Abstract
Plants with a winter growth habit flower earlier when exposed for several weeks to cold temperatures, a process called vernalization. We report here the positional cloning of the wheat vernalization gene VRN2, a dominant repressor of flowering that is downregulated by vernalization. Loss of function of VRN2, whether by natural mutations or deletions, resulted in spring lines, which do not require vernalization to flower. Reduction of the RNA level of VRN2 by RNA interference accelerated flowering time of transgenic winter wheat plants more than a month.
Common wheat (Triticum aestivum L.) is one of the primary grains consumed by humans and is grown in very different environments. This wide adaptability has been favored by the existence of wheat varieties with different growth habits. Winter wheats require a long exposure to low temperatures to flower (vernalization) and are sown in the fall, whereas spring wheats do not have a vernalization requirement and can be planted in spring or fall. The genes from the vernalization pathway prevent flower development during the winter, providing protection for the temperature-sensitive floral organs against the cold.
VRN1 and VRN2 are the central genes in the vernalization pathway in wheat, barley, and other temperate cereals. These two genes have strong epistatic interactions and are likely part of the same regulatory pathway (1, 2). In both diploid wheat (Triticum monococcum L.) and barley, VRN1 is dominant for spring growth habit whereas VRN2 is dominant for winter growth habit. Similar epistatic interactions and map locations indicate that wheat and barley vernalization genes are orthologous (3, 4).
The VRN1 gene from wheat is similar to the Arabidopsis meristem identity gene APETALA1 (AP1) (5), which initiates the transition from the vegetative to reproductive apex. Natural allelic variation at the AP1 locus is associated with differences in vernalization requirement in wheat but not in Arabidopsis (5). In Arabidopsis, natural variation for vernalization requirement has arisen through the generation of non-functional or weak FRI and FLC alleles (6).
The Arabidopsis FLC gene is a repressor of flowering that integrates the signals from the autonomous flowering pathway with those from extended cold treatment. FRI elevates expression of FLC to levels that inhibit flowering, whereas vernalization produces a permanent downregulation of FLC and induces flowering (7–9).
Induced mutations have been used to identify additional vernalization genes in Arabidopsis. VIN3 is induced by vernalization and is involved in the transient repression of FLC by histone deacetylation (10). Two additional Arabidopsis genes, designated VRN1 and VRN2, are required to keep FLC in its repressed state, but not for its initial repression by cold (11, 12). Arabidopsis VRN1 and VRN2 are different from the wheat genes with the same names. The VRN1 and VRN2 names are standard designations for the wheat vernalization loci, and we will use them hereafter to refer to the cereal genes.
Positional cloning of the wheat vernalization gene VRN2
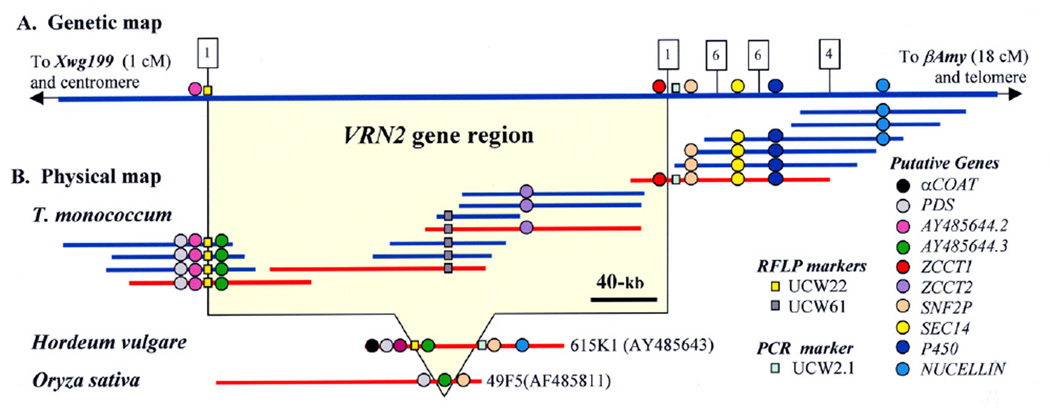
The VRN2 gene was initially mapped on chromosome 5A in a small F2 population from the cross between spring (DV92) and winter (G3116) accessions of diploid wheat T. monococcum (4). We used the same parental lines to develop a high-density map based on 5,698 gametes (Supporting Online Material, SOM-1). Screening of this population with VRN2 flanking markers NUCELLIN and UCW22 resulted in the identification of 18 recombination events within this region (Fig. 1 and SOM-1). Progeny tests for vernalization requirement were performed for these 18 plants. We generated additional markers from the BAC clones included in the physical map (Fig. 1) and defined the location of the two crossovers flanking VRN2 (SOM, Figs. S2, S3 and S4). The VRN2 gene was mapped into a 0.04-cM interval flanked by RFLP marker UCW22 and PCR marker UCW2.1 (Fig. 1).
