Abstract
The adenosine A2A receptor (A2AR) plays a key role in transmembrane signaling mediated by the endogenous agonist adenosine. Here, we describe the crystal structure of human A2AR thermostabilized in an active-like conformation bound to the selective agonist 2-[p-(2-carboxyethyl)phenylethyl-amino]-5′-N-ethylcarboxamido adenosine (CGS21680) at a resolution of 2.6 Å. Comparison of A2AR structures bound to either CGS21680, 5′-N-ethylcarboxamido adenosine (NECA), UK432097 [6-(2,2-diphenylethylamino)-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-tetrahydrofuran-2-yl]-N-[2-[[1-(2-pyridyl)-4-piperidyl]carbamoylamino]ethyl]purine-2-carboxamide], or adenosine shows that the adenosine moiety of the ligands binds to the receptor in an identical fashion. However, an extension in CGS21680 compared with adenosine, the (2-carboxyethyl)phenylethylamino group, binds in an extended vestibule formed from transmembrane regions 2 and 7 (TM2 and TM7) and extracellular loops 2 and 3 (EL2 and EL3). The (2-carboxyethyl)phenylethylamino group makes van der Waals contacts with side chains of amino acid residues Glu169EL2, His264EL3, Leu2677.32, and Ile2747.39, and the amine group forms a hydrogen bond with the side chain of Ser672.65. Of these residues, only Ile2747.39 is absolutely conserved across the human adenosine receptor subfamily. The major difference between the structures of A2AR bound to either adenosine or CGS21680 is that the binding pocket narrows at the extracellular surface when CGS21680 is bound, due to an inward tilt of TM2 in that region. This conformation is stabilized by hydrogen bonds formed by the side chain of Ser672.65 to CGS21680, either directly or via an ordered water molecule. Mutation of amino acid residues Ser672.65, Glu169EL2, and His264EL3, and analysis of receptor activation either in the presence or absence of ligands implicates this region in modulating the level of basal activity of A2AR.
Introduction
Innovative strategies in protein engineering, such as the construction of T4 lysozyme (T4L) fusion proteins and the identification of thermostabilizing point mutations (Tate and Schertler, 2009; Tate, 2012), have led to the structure determination of over 25 G protein–coupled receptors (GPCRs) bound to ligands with a wide range of efficacy (Venkatakrishnan et al., 2013). The first human adenosine A2A receptor (A2AR) structure was determined after engineering a T4L fusion and crystallizing it in lipidic cubic phase in complex with the inverse agonist 4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385; PDB ID 3EML; Jaakola et al., 2008). Subsequently, the structure of a thermostabilized version of A2AR was determined, also with ZM241385 bound (PDB ID 3PWH; Dore et al., 2011), and the high similarity between the structures [root mean square deviation (RMSD) 0.6 Å for 284 residues and 1648 atoms aligned in PyMOL (Schrödinger, San Diego, CA)] showed that neither the thermostabilizing mutations nor the T4L fusion had any significant impact on the structure of the receptor (Tate, 2012). A total of 12 X-ray structures are now available for A2AR, bound to the following ligands: inverse agonist ZM241385 (Jaakola et al., 2008; Dore et al., 2011; Hino et al., 2012); the full agonists adenosine (Lebon et al., 2011b), 5′-N-ethylcarboxamido adenosine (NECA; Lebon et al., 2011b), and UK432097 [6-(2,2-diphenylethylamino)-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-tetrahydrofuran-2-yl]-N-[2-[[1-(2-pyridyl)-4-piperidyl]carbamoylamino]ethyl]purine-2-carboxamide] (Xu et al., 2011); the neutral antagonists caffeine and xanthine amine congener (Dore et al., 2011); and to novel compounds developed as preclinical candidates for the treatment of Parkinson’s disease (Congreve et al., 2012; Langmead et al., 2012). Recently, the mechanism of the negative allosteric modulation of A2AR by sodium for agonist binding was elucidated following the determination of a 1.8-Å resolution structure of the receptor (Liu et al., 2012). The crystal structures have revealed a common set of amino acid residues that interact with ligands—namely, Leu853.33, Phe168EL2, Glu169EL2, Met1775.38, Trp2466.48, Leu2496.51, Asn2536.55, and Ile2747.39 (superscripts refer to the Ballesteros Weinstein nomenclature; Ballesteros and Weinstein, 1995). Both Asn2536.55 and Phe168EL2 make strong interactions with the adenine moiety in agonists and the equivalent triazolotriazine moiety in inverse agonists (see Fig. 1 for ligand structures) via two hydrogen bonds and by π-electron stacking, respectively. All of the structures of A2AR bound to agonists (NECA, adenosine, UK432097) identified hydrogen bonds between the ribose and the side chains of Ser2777.42 and His2787.43 in transmembrane region 7 (TM7; Lebon et al., 2011b; Xu et al., 2011). Residues in TM3, Val843.32, and Thr883.36 are also important for both agonist binding and receptor activation. Interestingly, structure-based drug design led to the development of new triazine derivatives that are inverse agonists, but which interact with an amino acid residue, His2787.43, previously identified as interacting with the ribose moiety of the agonist adenosine (Congreve et al., 2012).
Fig. 1.
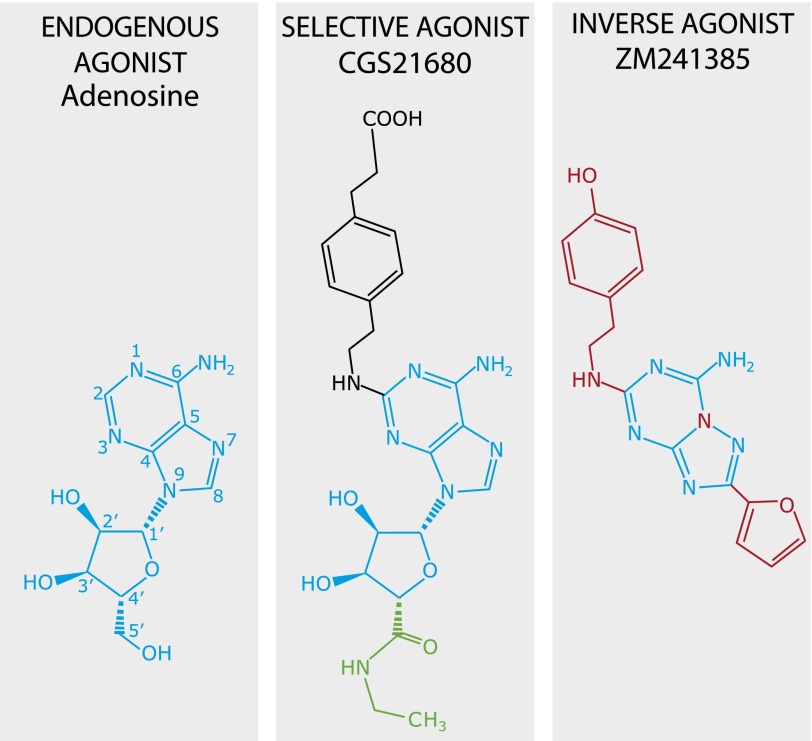
Structures of selected ligands for the adenosine A2A receptor. The structure of the endogenous agonist adenosine is shown in light blue, and structural elements conserved between adenosine, CGS21680, and ZM241385 are likewise in blue. The (2-carboxyethyl)phenylethylamino group of CGS21680 is depicted in black, whereas the N-ethylcarboxyamino group is cultured green. The structure of the agonist NECA is composed of the blue and green portions of CGS21680. Regions in ZM241385 that differ from adenosine are shown in red.
