). Thus, the question of whether AID specifically mediates point mutation during SHM and DSBs during CSR via distinct mechanisms has remained unresolved. Correspondingly, whether or not most V exon DSBs are directly linked to AID-initiated SHM remains an important question, given that deletions (and related insertions) can contribute to antibody specificity, for example in certain extensively mutated anti-HIV-1 broadly neutralizing antibodies (bnAb) (Wu et al., 2011; Mascola and Haynes, 2013; Kepler et al., 2014).
Intestinal Peyer’s Patch (PP) GCs are chronically present in mice in the absence of specific immunization due to continual stimulation by commensals and gut antigens (Casola et al., 2004). Correspondingly, PP GC B cells with Vλ exon SHMs are found robustly in BCR-deficient mice that express the Epstein Barr Virus protein LMP2a as a BCR signaling surrogate, indicating that SHM occurs in PP GC B cells in the absence of BCR engagement with antigen (Casola et al, 2004). In this regard, intestinal B cell development and primary antibody diversification occurs in sheep, cattle, pigs, chicken, and rabbits (Lanning and Knight, 2005) and has also been implicated in the mouse (Wesemann et al., 2013). Thus, it is conceivable that mouse PP GCs, beyond initiating antigen-specific responses, may also undergo SHM via antigen-independent stimulation to further expand primary BCR repertoires (Lanning and Knight, 2005; Casola et al., 2004). However, the degree to which SHMs are selected by specific antigens in V exon repertoires of PP GC B cells that express a functional BCR has not been addressed.
To elucidate roles of V(D)J exon and S region sequences in AID-targeting and DSB versus mutational outcomes in mouse GC B and CSR-activated B cells, we developed an efficient approach to measure AID activity on a fixed "productive" IgH V(D)J exon allele, versus an array of test sequences driven by the same promoter on the other “passenger" allele. This approach has provided unanticipated insights into long-standing questions regarding AID targeting and outcome during SHM and CSR.
RESULTS
A V(D)J Replacement Passenger Allele System
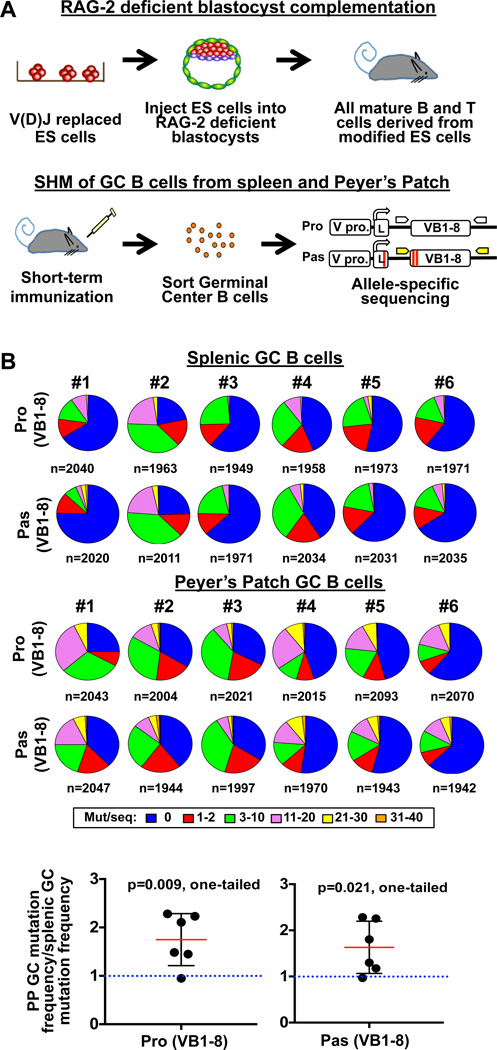
We developed an efficient V(D)J passenger allele system to test influences of substrate sequences on AID targeting and the outcome of such activity in mouse GC B cells or CSR-activated cultured B cells (Figures 1A and S1A). For this approach, we generated an ES cell line with a productive pre-assembled V(D)J allele encoding the IgH V region of the 4-hydroxy-3-nitrophenylacetyl (NP)-binding B1–8 antibody (VB1–8) inserted in place of the 4 IgH JH segments (Sonoda et al., 1997). On the other "passenger" IgH allele of these "VB1–8" ES cells, we inserted a puromycin-resistance cassette in place of the 4 JHs. To generate VB1–8 ES cells with different test passenger sequences, we replaced the inserted puromycin-resistance cassette with a "passenger" cassette, which consists of a VB1–8 promoter and leader sequence containing a translation termination codon, followed by test sequences (Figure 1A, bottom). Thus, as the passenger test sequences cannot encode proteins, their SHM patterns are not biased by antigen-specific B cell selection forces. VB1–8/passenger ES clones are used for RAG-2 deficient blastocyst complementation ("RDBC"; Chen et al., 1993) to generate chimeric mice in which all peripheral lymphocytes are derived from the injected ES cells (Figure 1A, top). We induce SHM in splenic GC B cells of VB1–8/passenger chimeras by short-term immunization (10 days) with NP-chicken gamma globulin (NP-CGG) or other immunogens to activate GC formation in the absence of appreciable antigen-specific B cell selection (Weiss et al., 1992). Following immunization, we purify GC B cells from spleens and PP of individual RDBC chimeras and amplify their productive VB1–8 and the passenger allele sequences via allele-specific primers, sequence the products via next-generation high-throughput sequencing, and assay them for SHMs (Figures 1A, bottom and S1B).
Figure 1. V(D)J Replacement Passenger Allele System.
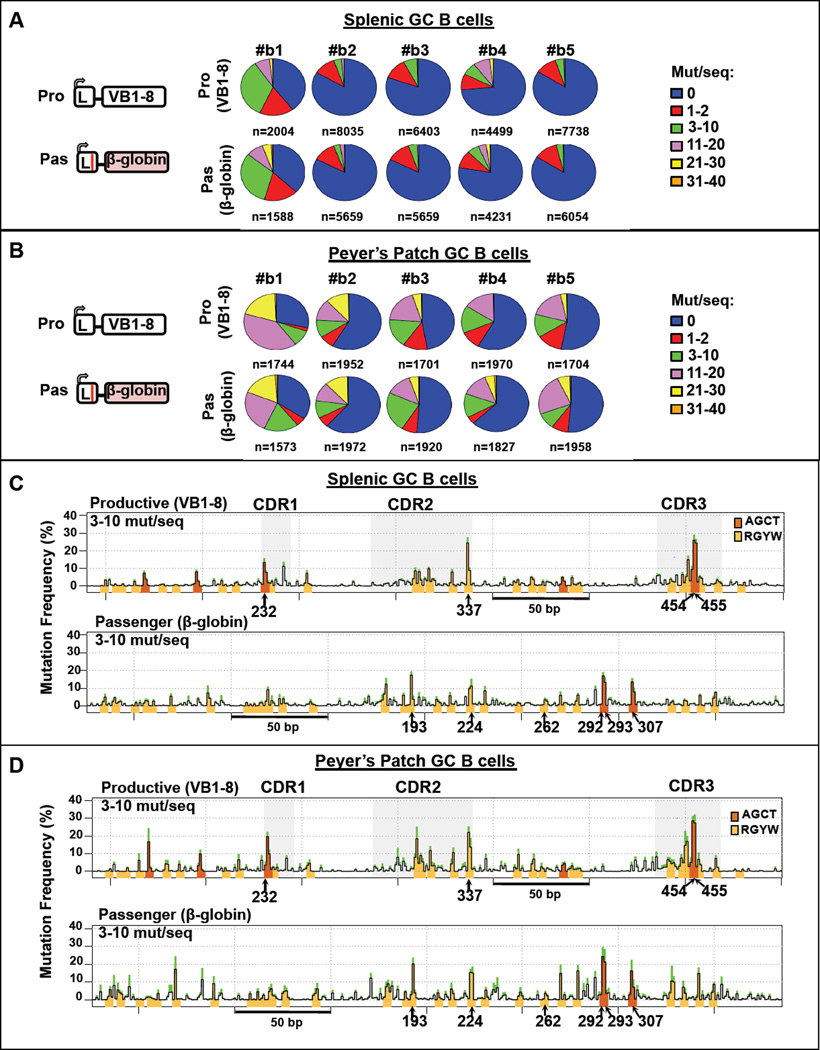
(A) Top: RDBC generates chimeric mice whose mature B cells carry a fixed VB1–8 productive allele and test sequences in the V(D)J passenger allele. Bottom: assay of SHM of GC B cells from spleen and PP of immunized mice. Pro, pas, V pro and L represent productive allele, passenger allele, V promoter and leader sequence, respectively. Red lines across the leader sequence and VB1–8 in the passenger allele represents a termination codon and two nucleotide changes from productive VB1–8, respectively. (B) Pie charts showing the proportion of sequences that have the indicated number of mutations per sequence in productive and passenger VB1–8 alleles from splenic GC B cells (top) and PP GC B cells (middle) of six independent mice. Total number of sequence reads from each mouse is indicated below each pie chart. Bottom: Ratios of mutation frequencies of PP GC B cells to mutation frequencies of splenic GC B cells (calculated within each mouse), for productive VB1–8 (left) and passenger VB1–8 alleles (right). Ratios were calculated for each of the six mice separately and displayed as the mean ± standard deviation (SD) of the six mice. One-sample student t-test was performed to test for significance of difference of the mean from a hypothetical mean of 1.0 (labeled in blue dotted line). One-tailed p value is shown. See also Figure S1 and Table S1.
