CMV seroprevalence increases with age and is significantly associated with migration background, country of origin, and place of birth. We identified the birth order of siblings, breastfeeding, early day care attendance and living in East Germany as further risk factors.
Keywords: children and adolescents, cytomegalovirus, risk factors, seroprevalence, transmission
Abstract
Background. Congenital cytomegalovirus (CMV) infection can cause severe birth defects. The majority of children with congenital CMV are born to CMV-seropositive women; however, transmission from mother to fetus and resulting defects are more likely to occur when mothers experience seroconversion during pregnancy. The objective of this study was to provide a population-based estimate of CMV seropositivity and to identify factors that correlate with the detection of CMV-immunoglobulin (Ig)G antibodies.
Methods. Cytomegalovirus-specific IgG antibodies were determined by enzyme-linked immunosorbent assay in 13 876 serum samples from children and adolescents (aged 1–17 years). Cytomegalovirus seroprevalence was correlated with children's age, gender, migration background, country of origin, place of birth, socioeconomic status, breast feeding, daycare attendance, order and number of siblings, and residence in East versus West Germany.
Results. Age-adjusted seroprevalence was 27.4% (95% confidence interval, 25.8–29.0). Cytomegalovirus seroprevalence increased with age (21.5% at ages 1–2; 32.0% at ages 14–17). Cytomegalovirus seropositivity was significantly associated with migration background, country of origin and place of birth, and (among migrants only) with low socioeconomic status. Risk factors for CMV acquisition included the birth order of siblings, breastfeeding, early daycare attendance, and living in East Germany.
Conclusions. In Germany, CMV seroprevalence increases with age, irrespective of gender. These data highlight risk factors associated with seroprevalence and help to identify a target age for the application of a CMV vaccine.
Cytomegalovirus (CMV), a member of the Herpesviridae, can cause life-threatening disease in immunocompromised individuals as well as in fetuses [1]. The highest risk of transmission and fetal infection occurs if a seronegative mother acquires CMV during pregnancy. This intrauterine transmission can result in microcephaly, mental retardation, developmental delay, visual impairment/retinitis, convulsions, and frequently hearing loss [2]. For Germany, an annual number of 3500 congenitally CMV-infected infants has been estimated [3]. After birth, CMV is transferred from mother to child by breast feeding [4, 5], and CMV immunoglobulin (Ig)G seroprevalence increases with age as children enter daycare facilities. Seropositive children can shed the virus for months and represent a source of infection for seronegative children and caregivers [6–9].
During 2003–2006, the Robert Koch Institute conducted the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) [10]. This nationally representative population-based survey collected data from 16 706 individuals aged 1–17 years. A total of 13 876 sera were tested for CMV IgG antibodies to obtain an estimate of CMV IgG seroprevalence in children and adolescents in Germany. We analyzed the association between CMV seroprevalence and potential factors influencing infection such as household members including siblings, breast feeding, and daycare attendance to identify (1) factors/sources of infection before, during, and after puberty and (2) risk factors for virus transmission that might result in congenital infection.
METHODS
Survey Sample and Design
The KiGGS study is a cross-sectional national health survey among children and adolescents aged 1–17 years living in Germany, conducted by the Robert Koch Institute [10]. Children and adolescents were recruited in 167 sample points (communities) throughout Germany, stratified by federal state and community type. Special care was taken to include children and adolescents with a migration background. The overall response rate was 66.6%. Blood samples were collected starting at age 1. The study was approved by the Charité-Universitätsmedizin Berlin ethics committee and the Federal Office for Data Protection.
Serological Testing
Serological testing of CMV IgG in patient samples was performed in the German reference laboratory for measles, mumps, and rubella at the Robert Koch Institute. Cytomegalovirus IgG was evaluated in study subjects older than 1 year to rule out measurement of maternal antibodies.
Cytomegalovirus IgG titer of all serum samples was determined by the Euroimmune anti-CMV-virus-AT-enzyme-linked immunosorbent assay (ELISA) (IgG) (Euroimmun, Luebeck, Germany) based upon lysates from CMV-infected cells. An automated processor (Tecan Evolyzer, Crailsheim, Germany) was used. All samples were tested with kits of the same lot number. The result of the ELISA was calculated by correlation to a standard curve and expressed in relative units (RU)/mL. This result was interpreted according to the manufacturer's recommendations as negative for titers <16 RU/mL, equivocal for titers ≥16 and <22 RU/mL, and positive for titers ≥22 RU/mL.