Most of the residues of the orthosteric binding site engaged by adenosine are conserved between the different receptor subtypes (Lane et al., 2011; Lebon et al., 2011b). It is consequently difficult to design selective ligands for an adenosine receptor subtype, unless extensions are made into regions of the receptor that are less well conserved, although recently structure-based drug design has facilitated the development of A2AR-specific ligands that bind deeply in the orthosteric binding pocket (Congreve et al., 2012). Before structures were available, a large number of ligands were developed with modifications at position 2 of the adenine ring (see Fig. 1), some of which display high potency and selectivity toward one receptor subtype (Jacobson and Gao, 2006). 2-[p-(2-carboxyethyl)phenylethyl-amino]-5′-N-ethylcarboxamido adenosine (CGS21680) was the first highly selective ligand allowing the discrimination of A2AR from the A2B and A1 receptors in rat brain (Jarvis et al., 1989; Jarvis and Williams, 1989). CGS21680 was predicted to extend out of the adenosine binding pocket into the extracellular loops (ELs) of the receptor, which represents a region of significant sequence diversity and the proposed site of binding of allosteric ligands in the adenosine A1 receptor (Peeters et al., 2012). In the structures of A2AR bound to adenosine or NECA, EL2 and EL3 are in close proximity with a hydrogen bond between His264EL3 and Glu169EL2. Different conformations of EL2 and EL3 are observed in A2AR bound to the agonist UK432097, where the bulky extensions from position 2 on the adenosine ring sterically block close interactions between EL2 and EL3, resulting in His264EL3 and Glu169EL2 being 3.8 Å further apart (distance between Cα atoms) than observed in the structures bound to adenosine or NECA (Lebon et al., 2011b; Xu et al., 2011).
In efforts to understand the molecular determinants of CGS21680 binding and to provide a foundation for understanding subtype specificity, we report here the high-resolution crystal structure of the subtype-selective agonist CGS21680 bound to the thermostabilized human A2A receptor, A2AR-GL31.
Materials and Methods
Adenosine deaminase, ZM241385, CGS21680, NECA, and 4-(3-butoxy-4-methoxybenzyl)imidazolidin-2-one (Ro20-1724) were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). n-Decyl-β-d-maltopyranoside (DM) was purchased from Anatrace (Maumee, OH). Monoolein was from Nu-Chek Prep (Elysian, MN). Cholesteryl hemisuccinate and cholesterol were purchased from Sigma-Aldrich (Dorset, England). Chloroalkane-Lumi4Tb and tag light reagent were from Cisbio Bioassays (Codolet, France). Lipofectamine 2000 was purchased from Invitrogen–Life Technologies (Saint Aubin, France).
Receptor Constructs.
Pharmacology of wild-type A2AR was determined using the full-length human A2AR cDNA cloned into pcDNA3.1 (pcDNA3.1_WT-A2AR; Bennett et al., 2013) provided by Kirstie A. Bennett from Heptares Therapeutics (Welwyn Garden City, England). A2AR mutants S67A, E169A, H264A, and L267A were produced by introducing single-point mutations into pcDNA3.1_WT-A2AR using the QuikChange II site-directed mutagenesis kit (Agilent Technologies, Stockport, England). Receptor expression at the cell surface was assessed using A2AR with a HaloTag at the N-terminus of the receptor, HALO-A2A, provided by Cisbio Bioassays. Construction of the thermostabilized agonist-bound conformation of the human adenosine receptor, A2AR-GL31, was described previously (Lebon et al., 2011a,b).
Cell Culture, Transient Transfections, and cAMP Assays.
Chinese hamster ovary (CHO) cells were grown to 80% confluence in Glutamax Dulbecco’s modified Eagle’s medium Ham’s F12 medium (Invitrogen, Paisley, UK) complemented with 10% fetal bovine serum (Labtech International, Uckfield, UK). For transfection, cells were seeded at a density of 12,500, 25,000, 50,000, or 100,000 cells/well in a volume of 100 μl in a 96-well plate and transfected with 50 ng of either wild-type A2AR or the mutants S67A, E169A, H264A, or L267A using Lipofectamine 2000 (Life Technologies, Paisley, UK). Dose-response curves were obtained by transfecting 25,000 cells/well with 50 ng of plasmid DNA. In brief, 50 ng of plasmid DNA was mixed with Lipofectamine 2000 in a total volume of 50 μl and incubated 30 minutes at room temperature, according to the manufacturer’s guidelines, and then added to the required number of cells. After 48 hours of receptor expression, cAMP production was measured using the Dynamic 2 cAMP HTRF kit (Cisbio Bioassays), according to the manufacturer’s protocol. In brief, the cells were washed in phosphate-buffered saline, then stimulated using different compound concentrations in a total volume of 50 μl, complemented with the phosphodiesterase inhibitor Ro20-1724 (50 μM) and adenosine deaminase (1 U/ml). Compounds were diluted in Dulbecco’s modified Eagle’s medium Ham’s F12 medium, without serum. After 30-minute incubation, 25 μl of cAMP-d2 conjugate and 25 μl of anti–cAMP-cryptate diluted in lysis buffer were added per well. The plates were incubated at room temperature for 1 hour, and then the fluorescence per well was recorded using the Pherastar 96-well time-resolved fluorescence plate reader (BMG Labtech, Champigny sur Marnee, France). Data were analyzed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Functional concentration response data were fitted to a four-parameter logistic equation. The pEC50 values are expressed as the mean ± S.E.M. Statistical analyses were performed using a one-way analysis of variance with Dunnett’s post test. Values were stated as significantly different at P < 0.05.
Expression, Purification, and Crystallization.
Cell-surface receptor expression levels in human embryonic kidney 293 cells were measured using the HALO-A2AR construct, which were labeled according to the manufacturer’s protocol (Cisbio Bioassays). In brief, cells were washed with 150 µl of Tag-Lite buffer (Cisbio Bioassays), incubated for 1 hour (37°C) with 100 nM Chloroalkane-Lumi4Tb (Cisbio Bioassays) diluted in Tag-Lite buffer (50 µl/well), washed three times with 100 µl of Tag-Lite buffer, and the fluorescence measured using a Pherastar 96-well time-resolved fluorescence plate reader.