Mutation Profiles of Productive and Passenger VB1–8 Alleles in Splenic GC B cells
To validate the RDBC-based VB1–8/passenger approach, we assayed a bona fide VB1–8 sequence as a passenger (Figure 1A, bottom). Given the essentially identical sequence and transcription control elements, we expected a similar frequency and pattern of SHMs in productive and passenger alleles of splenic GC B cells under non-selective immunization conditions. For analysis, we compared mutations per sequence in productive and passenger alleles from 6 different chimeras by breaking them into 6 different mutation strata, ranging from 0 to 31–40 mutations/sequence and using pie charts to visualize overall SHM levels for each mouse (Figures 1B and S1C). Both productive and passenger VB1–8 alleles were mutated substantially in splenic GC B cells (Figure 1B, top), with levels far above background (Figures S1D–E; Table S1). While overall SHM levels varied between different chimeras, productive and passenger VB1–8 alleles in splenic GC B cells of a given chimera had very similar SHM levels (Figures 1B, top and S1F, left). Moreover, productive and passenger VB1–8 alleles in GC B cells from given chimeras had remarkably similar distributions of sequences across the 6 tested SHM strata (Figure 1B, top).
Kinetic Analysis of Hotspot Mutation with Increasing Mutation Frequency
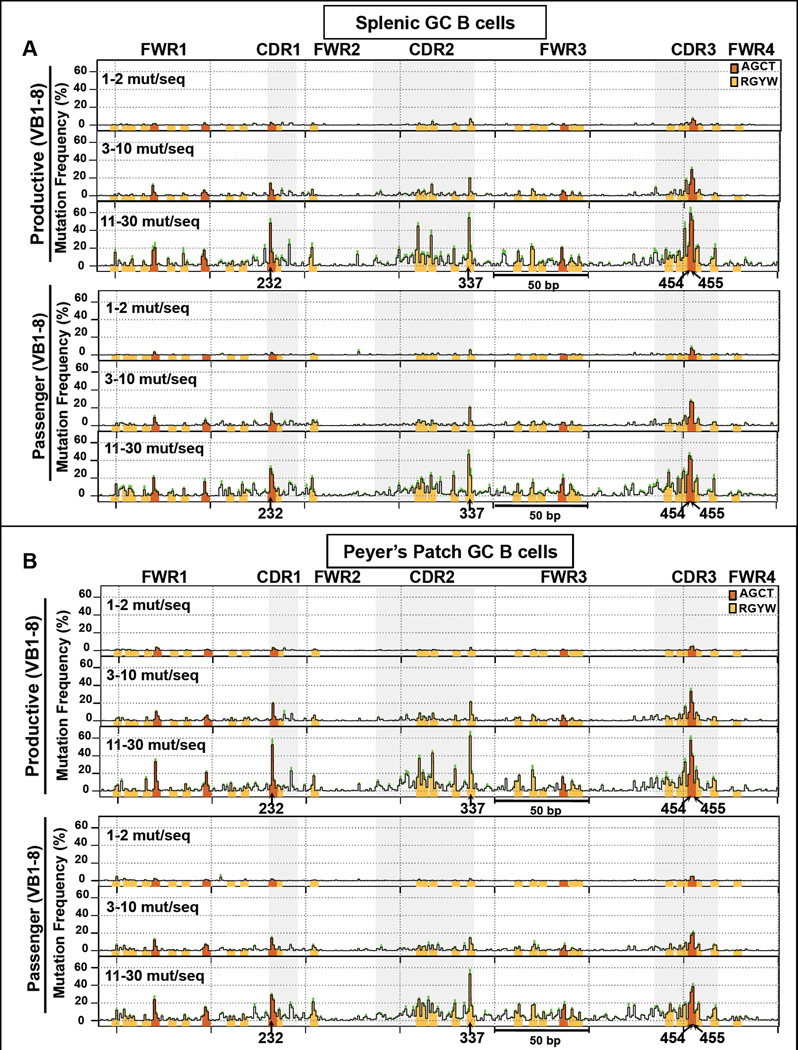
One of our major goals was to test for AID target hotspots in various passenger sequences by assaying for them in sequences with low to high mutations levels. In this regard, duplicate mutations that arise from independent targeting events at hotspots when mutation levels are low may be difficult to distinguish from PCR repeats and, therefore, difficult to capture by standard SHM analyses that exclude duplicates (e.g. Rada et al., 2004). To counter this limitation, we developed an analysis strategy that includes duplicates, but controls for PCR repeats by analyzing 5 to 6 independent mouse replicates for each experiment and then averaging mutation frequency of each nucleotide across replicates. In addition, comparing the mutation pattern of sequences in low to high strata allows us to visualize hotspot emergence and ongoing accumulation and, thus, serves as a surrogate SHM kinetic analysis. For ease of presentation, we present such “kinetic” analyses for productive and passenger alleles in three representative bins (Figure 2A). Stratification of sequences into narrower bins gave similar results and conclusions (Figure S2A, left).
Figure 2. SHM Profiles of VB1–8 Productive and Passenger Alleles.
(A and B) Map of mutations (SHM Profile) on the VB1–8 productive and passenger allele sequences in (A) splenic and (B) PP GC B cells. The y-axis indicates the mutation frequency at each nucleotide plotted as the mean % of sequences in the indicated strata that contain a mutation at the indicated nucleotide ± standard error of the mean (SEM) (green shading indicates top error bar) from 6 independent mice. Orange and yellow bars mark the positions of AGCT and other RGYW motifs, respectively. See also Figure S2.
Combined analyses of sequence reads revealed three robust hotspots in both productive and passenger VB1–8 alleles, including the two C nucleotides on opposite DNA strands of the AGCT motif in CDR3 (positions 454 and 455) and a C in an RGYW (AGCA) motif in CDR2 (position 337) (Figure S2B, top). These hotspots were apparent at low SHM levels (1–2 mutations/sequence) and further accumulated in sequences with intermediate (3–10 mutations/sequence) and high (11–30 mutations/sequence) SHM levels. In the latter, they are mutated in 40–60 % of sequences (Figure 2A). AGCT motifs in FWR1 and FW3, and certain RGYW motifs in CDR2 and CDR3 are lower level hotspots in both productive and passenger alleles, which are evident at intermediate mutation levels and more so in highly mutated sequences, reaching mutation in 20–40 % of sequences (Figure 2A). Within all productive and passenger sequences with high SHM levels, AGCT and RGYW motifs contribute most residues with mutations well above background; those above background in non-RGYW motifs tend to occur adjacent to CDRs where they may, in part, result from SHM spreading from initiating G/C base pairs (bp)(Figure 2A). Finally, the SHM frequency at each bp of the productive allele is highly correlated with that of the passenger allele at each strata (Pearson correlation, r =0.75–0.87) (Figure S2C). Overall, these findings support a “hierarchical” SHM process by which mutations are targeted preferentially to a few preferred CDR hotspot motifs, followed by accumulation of mutations in other AID target motifs sites and eventually in non-RGYW sequences that in part represent SHM spreading to other sites.