Variables
Information was collected in self-administered questionnaires filled in by the parents and by the adolescents themselves (starting at age 11). A participant was defined to have a 1-sided/2-sided migration background (MigB) if 1/both of the parents were not born in Germany and/or had no German citizenship [11]. For the present analysis, country of origin was defined to be the country where the mother was born; if this information was missing or the 1-sided MigB was from the father's side, the father's country of birth was used. The country of origin was grouped as follows: Turkey; Russian Federation/former Soviet Union (eg, Lithuania, Ukraine, Belarus, Kasachstan); Central/Southern Europe (eg, Bosnia, Bulgaria, Greece, Spain, Romania); Western Europe etc (eg, Belgium, Denmark, France, Slovakia, Switzerland, Hungary, Canada, Unites States of America, Australia); Poland; Arabic-Islamic countries (eg, Lebanon, Marocco, Pakistan, Senegal, Guinea); other countries (eg, Argentina, Brazil, Vietnam, the Philippines, China, Angola, Eritrea) [12]. In the descriptive analyses, nonmigrants and migrants with a 1-sided MigB were grouped together. The place of birth of the children themselves was taken from the adolescents' questionnaire and classified as Germany yes/no. Children aged 1–10 were assumed to have been born in Germany if the year when the mother migrated to Germany was before the child's birth year, which introduces some uncertainties in very young children. Socioeconomic status (SES) was defined based on the parents' highest educational status, the parents' highest professional status, and household income, and classified into 3 categories [10, 13]. Region was categorized as East Germany (including Berlin) versus West Germany. Sibling order was defined based on siblings living in the same household because this provided somewhat larger associations in preliminary analyses than using the order of biological siblings. Children and adolescents were classified as (1) having no siblings or being a middle child (ie, having both younger and older siblings living in the household), (2) being the oldest child, or (3) being the youngest child in the household. Daycare attendance was categorized based on the age when daycare attendance started (0 or 1 years vs 2 years vs 3 years or older). Breastfeeding was classified as never breastfed versus ever, but not fully breastfed versus ever breastfed with missing information on whether this was full breastfeeding or not versus fully breastfed, but not until the 4th month versus fully breastfed until the 4th month or longer. “Full breastfeeding” means exclusive or predominant breastfeeding, ie, breastfeeding with possible additional intake of water or tea [14].
Statistical Analysis
All analyses were done using the KiGGS sampling weights for the population with a blood sample available. This weight accounted for the unequal sampling probabilities due to the design and included an adjustment to the German population statistics (as of December 31, 2004) by age, sex, region, and nationality (German vs non-German) [10]. In sensitivity analyses, an extended weighting factor was used that additionally adjusted for parental education × MigB. Standard errors were calculated by Taylor linearization and taking the weighting and the clustering in sample points into account, using the survey procedures in SAS version 12.3 (2012; SAS Institute, Cary, NC). Confidence intervals (CIs) were calculated on the logit scale.
A multivariable logistic regression model was built to analyze the association of various factors with CMV IgG seropositivity. Only participants with complete information on all model variables were included in the model. The final model included sex, age group, region, MigB, country of origin, place of birth, SES (low vs medium or high SES), sibling order, daycare attendance, breastfeeding, and the interaction of MigB and SES. Variable selection was done based on the Akaike information criterion and the significance level (P = .05). In addition to the adjusted odds ratios (aOR) and their 95% CIs, predictive margins were calculated to give an illustration of the aOR on the prevalence scale. The predictive margins are the averaged probabilities of CMV IgG seropositivity predicted by the model, comparing, eg, East and West Germany and leaving all other variables unchanged [15–17]. Thus, the predictive margins can be thought of as adjusted prevalences reflecting only the effect of the variable in question, in this case region, assuming that all other variables, eg, MigB, show the same distribution across regions. The predictive margin for a particular country of origin was calculated according to the weighted proportion of 1-sided/2-sided MigB among all migrants. The predictive margins for 1-sided/2-sided MigB were calculated once for Turkey, the reference category of the country of origin, and once for the marginal distribution of the country of origin among all migrants.
RESULTS
Descriptive Analysis
During 2003–2006, 16 706 individuals aged 1–17 years in Germany were interviewed for the KiGGS study. Of these, 13 876 (83%) children and adolescents were screened for CMV IgG antibodies (Table 1).
Table 1.
Study Population and CMV IgG Seroprevalence in 1- to 17-Year-Olds by Age Group and Sex, KiGGS Study, Germany 2003–2006
| Age | Sex | Total No. | No. With CMV Result | Percent With CMV Result | CMV Seroprevalence (95% CI) |
|---|---|---|---|---|---|
| 1–2 yrs | Boys | 936 | 535 | 57% | 21.4% (17.5–25.9) |
| 1–2 yrs | Girls | 934 | 523 | 56% | 21.7% (17.5–26.5) |
| 1–2 yrs | Total | 1870 | 1058 | 57% | 21.5% (18.4–25.1) |
| 3–6 yrs | Boys | 1950 | 1486 | 76% | 24.6% (21.9–27.4) |
| 3–6 yrs | Girls | 1925 | 1406 | 73% | 24.6% (21.7–27.7) |
| 3–6 yrs | Total | 3875 | 2892 | 75% | 24.6% (22.3–27.0) |
| 7–10 yrs | Boys | 2127 | 1860 | 87% | 25.8% (23.5–28.3) |
| 7–10 yrs | Girls | 2021 | 1739 | 86% | 25.8% (23.1–28.7) |
| 7–10 yrs | Total | 4148 | 3599 | 87% | 25.8% (23.9–27.8) |
| 11–13 yrs | Boys | 1588 | 1467 | 92% | 28.0% (24.9–31.2) |
| 11–13 yrs | Girls | 1488 | 1382 | 93% | 29.1% (26.3–32.2) |
| 11–13 yrs | Total | 3076 | 2849 | 93% | 28.5% (26.1–31.1) |
| 14–17 yrs | Boys | 1904 | 1775 | 93% | 30.5% (27.9–33.4) |
| 14–17 yrs | Girls | 1833 | 1703 | 93% | 33.5% (30.8–36.3) |
| 14–17 yrs | Total | 3737 | 3478 | 93% | 32.0% (29.9–34.1) |
| Total | Boys | 8505 | 7123 | 84% | 26.8% (25.1–28.6) |
| Total | Girls | 8201 | 6753 | 82% | 27.9% (26.1–29.8) |
| Total | Total | 16 706 | 13 876 | 83% | 27.4% (25.8–29.0) |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; Ig, immunoglobulin; KiGGS, German Health Interview and Examination Survey for Children and Adolescents.