Fig. 1.
A. Genetic map of the VRN2 region on chromosome 5Am of T. monococcum based on 5,698 gametes. Numbers of crossovers in the critical recombinant plants are indicated in boxes. B. Physical map of the wheat VRN2 region in T. monococcum and in colinear regions from barley and rice. BAC clones indicated in red have been sequenced (438,828-bp, AY485644). The order of BAC clones from left to right is: 374A18, 94E8, 304H18, 258C22, 301G15, 615O6, 650N20, 405L8, 271O11, 275P20, 157P20, 455C17, 322L23, 702K8, 32A1, 533H16 and 324G2 (bold letters indicate sequenced BACs). Additional information for the markers used in this figure has been deposited in the SOM.
Markers UCW22 and UCW2.1 were used to screen the BAC library of T. monococcum accession DV92 (13). A physical map of the VRN2 region was constructed by chromosome walking (Fig. 1). Overlapping BACs 258C22, 301G15, 405L8, and 455C17 were sequenced (438,828-bp), annotated and deposited in GenBank (AY485644). We also sequenced orthologous BAC 615K1 from barley variety Morex (AY485643) and BAC 49F5 from rice variety Nipponbare (AF485811).
Eight genes and one pseudogene were detected within the sequenced region indicating a gene density of one gene per 55-kb and a ratio between genetic and physical distances of approximately 1.7-Mb/cM. Five of these genes were found in the same order and orientation in the barley BAC and three in the rice BAC, confirming the colinearity of this region among cereals (Fig. 1). The closest common genes flanking the VRN2 gene, PDS and SNF2P, were 7-kb apart in rice, 26-kb apart in barley, and 328-kb apart in T. monococcum (Fig.1).
The sequences from markers UCW22 and UCW2.1 flanking the VRN2 gene in the genetic map (Fig. 1) were used to delimit a 315-kb candidate region within AY485644. Approximately 75% of this sequence was annotated as repetitive elements. Within the non-repetitive region only three genes were completely linked to VRN2. The first gene, designated AY485644.3, encoded a predicted 254-amino acid protein that was 87% and 96% similar to the putative orthologous proteins in the colinear BACs from rice and barley respectively (Fig. 1). The function of gene AY485644.3 is currently unknown.
The other two genes completely linked to VRN2 encoded proteins that were 76% identical, suggesting a duplication event that occurred approximately 14 ± 3 million years ago (SOM-2). These two proteins had similarities with CO and CO-like proteins of Arabidopsis (E= 2e−11) and rice (AP005307, OsIE= 3e−16; AAL79780, OsHE= 2e−16). This similarity was restricted to the 43-amino acids of the CCT (CO, CO-like, and TOC1) domain present in all these proteins (SOM-3, Fig. S10). This domain determines the nuclear localization of CO, the key gene in Arabidopsis photoperiod pathway (14, 15) and may have a similar function in these two genes. We named these two genes ZCCT1 and ZCCT2 based on the presence of a putative zinc finger in the first exon and the CCT domain in the second exon.
Evolutionary relationships between the ZCCT and CO-like genes
We isolated similar ZCCT genes from the A genome of tetraploid wheat (AY485979, AY485980) and from winter barley variety Diarokkaku (AY485977, AY485978), and compared their CCT domains with those from other CO-like genes (SOM-3, Fig. S10). We performed a Neighbor Joining cluster analysis using the CCT motifs from the ZCCT proteins and from members of each of the four major classes (I to IV) of CO-like proteins (16) (SOM-3, Fig. S11). CCT motifs from Group III (AtCOL9 and OsN) were used as an out-group. The ZCCT proteins formed a separate group that did not include any rice or Arabidopsis protein. This group was related to Group IV proteins (HvCO9, OsI, OsH), which included proteins only from grass species. Proteins from both Arabidopsis and grass species were present in the separate Groups I (AtCO, OsHD1) and II (AtCOL6, OsJ), including proteins involved in the regulation of flowering by photoperiod (SOM-3, Fig. S11). Analysis of the putative zinc fingers confirmed the classification based on the CCT domains (SOM-3, Fig. S12). CO-like proteins from Groups I and II have one or two B-box zinc fingers whereas the ZCCT proteins showed one C2H2 zinc finger. Group IV zinc fingers were more similar, although not identical, to those from the ZCCT proteins (SOM-3, Fig. S12).