Peyer’s Patches GC B cells Contain Highly Mutated but Unselected BCR Repertoire
We also examined the VB1–8 productive and passenger alleles in purified PP GC B cells from the same short-term immunized chimeras described above. Again, productive and passenger VB1–8 alleles in PP GC B cells of a given chimera had very similar overall SHM levels (Figures 1B, middle and S1F, left). A major difference between SHM of productive and passenger VB1–8 sequences between splenic and PP GC B cells was that frequency of SHMs on each was substantially higher in PP GC B cells than in splenic B cells (Figure 1B, bottom). PP GC B cells also had a significantly greater percentage of highly mutated sequences (>20 mutations per sequence) than splenic GC B cells (Figure S1F, right). Notably, despite having accumulated higher levels of SHMs, PP GC B cells exhibited very similar SHM patterns to those of splenic GC B cells on both productive and passenger alleles (Figures 2B, S2A, right and S2B, bottom). In particular, productive and passenger alleles in PP GC B cells had the same major and lower level hotspots (Figure 2B) with the only minor difference being a slight dampening of the more major hotspot levels on passenger versus productive allele sequences (Figure 2B), which may reflect loss of some via AID-initiated deletions (see below). Nevertheless, in PP GC B cells there were high correlations between the mutation frequencies at each bp across each strata when comparing productive to the passenger alleles (Pearson correlation, r = 0.71–0.85) (Figure S2D), or when comparing the productive allele on PP GC B cells to the productive allele in splenic GC B cells (Pearson correlation, r=0.80–0.90) (Figure S2E). Thus, in both splenic and PP GC B cells, SHM patterns reflect preferred sites of AID activity on the VB1–8 sequence rather than selection for a specific response. PP GC B cells of 4 unimmunized mice showed similar hotspots and highly correlated SHM profiles (Figures S1C and S2F–G). These findings suggest that the BCR repertoire of PP GC B cells may reflect SHM activity on a germline V(D)J repertoire in the absence of antigen selection.
Regions That Are Highly Targeted for SHM Are Also Highly Targeted for DSBs
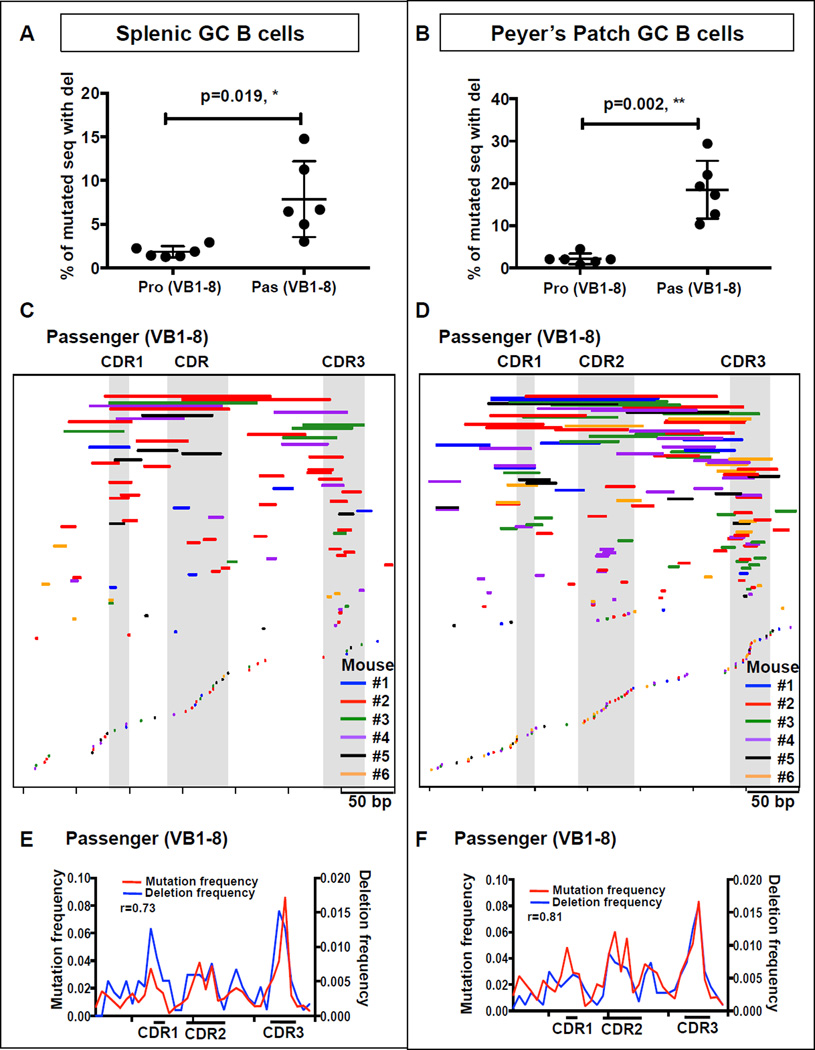
We found deletions on average in about 8 % of splenic and 19 % of PP GC B cell mutated passenger allele sequences, but only in about 2 % of the corresponding productive sequences (Figures 3A–B; Table S2A). Given that VB1–8 passenger and productive alleles accumulated SHMs similarly, relative absence of deletions in productive alleles likely represents negative cellular selection against B cells with BCR-inactivating deletions. We also did similar analysis of unique (excluding duplicates) sequences and reached the same conclusions (Figures S3A–B; Table S2B). For both splenic and PP GC passenger VB1–8 sequences, deletion frequency was directly related to SHM frequency as sequences with higher levels of mutations had higher levels of deletions (Figures S3C–D). In this regard, many unique deletions in passenger VB1–8 alleles (Figures 3C–D) (and productive alleles, Figures S3E–F) have endpoints that occur in and around VB1–8 CDR sequences, the sites that harbor the highest SHM levels (Figure 2). The location of deletions endpoints versus SHMs along VB1–8 passenger sequences was highly correlated for both splenic GC B cell sequences (Figure 3E) and PP GC B cell sequences (Figure 3F). Thus, AID-targeting for SHMs in VB1–8 frequently generates DSBs that are the precursors for deletions. Finally, we found very low levels of insertions in the assayed VB1–8 productive and passenger allele sequences (Figures S3G–H).
Figure 3. Deletions in VB1–8 Passenger Allele.
(A and B) Deletion frequency, calculated as the % of all mutated sequences that contain deletions of VB1–8 productive and passenger alleles in (A) splenic and (B) PP GC B cells. Data are represented as mean ± SD from 6 mice. Two-tailed, paired t test p values are indicated. (C and D) Map of unique deletions in VB1–8 passenger allele from (C) splenic and (D) PP GC B cells. Deletions are represented by lines whose start and end indicate the start and end of the deletion. Deletions from each of the 6 mice are displayed with a line of a different color. (E and F) The location of SHMs compared to the location of deletion endpoints. Pearson correlation coefficient (r) between SHM frequency and deletion (endpoint) frequency of each bin in (E) splenic and (F) PP GC B cells are indicated. See also Figure S3 and Table S2.
