Azole resistance in Aspergillus fumigatus has now been reported from 6 continents and is emerging as a global health problem. The epidemiology, spread of azole resistance, the clinical implications, and directions of research are highlighted.
Keywords: emergence of azole resistance, azole fungicides, aspergilloma, invasive aspergillosis, chronic pulmonary aspergillosis
Abstract
Azole resistance in Aspergillus fumigatus has emerged as a global health problem. Although the number of cases of azole-resistant aspergillosis is still limited, resistance mechanisms continue to emerge, thereby threatening the role of the azole class in the management of diseases caused by Aspergillus. The majority of cases of azole-resistant disease are due to resistant A. fumigatus originating from the environment. Patient management is difficult due to the absence of patient risk factors, delayed diagnosis, and limited treatment options, resulting in poor treatment outcome. International and collaborative efforts are required to understand how resistance develops in the environment to allow effective measures to be implemented aimed at retaining the use of azoles both for food production and human medicine.
Aspergillus fumigatus is a saprophytic mold that causes allergic, chronic, and acute invasive diseases in humans and animals [1]. The fungus is ubiquitous due to an abundant asexual reproduction cycle, producing many billions of spores, and its ability to survive in very different environments. The fungus is thermotolerant, able to resist temperatures as high as 60°C, and is important for the degradation of organic matter. Although A. fumigatus is not a primary pathogen for living animals or plants, it has evolved as an important cause of opportunistic fungal diseases in humans. Several decades ago, invasive aspergillosis was a much-feared complication of immunosuppressive treatments as the disease was associated with high morbidity and mortality [2–4]. The survival rates of immunocompromised patients with invasive aspergillosis have improved dramatically due to many factors, one of which is the availability of azole antifungal drugs. This class comprises a number of agents with activity against aspergilli, including itraconazole (available for clinical use since 1997), voriconazole (since 2002), posaconazole (since 2006), and, most recently, isavuconazole [5]. Each of these agents has proved beneficial for the treatment of acute invasive and chronic pulmonary aspergillosis, the prevention of invasive aspergillosis, and for difficult-to-treat disease, such as central nervous system Aspergillus disease [6, 7]. Recent studies also show that there is a role for azole therapy in patients with severe asthma with fungal sensitization as its use improved their quality of life and pulmonary function [8]. Moreover, azole drugs are the only anti-Aspergillus agents that are orally available, and therefore play an important role in long-term or ambulatory therapy such as for chronic pulmonary aspergillosis [9].
However, the clinical advances that have been made possible through the use of azole drugs might be threatened by the emergence of azole resistance in A. fumigatus [10–12]. We aim to describe the epidemiology and spread of azole resistance in A. fumigatus, the clinical implications, and directions of research that will help to understand and possibly contain this problem.
RESISTANCE DEVELOPMENT IN A. FUMIGATUS
Generally, 2 routes of resistance development are distinguished: through long-term azole patient therapy and via the application of azole compounds in the environment [13, 14]. Although the clinical characteristics of these routes are very different (Table 1), the fundamental prerequisites for azole resistance development are the same: Any setting that brings together actively reproducing Aspergillus and azole compounds has a risk of mutations developing that confer resistance to azole compounds [14, 17]. Such conditions could be present in a patient with an aspergilloma receiving azole therapy. Within the pulmonary cavity, asexual reproduction of A. fumigatus occurs and spores are produced abundantly, many of which may harbor azole resistance mutations. Genetic analysis of A. fumigatus from dissected aspergillomas and clinical cultures from patients with aspergilloma indeed confirm that A. fumigatus undergoes multiple genetic changes during infection, including those conferring azole resistance [14]. This may be reflected in diagnostic specimens as multiple azole resistance mechanisms may be present in culture, concomitant with azole-susceptible colonies [17]. Although A. fumigatus is a eukaryotic microorganism, the complex cellular composition does not preclude rapid resistance development in response to antimicrobial exposure, as seen with bacterial pathogens. However, horizontal gene transfer, which is common in the spread of bacterial resistance, is not commonly seen in fungi. Acquired resistance has been exclusively described in patients with a cavity or aspergilloma [14, 17]. Resistance mechanisms that are recovered in culture are characterized by point mutations in the Cyp51A gene, which is the target of the antifungal azoles (Table 1). However, although the Cyp51A gene is considered a hot-spot for resistance mutations, many isolates with an azole-resistant phenotype are found to have no mutations in this gene, which suggests that other resistance mechanisms are present, some of which have been identified but many of which remain unknown [18].