These results suggest that the ancestor of the ZCCT and Group IV proteins originated in the grasses and that the ZCCT proteins diverged substantially in the temperate cereals species adapted to the cold regions. The divergence of the ZCCT proteins might have been favored by a duplication of the ancestral Group IV protein in the temperate cereals. This is suggested by the absence of ZCCT orthologues in rice in the colinear region between AY485644.3 and SNF2P (Fig. 1).
Variation of the RNA levels of the candidate genes during vernalization
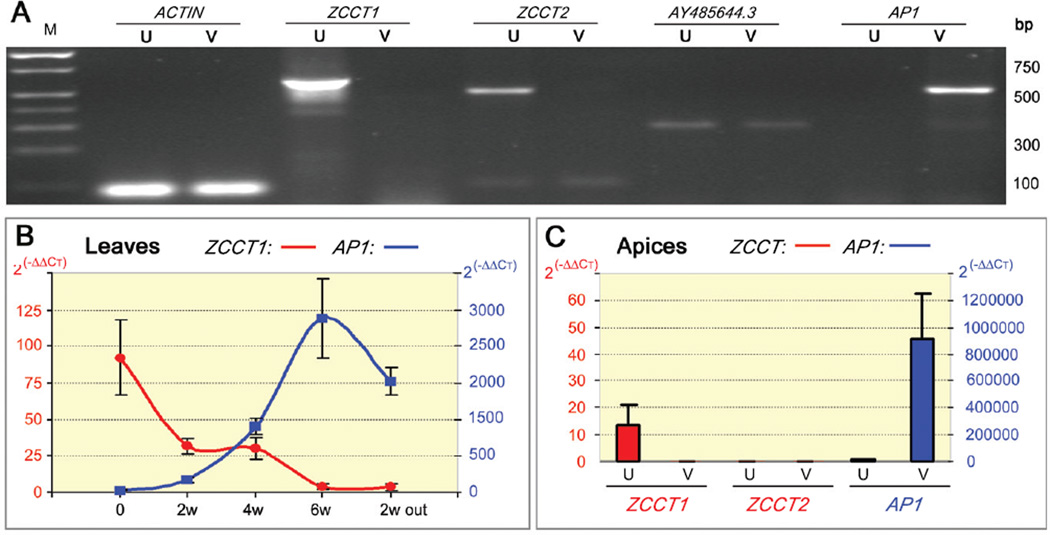
The RNA levels of the three genes completely linked to VRN2 were investigated during vernalization. RNA amounts of the AY485644.3 gene were not affected by vernalization (Fig. 2A), and were similar in spring and winter genotypes. Numerous Triticeae ESTs from different cDNA libraries showed similarity to AY485644.3 (E< e−100), suggesting relatively high mRNA levels in different tissues.
Fig. 2.
A. RT-PCR from leaves of unvernalized (U) or vernalized (V) G3116 winter wheat plants. RNA samples from the vernalized plants (6 weeks at 4°C) were collected 5 days after returning the plants to room temperature. Among the three genes completely linked to VRN2, RNA levels of ZCCT1 and ZCCT2 were down regulated by vernalization and those of AY485644.3 were not affected by vernalization. In the same cDNA samples, AP1 RNA levels were up regulated by vernalization. M indicates molecular weight DNA marker. B-C) Quantitative PCR. B. Leaves: Transcript levels of ZCCT1 (red scale) and AP1 (blue scale) relative to UBIQUITIN in G3116 (averages of 5 plants ± SE): 0: before 4°C; 2w, 4w, 6w: weeks at 4°C; 2w out: 2 weeks at room temperature after vernalization. C. Apices: Transcript levels of ZCCT1, ZCCT2 and AP1 (=VRN1) relative to ACTIN in G3116 (averages of 3 pools of apices from 5 plants each ± SE). U= unvernalized, V= 3–5 days at room temperature after 6 weeks of vernalization. Units are linearized values using the 2(−ΔΔCT) method, where CT is the threshold cycle.