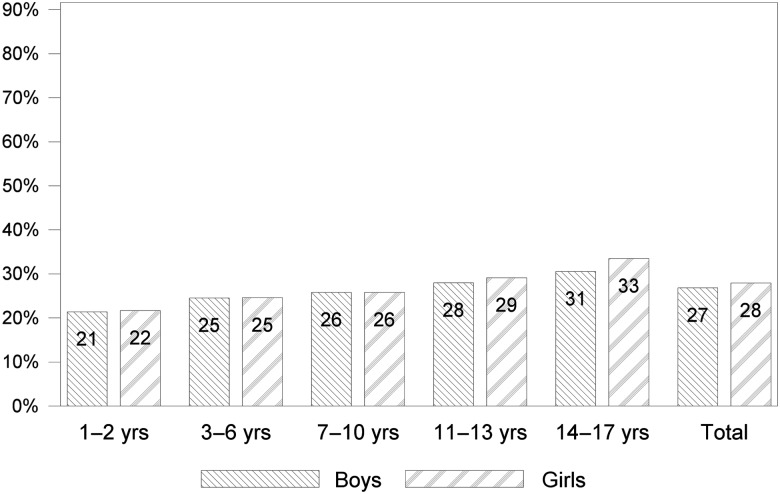
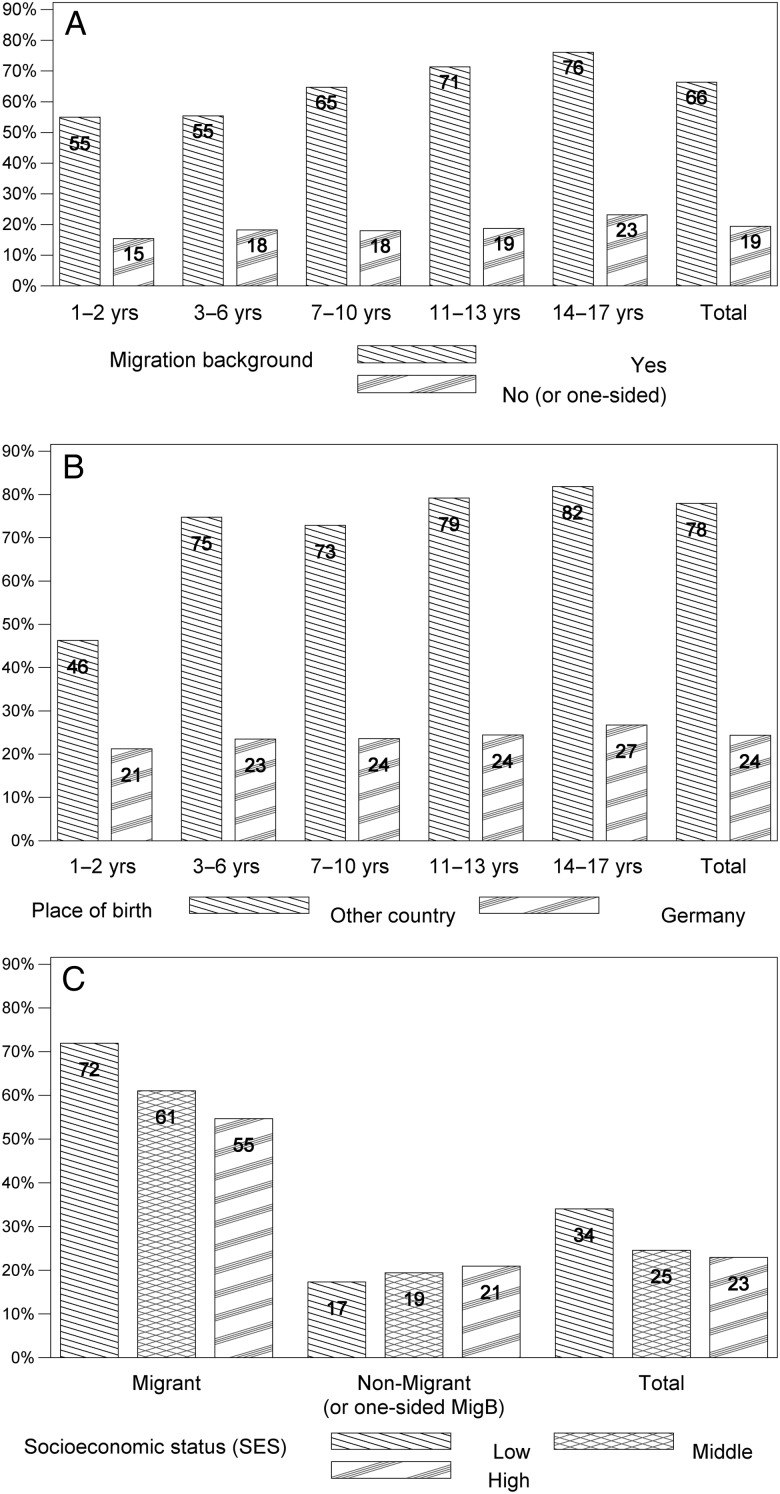
The overall CMV IgG seroprevalence was 27% (95% CI, 26–29). There was no significant difference in CMV seroprevalence between same-aged boys and girls throughout childhood and teenage years, and seroprevalence increased gradually from infancy to early adulthood (Figure 1). Through all ages, children and adolescents with a 2-sided MigB had significantly higher percentages of CMV IgG antibodies than those without MigB, ranging from 55% in 1- to 2-year-olds to 76% in 14- to 17-year-olds (Figure 2a). The overall prevalence of CMV IgG was 66% (95% CI, 64−69) in individuals with a 2-sided MigB; in contrast, only 19% (95% CI, 18−21) of those without a MigB or with a 1-sided MigB were CMV IgG-positive. A higher prevalence of CMV IgG in children born outside Germany (78%; 95% CI, 74−81) compared with those born in Germany (24%; 95% CI, 23−26) (Figure 2b) was observed. Apart from the youngest age group, where the place of birth could not be ascertained precisely, the increase in CMV IgG seropositivity with age was less pronounced when stratifying on place of birth than when stratifying on MigB. The association of CMV IgG seropositivity with SES differed according to MigB. Migrants with low SES had statistically significantly higher CMV antibody frequencies compared with those with middle or high SES. Nonmigrants or those with a 1-sided MigB with a low SES had lower CMV antibody frequencies than those with middle or high SES (Figure 2c). Overall, CMV IgG seroprevalence was higher among those with a low SES (34%; 95% CI, 32−37) than among those with a middle or high SES (25% and 23%; 95% CI, 23−26 and 21−25) (Figure 2c). Cytomegalovirus IgG seroprevalence changed by at most 2 percentage points when the extended weighting factor was used (results not shown), with the exception of the 1- to 2-year-olds born outside Germany where the prevalence rose from 46% to 53% with the extended weighting factor.
Figure 1.
CMV seroprevalence (in percent) in children and adolescents in Germany, by age group and sex (boys left, girls right). In addition, total seroprevalence is shown.
Figure 2.
Variables associated with CMV seroprevalence (in percent). For all age groups, CMV seroprevalence is higher in children and adolescents with migration background (A) and if the place of birth was outside Germany (B). In migrants, CMV seroprevalence increases with lower socioeconomic status (SES), which is not observed in non-migrants (C).