Non-Ig Sequences Target as Robust SHM as V exons
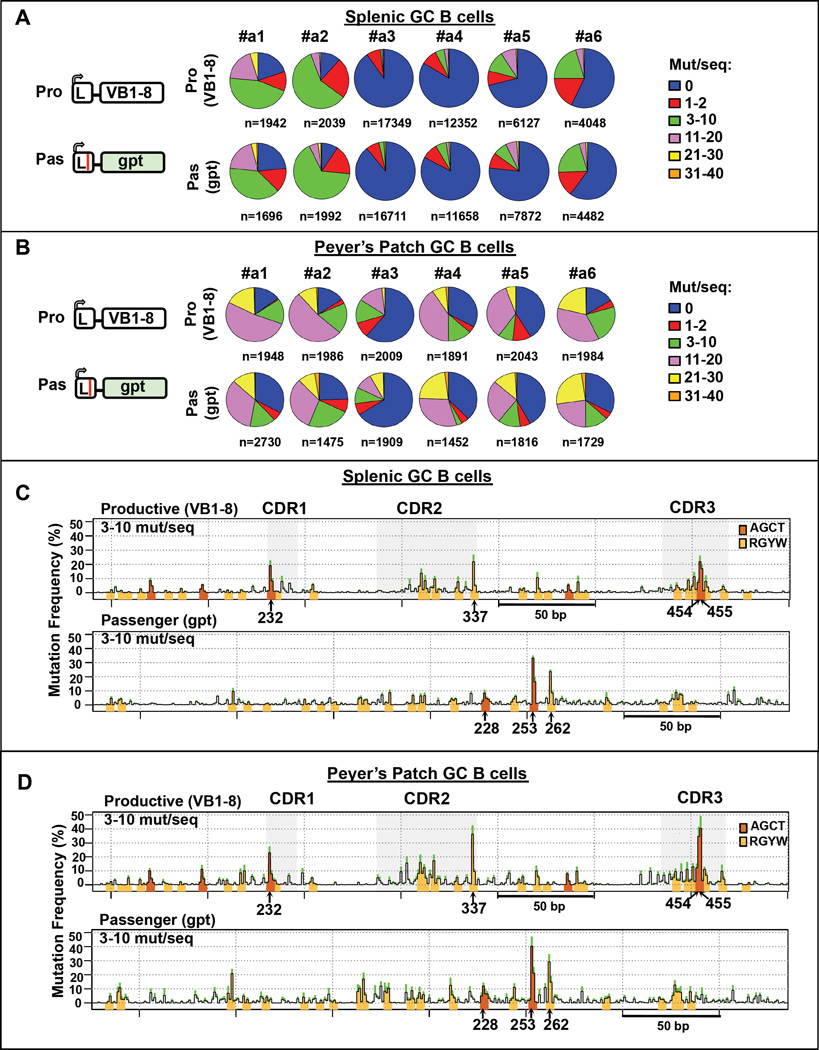
To address the question of whether high-level SHM targeting is unique to V exon CDR sequences, we assayed SHM activity on two non-Ig passenger allele substrates, an E. coli gpt gene (Figures 4 and S4) and a human β-globin gene (Figures 5 and S5), and compared SHM frequency on each passenger to that of its corresponding productive VB1–8 allele. These non-Ig sequence passengers were matched to the VB1–8 sequence in size (360 bp) and also contained similar densities of AID targeting motifs (23 RGYWs in the gpt sequence and 30 RGYWs in the β-globin sequence, compared to 31 RGYWs in the VB1–8 sequence). Both bacterial gpt and β-globin passengers undergo SHM and have remarkably similar frequency distributions of mutations per sequence and mutation frequencies to the productive V(D)J allele (Figures 4A–B, 5A–B, S4A and S5A). The mutation frequency of each type of base substitution also was similar between non-Ig passengers and their corresponding VB1–8 productive alleles (Figures S4B, top and S5B, top). As for VB1–8 passengers, non-Ig passengers were more highly mutated in PP GC B cells (Figures S4C and S5C). SHM profiles of passenger gpt and β-globin sequences show that hotspots occurred mainly on RGYW motifs including AGCTs and that the same hotspots were identified in non-Ig passenger sequences from splenic and PP GC B cells (Figures 4C–D and 5C–D). SHM of the most robust hotspots in the non-Ig passengers (e.g. nucleotides G of AGCT motif at position 253 in gpt and in position 292 in β-globin) showed mutation frequencies comparable to those of VB1–8 AGCTs in CDRs (e.g. nucleotides G at position 454 or 232) (Figures 4C–D, 5C–D, S4B, bottom and S5B, bottom), with very similar "kinetics" of accumulation at different strata (Figures S4D–E and S5D–E). As for VB1–8 passengers, SHM patterns in splenic GC B cells and PP GC B cell sequence were very similar, despite the latter accumulating higher levels of mutations (Figures 4C–D, 5C–D, S4D–E and S5D–E). Also like the VB1–8 passenger, gpt and β-globin passenger sequences underwent substantial levels of deletions (Figures S4F–G and S5F–G), with endpoints highly correlated with the location of SHMs (Figures S4H–I and S5H–I).
Figure 4. E.coli gpt Sequence Mutates as Frequently as VB1–8 Sequence.
(A–B) Left: Schematic of productive VB1–8 and passenger gpt alleles. Middle: Pie charts showing proportion of sequences that have the indicated (see legend, right) number of mutations per sequence in productive VB1–8 and passenger gpt allele from (A) splenic and (B) PP GC B cells of 6 independent mice. (C–D) SHM profiles of productive VB1–8 and passenger gpt allele from (C) splenic and (D) PP GC B cells from 6 mice. The y-axis and other details are as described for Figure 2. Data from mutation strata 3–10 mutation/sequence is shown. See also Figure S4.
Figure 5. Human β-globin Sequence Mutates as Frequently as VB1–8 Sequence.
(A–B) Left: Schematic of productive VB1–8 and passenger β-globin alleles. Middle: Pie charts showing proportion of sequences that have the indicated (see legend, right) number of mutations per sequence in productive VB1–8 and passenger β-globin allele from (A) splenic and (B) PP GC B cells of 5 independent mice. (C–D) SHM profiles of productive VB1–8 and passenger β-globin allele from (C) splenic and (D) PP GC B cells from 5 mice. The y-axis and other details are as described for Figure 2. Data from mutation strata 3–10 mutation/sequence is shown. See also Figure S5.