In contrast, the absence of ESTs corresponding to the ZCCT genes in the extensive wheat and barley collections suggested low transcript levels. ZCCT1 and ZCCT2 transcripts were detected by RT-PCR in the leaves before but not after vernalization (Fig. 2A). The mRNA levels of ZCCT1 and ZCCT2 in diploid wheat were quantified using TaqMan systems (SOM-4) and ACTIN and UBIQUITIN as endogenous controls (5). A progressive decrease of ZCCT1 (Fig. 2B) and ZCCT2 transcripts (SOM-4, Fig. S17) was observed in the leaves during vernalization. Control winter plants kept at room temperature maintained stable ZCCT RNA levels. ZCCT transcription was not restored after the plants were removed from the cold room (4°C) after six weeks of vernalization (Fig. 2B, 2w out). Downregulation of ZCCT1 after vernalization was also confirmed in common winter wheat variety Jagger (data not shown).
The downregulation of the ZCCT genes during vernalization was concomitant with an increase of wheat VRN1 (=AP1) transcription (Fig. 2B). These opposite transcription profiles are consistent with the epistatic interactions between VRN1 and VRN2 (2, 5).
ZCCT1 transcripts were present in the apices from the unvernalized winter plants but were not detected after vernalization (Fig. 2C). VRN1 transcripts in the apices showed the same pattern as in the leaves, being induced after vernalization (Fig. 2C). Using the same RNA samples, we did not detect transcripts of ZCCT2. These results suggested that either ZCCT2 was not expressed in the apices or its transcription level was below our detection threshold. Since apices are the critical points for the transition between the vegetative and reproductive phases, these observations suggested that ZCCT1 was a better candidate for VRN2 than was ZCCT2.
Allelic variation of candidate genes among cultivated T. monococcum accessions
ZCCT1 transcription was downregulated during vernalization in both winter G3116 and spring DV92 plants, suggesting that the differences in growth habit were not caused by differences in the transcriptional regulation of ZCCT1. To test this hypothesis we compared the sequences of the promoter and coding regions from the three VRN2 candidate genes between spring and winter accessions of cultivated T. monococcum (SOM, Table S1).
We observed no differences in the AY485644.3 protein between vrn2-spring accession DV92 and Vrn2-winter accessions PI355532 and PI277133 (AY485962, AY485961). Similarly, no differences were found in the predicted ZCCT2 proteins between vrn2-spring accession DV92 and Vrn2-winter accessions PI272561 and PI277133 (AY485976, AY485975). In addition, no differences were found between DV92 and winter accession PI272561 in the first 1-kb of the promoter or in the 763-bp of the 3’ end region of the ZCCT2 gene (SOM-5.2). These results suggested that the differences in vernalization requirement were not caused by differences in the coding sequences of these two genes or in the regulatory sequences of ZCCT2.
No differences were found in the promoter region of ZCCT1 between DV92 and winter accession PI272561 (SOM-5.3). However, comparison of the ZCCT1 coding region between DV92 and 16 T. monococcum accessions with winter growth habit (SOM, Table S1) provided good evidence that ZCCT1 was the VRN2 gene. The spring accession DV92 carried a point mutation at position 35 of the CCT domain that replaced an arginine (R) amino acid by a tryptophan (W). This arginine is conserved in all of the ZCCT proteins (SOM-3, Fig. S10) and in all of the CO-like proteins from Arabidopsis, rice and barley (16). A point mutation at the same position in the CCT domain of CO in Arabidopsis EMS mutant co-7 did not affect the nuclear localization of the CO protein but produced a severe effect on flowering time (15). Kurup et al. (17) suggested that the CCT domain might be involved in protein-protein interactions, and that a mutation within this domain can disrupt these interactions. The conservation of the 35-R amino acid in all the CCT domains and the strong effect of its mutation on flowering time in Arabidopsis suggest that this amino acid is essential for the correct function of the CCT domain, and that the point mutation observed in DV92-ZCCT1 is the likely cause of its spring growth habit.