To identify factors associated with CMV seroprevalence, we analyzed different variables in a multivariable logistic regression model that was additionally adjusted for age group (P < .01) and sex (P = .14) (Table 2). The risk of being CMV seropositive was higher in East Germany (including Berlin) than in West Germany (aOR = 1.38; 95% CI, 1.18–1.63), corresponding to a difference in the average predicted probability of being CMV seropositive of 29% versus 24%.
Table 2.
Results of Multivariable Logistic Regression Model for CMV IgG Seropositivity in 1- to 17-Year-Olds, KiGGS Study, Germany 2003–2006
| Variable | Category | Total No. | No. With CMV Result | Percent With CMV Result | No. in Model | Percent in Model | P Value for Variable | P Value for Category | aORa | (95% CI) | Predictive Margin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | East Germany (including Berlin) | 5569 | 4714 | 85% | 4221 | 76% | <.01 | <.01 | 1.38 | (1.18–1.63) | 29% |
| West Germany | 11 137 | 9162 | 82% | 8011 | 72% | Ref. | 24% | ||||
| MigB, stratified by SESb | Nonmigrant, low SES | 2984 | 2410 | 81% | 2179 | 73% | <.01 | Ref. | 17% | ||
| 1-sided MigB, low SES | 327 | 255 | 78% | 203 | 62% | <.01 | 7.95c | (5.09–12.4) | 59%/39% d | ||
| 2-sided MigB, low SES | 1193 | 950 | 80% | 668 | 56% | <.01 | 23.9c | (17.4–32.8) | 80%/61%d | ||
| Nonmigrant, middle/high SES | 9865 | 8370 | 85% | 7935 | 80% | Ref. | 19% | ||||
| 1-sided MigB, middle/high SES | 868 | 695 | 80% | 594 | 68% | <.01 | 7.22c | (4.85–10.7) | 60%/39%d | ||
| 2-sided MigB, middle/high SES | 1013 | 831 | 82% | 653 | 64% | <.01 | 16.0c | (11.3–22.7) | 76%/55%d | ||
| MigB and/or SES missing | 456 | 365 | 80% | 0 | |||||||
| Country of origin | Turkey | 765 | 591 | 77% | 411 | 54% | <.01 | Ref. | 70% | ||
| Russian Fed., former SU | 651 | 538 | 83% | 395 | 61% | .10 | 0.70 | (0.46–1.07) | 63% | ||
| Central/Southern Europe | 631 | 513 | 81% | 387 | 61% | <.01 | 0.28 | (0.19–0.41) | 43% | ||
| Western Europe, USA etc | 383 | 322 | 84% | 307 | 80% | <.01 | 0.09 | (0.05–0.15) | 21% | ||
| Poland | 364 | 315 | 87% | 269 | 74% | <.01 | 0.20 | (0.13–0.30) | 36% | ||
| Arabic-Islamic | 295 | 230 | 78% | 163 | 55% | <.01 | 0.55 | (0.38–0.79) | 58% | ||
| Other | 324 | 252 | 78% | 186 | 57% | <.01 | 0.45 | (0.28–0.71) | 54% | ||
| Missing | 481 | 354 | 74% | 0 | |||||||
| Place of birth | Other country | 778 | 680 | 87% | 431 | 55% | <.01 | <.01 | 2.14 | (1.54–2.96) | 38% |
| Germany | 15 776 | 13 085 | 83% | 11 801 | 75% | Ref. | 25% | ||||
| Missing | 152 | 111 | 73% | 0 | |||||||
| SES, stratified by MigBb | Low SES, nonmigrant | 2984 | 2410 | 81% | 2179 | 73% | .06 | .20 | 0.90 | (0.78–1.05) | 17% |
| Middle/high SES, nonmigrant | 9865 | 8370 | 85% | 7935 | 80% | Ref. | 19% | ||||
| Low SES, 1-sided MigB | 327 | 255 | 78% | 203 | 62% | .98 | 1.00 | (0.64–1.55) | 39%e | ||