The A2AR construct, A2AR-GL31, is an engineered version of human A2AR. A2AR-GL31 contains four thermostabilizing mutations, L48A, A54L, T65A, and Q89A, that improve thermostability of the agonist-bound conformation of human A2AR (Lebon et al., 2011a). In addition, to facilitate crystallization, the C-terminus was deleted after Ala316, and the point mutation N154A in EL2 was introduced to remove the N-linked glycosylation site. A proteolytically cleavable His10 tag was introduced at the C-terminus to facilitate purification. Baculovirus expression and purification were performed exactly as previously described, except that the detergent DM was used throughout at a final concentration of 0.15%. Purified A2AR-GL31 used for crystallization was in a buffer containing 25 mM Hepes (pH 7.4), 0.1 M NaCl, 100 μM CGS21680, and 0.15% DM. Purified receptor fractions were concentrated to 15–25 mg/ml in a final volume of 50–60 μl. Determination of the receptor concentration was performed using the amido black assay (Schaffner and Weissmann, 1973).
The A2AR-GL31–CGS21680 complex was crystallized using the lipidic cubic phase (LCP) crystallization method (Cherezov et al., 2006; Caffrey and Cherezov, 2009). LCP crystallization setups contained a 2:3 (v/v) receptor-to-monoolein ratio, which was dispensed in 25-nl aliquots using a lipid-handling instrument, the mosquito-LCP from TTP Labtech (Melbourn, England). High-quality diffraction crystals were obtained in two different crystallization conditions, leading to two different crystal forms, monoclinic and orthorhombic. Monoclinic crystals were grown in 0.05 M acetate-HCl (pH 4.8), 6% sucrose, 21.9% pentaethylene glycol with the monoolein supplemented with 10% (w/w) cholesterol before mixing with the purified receptor. Orthorhombic crystals were grown in 0.1 M ADA (pH 7.0) and 21.6% PEG 600, with cholesterol hemisuccinate added to the purified receptor [10% (w/w) cholesteryl hemisuccinate: A2AR] before mixing with the monoolein. All LCP crystallization trials contained 800 μl of precipitant per well (lipidic cubic phase kit; Molecular Dimensions Ltd., Newmarket, UK). Crystals were grown at 22°C, harvested with cryo-loops, and cryo-cooled in liquid nitrogen.
Data Collection, Structure Solution, and Refinement.
Diffraction data for the CGS21680 complex were collected at the European Synchrotron Radiation Facility (Grenoble, France) on the microfocus beamline ID23-2 (wavelength 0.8726 Å) using a 10-μm focused beam and a Mar 225CCD detector. The microfocus beam was essential for the location of the best diffracting parts of single crystals and the collection of several wedges of data from different positions on the crystal. Images were processed with MOSFLM (Leslie, 2006) and SCALA (Evans, 2006). The CGS21680 complex was solved by molecular replacement with PHASER (McCoy et al., 2007) using the adenosine-bound A2AR-GL31 crystal structure (PDB ID 2YDO) as a search model after removal of all solvent molecules and the ligand. Two protein chains were found per asymmetric unit in the orthorhombic crystal form and one protein chain per asymmetric unit in the monoclinic crystal form. Refinement and rebuilding were carried out using REFMAC (Murshudov et al., 1997) and COOT (Emsley and Cowtan, 2004), respectively. Validation of the final refined models was performed using Molprobity. All figures in the manuscript were generated using either PyMOL or CCPmg (Potterton et al., 2004).
Results
Crystallization of the Selective Agonist CGS21680 Bound to Thermostabilized A2AR-GL31.
A2AR-GL31 is an engineered version of the human A2AR with improved thermostability in the agonist-bound conformation through the introduction of four point mutations, L48A, A54L, T65A, and Q89A (Lebon et al., 2011a). In addition, to facilitate crystallization, the C-terminus was deleted after Ala316, and the point mutation N154A in EL2 was introduced to remove the N-linked glycosylation site. A2AR-GL31 has been successfully cocrystallized with the endogenous agonist adenosine and the synthetic agonist NECA (Lebon et al., 2011b) (Fig. 1). Pharmacological characterization was previously performed showing that the affinity of A2AR-GL31 for agonist is identical to wild-type A2AR, although the signaling properties of the receptor are impaired due to the orthosteric binding pocket being uncoupled from the G protein binding site (Lebon et al., 2011b).
CGS21680-bound A2AR-GL31 was purified as described previously for NECA-bound A2AR-GL31, and the purified receptor was of high quality with a monodisperse gel filtration profile. No crystals were obtained using vapor diffusion, even when crystallization conditions were used similar to those previously identified for the crystallization of A2AR-GL31 bound to either NECA or adenosine. However, crystallization trials in LCP were successful and yielded two different crystal forms, with space groups P21 (monoclinic) and P212121 (orthorhombic). Complete data sets to 2.6 Å resolution were obtained for each crystal form at European Synchrotron Radiation Facility using the microfocus beamline ID23-2. The structures were solved by molecular replacement using the adenosine-bound conformation of A2AR-GL31 (PDB ID 2YDO) (Table 1).
TABLE 1.
Data collection and refinement statistics
| A2AR-GL31 CGS21680 Monoclinic (Two Crystals) | A2AR-GL31 CGS21680 Orthorhombic (Two Crystals) | |
|---|---|---|
| Data collectiona | ||
| Space group | P21 | P212121 |
| Cell dimensions | ||
| a, b, c (Å) | 60.4, 59.2, 65.1 | 57.2, 105.9, 125.9 |
| α, β, γ (°) | 90.0, 92.8, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å)b | 45.34–2.60 (2.74–2.60) | 54.10–2.60 (2.74–2.60) |
| Rsym or Rmerge | 0.100 (0.560) | 0.194 (1.039) |
| I/σI | 7.4 (1.9) | 9.6 (2.3) |
| Completeness (%) | 95.1 (97.0) | 99.2 (99.0) |
| Redundancy | 3.4 (3.4) | 9.7 (9.8) |
| Refinement | ||
| Resolution (Å) | 64.9–2.6 | 125.8–2.6 |
| No. reflections | 13562 | 23995 |
| Rwork/ Rfree | 0.259/0.312 | 0.239/0.271 |
| No. atoms | ||
| All | 2446 | 4542 |
| Proteins | 2401 | 4404 |
| Ligands | 36 | 72 |
| Lipids | 0 | 50 |
| Waters | 9 | 16 |
| B factors | ||
| All | 55.0 | 39.6 |
| Proteins | 54.9 | 39.6 |
| Ligands | 62.9 | 33.3 |
| Lipids | n/a | 49.1 |
| Waters | 43.0 | 33.6 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.006 | 0.006 |
| Bond angles (°) | 1.02 | 1.02 |
n/a, not applicable; R.m.s., root mean square.
Structures were determined from data collected from two crystals for each space group.
Highest resolution shell is shown in parentheses.
The monoclinic crystal form contained one molecule of A2AR-GL31 per asymmetric unit, whereas the orthorhombic crystal form contained two molecules of A2AR-GL31 (referred to as molecule A and molecule B) arranged as a nonphysiologic antiparallel dimer. Both structures are very similar to the adenosine-bound and NECA-bound structures with overall RMSDs of 0.4 Å for all residues. Overall, the electron density was well defined for receptors crystallized in both space groups, with the exception of the extracellular surface. In the orthorhombic form, electron density for EL2, EL3, and CGS21680 was clearly resolved (Fig. 2), although density for residues 148–164 in EL2 of molecule A was very weak, and this region was not modeled. In the monoclinic form, residues 155–157 in EL2 and 263 in EL3 could not be modeled, and no density was observed for the (2-carboxyethyl)phenylethylamino group of CGS21680 (Supplemental Fig. 1). In addition, in the monoclinic space group, the electron density for the Glu169EL2 side chain was weak, and TM2 appears to be in a conformation similar to the NECA-bound A2AR-GL31 structure. As the model for molecule A of the orthorhombic form was the most complete, and as the structures from the two different space groups were very similar (overall RMSD 0.25 Å), further discussion will focus on molecule A of the CGS21680-bound A2AR-GL31 structure determined from the orthorhombic space group, as this was considered to be the most representative structure of A2AR-GL31 bound to CGS21680.