Table 1.
Characteristics of Patient-Acquired Resistance and Environmental Resistance in Aspergillus fumigatus
| Patient-Acquired Resistance | Environmental Resistance |
|---|---|
| Chronic pulmonary aspergillosis with cavitary lesion or aspergilloma | All Aspergillus diseases, including allergic bronchopulmonary aspergillosis, acute invasive aspergillosis, chronic colonization in cystic fibrosis |
| Previous or ongoing azole therapy in all patients | Two-thirds of patients have no history of azole therapy |
| Clinical failures to azole therapy | Clinical failures to azole therapy |
| Multiple resistance mutations may be present in a single clinical sample | Only 1 azole resistance mechanism present in most patients |
| Both azole-susceptible and azole-resistant phenotypes simultaneously present in culture | Both azole-susceptible and azole-resistant phenotypes simultaneously present in culture |
| Multiazole and panazole resistance phenotypes | Multiazole and panazole resistance phenotypes |
| Point mutations in the Cyp51A gene, including substitutions at G54, P216, F219, M220, G138, Y431, and G448 non-Cyp51A-mediated resistance mechanisms: HapE unknown resistance mechanisms | Mutations in the Cyp51A gene in combination with a transcriptional enhancer (tandem repeat) in the promoter region of the gene: TR34/L98H, TR53, and TR46/Y121F/T289Aa |
| High genetic diversity between azole-resistant isolates from unrelated patients | Low genetic diversity between azole-resistant isolates from unrelated patients |
| Aspergillus fumigatus colonies may show an abnormal colony morphology, lack of sporulation or reduced growth rate | No apparent fitness cost |
Resistance mutations are also believed to develop in the environment when the fungus is exposed to azole compounds that exhibit anti-Aspergillus activity [18]. Although A. fumigatus is not a phytopathogen, many azole fungicides were found to have activity against A. fumigatus isolates [19, 20]. Some of the azole fungicides are of the triazole class and have a similar molecule structure to the medical triazoles [20]. It was hypothesized that A. fumigatus develops resistance due to use of azole fungicides to combat phytopathogens for crop protection. Because of the molecule similarity of fungicides with medical triazoles, the latter also lose activity. In addition to abundant asexual reproduction, parasexual and sexual reproduction probably also occurs in the environment, thereby increasing the fungus's ability to undergo genetic recombination and thus overcome cellular stress caused by fungicide exposure. Azole fungicides have a broad range of applications, including plant and crop protection, prevention of postharvest spoilage, and preservation of materials. Azole fungicides are used globally, thus creating an environment where azole-resistant A. fumigatus can thrive. In contrast to the United States, where environmental azole resistance in A. fumigatus appears to be uncommon [21], health authorities in Europe have been called to action. The European Centre for Disease Prevention and Control brought together experts from agricultural, veterinary, and medical fields to discuss the problem of emerging azole resistance in Aspergillus [22].
Clinically, environmental resistance is characterized by a complete lack of patient risk factors. Only residency in or visiting of a geographic area with known environmental resistance can be considered a risk.