The R/W mutation in DV92 created an NcoI restriction site (SOM, Fig. S3) that was absent in the wild allele. This polymorphism was used to screen a germplasm collection of 65 accessions of cultivated T. monococcum from different parts of the world (SOM, Table S1). The R/W mutation was absent in all 16 accessions with a winter growth habit, but present in 22 of the 49 spring accessions (SOM, Table S1). Screening of the remaining 27 spring accessions by hybridization with ZCCT1 showed that 17 accessions had a complete deletion of ZCCT1 and ZCCT2. Seven of the ten remaining spring accessions showed deletions in the VRN1 promoter that explained their spring growth habit (5). The spring growth habit of the last three accessions remains unexplained. In summary, the mutations or deletions at the ZCCT1 gene and the VRN1 promoter described so far were sufficient to explain the spring growth habit of 46 out of the 49 cultivated T. monococcum accessions analyzed in this study (SOM, Table S1).
Allelic variation in barley
The absence of ZCCT genes in the orthologous BAC from spring barley variety Morex (Fig. 1) suggested that this variety carries a recessive vrn2 allele. Hybridization of XbaI digested barley genomic DNA with a wheat ZCCT1 probe showed no hybridization in Morex, but three RFLP fragments in winter H. spontaneum. The analysis of 102 F2 plants from a cross between the spring variety Morex and the winter accession of H. spontaneum showed that all the F2 plants homozygous for the deletion had a spring growth habit. In addition, the three RFLP fragments were completely linked to VRN2 flanking gene SNF2P, demonstrating that Morex has a recessive vrn2 allele completely linked to the ZCCT deletion.
To study the distribution of the deletion of the ZCCT genes in barley and its association with the vrn2 allele, we screened a collection of 85 barley varieties from different parts of the world that were previously characterized genetically for their vernalization alleles (18). Hybridization of Southern blots of DNAs from these varieties with the wheat ZCCT1 probe showed the presence of the ZCCT genes in the 23 winter barley varieties and complete deletion of all ZCCT genes in 61 vrn2-spring barley varieties (19). Spring barley variety Fan (vrn2) showed only one ZCCT gene, indicating a different deletion (19).
Validation of ZCCT1 as VRN2 by RNAi transgenic wheats
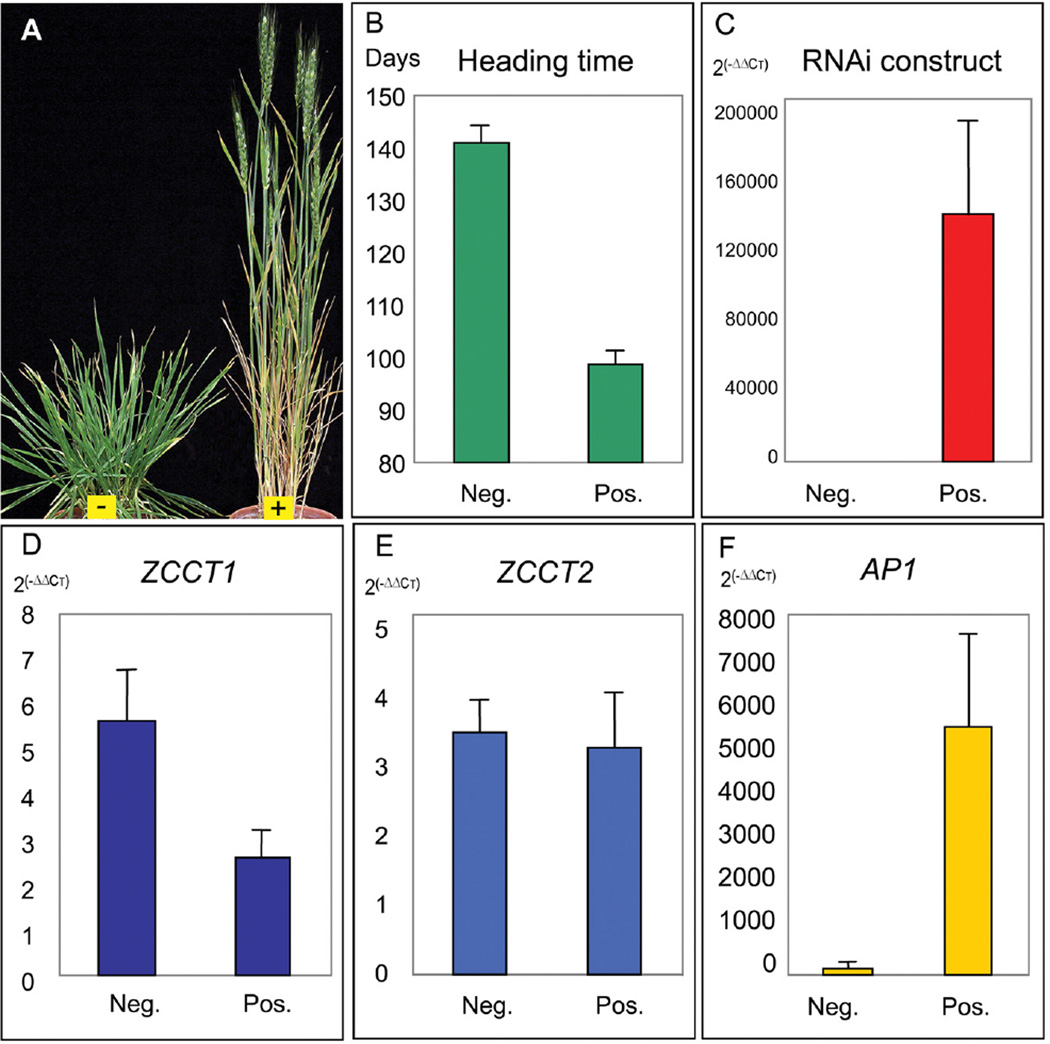
Transformation experiments were performed in winter hexaploid wheat variety Jagger because it is currently not possible to transform efficiently diploid T. monococcum. Transformation was performed by bombardment with an RNA interference (RNAi) construct including a 347-bp segment from the T. monococcum ZCCT1 gene (SOM-6). We identified three independent transgenic plants by PCR (SOM-6), but only one of them showed the expected acceleration of flowering relative to the negative controls and was analyzed further.