| Middle/high SES, 1-sided MigB | 868 | 695 | 80% | 594 | 68% | Ref. | 39%e | ||||
| Low SES, 2-sided MigB | 1193 | 950 | 80% | 668 | 56% | .02 | 1.35 | (1.05–1.73) | 61%e | ||
| Middle/high SES, 2-sided MigB | 1013 | 831 | 82% | 653 | 64% | Ref. | 55%e | ||||
| SES and/or MigB missing | 456 | 365 | 80% | 0 | |||||||
| Sibling order | Middle child/no siblings | 5404 | 4474 | 83% | 4149 | 77% | <.01 | Ref. | 25% | ||
| Oldest child | 4357 | 3727 | 86% | 3503 | 80% | <.01 | 1.36 | (1.20–1.54) | 30% | ||
| Youngest child | 5978 | 4902 | 82% | 4580 | 77% | <.01 | 0.77 | (0.68–0.86) | 21% | ||
| Missing | 967 | 773 | 80% | 0 | |||||||
| Daycare | Daycare started at age 0 or 1 yrs | 2942 | 2424 | 82% | 2286 | 78% | <.01 | <.01 | 1.35 | (1.15–1.59) | 29% |
| Daycare started at age 2 yrs | 2076 | 1782 | 86% | 1675 | 81% | .16 | 1.15 | (0.95–1.39) | 26% | ||
| No daycare before age 3 | 10 702 | 8903 | 83% | 8271 | 77% | Ref. | 24% | ||||
| Missing | 986 | 767 | 78% | 0 | |||||||
| Breastfeeding | Never breastfed | 3531 | 2905 | 82% | 2620 | 74% | <.01 | Ref. | 16% | ||
| Ever (but not fully) breastfed | 1299 | 1106 | 85% | 1013 | 78% | <.01 | 1.38 | (1.11–1.72) | 20% | ||
| Ever breastfed (no info on full breastfeeding given) | 1125 | 953 | 85% | 795 | 71% | <.01 | 2.30 | (1.81–2.91) | 27% | ||
| Fully breastfed (not until the 4th month) | 3567 | 3022 | 85% | 2768 | 78% | <.01 | 2.01 | (1.69–2.39) | 25% | ||
| Fully breastfed at least until the 4th month | 6592 | 5404 | 82% | 5036 | 76% | <.01 | 2.77 | (2.35–3.26) | 30% | ||
| Missing | 592 | 486 | 82% | 0 | |||||||
| Total | 999 | 16 706 | 13 876 | 83% | 12 232 | 73% | 25% |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CMV, cytomegalovirus; Fed., Federation; Ig, immunoglobulin; KiGGS, German Health Interview and Examination Survey for Children and Adolescents; MigB, migration background; Ref., reference category; SES, socioeconomic status; SU, Soviet Union; USA, United States of America.
a Odds ratios adjusted for sex, age group, and all the variables contained in the table.
b P for interaction migration background; SES = 0.0231.
c The aOR refers to country of origin = Turkey. For other countries of origin, the aOR must be multiplied by the aOR for the corresponding country of origin.
d The first predictive margin refers to country of origin = Turkey. The second predictive margin refers to the marginal distribution of the country of origin among all migrants with 1-sided or 2-sided migration background.
e The predictive margin refers to the marginal distribution of the country of origin among all migrants with 1-sided or 2-sided migration background.
