Abstract
A systems approach is being applied in many areas of the biological sciences, particularly in cancer research. The coordinated, simultaneous extraction of DNA, RNA, and proteins from a single sample is crucial for accurate correlations between genomic aberrations and their consequences on the transcriptome and proteome. We present an approach to extract and completely solubilize up to 98% of the total protein recovered from archived samples following TRIzol® isolation of RNA and DNA. We also demonstrate using polyacrylamide gel electrophoresis (PAGE) and Western blot analysis that the proteins, representing both a wide molecular weight range and some posttranslational modifications, such as protein phosphorylation, remain stable in phenol-ethanol for up to 3 years at −20°C.
INTRODUCTION
Extraction of DNA, RNA, and proteins from biological samples is a common procedure in molecular biology laboratories for analysis of the genome, transcriptome, and proteome, respectively. TRIzol®, a monophasic solution of phenol and guanidine isothiocyanate, is designed as a one-stop reagent for the extraction of RNA, DNA, and proteins from tissues or cells (1). While there are numerous publications documenting its utility for the extraction of nucleic acids, fewer reports describe its application to the extraction of proteins. This is mainly due to difficulties in resolubilizing the protein fraction; therefore, the more common procedure is to divide the sample and treat one portion with TRIzol reagent for RNA and DNA extraction and subject the second portion to a lysis buffer for recovery of the proteins. However, when dealing with small samples such as tumor biopsies, a single extraction reagent is crucial in order to obtain enough material for subsequent analyses. The additional advantage is that all analyses can be performed on the same cell mass; this facilitates direct comparisons of alterations in the genome, transcriptome, and proteome. In this report, we present a method to efficiently extract and solubilize proteins from tissue samples using TRIzol reagent subsequent to the sequential extraction of RNA and DNA. Additionally, we provide evidence that the proteins and some posttranslational modifications remain stable in phenol-ethanol for up to 3 years at −20°C.
MATERIALS AND METHODS
Samples
HCT 116 cells were obtained from ATCC (accession no. CCL-247; Manassas, VA, USA). Cells were cultured in T75 flasks to about 80% confluence in McCoy’s 5A Media (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Invitrogen). Prior to harvesting, cells were rinsed with phosphate-buffered saline (PBS). For cells harvested with TRIzol (Invitrogen), 8 mL TRIzol were added to each flask, the cells were removed with a cell scraper, and the suspension transferred to a polypropylene tube capable of withstanding high-speed centrifugation [maximum relative centrifugal force (RCF) of 7500× g]. Sequential RNA and DNA extraction was performed as described in the Invitrogen product information (www.invitrogen.com/content/sfs/manuals/15596026.pdf). Protein pellets isolated from phenol-ethanol supernatants were dissolved in 200 μL solvent. In the cases where two solvents were used, 100 μL each solvent were used.
For proteins isolated with a lysis buffer, cells were rinsed with PBS, then 2 mL trypsin (Invitrogen) were added. After a 3-min incubation at 37°C, the cells were rinsed from the flask in 10 mL media containing 10% FBS, transferred to a 15-mL conical tube, and centrifuged and pelleted at 250× g for 10 min. The media was aspirated, and the cells were resuspended and rinsed in PBS. Cells were pelleted again and placed on ice. The PBS was aspirated, and the pellet resuspended in 100 μL TNE lysis buffer [0.25 mL 50 mM Tris, pH 7.5, 40 μL 2 mM EDTA, 87.7 mg NaCL, 22.3 mg Na4P2O7, 2.1 mg NaF, 0.2 mg Na3VO4, 100 μL 1% Nonidet® P40 (NP40), and 9.61 mL water, sterile filtered with a 0.2-μm filter, and stored at 4°C]. Since Na4P2O7, NaF, and Na3VO4 function as phosphatase inhibitors, they were added to each solvent in the same relative amounts as in the TNE lysis buffer. One Complete™ Mini Protease Inhibitor Cocktail Tablet (Roche Applied Science, Indianapolis, IN, USA) was added for every 10 mL TNE lysis buffer. Cells were lysed for 30 min on ice. Each sample described represents the proteins from one harvested T75 flask.
Protein concentrations were determined using the BCA™ Protein Assay Reagent kit (Pierce, Rockford, IL, USA) and fraction V, protease-free bovine serum albumin (BSA; Roche Applied Science) as the protein standard. For nonaqueous protein solutions, a 1:50 dilution in water was measured, and the corresponding concentration calculated.