Fig. 2.
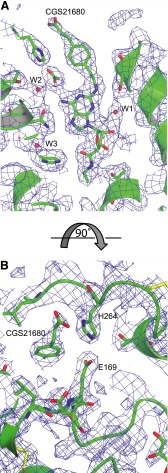
Electron density for the CGS21680 ligand binding site in the orthorhombic crystal form. 2Fo-Fc electron density map of molecule A viewed from two different perspectives: viewed parallel to the membrane plane to show the density for CGS21680 (A) and viewed from the extracellular side to show Glu169EL2 and His264EL3 (B). Side chains are shown in stick representation (carbon, green; oxygen, red; nitrogen, blue; sulfur, yellow). Figures were made using CCP4mg and the contour level is 1.2 σ.
The overall conformation of CGS21680-bound A2AR-GL31 is the same, within experimental error, as the structures of A2AR-GL31 bound to either adenosine or NECA, which, with the exception of the extracellular loops, are virtually identical to the conformation of the wild-type receptor bound to UK432097. This conformation of the receptor is best described as “active-like” (Lebon et al., 2011b). The major internal rearrangements of side chains and α-helices characteristic of agonist binding are observed in the receptor core within all of these structures, but the opening of the cytoplasmic face to allow G protein coupling has not occurred to the same extent as observed in the β2-adrenergic receptor bound to a heterotrimeric G protein (Kobilka, 2011; Rasmussen et al., 2011b; Lebon et al., 2012). Thus, the structures of agonist-bound A2AR represent a thermodynamically stable conformation primed to bind G proteins upon movement of the cytoplasmic end of TM6, presumably through either thermal fluctuations or by induced fit.
The Binding Mode of CGS21680.
The synthetic agonist CGS21680 is an analog of the natural agonist adenosine with a (2-carboxyethyl)phenylethylamino group on the C2 position of adenine and an N-ethylcarboxyamido group on C5′ of the ribose (Fig. 1). CGS21680 is a selective agonist of human A2AR that shows weak binding and activation of the human A1 and A2B receptors (Jacobson and Gao, 2006). The N-ethylcarboxyamido adenosine moiety of CGS21680 occupies the same binding site as described for NECA, with hydrogen bonds between Ser2777.42 and His2787.43 and the 3′ and 2′ hydroxyls of the ribose moiety, respectively. N6 of the adenosine moiety makes hydrogen bonds with Asn2536.55 and Glu169EL2, whereas Phe168EL2 makes strong π-stacking interactions with the adenine ring. Further hydrophobic interactions involving Leu853.33, Met1775.38, Trp2466.48, Leu2496.51, Met2707.35, and Ile2747.39 complete the orthosteric ligand binding pocket (Fig. 3; Supplemental Table 1). The binding mode of the adenosine moiety in CGS21680 is thus identical to the mode of binding of the analogous regions of the agonists adenosine, NECA (Lebon et al., 2011b), and UK432097 (Xu et al., 2011).
Fig. 3.
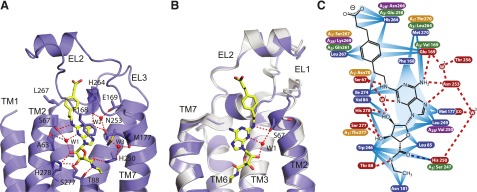
Ligand binding mode of the selective agonist CGS21680. (A) Structure of the extracellular portion of A2AR-GL31 bound to the selective agonist CGS21680. Elements of the structure are depicted as follows: A2AR-GL31, illustration (blue ribbons); specific amino acid side chains, sticks (carbon, blue; oxygen, red; nitrogen, dark blue; sulfur, yellow); CGS21680, sticks (carbon, yellow; nitrogen, dark blue; oxygen, red); ordered water molecules, red spheres; favorable hydrogen bonds, red dashed line; weak hydrogen bonds, blue dashed line (discussed later). (B) Superposition of CGS21680-bound A2AR-GL31 (blue) with NECA-bound A2AR-GL31 (gray). Note the differences at the extracellular end of TM2 and the different rotameric positions of Ser672.64. (C) Polar and nonpolar interactions between the receptor and CGS21680 are shown for molecule A (see Supplemental Fig. 2 for the comparison with molecule B). Amino acid residues within 3.9 Å of the ligands are depicted and make the following interactions: van der Waals contacts (blue rays), potential hydrogen bonds with favorable geometry (red dashed lines, as identified by HBPLUS (http://www.ebi.ac.uk/thornton-srv/software/HBPLUS/); see Materials and Methods), hydrogen bonds with unfavorable geometry (blue dashed lines, donor acceptor distance more than 3.6 Å). Where the amino acid residue differs between human A2AR and human A1R, A2BR, or A3R, the equivalent residue is shown highlighted in orange, purple, or green, respectively. (A) and (B) were generated using PyMOL.
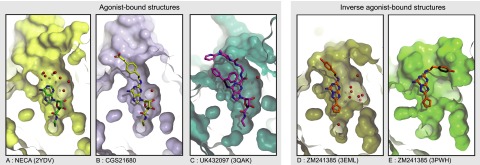
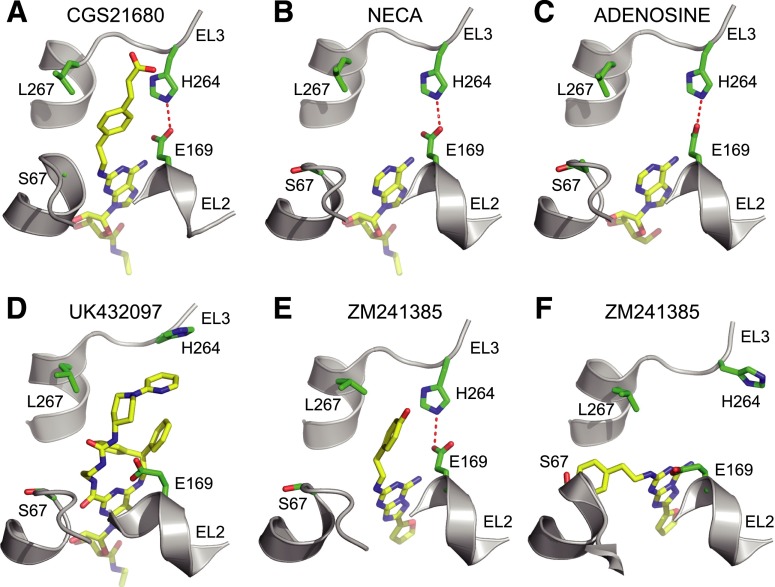
Despite the overall similarity in the binding mode of the adenosine moieties of A2AR agonists, the shape of the binding pocket at the extracellular surface differs significantly between the various ligand-bound structures (Fig. 4). In the NECA-bound A2AR structure, seven ordered water molecules form a hydrogen bond network in a wide opening at the extracellular entrance to the ligand binding pocket (Lebon et al., 2011b). In contrast, only a single water molecule resides in the equivalent position of the CGS21680 binding site, which, along with significant van der Waals interactions between the receptor and the (2-carboxyethyl)phenylethylamino moiety, causes the entrance of the ligand pocket to narrow. This is the opposite of that observed in the UK432097 structure, where the bulky extensions to the adenosine moiety cause the entrance to the ligand binding pocket to widen appreciably compared with the NECA-bound structure (Xu et al., 2011). The entrance to the ligand binding pocket is also very open when the inverse agonist ZM241385 is bound, particularly in the structure determined of A2AR in detergent (3PWH; Dore et al., 2011) in comparison with the structure determined in LCP (3EML; Jaakola et al., 2008), which may reflect the different poses of the phenylethylamino moiety observed between the two structures (Fig. 4).