The odds ratio (OR) for MigB differed by SES (P for interaction = .02). In case of a low SES, the aOR for a 1-sided MigB versus none was 7.95 (95% CI, 5.09–12.4), and the aOR for a 2-sided MigB versus none was 23.9 (95% CI, 17.4−32.8); in case of a middle or high SES, the aOR was lower at 7.22 (95% CI, 4.85−10.7) for a 1-sided and 16.0 (95% CI, 11.3−22.7) for a 2-sided MigB. It should be noted that these OR refer to Turkey as the country of origin, the country with the highest aOR. For other countries of origin, the aOR must be multiplied by the aOR for the respective country; for example, for children and adolescents with a middle or high SES and a 1-sided MigB from Poland, the aOR compared with nonmigrants is 7.22 × 0.20 = 1.46. The predictive margins were highest for Turkey (70%), Russia and the former Soviet Union (63%), and Arabic-Islamic countries (58%) and lowest for Poland (36%) and Western Europe, North America, etc (21%). For all countries of origin taken together, the predictive margins were 39% for a 1-sided and 61% for a 2-sided MigB, respectively, versus 17% for nonmigrants in case of a low SES. In case of a middle or high SES, the predictive margins were 39% for a 1-sided and 55% for a 2-sided MigB, versus 19% for nonmigrants. The risk for CMV seropositivity was further increased when a child was born outside Germany (aOR = 2.11; 95% CI, 1.51–2.94), corresponding to a predictive margin of 38% (vs 25% for children born in Germany).
An association of SES and CMV seropositivity was only found among children and adolescents with a 2-sided MigB (P for interaction = .02), where the aOR for a low SES was 1.35 (95% CI, 1.05–1.73) compared with migrants with a middle or high SES, with predictive margins of 61% and 55%, respectively.
First-born children and adolescents had a higher risk of being CMV seropositive (aOR = 1.36; 95% CI, 1.20–1.54; predictive margin 30%) compared with “middle children” or children and adolescents without siblings (predictive margin 25%). Youngest children, on the other hand, had a lower risk (aOR = 0.77; 95% CI, 0.68–0.86; predictive margin 21%) than those without siblings. When the number of siblings was added to the model, the risk increased by aOR = 1.08 per sibling (P = .06), but the protective effect for the youngest child remained unless the number of siblings was 3 or more where the risk was similar to children without siblings (results not shown).
Children enrolled in daycare before the age of 2 had a higher risk for CMV seropositivity with an aOR of 1.35 (95% CI, 1.15–1.59; predictive margin 29%) compared with children who were at home or only attended daycare at 3 years or later (predictive margin 24%). A nonsignificantly elevated aOR of 1.15 (95% CI, 0.95–1.39; predictive margin 26%) was observed when daycare started at age 2.
Finally, breastfeeding increased the risk of being CMV seropositive, with the OR compared with children never breastfed (predictive margin 16%) increasing with the extent and duration of breastfeeding. For children not fully breastfed, the aOR was 1.38 (95% CI, 1.11–1.72; predictive margin 20%), and it increased to aOR = 2.77 (95% CI, 2.35–3.26) and a predictive margin of 30% in children fully breastfed until the 4th month or longer. Sensitivity analyses revealed a further increase in risk when breastfeeding continued until the 6th month or longer (aOR = 2.95 compared with aOR = 2.57 for breastfeeding until the 4th or 5th month). Sensitivity analyses with the extended weighting factor or including missing covariate values as valid values in the model did not change the results appreciably.
DISCUSSION
In our study population of 13 876 children and adolescents, CMV IgG seroprevalence ranged between 21.4% (boys at 1–2 years of age) and 33.5% (girls at 14–17 years of age) with an overall seroprevalence of 27%. For children 1–5 years of age, we detected CMV IgG seroprevalence between 21% and 25%. These results are comparable with data obtained from similar age groups from the National Health and Nutrition Examination Survey (NHANES) of 2011–2012 in the United States (CMV IgG seroprevalence of 20.7%); however, for 1-year-olds, 12.3% was reported for the United States compared with 22% in Germany [18]. Our numbers are higher than those reported for the same age group from a single center 30 years ago [8]. In Germany, CMV seroprevalence among adolescent females and males aged 11–17 years ranged between 29% and 33% and 28% and 31%, respectively, and are lower than those found in smaller cohorts from the United States [19, 20]. Because this survey was conducted among children and adolescents, no data on maternal CMV serostatus are available, although variations in maternal serostatus are likely to contribute to differences in our subgroups.
Consistent with earlier published NHANES data [21], we found increased CMV IgG seroprevalence in older children, in those with MigB, birth outside Germany, and lower SES. An age-dependent increase in seroprevalence has been documented in several studies [21–27]; however, a study from Norway reported a high regional CMV seroprevalence in young pregnant women [28]. Data from 40 324 pregnant women in Germany showed that seroprevalence of CMV IgG was 42.3% and higher among young women [29]. This might be attributed to increased attendance of daycare facilities and higher rates of breastfeeding but also to different demographics of young pregnant women because unmarried women and women with lower SES or foreign nationality are overrepresented in this group. In a group of 24 260 healthy blood donors in Germany, CMV seroprevalence was reported to be higher in female blood donors with a highest rate of CMV seroconversion in those donors between 30 and 35 years of age [30]. Although these data might not be representative, approximately 30% of live births in Germany are born to women between 30 and 35 years of age, which represents the group with a higher seroconversion rate according to the data from Hecker et al [30], and these women are exposed to their young children. The relatively low level of maternal CMV seroprevalence in Germany is likely to contribute to a lower overall seroprevalence, especially on our cohort of children and adolescents.
In our study, daycare attendance starting before the age of 3 increased the risk of CMV seropositivity. Although this has been reported previously [31, 32], little association between daycare attendance and CMV seroprevalence has been documented by other groups [6, 33–35]. This might be due to the amount of time children spend in the facility at young age but might be influenced by ethnicity and/or household circumstances.