Tumor biopsies were collected and immediately stored in RNAlater® (Ambion, Austin, TX, USA). Sequential RNA and DNA extraction was done with TRIzol following the manufacturer’s protocol. While evaluations of the RNA by microarray and reverse transcription PCR (RT-PCR) analysis and the DNA by conventional chromosome comparative genomic hybridization (CGH) were successfully performed, the protein fractions were stored at −20°C for 3 years as phenol-ethanol supernatants awaiting subsequent analysis (2,3).
Dialysis of Phenol-Ethanol Supernatants
The phenol-ethanol supernatants were loaded into Spectra/Por® 6 regenerated cellulose (RC) dialysis membranes (MWCO 2000; Spectrum Laboratories, Rancho Dominguez, CA, USA) and dialyzed against three changes of an aqueous 0.1% sodium dodecyl sulfate (SDS) solution at 4°C, changing the solution first after 16 h, then after 4 h, and again after 2 h, respectively. For every 1 mL phenol-ethanol supernatant, 100 mL 0.1% SDS solution were used. During dialysis, the samples partitioned into three phases: (i) a colorless supernatant (approximately 85% volume), (ii) a globular mass (approximately 10% volume), and (iii) a colorless, viscous liquid (approximately 5% volume).
To concentrate the supernatant in the dialysis membrane for the purposes of protein content determination, samples were removed from the dialysis membrane, loaded into iCON™ Concentrators (20 mL capacity, 9K MWCO; Pierce) and centrifuged at 6000× g at room temperature for 20 min in a swinging-bucket rotor to reduce the volumes from 12 mL to 100 μL. The globular mass, containing the bulk of the proteins, was resuspended in 200 μL total solvent either 8 M urea in Tris-HCl, pH 8.0, 1% SDS in molecular biology-grade water or a 1:1 combination of the two.
Protein extracts isolated under the various conditions and from the different phases were analyzed on polyacrylamide gels (4% 12% or 10% NuPAGE® Bis-Tris Gel; Invitrogen) and stained with Coomassie™ Brilliant Blue R-250 (Bio-Rad Laboratories, Hercules, CA, USA). For Western blot analyses, protein extracts were first resolved on polyacrylamide gels (NuPAGE 10% Bis-Tris Gel) and transferred to a Sequi-Blot™ polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories). Mouse anti-γ-tubulin (Sigma-Aldrich, St. Louis, MO, USA), rabbit anti-c-Myc, or rabbit anti-phospho c-Myc (Epitomics, Burlingame, CA, USA) were used as the primary antibodies, and the blots were detected with ECL™ Western blotting detection reagents and analysis system (GE Healthcare, Piscataway, NJ, USA). The SeeBlue® Plus2 Pre-Stained Standard (Invitrogen) was used as the molecular weight marker for SDS-polyacrylamide gel electrophoresis (SDS-PAGE); the ECL protein molecular weight markers (GE Healthcare) and the MagicMark™ XP Western Protein Standard (Invitrogen) were used for the Western blot analyses.
RESULTS AND DISCUSSION
In the TRIzol product information (www.invitrogen.com/content/sfs/manuals/15596026.pdf) (4), the recommended approach begins with homogenization of the tissue sample followed by a phase separation from the TRIzol reagent after the addition of chloroform. While the RNA is in the upper, aqueous phase, the DNA and proteins are in the lower, organic phenol-chloroform phase. The DNA is then precipitated by the addition of ethanol, and the proteins remain in the phenol-ethanol supernatant. The next recommended steps in the protocol involve precipitating the proteins with isopropyl alcohol or acetone followed by centrifugation. The protein pellet is then washed with 0.3 M guanidine hydrochloride in 95% ethanol and centrifuged again. Following this procedure yields a sizable, tightly packed protein pellet.
In the TRIzol product information, the recommendation for solubilization of the pellet is to pipet the sample in aqueous 1% SDS. If pipeting does not achieve complete dissolution, incubation of the sample at 50°C is suggested. We generated protein pellets with the precipitation method described in the product information and attempted to solubilize them in aqueous 1% SDS as recommended. Even after a 30-min incubation at 50°C, the protein pellets were not dissolved by pipeting. We then attempted to solubilize the pellets by vortex mixing and sonicating for 3 min and achieved only marginally improved solubilization. This result is consistent with previously published reports, which also described difficulty resuspending centrifuged pellets in 1% SDS alone and reported improved solubilization schemes to resuspend precipitated pellets. Some of the solvents described in these reports include sarkosyl with SDS at high pH (5), 9.5 M urea in 2% CHAPS (6), or a triethanolamine-glycerol buffer with CHAPS (7); a recent report describes near complete solubilization of precipitated pellets with 2% diethylamine (8). While these methods result in increased solubility of the proteins, no method has been reported thus far that completely resuspends the centrifuged pellets, and it has not been explored to which extent protein integrity is maintained after prolonged exposure to TRIzol.