Fig. 4.
Comparison of the ligand binding site of the human A2AR bound to various ligands (PDB IDs are shown in parentheses): NECA (2YDV) (A), CGS21680 (B), UK432097 (3QAK) (C), and ZM241385 (3EML and 3PWH, respectively) (D and E). The receptor is viewed as a slice perpendicular to the membrane plane through the binding pocket, with surfaces shown in color and water molecules represented as red spheres. Figures were generated using PyMOL.
The (2-carboxyethyl)phenylethylamino group of CGS21680 makes van der Waals interactions with mainly three side chains—namely, Leu2677.32 at the top of TM7, Glu169EL2, and His264EL3 in EL3 (Fig. 3). His264EL3 also makes a hydrogen bond with Glu169EL2, thus potentially stabilizing the extracellular structure of the receptor. It is interesting to observe in the structure of A2AR-GL31 in the monoclinic crystal form that the geometry of the His264EL3- Glu169EL2 hydrogen bond is much less favorable, and there is only weak density for His264EL3, Glu169EL2, and the (2-carboxyethyl)phenylethylamino group of CGS21680 (Supplemental Fig. 1). It is clear that the extracellular region can adopt a number of different conformations in different crystal structures (Fig. 5). It is not unknown for structures of GPCRs to exhibit slightly different conformations, even in the same crystal (Moukhametzianov et al., 2011), and it is also known that the extracellular region of many GPCRs is the site at which allosteric modulators of receptors can bind (Wheatley et al., 2012). Thus, the subtle differences in structure observed between CGS21680-bound A2AR in the two different crystal forms may be of some biologic relevance in affecting the equilibrium between the inactive and active states of the receptor.
Fig. 5.
Comparison of receptor-ligand interactions in EL2 and EL3. Structures shown are a portion of the extracellular region of A2AR (gray illustration) and the bound ligand (sticks: carbon, yellow; nitrogen, blue; oxygen, red) (PDB IDs are shown in parentheses): CGS21680 (A); NECA (2YDV) (B); adenosine (2YDO) (C); UK432097 (3QAK) (D); ZM241385 (3EML) (E); and ZM241385 (3PWH) (F). Selected amino acid residues in van der Waals contact with CGS21680 are shown in all of the structures (Ser672.65, Leu2677.32, His264EL3, Glu169EL2). His264EL3 and Glu169EL2 interact through a hydrogen bond that is absent in UK432097-bound A2AR, due to the steric effects of the large ligand, and in one of the ZM241385-bound structures (3PWH). Figures were generated using PyMOL.
There are three water molecules (W1, W2, and W3) in the CGS21680-bound A2AR-GL31 structure that have clear electron density and low B factors (Figs. 2 and 3; Supplemental Fig. 3). The water molecule W1 forms hydrogen bonds to the side chain of Ser672.64 and the ribose and adenine moieties of CGS21680 (Fig. 3), and forms a weaker polar interaction with the backbone carbonyl of Ala63, thus forming a bridge between the receptor and the ligand. The top of TM2 is tilted toward the ligand in comparison with the adenosine-bound A2AR structure, with the Cα of Ser672.65 shifted by 2 Å. In addition, the rotamer of Ser672.65 has changed with respect to the adenosine-bound structure so that the hydroxyl group is oriented toward the ligand. The water molecule W1 is not resolved in the structure solved from the monoclinic crystal form, in which Ser672.65 adopts a similar position seen in the adenosine-bound structure. The water molecule W2 forms hydrogen bonds with the side chains of amino acid residues Glu169EL2, Asn2536.55, and Thr2566.58, whereas the water molecule W3 forms hydrogen bonds with Met1775.38, His2506.52, and Asn2536.55. W2 and W3 both form hydrogen bonds with Asn2536.55, which probably helps to orient the side chain and position it optimally to form hydrogen bonds with the adenine moiety of the ligand. This is supported by mutagenesis data, which suggest that Asn2536.55 is one of the most important amino acid residues for the binding mode of ligands to human A2AR (Kim et al., 2006; Lane et al., 2012). The positions of these three water molecules are also conserved between the structures of A2AR bound to either adenosine, NECA, or ZM241385 (Jaakola et al., 2008; Lebon et al., 2011b) (Supplemental Fig. 3), supporting their importance in ligand binding.
Functional Characterization of the CGS21680 Ligand Binding Site.