Family members of young children have been reported to be at high risk for CMV infection [36, 37]. This correlates with our finding that the oldest child in a family bears the highest risk of being CMV seropositive and strongly supports that household transmission influences CMV seroprevalence among preadolescent children. Likewise, breastfeeding increased the risk of CMV seropositivity. This is in accordance with data from non-Hispanic white children and Mexican American children with foreign-born parents in the United States [6]. Breastfeeding rates vary by ethnicity and MigB; for example, mothers from Eastern Europe tend to breastfeed their children more frequently and over longer time periods.
In previous studies, African American women caring for young children in the United States have been reported as being at risk for virus transmission that might result in congenital infection [19]. In our study, children with a 2-sided MigB or those who were born outside Germany had a higher CMV IgG seroprevalence; however, this subgroup represents only a relatively small proportion of the population (approximately 1 in 6 children have a 2-sided MigB). In studies comparing United States-born to non-United States-born children, CMV seroprevalence was shown to be higher in groups with MigB [21]. Immunoglobulin G seroprevalence was only associated with lower SES in children and adolescents with a 2-sided MigB in our cohort. This may be due to the fact that income is more equally distributed in Germany than in other countries, such as Brazil or the United States, so that living conditions are not so disparate among the native German population [38]. On the other hand, this may also reflect the substantial heterogeneity that is present within the migrant population.
The major strength of this study is that it provides nationally representative information on age-specific CMV seroprevalence and determines associated demographic factors. Due to its cross-sectional design, our study cannot clarify the actual onset of CMV infection and only examines variables that were documented at the time of study. Cytomegalovirus seroprevalence in children could not be correlated with the maternal serostatus, and we cannot provide data to identify age groups bearing a higher potential to serve as a source of infection. An evaluation regarding a possible correlation between onset of sexual activity and CMV serostatus is currently under investigation, evaluating whether exposure to young children results more easily in CMV infection than sexual behavior in adolescents, as stated previously [19].
CONCLUSIONS
In conclusion, our data provide important information on CMV seroprevalence in children and adolescents in Germany that might help to assess optimal age for CMV vaccination in a population with overall low CMV seroprevalence. Because young women in their reproductive years are more likely to become infected for the first time and thus are at particular risk of passing the virus to their fetus, they might be considered for interventional studies including vaccine trials. Therefore, these data might be useful on a regional level. However, the rate of CMV seroprevalence varies widely by country or even region, and in countries like Brazil where CMV seroprevalence is already high at young age [39], the data may not be helpful to identify the best target group for vaccination. To determine the latter, individual assessments are necessary.
Acknowledgments
We gratefully acknowledge Melanie Tobler, Petra Kurzendoerfer, Christine Schwerdtfeger, and Anne Wolbert for technical assistance.
Financial support. This work was supported by funds from the Robert Koch Institute. The laboratory investigation was supported by a grant from the Network of National Reference Centers and associated Consiliary Laboratories in Germany.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe D, Howley P, eds. Field's Virology. 5th ed Philadelphia, PA: Lippincott Williams and Wilkins, 2007: pp 2702–72. [Google Scholar]
- 2.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 3.Hamprecht K, Jahn G. Human cytomegalovirus and congenital virus infection (in German). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50:1379–92. [DOI] [PubMed] [Google Scholar]
- 4.Pass RF, Anderson B. Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. J Pediatric Infect Dis Soc 2014; 3(Suppl 1):S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagno S, Reynolds DW, Pass RF, Alford CA. Breast milk and the risk of cytomegalovirus infection. N Engl J Med 1980; 302:1073–6. [DOI] [PubMed] [Google Scholar]
- 6.Staras SA, Flanders WD, Dollard SC et al. Cytomegalovirus seroprevalence and childhood sources of infection: a population-based study among pre-adolescents in the United States. J Clin Virol 2008; 43:266–71. [DOI] [PubMed] [Google Scholar]
- 7.Pass RF, Hutto C. Group day care and cytomegaloviral infections of mothers and children. Rev Infect Dis 1986; 8:599–605. [DOI] [PubMed] [Google Scholar]
- 8.Pass RF, Hutto SC, Reynolds DW, Polhill RB. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics 1984; 74:121–6. [PubMed] [Google Scholar]
- 9.Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 2006; 118:e286–92. [DOI] [PubMed] [Google Scholar]
- 10.Kurth BM, Kamtsiuris P, Holling H et al. The challenge of comprehensively mapping children's health in a nation-wide health survey: design of the German KiGGS-Study. BMC Public Health 2008; 8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenk L, Ellert U, Neuhauser H. Children and adolescents in Germany with a migration background. Methodical aspects in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) (in German). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50:590–9. [DOI] [PubMed] [Google Scholar]
- 12.Schenk L, Neuhauser H, Ellert U et al. German Health Interview and Examination Survey for Children and Adolescents (KiGGS 2003–2006) (in German). Gesundheitsberichterstattung des Bundes. Robert Koch Institute, Berlin. 2008:14–25. Available at: http://www.rki.de/DE/Content/Gesundheitsmonitoring/Gesundheitsberichterstattung/GBEDownloadsB/KiGGS_migration.pdf;jsessionid=EA3523CA5F075318A0E73B07B540C385.2_cid298?__blob=publicationFile. Accessed 4 January 2016.