Dialysis is suggested as an alternative to precipitation in the protein isolation notes of the TRIzol product information, suggesting more efficient protein recovery. In this method, the phenol-ethanol is dialyzed against 0.1% SDS. Over 22 h of dialysis, the sample phase separates into three components within in the dialysis membrane: (i) a supernatant, (ii) a mass, and (iii) a viscous liquid. The product information indicates that the supernatant contains the proteins. We tested the concentration of proteins in the supernatant and the viscous liquid fraction following dialysis using the BCA assay, but were not able to detect any proteins. Only after concentration of the sample 120-fold (12 mL to 100 μL) were we able to detect proteins in the supernatant at a relatively low concentration (1.65 μg/μL). Protein determination in the remaining dialysis fractions reveals that 98% of the protein is in the pellet and only 2% remains in the supernatant, with no protein in the small volume of viscous liquid.
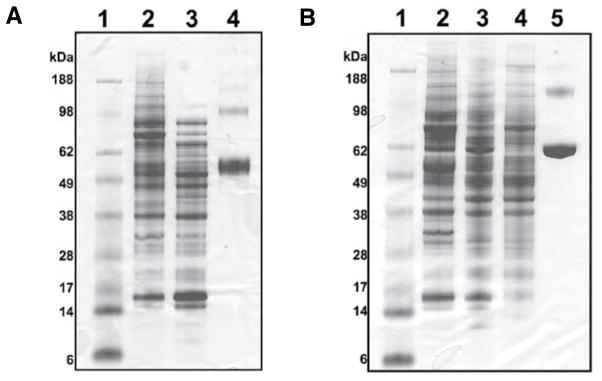
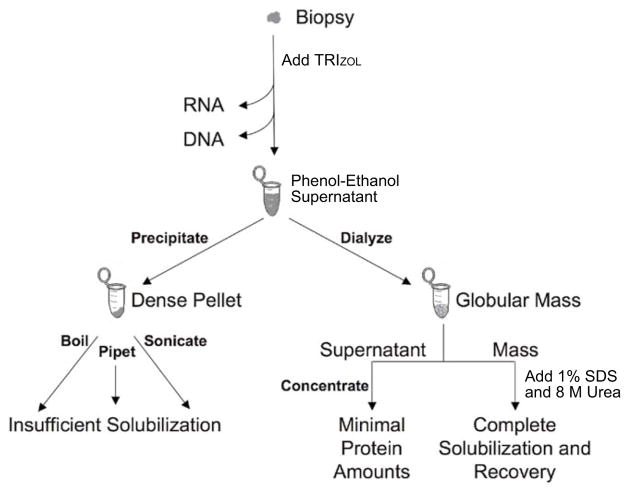
The globular mass recovered from the dialysis procedure is less compact and is therefore much easier to solubilize than the pellet recovered from the standard precipitation procedure. We tried a range of solvents to solubilize the proteins in the dialyzed pellets. The two solvents that resuspended the largest portion of the dialyzed pellets were 8 M urea in Tris-HCl, pH 8.0, and aqueous 1% SDS (Table 1 and Figure 1). Urea and SDS are frequently used for solubilizing proteins (9). Urea is a powerful protein denaturant; it breaks disulfide bonds and increases the solubility of some proteins, although the carbamylation of proteins has been demonstrated as a potential undesirable secondary effect (10). As a detergent, SDS aids in disrupting noncovalent bonds in the proteins, thereby denaturing them and causing the molecules to lose their native conformation. To facilitate protein solubilization in SDS, the solution can be boiled for short periods of time without protein degradation. While we tried all of these approaches, the best solubilization was achieved when the 8 M urea in Tris-HCl, pH 8.0, and aqueous 1% SDS were combined in a 1:1 ratio (Table 1 and Figure 1A, lane 2). A flowchart depicting the different protein extraction and solubilization protocols is shown in Figure 2.
Table 1.