SHM Profile of S Regions in GC B Cells
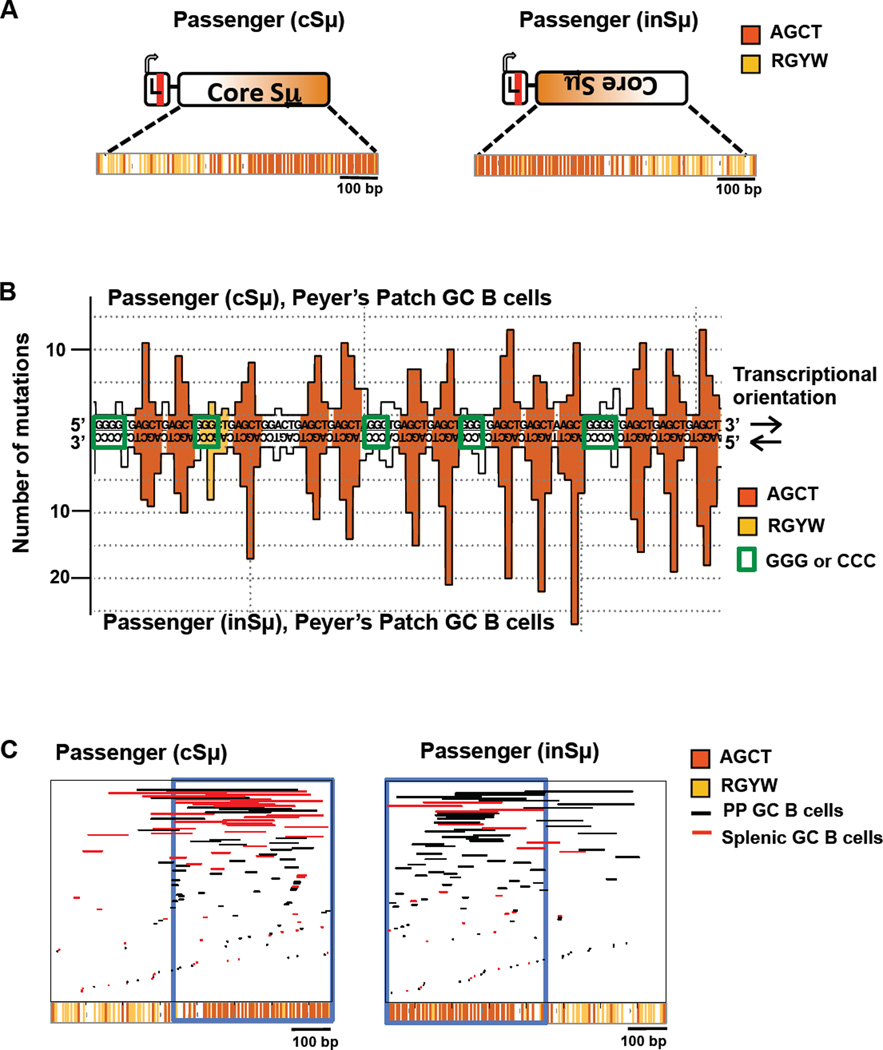
Analyses of SHM patterns of core mouse S regions have been hindered by their highly repetitive sequence (Rouaud et al., 2013). We circumvented this limitation by using a 756 bp truncation of Sμ, which includes 426 bp of the Sμ core as a passenger, in both direct and inverted orientations ("cSμ" and "inSμ" respectively; Figure 6A). For analyses, the cSμ and InSμ passengers were sequenced by Sanger method, since we could not optimize high-throughput sequencing through these highly repetitive sequences. Given the more limited number of reads obtained by this approach, we focused on SHM patterns, by pooling reads from six mice and plotting for unique sequences the total number of mutations at each bp. For both cSμ and InSμ passengers, each of the densely packed S region AGCT motifs are SHM hotspots in both PP GC B cells (Figures 6B, S6A and S6B) and splenic GC B cells (Figure S6C). The symmetrical AID targeting of SHMs of cytidines within AGCT palindromes in both transcriptional directions likely facilitates S region DSBs as proposed (Di Noia and Neuberger, 2007). Further supporting the importance of the AGCTs to DSB formation, the great majority of deletions in passenger cSμ and InSμ sequences map to regions of high AGCT density which occur on opposite ends of the cSμ and InSμ passenger sequences (Figures 6C and S6D–E). Finally, intervening G/C rich stretches between AGCT hotspots are rarely SHM targets in the passenger sequences (Figures 6B and S6C). As these G/C stretches are highly conserved in mammalian S regions, our findings suggest that they may provide functions other than serving as targets for AID-initiated lesions.
Figure 6. SHM Profiles and Deletion Maps of Switch Regions.
(A) Schematic of passenger core Sμ (cSμ) (left) and inverted Sμ (inSμ) (right) alleles. (B) Excerpt of SHM pattern of cSμ (top) and inSμ (bottom) passengers from PP GC B cells. The y-axis indicates the total number of mutations. Green open boxes show the positions of G-stretches and C-stretches on cSμ and inSμ alleles, respectively. (C) Location of unique deletions in passenger cSμ (left) and inSμ alleles (right). Deletions from splenic GC B cells are depicted with red lines and PP GC B cells with black lines. For (B) and (C), data are pooled from 6 mice. See also Figure S6.
SHM of V region in B cells Activated In Culture
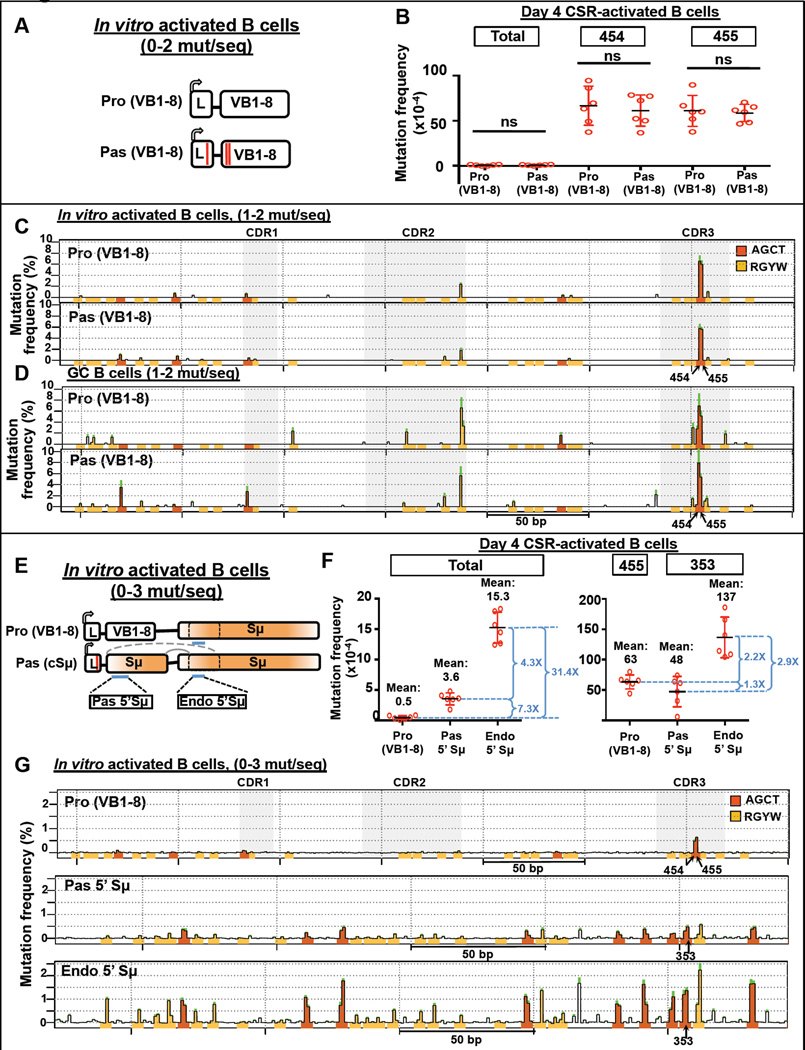
While S regions are AID targets in both GC and CSR-activated B cells in culture, V exons reportedly are targeted by AID only in GC B cells (Storb, 2014; Nagaoka et al., 2002; Maul et al., 2014), leading to the lingering question of how AID is targeted to S regions but not adjacent IgH V exons, given that both are transcribed (Storb, 2014). We re-evaluated this notion, now with the insight that SHMs of VB1–8 in GC B cell sequences with low SHM levels concentrate in two CDR3 AGCT hotspots (positions 454 and 455). Consistent with prior findings, SHM along the length of productive and passenger VB1–8 sequences is close to assay background in B cells stimulated in culture for 4 days with αCD40 plus IL-4 to induce CSR to Sγ1 and Sε (Figures 7A–B and S7A–C). However, examination of SHM at VB1–8 hotspot nucleotides 454 and 455 of productive and passenger VB1–8 alleles in CSR-activated B cells reveals that they undergo readily detectable and similar levels of SHM (Figure 7B) and deletions (Figures S7D–H). Indeed, comparison of SHM profiles of productive and passenger VB1–8 alleles of CSR-activated B cells with those of GC B cell sequences with similarly low SHM levels (1–2 mutations/sequence), reveals highly similar profiles and extent of hotspot nucleotide 454 and 455 mutation (Figures 7C–D).
Figure 7. SHM of the V exon and S region in B cells Activated in Culture.