EPIDEMIOLOGY
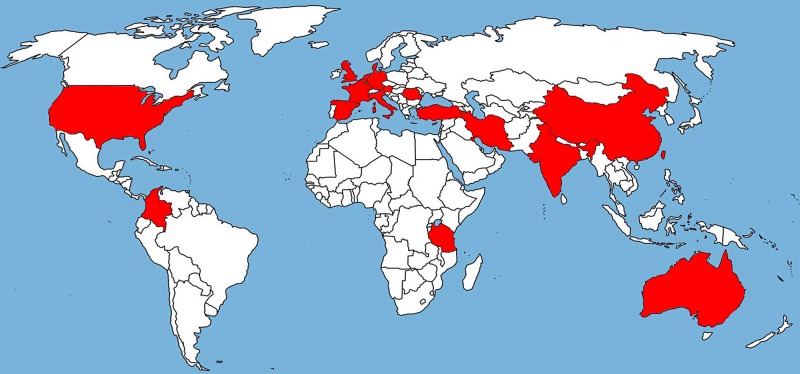
In vitro susceptibility testing of A. fumigatus isolates is not routinely performed in most clinical microbiology laboratories, thus underestimating the true prevalence of resistance. Studies that had investigated the frequency of azole resistance in Aspergillus culture collections report finding the first resistant isolates up to 17 years earlier [23]. TR34/L98H was first found in the Netherlands in 1998 [13], and recently a TR34/L98H isolate was reported from Italy, also originating from 1998 [23]. The TR46/Y121F/T289A resistance mechanism was also first reported in the Netherlands in 2009 [24], but a recent study reported the recovery of TR46/Y121F/T289A from a patient in the United States already in 2008 (Table 2) [25]. Surveillance studies and case series suggest the global presence of azole resistance in A. fumigatus [15, 16, 25–39], including reports from Europe, the Middle East, Asia, Africa, Australia and, most recently, North and South America (Figure 1) [25, 35].
Table 2.
Country and Year of First Recovery of TR34/L98H and TR46/Y121F/T289A Resistance Mechanisms in Aspergillus fumigatus and Year of Publicationa
| TR34/L98H |
TR46/Y121F/T289A |
||||||
|---|---|---|---|---|---|---|---|
| Country | First Case | Type of Isolate | Year of Publication [Reference] | Country | First Case | Type of Isolate | Year of Publication [Reference] |
| Netherlands | 1998 | C + E | 2008 [13] | United States | 2008 | C | 2015 [25] |
| Italy | 1998 | C + E | 2015 [23] | Netherlands | 2009 | C + E | 2013 [24] |
| Turkey | 2000 | C | 2015 [26] | Belgium | 2012 | C + E | 2012 [12, 27] |
| Spain | 2003 | C | 2013 [12, 28] | Germany | 2012 | C + E | 2015 [12, 15] |
| Australia | 2004 | C | 2015 [29] | India | 2012 | E | 2014 [30] |
| Iran | 2005 | C + E | 2013 [12, 30] | France | 2013 | C + E | 2015 [31] |
| Belgium | 2006 | C + E | 2012 [12, 27] | Tanzania | 2013 | E | 2014 [32] |
| Denmark | 2007 | C + E | 2010/2011 [12, 33] | Denmark | 2014 | C | 2015 [33] |
| China | 2008–2009 | C | 2011 [30, 34] | Spain | 2014 | C | 2015 [28] |
| India | 2008 | C + E | 2012 [12, 30] | Colombia | 2015 | E | 2015 [35] |
| United Kingdom | 2009–2011 | C + E | 2009 [12, 36] | ||||
| France | 2010 | C + E | 2012 [12] | ||||
| United States | 2010 | C | 2015 [25] | ||||
| Germany | 2012 | C + E | 2012 [12, 15] | ||||
| Taiwan | 2011 | C | 2015 [37] | ||||
| Kuwait | 2013 | C + E | 2015 [30, 38] | ||||
| Poland | 2006–2014 | C | 2015 [39] | ||||
| Colombia | 2015 | E | 2015 [35] | ||||
Abbreviations: C, clinical; E, environmental.
a Due to space restriction, we were not able to include all individual publications. We have cited reviews, which included reports from individual countries over the years.
Figure 1.
Shaded areas show countries that have reported the TR34/L98H and TR46/Y121F/T289A resistance mechanism in clinical or environmental Aspergillus fumigatus isolates.