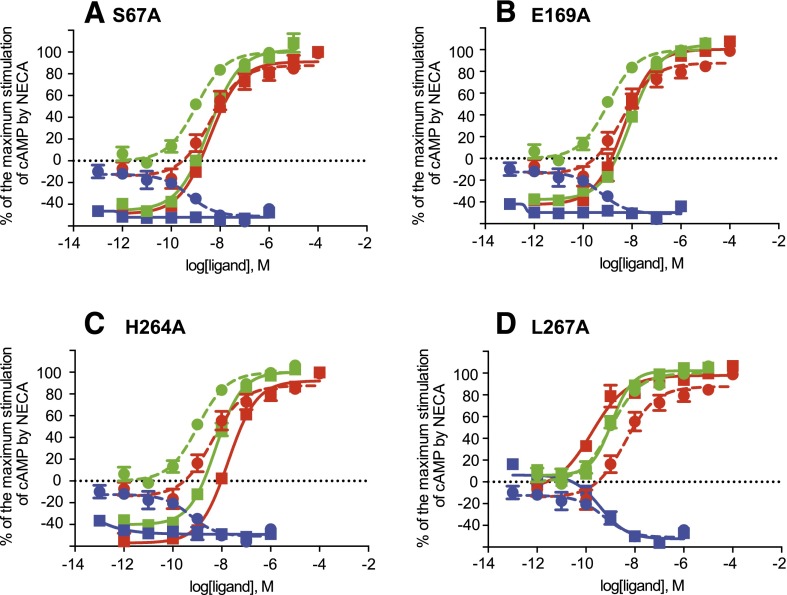
The structure of CGS21680-bound A2AR-GL31 identified the residues that make direct interactions with the ligand (Fig. 3), which is consistent with the mutagenesis of amino acid residues Leu853.33, Thr883.36, Phe168EL2, Leu2496.51, His2506.52, Asn2536.55, Ile2747.39, Ser2777.42, and His2787.43, which significantly reduced the affinity of CGS21680 and/or impaired G protein coupling (Kim et al., 1995; Jiang et al., 1997; Gao et al., 2000; Jaakola et al., 2010). However, the binding mode of the (2-carboxyethyl)phenylethylamino group of CGS21680 was previously unknown. The structure reported here identified contacts between this region of the selective agonist CGS21680 and Ser672.65, Glu169EL2, His264EL3, and Leu2677.32. These residues were subsequently mutated and the signaling properties of A2AR analyzed to define the contribution of each residue to the activity of CGS21680 (Fig. 6). Mutants of the full-length, wild-type A2AR (S67A2.65, E169AEL2, H264AEL3, and L267A7.32) were transiently transfected into CHO cells, and the level of basal activity was determined in the absence of ligand and the level of G protein coupling was determined in the presence of the full agonists NECA and CGS21680 and the inverse agonist ZM241385. Of the four mutations tested, the mutant L267A7.32 showed the biggest reduction (24-fold) in CGS21680 potency, with a nearly 6-fold reduction in potency observed in the mutant H264AEL3 (Table 2). There was no significant effect on CGS21680 potency in the mutants S67A2.65 or E169AEL2, which was particularly surprising given the reduction in potency observed upon NECA binding (3-fold and 15-fold, respectively). Only the mutant H264AEL3 gave similar reductions in potency for both NECA and CGS21680.
Fig. 6.
Effects of the agonists CGS21680 and NECA and the inverse agonist ZM241385 on cAMP production in CHO cells transiently transfected with A2AR. Each panel shows the response of the wild-type receptor (circles, dashed lines) compared with the mutant receptor specified (squares, continuous lines): S67A2.65 (A), E169AEL2 (B), H264AEL3 (C), and L267A7.32 (D). In each panel, the dose-response curves for the mutant are compared with the wild-type receptor for the following ligands: NECA (green), CGS21680 (red), and the inverse agonist ZM241385 (blue). Data are normalized as a percentage of the maximum and minimum responses observed in the NECA-fitted dose-response curve.
TABLE 2.
Comparison of the cAMP dose response induced by NECA and CGS21680 in A2AR mutants
| NECA |
CGS21680 |
|||
|---|---|---|---|---|
| pEC50a | Change in EC50 Relative to WTb | pEC50a | Change in EC50 Relative to WT | |
| WT | 9.05 ± 0.05 | 1 | 8.27 ± 0.29 | 1 |
| S67A | 8.50 ± 0.14 | 3.4 | 8.40 ± 0.23 | 0.62 |
| E169A | 7.87 ± 0.18**** | 15.1 | 8.07 ± 0.18 | 1.58 |
| H264A | 8.18 ± 0.17** | 7.4 | 7.52 ± 0.20 | 5.62 |
| L267A | 8.81 ± 0.08 | 1.7 | 9.66 ± 0.19** | 0.04 |
WT, wild type.
pEC50 values are expressed as the mean ± S.E.M. calculated from four independent experiments, each performed in triplicate. Values are stated as significantly different from the wild-type receptor using a one-way analysis of variance with Dunnett’s post test.
P < 0.005; ****P < 0.0001.
A2AR expressed in CHO cells displayed basal activity that was totally inhibited by binding of the potent inverse agonist ZM241385 (Fig. 6). In contrast, the mutants S67A2.65, E169AEL2, and H264AEL3 all showed impaired basal activity similar to the levels observed for ZM241385-bound A2AR (Fig. 6). The basal activity of A2AR observed in CHO cells is expected to be directly proportional to the expression levels of cell surface receptors; thus, increasing the number of receptors per cell increased the basal activity measured (Fig. 6; Supplemental Figs. 4 and 5). However, when the expression levels of the mutants S67A2.65, E169AEL2, and H264AEL3 were increased, there was no detectable increase in basal activity, and it remained at levels similar to that observed for ZM241385-bound A2AR (Fig. 6; Supplemental Fig. 5). The effect of the mutation L267A7.32 was less pronounced than that observed for the other three mutations, but was still significant. These data suggest that residues in the extracellular region of the A2AR play an important role in dictating the basal activity of the receptor.
Discussion
The structure of CGS21680-bound A2AR was determined by crystallization of the thermostable mutant A2AR-GL31 using the LCP technique. T4L or BRIL fusion proteins were not required, as was demonstrated previously for the crystallization of the β1-adrenergic receptor in LCP (Miller-Gallacher et al., 2014). The chemical structure of CGS21680 is very similar to NECA, except that there is a (2-carboxyethyl)phenylethylamino extension that, when the ligand is bound to A2AR, protrudes out of the adenosine binding pocket into a pocket formed by EL2, EL3, and the extracellular portions of TM2 and TM7. The extracellular regions are the most divergent in sequence between adenosine receptor subtypes (Supplemental Fig. 6), so it is not entirely surprising that CGS21680 may gain at least some of its subtype specificity from binding to amino acid residue side chains that are nonconserved (Ser672.65, Glu169EL2, His264EL3, and Leu2677.32). This is highlighted by the high selectivity of CGS21680 for A2AR compared with A2BR, which is due mainly to differences in the amino acid sequences of EL2. Remarkably, replacement of EL2 in A2BR by EL2 from A2AR allowed the recovery of some binding affinity for CGS21680 and led to activation of heterotrimeric G protein (Seibt et al., 2013). However, defining the relative roles of individual amino acid residues in determining subtype specificity is not clear cut. The mutations S67A2.65 and E169AEL2 did not affect the potency of CGS21680, although there were 3-fold and 24-fold reductions in potency for the mutations H264AEL3 and L267A7.32, as previously reported for the H264AEL3 and E169AEL2 mutants (Lane et al., 2012). In addition, the results were different when the effect on the potency of NECA was measured: S67A2.65, E169AEL2, and H264AEL3 all affected potency, whereas L267A7.32 did not. The different results obtained for CGS21680 and NECA are probably partly a reflection of the subtly different conformations of the extracellular region of A2AR when bound to either ligand, such as the inward tilt of the extracellular region of TM2 when CGS21680 is bound that narrows the entrance to the ligand binding pocket. However, the dynamics of the whole extracellular region may have a greater influence.