(A) Naïve B cells of VB1–8 passenger mice were stimulated in culture for 4 days with αCD40 and IL4. (B) Mutation frequency of total nucleotides, nucleotide 454 and 455 of VB1–8 productive and passenger alleles at day 4. All sequences that have 0–2 mutations per sequence were included in the analysis. Unpaired t-test was performed. (C–D) SHM profiles of matched VB1–8 productive and passenger alleles in sequences that contain 1–2 mutations/sequence from (C) day 4 CSR-activated B cells and (D) splenic GC B cells shown in Figure 2A. Peaks that have a SD greater than the mean were excluded. (E) Naïve B cells of cSμ passenger mice were stimulated in culture for 4 days with αCD40 and IL4. The 5’ region immediately upstream of the core in passenger Sμ (Pas 5’ Sμ) and endogenous Sμ (Endo 5’ Sμ), indicated in blue lines, were analysed. (F) Mutation frequency over entire indicated allele/region (left), and at individual nucleotides indicated (right). All sequences that have 0–3 mutations/sequence were included in the analysis. The mean values and fold change between each mean are indicated. (G) SHM profiles of VB1–8 productive allele, Pas 5’ Sμ and Endo 5’ Sμ of data shown in (F). For (B and F) data represent mean ± SD of 6 independent stimulation of cells from 6 independent mice. For (C, D and G) data represent mean frequency ± SEM of 6 independent mice. The y-axis and other details are as described for Figure 2. For (B, C F, G), data shown are mutation frequency at day 4 after subtraction of mutation frequency at day 0. See also Figure S7 and Supplemental Experimental Procedures.
To compare mutation frequencies of hotspots in VB1–8 to those in S regions, we assayed 4 day CSR-activated B cells that harbored a productive VB1–8 and a passenger Sμ For these analyses, we sequenced a portion of passenger cSμ amenable to high throughput analysis ("Pas 5’ Sμ") and also the same portion of the endogenous Sμ ("Endo 5’ Sμ") (Figure 7E). The overall mutation frequency of the Pas 5’ Sμ was much greater (more than 7-fold) than that of the productive VB1–8 exon (Figures 7F, left and S7I–K). However, comparison of the SHM level of VB1–8 nucleotide 455 to that of a representative S region AGCT hotspot at nucleotide 353 revealed them to be quite similar (Figure 7F, right). Thus, the increased frequency of overall SHM of Pas 5'Sμ compared to that of VB1–8 is generated by the much larger number of AGCT (and related) hotspots in Pas 5' Sμ, rather than increased AID-activity at a given hotspot (Figure 7G). Notably, the endogenous 5’ Sμ sequence undergoes SHM at AGCT hotspots at somewhat increased levels (approximately 3 fold) compared to that of AGCT motifs in the Pas 5'Sμ (Figures 7F, right and 7G). This increase could result from different transcriptional promoters and/or effects of having an adjacent full length S region that could influence AID recruitment via transcription-associated mechanisms and/or by promoting longer stable R loops that increase AID access. Finally, cSμ passenger sequences undergo internal deletions (Figures S7L–P) and support robust CSR-like events to Sε in about 20 % of the day 4 activated B cells (Figures S7Q–R). As Sanger sequencing in GC B cells indicates that SHM frequency 5’ Sμ AGCT hotspots are similar to that of such hotspots across core Sμ (Figure S6A), we conclude that relatively low SHM levels of individual AGCT motifs in cSμ collectively are sufficient to drive CSR.
DISCUSSION
We describe a rapid passenger allele approach to test intrinsic capacity and preferences of AID to induce SHMs and DSBs within test sequences that replace a non-productive IgH V exon. A key feature of the approach is lack of cellular selection for or against mutations and deletions, enabling insights into roles of substrate sequences in AID-targeting. In this regard, the assay uses short immunization protocols that do not activate affinity maturation; thus, SHM patterns on identical productive and passenger V exon sequences in GC B cells are remarkably similar, both in extent and detail. This assay feature allows use of the same productive allele to standardize overall SHM experienced by different passengers in different experiments. Additional assay innovations include retaining all recovered sequences for analysis and relying on multiple biological replicates to validate conclusions, allowing detection of AID hotspots even at low overall SHM levels, such as those early in an immune response or the previously unappreciated V exon SHMs in cultured CSR-activated B cells. Also, by dividing recovered sequences into mutational strata, we can estimate relative mutational kinetics at which SHMs of a particular nucleotide appear, revealing early and late hotspots, as well as “spreading” of SHMs from CDR hotspots to adjacent regions. This overall approach allowed us to address long-standing questions in the SHM and CSR field (see below). Finally, many of these powerful assay features could be extended to dissect contributions of sequence-intrinsic AID targeting to affinity maturation and antibody responses by using an antigen-specific productive V(D)J exon and a matched passenger V(D)J exons in the context of longer term immunizations.
Mechanisms That Target SHM Activity
We addressed the long-standing question of whether V exon sequences are required to recruit high-level SHM activity. Our studies reveal that single-copy bacterial gpt and human β-globin sequences in the endogenous V exon location have overall SHM frequencies similar to V exons and that some of their AID target motifs mutate at similar frequencies as the same motifs in V exon CDR sequences. These findings prove that high level SHM targeting in GC B cells is not specific to V exons, or even CDR sequences; establishing definitively that the V exon location is privileged for SHM. Mechanisms that make V exon location privileged for AID targeting may involve linked enhancers (Odegard and Schatz, 2006; Buerstedde et al., 2014; Rouaud et al., 2013), adjacent AID recruitment motifs (Storb, 2014), or transcriptional-related mechanisms, such as convergent sense/antisense transcription (Meng et al., 2014). SHM versus CSR specific AID-targeting factors were hypothesized based on the notion that V exons do not undergo SHM in CSR-activated B cells (Muramatsu et al., 2007); however, our finding that V exons are targeted for SHM in CSR-activated B cells obviates a strict requirement for such factors (see below).
It has generally been thought that CDRs are highly evolved to target high SHM levels (e.g. Wei et al., 2015). Consistent with this notion, AGCT or AGCA motifs in the V(D)J exon CDRs are hotspots while the same motifs in FWRs are not (Figure 2). However, we find similar examples in the bacterial gpt sequence where one AGCT (position 253) is as strong a hotspot as those in VB1–8 CDRs, while another is not (position 228) (Figures 4C–D). Likewise, in the β-globin sequence, AACC at position 193 is a major hotspot, but the identical motif at position 262 is not (Figures 5C–D). Thus, elements that can enhance SHM at CDRs and/or suppress SHM at FWRs are not specialized to V exons or to CDR sequences. Such relatively recurrent hotspot sequence combinations are likely evolutionarily selected for in CDRs and against in FWRs. In ongoing studies, we are using the passenger system to test additional sequence combinations flanking AGCT hotspots within otherwise randomized synthetic sequences to ultimately elucidate sequences that define an AID hotspot in the V exon location.
SHMs and DSBs are Targeted by the Same Mechanism
We now show that deletions occur frequently on highly mutated V(D)J exons as part of a normal SHM process. In this regard, we demonstrate a clear and direct relationship between SHM activity and DSB activity on a V exon, with the highest density and the majority of deletion endpoints focused at locations of highest level SHM (e.g. at AGCTs within CDR sequences). In addition, we find that this same relationship holds for non-Ig passenger sequences. Taken together, our findings reveal that DSB generation within V(D)J exons is a common and normal outcome of SHM activity. These findings answer a long-standing question in the vaccine field regarding how bnAbs with Ig chains encoded by V exons harboring deletions arise by showing that specialized mechanisms are not necessary. Our findings that highly mutated PP GC B cells contain abundant V exon deletions offers the potential of developing new strategies to elicit such antibodies (see below).
Features That Optimize S region sequences for CSR
By using a passenger Sμ region we are able to view in depth the SHM and DSB (deletion) pattern of core S regions. Mammalian Sμ contains large numbers of densely packed repeats closely related to the sequence GGGGTGAGCT. Strikingly, within such core Sμ repeats, Cs on both strands of the canonical AGCT hotspot duplexes were robustly targeted in both transcriptional orientations in GC B cells. In this regard, DSBs/deletions in passenger core Sμ were highly enriched in regions of high AGCT density, independent of transcriptional orientation, providing direct support for the model that the high density of these palindromic targets allows generation of closely spaced AID-initiated lesions on both strands that then promote DSBs (Di Noia and Neuberger, 2007). Notably, however, we find little or no targeting of Cs in Sμ passengers that occur opposite the conserved template strand Sμ repeat G-stretches, even if Sμ transcriptional orientation is reversed. This latter finding is consistent with adjunct functions, perhaps in AID recruitment via R-loops (Shinkura et al., 2003), RNA-mediated mechanisms (Zheng et al, 2015), and/or potential roles in S region synapsis (Maizels and Gray, 2013).