Solubilization of Protein Pellets Following Dialysis or Precipitation
| Extraction Methoda | Solventb | Boiled 3 min | Concentration of Solubilized Proteins (μg/μL) |
|---|---|---|---|
| Dialysis | 100 μL SDS and 100 μL urea | 10.46 | |
| Dialysis | 200 μL urea | 9.86 | |
| Dialysis | 200 μL SDS | Yes | 5.99 |
| Precipitation | 100 μL SDS and 100 μL urea | 4.83 | |
| Dialysis | 200 μL SDS | 1.25 | |
| Lysis buffer | 200 μL Lysis buffer | 12.20 |
SDS, sodium dodecyl sulfate.
Dialysis against 0.1% SDS; precipitation with isopropanol and centrifugation.
SDS indicates 1% SDS; urea indicates 8 M urea in Tris-HCl, pH 8.0.
Figure 1. Solubilization of protein pellets.
Equivalent volumes of each reagent were used for solubilization of the pellets. Differences in the amount of protein observed in each lane reflects the amount of protein solubilized. Ten microliters were loaded in each lane. (A) Dialyzed and precipitated pellets are compared after TRIzol extraction. Lane 1, SeeBlue Plus2 Pre-Stained Standard; lane 2, dialyzed, solubilized in 0.5% sodium dodecyl sulfate (SDS) and 4 M urea in Tris-HCl, pH 8.0; lane 3, precipitated, solubilized in 0.5% SDS and 4 M urea in Tris-HCl, pH 8.0; lane 4, bovine serum albumin (BSA; 4 μg). (B) Comparison of proteins extracted from HCT 116 cells using TRIzol and with TNE lysis buffer. Lane 1, ladder; lane 2, TRIzol extraction, dialyzed, solubilized in 0.5% SDS and 4 M urea in Tris-HCl, pH 8.0; lane 3, TRIzol extraction, dialyzed, solubilized in 1% SDS and boiled for 3 min; lane 4, TNE lysis buffer extraction; lane 5, BSA (4 μg).
Figure 2.
Protein extraction and solubilization from a biopsy using TRIzol.
We also compared the amount of protein we were able to extract using the dialysis approach with the recovery from the precipitation procedure (Figure 1A) and that obtained from a standard cell lysis procedure (Figure 1B). More protein, especially high molecular weight proteins, was obtained from the dialyzed pellets than from the precipitated pellets (Figure 1A). The amount of protein extracted with lysis buffer was slightly greater (Table 1 and Figure 1B) compared to extraction with TRIzol.
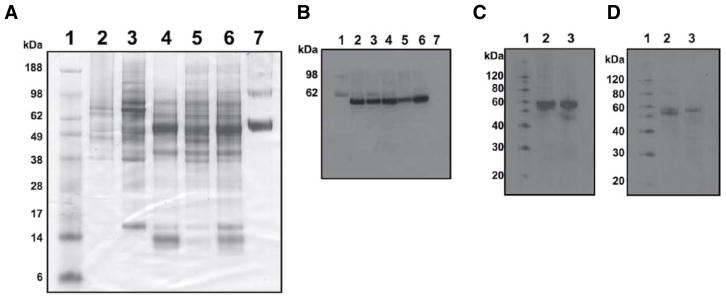
To test the stability of proteins in phenol-ethanol supernatants, we extracted proteins from three patient biopsy samples that were stored at −20°C for 3 years. Significant amounts of protein were recovered from all of the samples (Figure 3A). For example, following RNA and DNA extraction, 1.9 mg protein were extracted from a 40-mg biopsy using our approach. Using SDS-PAGE, we detected numerous proteins ranging in molecular weight from 200 5 kDa. Although numerous distinct protein bands were present, we decided to further examine the samples for possible degradation by evaluating individual proteins by Western blot analysis. As a relatively stable structural protein, γ-tubulin was selected as a positive control to assess total amounts of protein in the samples. The 3-year-old samples showed amounts of γ-tubulin comparable to the colorectal cancer cell line HCT 116 when the total amount of protein loaded in each lane was identical (Figure 3B). Similarly, we could detect equivalent levels of the transcription factor c-Myc in the 3-year-old TRIzol samples and the fresh HCT 116 isolates. Additionally, we were also able to detect both the unphosphorylated (cytoplasmic) and phosphorylated (nuclear) forms of this protein (Figure 3C), indicating that some posttranslational modifications survive TRIzol extraction. While we feel confident that the proteins remained intact after 3 years in the phenol-ethanol supernatant, other parameters of protein integrity, such as the presence of many other forms of posttranslational modifications, have yet to be assessed.
Figure 3. Comparison of proteins from patient samples and cell lines.