We self-pollinated the early-flowering transgenic T0 plant and determined the presence or absence of the transgene in 42 plants from the T1 progeny by Southern blots. The plants carrying the transgene flowered on average 42 days earlier than the negative plants (P<0.001, Fig. 3A-B). Quantitative PCR analysis of eight negative and eight positive transgenic T1 plants (Fig. 3C-F) showed reduction of the endogenous RNA levels of ZCCT1 (P<0.05, Fig. 3D) but not of ZCCT2 (P=0.79, Fig. 3E). The positive transgenic T1 plants showed higher AP1 RNA levels (P<0.001, Fig. 3F). This experiment confirmed that the reduction of the RNA level of ZCCT1 is associated with the acceleration of flowering time and that RNAi can be used successfully in polyploidy wheat, which carries multiple homoeologous copies of ZCCT1. This experiment demonstrated that VRN2 also regulates flowering by vernalization in polyploid winter wheats, despite observations of allelic variation at this locus in diploid wheat but not in polyploid wheat (4).
Fig. 3.
A. Transgenic Jagger T1 plants segregating for the presence and absence of the RNA interference construct for ZCCT1 and for flowering time. B. Average heading date of T1 plants carrying the transgene (31 plants) and without the transgene (11 plants). C-F. Average RNA level of 8 positive and 8 negative T1 plants from the progeny of the early flowering T0 plant. Units are linearized values using the 2(−ΔΔCT) method, where CT is the threshold cycle. C. RNA level of the RNAi construct; D-F. Endogenous RNA levels of D. ZCCT1, E. ZCCT2, F. AP1. Error bars indicate one standard error of the mean.
The vernalization response in temperate cereals
The complete linkage between ZCCT1 and VRN2 in a large mapping population, its gradual and stable transcriptional downregulation by vernalization, its opposite transcription profile to VRN1, the association between natural allelic variation at ZCCT1 and spring growth habit, and the acceleration of flowering time by RNAi of ZCCT1 transcripts all demonstrate that ZCCT1 is VRN2.
The ZCCT1 gene belongs to a different family of transcription factors than the Arabidopsis MADS-box gene FLC but has an analogous function. Both genes are dominant repressors of flowering downregulated by vernalization. Similarities between ZCCT1 and Arabidopsis proteins are restricted to the conserved CCT domains present in CO and CO-like proteins. However, the CCT domains from the ZCCT genes belong to a group that does not include any Arabidopsis protein (SOM-3, Fig.S11).
An additional difference between wheat and Arabidopsis is the frequent association between natural differences in growth habit and allelic variation at the AP1 locus in wheat (5), but not in Arabidopsis. Even in the extensive collection of Arabidopsis mutants there are no reports of differences in vernalization requirement associated with the AP1 gene.