Prior studies concluded that V exons are not SHM targets in B cell activated in culture. Consistent with this notion, we found the passenger Sμ had an approximately 7-fold greater overall mutation frequency than the V exon in CSR-activated B cells. However, AGCT hotspot motifs in V exons and S region passengers were targeted at similar levels in these cells with the increased overall SHM frequency of the S region passenger reflecting a greater number of AGCT hotpots. Indeed, endogenous Sμ region AGCT motifs are targeted only about 2-fold more frequently than AGCT hotspots in passenger V exons. Notably, the pattern and extent of V exon hotspot mutations in CSR-activated B cells is similar to that of GC B cells early in the process of SHM accumulation. Thus, we suggest that SHM is limited in CSR-activated B cells because they have not been exposed to sufficient AID-activity, either due to AID levels, length of exposure, or both. Still, Sμ passengers, while having similarly low-level SHM at AID hotspot motifs as GC B cells early in the process, are targeted for sufficient DSBs to promote relatively robust joining to downstream S region sequences in a CSR-like process. Thus, the great abundance of palindromic AGCT hotspots make S regions highly sensitive substrates for generating CSR-initiating DSBs at relatively low-level AID exposure. Accordingly, the limited number of highly targeted AGCT motifs in the V exon results in fewer deletions/DSBs in the V exon compared to the S region. Based on our findings, we propose that ability of S regions to undergo DSBs with limited AID exposure evolved to allow efficient IgH CSR, without generating substantial SHM of adjacent V exons that could affect antibody affinity or specificity.
Implications for Generation of Highly Mutated Antibodies During an Immune Response
Microbial antigens promote formation of PP GCs and IgL V exon SHM without immunization (González-Fernández and Milstein, 1993) and even in the absence of antigen recognition via IgH or IgL chains in the context of a BCR (Casola et al., 2004). The latter studies led to the hypothesis that chronic activation of PP GC B cells expands primary V(D)J recombination-generated antibody repertoires via extensive SHM in the absence of antigenic engagement of BCR (Casola et al., 2004). Our studies support this hypothesis by showing that productive and passenger IgH VB1–8 alleles in PP GC B cells undergo similarly high levels and specific patterns of SHMs, with the same set of major hotspots that reflect preferred AID SHM targets. Indeed, under short-term immunization conditions, SHM patterns of VB1–8 productive and passenger sequences were highly similar between splenic and PP GC B cells. Moreover, each Ig or non-Ig passenger, respectively, had similar SHM and deletion patterns in splenic versus PP GC B cells, except that levels were higher in the latter. Notably, primary hotspots in PP GC B cell productive and passenger alleles become saturated at high SHM levels, with additional SHMs accumulating in secondary hotspots or non-hotspot regions, allowing further diversification that might be enhanced by primary hotspot saturation. While our current studies do not assay for theoretical contributions of endogenous IgL chains, they clearly show that PP GC B cells harbor a diverse IgH VB1–8 repertoire via high level SHM in the absence of obvious selection. This process could generate B cell lineages that provide V exons with high levels of mutations and deletions as substrates for further mutation and selection via antigen-specific responses, providing greater diversity for additional mutational and selective forces. Our findings, coupled with prior findings (Casola et al, 2004) raise the possibility of tapping the highly mutated PP GC B cell antibody reservoir as an approach to elicit anti-HIV1 bnAbs or other antibodies with abundant SHMs and/or deletions.
EXPERIMENTAL PROCEDURES
Generation of Targeted ES cells
An ES cell line containing a VB1–8 productive allele (Sonoda et al., 1997) and a puromycin cassette in place of the V(D)J exon on the other allele was generated. ES cell lines containing passenger alleles were generated by replacing the puromycin cassette with the respective passenger sequences (see Supplemental Experimental Procedures for details). Chimeric mice were generated by RAG-2 blastocyst complementation (Chen et al., 1993).
Assays for SHM
GC B cells from spleen and PP were collected from 8–12 week old mice immunized with NPCGG (N-5055A, Biosearch Technologies) for 10 days. GC B cells were isolated from PPs and spleens of chimeras by flow cytometry sorting of cells that are B220+ and Peanut Agglutinin (PNA) hi. B220+ PNA lo cells were also collected during each sort and (along with ES cell DNA) served as negative controls for SHM. For SHM analysis of CSR-activated B cells in vitro, naïve B cells collected from the spleen of 6–8 week old mice were stimulated with αCD40 and IL4 for 4 days (see Supplemental Experimental Procedures for details).
PCR and Sequencing
The productive and passenger alleles were amplified in separate PCR reactions using allele-specific primers. The products were sequenced by 2 × 250bp Illumina MiSeq high throughput sequencing (Illumina) or Sanger sequencing systems where indicated in the text. For Illumina high throughput sequencing, sequencing adapters and barcodes were added to the end of DNA fragments by a second round of PCR (see Supplemental Experimental Procedures for details).
Data Analysis
A custom pipeline was used to process the sequencing data and to call mutations. To compare the location of deletion endpoints versus SHMs along passenger sequences, we measured the frequency of each within 10 bp bins and calculated the Pearson correlation (r) between deletion endpoint frequency and mutation frequency (see Supplemental Experimental Procedures for details). Statistical analysis is as stated in Figure Legends.
Supplementary Material
Acknowledgments
The authors thank Dr. Kefei Yu for the RMCE exchange plasmid, Klaus Rajewsky for the JH targeting construct, Pei-Yi Huang and Yuko Fujiwara for generating the chimeras, Kenneth Ketman and Natasha Barteneva for help with FACS sorting, and Garnett Kelsoe for helpful discussions. This work was supported by NIH grants R01AI077595 and CHAVI-ID 5UM1AI100645 (to FWA) and R21AI110777-01A1, R21CA184707-01A1, and R01CA166325-01A1 (to JHW). J.K.H. was supported by NIH grant F30AI114179-01A1. D.N. is supported by Dana Farber/ Harvard Cancer Center Core Grant 5P30 CA006516. J.H.W. is also supported by a Boettcher Foundation Webb-Waring Biomedical Research Award, an American Society of Hematology Scholar Award, and the Cancer League of Colorado. FWA is an investigator and ZD a postdoctoral fellow of the Howard Hughes Medical Institute. L.S.Y. was a Cancer Research Institute postdoctoral fellow and F.L.M. is a Lymphoma Research Foundation postdoctoral fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Number
The NCBI Sequence Read Archive accession number for next-generation sequencing data reported in this paper is SRP061422.
AUTHOR CONTRIBUTIONS
L.S.Y., J.K.H., J.H.W. and F.W.A. designed the study. L.S.Y, J.K.H, F.L.M., A.J., M.L., V.M. and J.H.W performed experiments. Z.D. and R.M.M. performed bioinformatics analyses, and R.M.M. designed the SHM pipeline. L.S.Y, J.K.H, J.H.W., Z.D. and F.W.A. analyzed and interpreted data. D.N. and T.B.K. advised on statistical analysis and various other aspects of data analysis. L.S.Y., J.K.H., Z.D., R.M.M. and F.W.A. designed figures. L.S.Y., J.K.H. and F.W.A. drafted the manuscript, and L.S.Y., J.K.H., Z.D., R.M.M., T.B.K., J.H.W. and F.W.A. polished the manuscript.