(A) Comparison of proteins extracted from fresh HCT 116 protein extracts (with TRIzol or TNE lysis buffer) and tumor biopsies stored in TRIzol-ethanol supernatant at −20°C for 3 years. Biopsies from three different patients are shown. Lane 1, SeeBlue Plus2 Pre-Stained Standard; lane 2, HCT 116 cells with TNE lysis buffer extraction; lane 3, HCT 116 cells, dialyzed, solubilized in 8 M urea in Tris-HCl, pH 8.0; lanes 4 6, patient biopsies, dialyzed, solubilized in 8 M urea in Tris-HCl, pH 8.0; lane 7, bovine serum albumin (BSA; 4 μg). (B) Western blot analysis with mouse anti-γ-tubulin, with the same samples as in panel A. (C) Western blot analysis with rabbit anti-c-Myc (left) and rabbit anti-phospho c-Myc (right). The Western blot analyses have been enhanced for brightness, but not otherwise altered. Lane 1, ECL protein molecular weight markers; lane 2, patient biopsy with TRIzol extraction, dialyzed, solubilized in 8 M urea in Tris-HCl, pH 8.0; lane 3, HCT 116 cells with TNE lysis buffer extraction.
Conclusions
TRIzol reagent can be effectively used to isolate not only RNA and DNA, but also protein, as we demonstrate here. This property is extremely useful when one wants to directly compare the genome, transcriptome, and proteome from the same small surgically obtained tissue biopsy where division of the sample for nucleic acid and protein extraction is not feasible. Protein can be recovered from phenol-ethanol supernatants by either precipitation or by dialysis, although the dialyzed pellets are significantly easier to solubilize and thereby result in better recovery efficiency. A combination of 0.05% SDS and 4 M urea in Tris-HCl, pH 8.0, allows the most complete solubilization of dialyzed pellets without apparent decrease in the integrity of the isolated proteins. In addition, we have demonstrated that proteins isolated from samples stored as phenol-ethanol supernatants for up to 3 years at −20°C do not suffer from significant degradation as assessed using PAGE and Western blot analysis.
Acknowledgments
We thank Linda Barenboim Stapleton, Kundan Sengupta, and Soujanya Pavani for experimental advice.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
To purchase reprints of this article, contact: Reprints@BioTechniques.com
References
- 1.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- 2.Ghadimi BM, Grade M, Difilippantonio MJ, Varma S, Simon R, Montagna C, Fuzesi L, Langer C, et al. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol. 2005;23:1826–1838. doi: 10.1200/JCO.2005.00.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grade M, Ghadimi BM, Varma S, Simon R, Wangsa D, Barenboim-Stapleton L, Liersch T, Becker H, et al. Aneuploidy-dependent massive deregulation of the cellular transcriptome and apparent divergence of the Wnt/beta-catenin signaling pathway in human rectal carcinomas. Cancer Res. 2006;66:267–282. doi: 10.1158/0008-5472.CAN-05-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu LC. Isolation and long-term storage of proteins from tissues and cells using TRIzol® reagent. Invitrogen Focus. 1997;17:98–100. [Google Scholar]
- 5.Banerjee S, Smallwood A, Chambers AE, Nicolaides K. Quantitative recovery of immunoreactive proteins from clinical samples following RNA and DNA isolation. BioTechniques. 2003;35:450–456. doi: 10.2144/03353bm02. [DOI] [PubMed] [Google Scholar]
- 6.Man TK, Li Y, Dang TA, Shen J, Perlaky L, Lau CC. Optimising the use of TRIzol-extracted proteins in surface enhanced laser desorption/ionization (SELDI) analysis. Proteome Sci. 2006;4:3. doi: 10.1186/1477-5956-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkland PA, Busby J, Stevens S, Jr, Maupin-Furlow JA. Trizol-based method for sample preparation and iso-electric focusing of halophilic proteins. Anal Biochem. 2006;351:254–259. doi: 10.1016/j.ab.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Nolan RL, Teller JK. Diethylamine extraction of proteins and peptides isolated with a mono-phasic solution of phenol and guanidine isothiocyanate. J Biochem Biophys Methods. 2006;68:127–131. doi: 10.1016/j.jbbm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Adams LD, Gallagher SR. Solubilization and preparation of proteins in tissue samples. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons; Cambridge, MA: 1992. pp. 10.14.11–10.14.19. [Google Scholar]
- 10.Roxborough HE, I, Young S. Carbamylation of proteins and atherogenesis in renal failure. Med Hypotheses. 1995;45:125–128. doi: 10.1016/0306-9877(95)90059-4. [DOI] [PubMed] [Google Scholar]