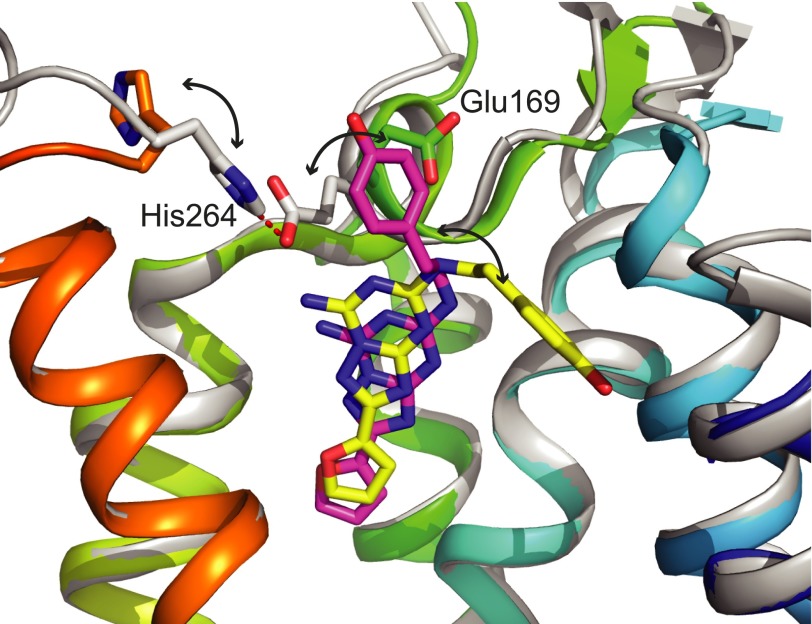
The dynamic nature of the extracellular region was suggested from analysis of the monoclinic crystal form of CGS21680-bound A2AR. CGS21680 was bound in a slightly different conformation compared with that observed in the orthorhombic crystal form, similar to that observed in NECA-bound A2AR, and the density for the extracellular region was very weak, suggesting that this region was poorly ordered, presumably due to its high flexibility. This is consistent with the weak reduction in affinity for CGS21680 binding when amino acid residues making contacts with the (2-carboxyethyl)phenylethylamino group were mutated to alanine. That the extracellular region can adopt multiple conformations, and that this can have an impact on ligand binding is observed upon comparison of the two structures of A2AR bound to ZM241385 (PDB IDs 3EML and 3PWH; Jaakola et al., 2008; Dore et al., 2011). ZM241385 contains a phenylethylamino extension from the triazolotriazine core of the ligand, analogous to the (2-carboxyethyl)phenylethylamino extension on CGS21680, which adopts different conformations in the two A2AR crystal structures (Fig. 7). This may be a result of the potential pH sensitivity of the salt bridge between Glu169EL2 and His264EL3 that is present in the A2AR structure determined at pH 5.5–6.5 (3EML) but is broken in the structure determined at pH 8–8.75 (3PWH). It is known that agonist binding to the A2AR is pH sensitive (Askalan and Richardson, 1994; Hiley et al., 1995), with higher affinities observed at a lower pH, and with the pH-sensitive amino acid residue proposed to be a histidine based on the implied pK value of 7.0 (Askalan and Richardson, 1994). Further work will be required to establish whether His264EL3 is indeed mediating the pH effect observed in A2AR. Consideration of the previous factors thus suggests that it is conceivable that the crystal structure of CGS21680-bound A2AR represents only one of a number of different poses of the (2-carboxyethyl)phenylethylamino moiety, which may go some way to explain the negligible effect of the alanine substitutions of Ser672.65 and Glu169EL2 upon the binding of CGS21680. Another confounding factor that precludes a full understanding of the reasons for the specificity of CGS21680 is that the high sequence divergence of the extracellular regions of the A1, A2A, A2B, and A3 receptors suggests that the structures of these regions may be different from the extracellular region of A2AR. Thus, further insights will only be gained once the structures of the A1, A2B, and A3 receptors have been determined.
Fig. 7.
Comparison of the binding mode of ZM241385 in two different A2AR structures. Structures of ZM241385-bound A2AR crystallized at either pH 5.5–6.5 (PDB ID 3EML; receptor in gray and ZM241385 in pink) or pH 8–8.75 (PDB ID 3PWH; receptor in rainbow coloration and ZM241385 in yellow) were compared in PyMOL and displayed in the illustrated representation. The different conformations of the ligands and the residues involved in salt bridge formation are highlighted by double-headed arrows.
Analysis of the A2AR mutants in the extracellular region of the receptor resulted in the surprising observation that the basal activity of the receptor could be abolished by single amino acid changes. Specifically, the mutants S67A2.65, E169AEL2, and H264AEL3 all showed virtually no basal activity even when expression levels of the receptor were highly elevated. A lesser effect was observed for the mutant L267A7.32. This is consistent with the concept of allosteric modulators of receptors binding to analogous regions at the top of the orthosteric binding site in other receptors, such as the adenosine A1 receptor (Peeters et al., 2012) and the muscarinic M2 receptor (Kruse et al., 2013). It is difficult to rationalize why these mutations significantly reduce basal activity, but it has been consistently observed that the binding of small agonists (adenosine and NECA) results in a contraction of the ligand binding pocket (Lebon et al., 2011b), as observed in the β-adrenergic receptors (Rasmussen et al., 2011a; Warne et al., 2011), and the closure of the extracellular loops over the entrance of the binding pocket. This may be facilitated by the interaction between Glu169EL2 and His264EL3 that form a hydrogen bond upon agonist binding, which would clearly be absent upon mutating either residue to Ala. Orthologous techniques for crystallography such as NMR, spin labeling, and molecular dynamics simulations will be needed to further study the dynamics of the extracellular region for additional insights into this interesting phenomenon.
Coordinates and structure factors have been deposited with the Protein Data Bank, PDB ID 4UG2 for the orthorhombic crystal form and 4UHR for the monoclinic crystal form.
Supplementary Material
Acknowledgments
The authors thank Julie Kniazeff for comments on this work and constructive discussion. The intracellular cAMP assay experiments were performed on the ARPEGE (Pharmacology Screening-Interactome) platform facility at the Institut de Génomique Fonctionnelle.
Abbreviations
- A2AR
adenosine A2A receptor
- A2AR-GL31
thermostabilized A2AR
- CGS21680
2-[p-(2-carboxyethyl)phenylethyl-amino]-5′-N-ethylcarboxamido adenosine
- CHO
Chinese hamster ovary
- DM
n-decyl-β-d-maltopyranoside
- EL
extracellular loop
- GPCR
G protein–coupled receptor
- LCP
lipidic cubic phase
- NECA
5′-N-ethylcarboxamido adenosine
- RMSD
root mean square deviation
- Ro20-1724
4-(3-butoxy-4-methoxybenzyl)imidazolidin-2-one
- T4L
T4 lysozyme
- TM
transmembrane region
- UK432097
6-(2,2-diphenylethylamino)-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-tetrahydrofuran-2-yl]-N-[2-[[1-(2-pyridyl)-4-piperidyl]carbamoylamino]ethyl]purine-2-carboxamide
- ZM241385
4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
Authorship Contributions
Participated in research design: Lebon, Tate.
Conducted experiments: Lebon, Edwards.
Performed data analysis: Lebon, Leslie.
Wrote or contributed to the writing of the manuscript: Lebon, Leslie, Tate.
Footnotes
C.G.T. is a shareholder and consultant for Heptares Therapeutics Ltd.