- 13.Lange M, Kamtsiuris P, Lange C et al. Sociodemographic characteristics in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) - operationalisation and public health significance, taking as an example the assessment of general state of health (in German). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50:578–89. [DOI] [PubMed] [Google Scholar]
- 14.Lange C, Schenk L, Bergmann R. Distribution, duration and temporal trend of breastfeeding in Germany. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) (in German). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50:624–33. [DOI] [PubMed] [Google Scholar]
- 15.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis 1982; 35:669–74. [DOI] [PubMed] [Google Scholar]
- 16.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics 1999; 55:652–9. [DOI] [PubMed] [Google Scholar]
- 17.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 2004; 160:301–5. [DOI] [PubMed] [Google Scholar]
- 18.Lanzieri TM, Kruszon-Moran D, Amin MM et al. Seroprevalence of cytomegalovirus among children 1 to 5 years of age in the United States from the National Health and Nutrition Examination Survey of 2011 to 2012. Clin Vaccine Immunol 2015; 22:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadler LP, Bernstein DI, Callahan ST et al. Seroprevalence and risk factors for cytomegalovirus infections in adolescent females. J Pediatric Infect Dis Soc 2013; 2:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadler LP, Bernstein DI, Callahan ST et al. Seroprevalence of cytomegalovirus (CMV) and risk factors for infection in adolescent males. Clin Infect Dis 2010; 51:e76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis 2010; 50:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubeck PR, Doerr HW, Rabenau HF. Epidemiology of human cytomegalovirus (HCMV) in an urban region of Germany: what has changed? Med Microbiol Immunol 2010; 199:53–60. [DOI] [PubMed] [Google Scholar]
- 23.Enders G, Bäder U, Bartelt U, Daiminger A. Prevalence of cytomegalovirus (CMV) antibodies and incidence of primary CMV infection in pregnant women in Germany (in German). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2003; 46:426–32. [Google Scholar]
- 24.de Ory F, Ramirez R, Garcia Comas L et al. Is there a change in cytomegalovirus seroepidemiology in Spain? Eur J Epidemiol 2004; 19:85–9. [DOI] [PubMed] [Google Scholar]
- 25.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 26.Gratacap-Cavallier B, Bosson JL, Morand P et al. Cytomegalovirus seroprevalence in French pregnant women: parity and place of birth as major predictive factors. Eur J Epidemiol 1998; 14:147–52. [DOI] [PubMed] [Google Scholar]
- 27.Korndewal MJ, Mollema L, Tcherniaeva I et al. Cytomegalovirus infection in the Netherlands: seroprevalence, risk factors, and implications. J Clin Virol 2015; 63:53–8. [DOI] [PubMed] [Google Scholar]
- 28.Odland ML, Strand KM, Nordbo SA et al. Changing patterns of cytomegalovirus seroprevalence among pregnant women in Norway between 1995 and 2009 examined in the Norwegian mother and child cohort study and two cohorts from Sor-Trondelag county: a cross-sectional study. BMJ Open 2013; 3:e003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enders G, Daiminger A, Lindemann L et al. Cytomegalovirus (CMV) seroprevalence in pregnant women, bone marrow donors and adolescents in Germany, 1996–2010. Med Microbiol Immunol 2012; 201:303–9. [DOI] [PubMed] [Google Scholar]
- 30.Hecker M, Qiu D, Marquardt K et al. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang 2004; 86:41–4. [DOI] [PubMed] [Google Scholar]
- 31.Adler SP. Molecular epidemiology of cytomegalovirus: viral transmission among children attending a day care center, their parents, and caretakers. J Pediatr 1988; 112:366–72. [DOI] [PubMed] [Google Scholar]
- 32.Hutto C, Ricks R, Garvie M, Pass RF. Epidemiology of cytomegalovirus infections in young children: day care vs. home care. Pediatr Infect Dis 1985; 4:149–52. [DOI] [PubMed] [Google Scholar]
- 33.Yow MD, White NH, Taber LH et al. Acquisition of cytomegalovirus infection from birth to 10 years: a longitudinal serologic study. J Pediatr 1987; 110:37–42. [DOI] [PubMed] [Google Scholar]
- 34.White NH, Yow MD, Demmler GJ et al. Prevalence of cytomegalovirus antibody in subjects between the ages of 6 and 22 years. J Infect Dis 1989; 159:1013–7. [DOI] [PubMed] [Google Scholar]
- 35.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect 2009; 137:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010; 20:311–26. [DOI] [PubMed] [Google Scholar]
- 37.Taber LH, Frank AL, Yow MD, Bagley A. Acquisition of cytomegaloviral infections in families with young children: a serological study. J Infect Dis 1985; 151:948–52. [DOI] [PubMed] [Google Scholar]
- 38.Ortiz I, Cummins M. Global inequality: Beyond the bottom billion - a rapid review of income distribution in 141 countries, 2011. Available at: http://www.unicef.org/socialpolicy/files/Global_Inequality.pdf Accessed 4 January 2016. [Google Scholar]
- 39.Yamamoto AY, Castellucci RA, Aragon DC, Mussi-Pinhata MM. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol Infect 2013; 141:2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]