Based on the previous observations, and the knowledge that temperate grasses evolved from subtropical primitive grasses that likely had no vernalization requirement (20), we conclude that Arabidopsis and the temperate grasses developed different vernalization pathways, including different genes downregulated by vernalization (ZCCT1 and FLC) and similar genes with different regulatory profiles (AP1).
The development of a vernalization response was an important step for the adaptation of the grasses to the cold regions. In most of the wild Triticeae species vernalization accelerates flowering, suggesting that the winter growth habit is the ancestral state in this group of species. However, these winter species retained the capacity to generate spring forms by loss of function mutations at two main vernalization loci. Independent mutations at these loci were maintained by natural selection in the wild species and by a strong selection pressure in the domesticated wheat and barley varieties. These results suggest that the wide adaptability of temperate cereals was favored by a flexible regulation system of flowering time.
Supplementary Material
Acknowledgments
The authors thank X. Zhang, A. Sanchez, Jeanie Lin, R. Shao, and C.S. Busso for excellent technical assistance, Dr. C.M. Leutenegger for his help with the TaqMan systems, Vicki Chandler for the pMCG161 vector, the US National Small Grain Collection for the T. monococcum seeds, and the Okayama University (Japan) and A. Kleinhofs for the barley seeds. This work was supported by the United States Department of Agriculture, National Research Initiative Grants 2000-1678 and 2003-00929 and by NSF PGRP #9975793.
Footnotes
Supporting results sections 1–6
Figs. S1 to S17, Table S1
References
- 1.Takahashi R, Yasuda S. Genetics of earliness and growth habit in barley. In: Nilan RA, editor. Proceedings of the 2nd International Barley Genetics Symposium. Washington: Washington State University Press; 1971. [Google Scholar]
- 2.Tranquilli GE, Dubcovsky J. J. Hered. 2000;91:304. doi: 10.1093/jhered/91.4.304. [DOI] [PubMed] [Google Scholar]
- 3.Laurie DA, Pratchett N, Bezant JH, Snape JW. Genome. 1995;38:575. doi: 10.1139/g95-074. [DOI] [PubMed] [Google Scholar]
- 4.Dubcovsky J, Lijavetzky D, Appendino L, Tranquilli G. Theor. Appl. Genet. 1998;97:968. [Google Scholar]
- 5.Yan L, et al. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6263. doi: 10.1073/pnas.0937399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazzani S, Gendall AR, Lister C, Dean C. Plant Physiol. 2003;132:1107. doi: 10.1104/pp.103.021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaels SD, Amasino RM. Plant Cell. 1999;11:949. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheldon CC, et al. Plant Cell. 1999;11:445. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johanson U, et al. Science. 2000;290:344. doi: 10.1126/science.290.5490.344. [DOI] [PubMed] [Google Scholar]
- 10.Sung SB, Amasino RM. Nature. 2004;427:159. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- 11.Gendall AR, Levy YY, Wilson A, Dean C. Cell. 2001;107:525. doi: 10.1016/s0092-8674(01)00573-6. [DOI] [PubMed] [Google Scholar]
- 12.Levy YY, Mesnage S, Mylne JS, Gendall AR, Dean C. Science. 2002;297:243. doi: 10.1126/science.1072147. [DOI] [PubMed] [Google Scholar]
- 13.Lijavetzky D, et al. Genome. 1999;42:1176. [PubMed] [Google Scholar]
- 14.Putterill J, Robson F, Lee K, Simon R, Coupland G. Cell. 1995;80:847. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 15.Robson F, et al. Plant J. 2001;28:619. doi: 10.1046/j.1365-313x.2001.01163.x. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths S, Dunford RP, Coupland G, Laurie DA. Plant Physiol. 2003;131:1855. doi: 10.1104/pp.102.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurup S, Jones HD, Holdsworth MJ. Plant J. 2000;21:143. doi: 10.1046/j.1365-313x.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi R. Catalogue of the barley germplasm preserved in Okayama University. Institute of Agricultural and Biological Sciences, Okayama University; 1983. [Google Scholar]
- 19.Chen C-L. MS, University of California. 2002. [Google Scholar]
- 20.Clayton WD, Renvoize SA. Genera Graminum. Grasses of the world. Kew, London: Royal Botanic Gardens; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.