REFERENCES
- Alt FW, Zhang Y, Meng FL, Guo C, Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–429. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AG, Neuberger MS, Milstein C. Discriminating intrinsic and antigen-selected mutational hotspots in immunoglobulin V genes. Immunol Today. 1993a;14:405–411. doi: 10.1016/0167-5699(93)90144-a. [DOI] [PubMed] [Google Scholar]
- Betz AG, Rada C, Pannell R, Milstein C, Neuberger MS. Passenger transgenes reveal intrinsic specificity of the antibody hypermutation mechanism: clustering, polarity, and specific hot spots. Proc Natl Acad Sci U S A. 1993b;90:2385–2388. doi: 10.1073/pnas.90.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Crowe JE., Jr Location and length distribution of somatic hypermutation-associated DNA insertions and deletions reveals regions of antibody structural plasticity. Genes Immun. 2012;13:523–529. doi: 10.1038/gene.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross L, Fukita Y, McBlane F, Demolliere C, Rajewsky K, Jacobs H. DNA double-strand breaks in immunoglobulin genes undergoing somatic hypermutation. Immunity. 2000;13:589–597. doi: 10.1016/s1074-7613(00)00059-5. [DOI] [PubMed] [Google Scholar]
- Buerstedde JM, Alinikula J, Arakawa H, McDonald JJ, Schatz DG. Targeting of somatic hypermutation by immunoglobulin enhancer and enhancer-like sequences. PLoS Biol. 2014;12(4):e1001831. doi: 10.1371/journal.pbio.1001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Chahwan R, Edelmann W, Scharff MD, Roa S. AIDing antibody diversity by error-prone mismatch repair. Semin Immunol. 2012;24:293–300. doi: 10.1016/j.smim.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- Dorner T, Brezinschek HP, Brezinschek RI, Foster SJ, Domiati-Saad R, Lipsky PE. Analysis of the frequency and pattern of somatic mutations within nonproductively rearranged human variable heavy chain genes. J Immunol. 1997;158:2779–2789. [PubMed] [Google Scholar]
- Goossens T, Klein U, Kuppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci U S A. 1998;95:2463–2468. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández A, Milstein C. Analysis of somatic hypermutation in mouse Peyer’s patches using immunoglobulin κ light-chain transgenes. Proc. Natl Acad Sci U S A. 1993;90:9862–9866. doi: 10.1073/pnas.90.21.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney JA, Misaghi S, Senger K, Garris C, Sun Y, Lorenzo MN, Zarrin AA. DNA targets of AID evolutionary link between antibody somatic hypermutation and class switch recombination. Adv Immunol. 2009;101:163–189. doi: 10.1016/S0065-2776(08)01005-5. [DOI] [PubMed] [Google Scholar]
- Han L, Masani S, Yu K. Overlapping activation-induced cytidine deaminase hotspot motifs in Ig class-switch recombination. Proc Natl Acad Sci U S A. 2011;108:11584–11589. doi: 10.1073/pnas.1018726108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JK, Alt FW, Yeap LS. Related Mechanisms of Antibody Somatic Hypermutation and Class Switch Recombination. Microbiol Spectr. 2015;3(1) doi: 10.1128/microbiolspec.MDNA3-0037-2014. MDNA3-0037-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler TB, Liao HX, Alam SM, Bhaskarabhatla R, Zhang R, Yandava C, Stewart S, Anasti K, Kelsoe G, Parks R, et al. Immunoglobulin gene insertions and deletions in the affinity maturation of HIV-1 broadly reactive neutralizing antibodies. Cell Host Microbe. 2014;16:304–313. doi: 10.1016/j.chom.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanning DK, Knight KL. Intestinal bacteria and development of the antibody repertoire. Discov Med. 2005;5:393–398. [PubMed] [Google Scholar]
- Liu M, Schatz DG. Balancing AID and DNA repair during somatic hypermutation. Trends Immunol. 2009;30:173–181. doi: 10.1016/j.it.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Maizels N, Gray LT. The G4 genome. PLoS Genet. 2013;9(4):e1003468. doi: 10.1371/journal.pgen.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul RW, Cao Z, Venkataraman L, Giorgetti CA, Press JL, Denizot Y, Du H, Sen R, Gearhart PJ. Spt5 accumulation at variable genes distinguishes somatic hypermutation in germinal center B cells from ex vivo-activated cells. J Exp Med. 2014;211:2297–2306. doi: 10.1084/jem.20131512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul RW, Gearhart PJ. AID and somatic hypermutation. Adv Immunol. 2010a;105:159–191. doi: 10.1016/S0065-2776(10)05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul RW, Gearhart PJ. Controlling somatic hypermutation in immunoglobulin variable and switch regions. Immunol Res. 2010b;47:113–122. doi: 10.1007/s12026-009-8142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng FL, Du Z, Federation A, Hu J, Wang Q, Kieffer-Kwon KR, Meyers RM, Amor C, Wasserman CR, Neuberg D, et al. Convergent transcription at intragenic super-enhancers targets AID-initiated genomic instability. Cell. 2014;159:1538–1548. doi: 10.1016/j.cell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol. 2007;94:1–36. doi: 10.1016/S0065-2776(06)94001-2. [DOI] [PubMed] [Google Scholar]
- Nagaoka H, Muramatsu M, Yamamura N, Kinoshita K, Honjo T. Activation-induced deaminase (AID)-directed hypermutation in the immunoglobulin Smu region: implication of AID involvement in a common step of class switch recombination and somatic hypermutation. J Exp Med. 2002;195:529–534. doi: 10.1084/jem.20012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol Cell. 2004;16:163–171. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Rouaud P, Vincent-Fabert C, Saintamand A, Fiancette R, Marquet M, Robert I, Reina-San-Martin B, Pinaud E, Cogne M, Denizot Y. The IgH 3' regulatory region controls somatic hypermutation in germinal center B cells. J Exp Med. 2013;210:1501–1507. doi: 10.1084/jem.20130072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saribasak H, Gearhart PJ. Does DNA repair occur during somatic hypermutation? Semin Immunol. 2012;24:287–292. doi: 10.1016/j.smim.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkura R, Tian M, Smith M, Chua K, Fujiwara Y, Alt FW. The influence of transcriptional orientation on endogenous switch region function. Nat Immunol. 2003;4:435–441. doi: 10.1038/ni918. [DOI] [PubMed] [Google Scholar]
- Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U. Why does somatic hypermutation by AID require transcription of its target genes? Adv Immunol. 2014;122:253–277. doi: 10.1016/B978-0-12-800267-4.00007-9. [DOI] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Wei L, Chahwan R, Wang S, Wang X, Pham PT, Goodman MF, Bergman A, Scharff MD, MacCarthy T. Overlapping hotspots in CDRs are critical sites for V region diversification. Proc Natl Acad Sci U S A. 2015;112:E728–E737. doi: 10.1073/pnas.1500788112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss U, Zoebelein R, Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992;22:511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501:112–115. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Schatz DG. Targeting of AID-mediated sequence diversification by cis-acting determinants. Adv Immunol. 2007;94:109–125. doi: 10.1016/S0065-2776(06)94004-8. [DOI] [PubMed] [Google Scholar]
- Yelamos J, Klix N, Goyenechea B, Lozano F, Chui YL, Gonzalez Fernandez A, Pannell R, Neuberger MS, Milstein C. Targeting of non-Ig sequences in place of the V segment by somatic hypermutation. Nature. 1995;376:225–229. doi: 10.1038/376225a0. [DOI] [PubMed] [Google Scholar]
- Zarrin AA, Alt FW, Chaudhuri J, Stokes N, Kaushal D, Du Pasquier L, Tian M. An evolutionarily conserved target motif for immunoglobulin class-switch recombination. Nat Immunol. 2004;5:1275–1281. doi: 10.1038/ni1137. [DOI] [PubMed] [Google Scholar]
- Zheng S, Vuong BQ, Vaidyanathan B, Lin JY, Huang FT, Chaudhuri J. Noncoding RNA Generated following Lariat Debranching Mediates Targeting of AID to DNA. Cell. 2015;161:762–773. doi: 10.1016/j.cell.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.