This work was funded in part by a grant from Heptares Therapeutics Ltd. and core funding from the Medical Research Council [Grant MRC U105197215]. Guillaume Lebon was funded by the program CNRS ATIP-AVENIR.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Askalan R, Richardson PJ. (1994) Role of histidine residues in the adenosine A2a receptor ligand binding site. J Neurochem 63:1477–1484. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. (1995) Integrated methods for the construction of three dimensional models and computational probing of structure function relations in G protein-coupled receptors Methods Neurosci 25:366–428. [Google Scholar]
- Bennett KA, Tehan B, Lebon G, Tate CG, Weir M, Marshall FH, Langmead CJ. (2013) Pharmacology and structure of isolated conformations of the adenosine A₂A receptor define ligand efficacy. Mol Pharmacol 83:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M, Cherezov V. (2009) Crystallizing membrane proteins using lipidic mesophases. Nat Protoc 4:706–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V, Clogston J, Papiz MZ, Caffrey M. (2006) Room to move: crystallizing membrane proteins in swollen lipidic mesophases. J Mol Biol 357:1605–1618. [DOI] [PubMed] [Google Scholar]
- Congreve M, Andrews SP, Doré AS, Hollenstein K, Hurrell E, Langmead CJ, Mason JS, Ng IW, Tehan B, Zhukov A, et al. (2012) Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. J Med Chem 55:1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, et al. (2011) Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. [DOI] [PubMed] [Google Scholar]
- Evans P. (2006) Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62:72–82. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Jiang Q, Jacobson KA, Ijzerman AP. (2000) Site-directed mutagenesis studies of human A(2A) adenosine receptors: involvement of glu(13) and his(278) in ligand binding and sodium modulation. Biochem Pharmacol 60:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Bottrill FE, Warnock J, Richardson PJ. (1995) Effects of pH on responses to adenosine, CGS 21680, carbachol and nitroprusside in the isolated perfused superior mesenteric arterial bed of the rat. Br J Pharmacol 116:2641–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino T, Arakawa T, Iwanari H, Yurugi-Kobayashi T, Ikeda-Suno C, Nakada-Nakura Y, Kusano-Arai O, Weyand S, Shimamura T, Nomura N, et al. (2012) G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 482:237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola VP, Lane JR, Lin JY, Katritch V, Ijzerman AP, Stevens RC. (2010) Ligand binding and subtype selectivity of the human A(2A) adenosine receptor: identification and characterization of essential amino acid residues. J Biol Chem 285:13032–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5:247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. (1989) [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther 251:888–893. [PubMed] [Google Scholar]
- Jarvis MF, Williams M. (1989) Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol 168:243–246. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee BX, Glashofer M, van Rhee AM, Jacobson KA. (1997) Mutagenesis reveals structure-activity parallels between human A2A adenosine receptors and biogenic amine G protein-coupled receptors. J Med Chem 40:2588–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wess J, van Rhee AM, Schöneberg T, Jacobson KA. (1995) Site-directed mutagenesis identifies residues involved in ligand recognition in the human A2a adenosine receptor. J Biol Chem 270:13987–13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Gao ZG, Jeong LS, Jacobson KA. (2006) Docking studies of agonists and antagonists suggest an activation pathway of the A3 adenosine receptor. J Mol Graph Model 25:562–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK. (2011) Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol Sci 32:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, Hübner H, Pardon E, Valant C, Sexton PM, et al. (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, Jaakola VP, Ijzerman AP. (2011) The structure of the adenosine receptors: implications for drug discovery. Adv Pharmacol 61:1–40. [DOI] [PubMed] [Google Scholar]
- Lane JR, Klein Herenbrink C, van Westen GJ, Spoorendonk JA, Hoffmann C, IJzerman AP. (2012) A novel nonribose agonist, LUF5834, engages residues that are distinct from those of adenosine-like ligands to activate the adenosine A(2a) receptor. Mol Pharmacol 81:475–487. [DOI] [PubMed] [Google Scholar]
- Langmead CJ, Andrews SP, Congreve M, Errey JC, Hurrell E, Marshall FH, Mason JS, Richardson CM, Robertson N, Zhukov A, et al. (2012) Identification of novel adenosine A(2A) receptor antagonists by virtual screening. J Med Chem 55:1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, Bennett K, Jazayeri A, Tate CG. (2011a) Thermostabilisation of an agonist-bound conformation of the human adenosine A(2A) receptor. J Mol Biol 409:298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. (2011b) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, Warne T, Tate CG. (2012) Agonist-bound structures of G protein-coupled receptors. Curr Opin Struct Biol 22:482–490. [DOI] [PubMed] [Google Scholar]
- Leslie AG. (2006) The integration of macromolecular diffraction data. Acta Crystallogr D Biol Crystallogr 62:48–57. [DOI] [PubMed] [Google Scholar]
- Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, IJzerman AP, et al. (2012) Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. (2007) Phaser crystallographic software. J Appl Cryst 40:658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Gallacher JL, Nehmé R, Warne T, Edwards PC, Schertler GF, Leslie AG, Tate CG. (2014) The 2.1 Å resolution structure of cyanopindolol-bound β1-adrenoceptor identifies an intramembrane Na+ ion that stabilises the ligand-free receptor. PLoS ONE 9:e92727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukhametzianov R, Warne T, Edwards PC, Serrano-Vega MJ, Leslie AG, Tate CG, Schertler GF. (2011) Two distinct conformations of helix 6 observed in antagonist-bound structures of a beta1-adrenergic receptor. Proc Natl Acad Sci USA 108:8228–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255. [DOI] [PubMed] [Google Scholar]
- Peeters MC, Wisse LE, Dinaj A, Vroling B, Vriend G, Ijzerman AP. (2012) The role of the second and third extracellular loops of the adenosine A1 receptor in activation and allosteric modulation. Biochem Pharmacol 84:76–87. [DOI] [PubMed] [Google Scholar]
- Potterton L, McNicholas S, Krissinel E, Gruber J, Cowtan K, Emsley P, Murshudov GN, Cohen S, Perrakis A, Noble M. (2004) Developments in the CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr 60:2288–2294. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011a) Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 469:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011b) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W, Weissmann C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem 56:502–514. [DOI] [PubMed] [Google Scholar]
- Seibt BF, Schiedel AC, Thimm D, Hinz S, Sherbiny FF, Müller CE. (2013) The second extracellular loop of GPCRs determines subtype-selectivity and controls efficacy as evidenced by loop exchange study at A2 adenosine receptors. Biochem Pharmacol 85:1317–1329. [DOI] [PubMed] [Google Scholar]
- Tate CG. (2012) A crystal clear solution for determining G-protein-coupled receptor structures. Trends Biochem Sci 37:343–352. [DOI] [PubMed] [Google Scholar]
- Tate CG, Schertler GF. (2009) Engineering G protein-coupled receptors to facilitate their structure determination. Curr Opin Struct Biol 19:386–395. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. (2013) Molecular signatures of G-protein-coupled receptors. Nature 494:185–194. [DOI] [PubMed] [Google Scholar]
- Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, Schertler GF, Tate CG. (2011) The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature 469:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley M, Wootten D, Conner MT, Simms J, Kendrick R, Logan RT, Poyner DR, Barwell J. (2012) Lifting the lid on GPCRs: the role of extracellular loops. Br J Pharmacol 165:1688–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.