It remains unclear when and where these resistance mechanisms first emerged, although genotyping of epidemiologically and geographically unrelated strains shows a lower genetic diversity among isolates harboring TR34/L98H and TR46/Y121F/T289A compared with wild-type isolates, which suggests that each mutation might have originated from a common ancestor [24, 40, 41]. Our current understanding is that resistance traits can migrate rapidly. Isolates harboring TR46/Y121F/T289A from the Netherlands were found to be genetically highly related to resistant isolates from India [30]. Whole-genome sequencing and population analysis indicated that azole-resistant alleles are segregating into diverse genetic backgrounds, which will result in increasing genetic diversity over time [42]. As far as we know, azole resistance, due to mutations in the Cyp51A gene, is not associated with a fitness cost [43]. The consequence is that resistant isolates would be predicted to compete with wild-type isolates in the field and persist in the environment.
Surveillance studies have shown that between 64% and 71% of patients with Aspergillus disease due to environmental azole-resistant A. fumigatus had no history of prior azole therapy [24, 44]. Furthermore, azole resistance may occur in any Aspergillus disease, including acute invasive aspergillosis, chronic pulmonary aspergillosis, or allergic manifestations such as allergic bronchopulmonary aspergillosis. It is possible that azole prophylaxis or azole monotherapy provides a selective advantage for azole-resistant A. fumigatus and might increase the risk for azole-resistant breakthrough infections [45]. Unfortunately, the environmental resistance route was found to be the dominant route for resistance cases. In the Netherlands, between 82% and 89% of azole-resistant cases were due to TR34/L98H and TR46/Y121F/T289A (P. Verweij, personal communication), whereas this was the case in 64% of cases in Belgium [27] and 87% of cases in Turkey [26].
DETECTION OF AZOLE-RESISTANT DISEASE
Azole-resistant Aspergillus disease is difficult to diagnose, as Aspergillus cultures are negative in the majority of patients. Biomarkers based on Aspergillus cell wall components, such as galactomannan or 1,3-β-D-glucan, are unable to detect azole resistance. At best, circulating biomarkers may indicate treatment failure if they continue to increase during azole therapy. Several investigators have used in-house molecular tests to detect azole resistance mutations directly in patient samples, using both tissue and respiratory secretions [46, 47]. Recently, a commercial polymerase chain reaction (PCR)–based assay became available (AsperGenius, PathoNostics, Maastricht, the Netherlands) that enables the detection of several Aspergillus species and includes markers for the detection of the TR34/L98H and TR46/Y121F/T289A resistance mechanisms. Preliminary clinical validation studies indicate that this approach is feasible when bronchoalveolar lavage fluid is tested, although only very few Aspergillus culture-negative and resistance PCR-positive cases have been described [48]. The sensitivity of the resistance PCR might be a limiting factor, as only a single copy of the Cyp51A gene is present in each Aspergillus cell, in contrast with the multigene targets that are commonly used for detection of Aspergillus species. This is especially a concern when only serum is tested. A negative resistance PCR may be due to the high detection limit of the test rather than the absence of resistance mutations, and therefore it will prove difficult to rule out resistance. Furthermore, at present, only 2 resistance mutations are detected, while at least 15 Cyp51A gene–mediated resistance mechanisms have been described [18].
Azole resistance can be tested when A. fumigatus is recovered through culture. However, even in culture-positive patients, resistance may be missed due to concomitant presence of azole-susceptible and azole-resistant colonies [38]. Furthermore, positive cultures may have to be sent to reference laboratories, due to limited availability or experience with fungal resistance testing on site, thus causing delay of effective therapy.
MANAGEMENT OF AZOLE-RESISTANT ASPERGILLUS DISEASES
All studies to date show that azole resistance is associated with treatment failure [24, 36, 44, 45]. Mortality rates in case series of patients with culture-positive azole-resistant invasive aspergillosis varied between 50% and 100% [24, 44, 45]. Preclinical experimental models also indicate that an elevated azole minimum inhibitory concentration (MIC) significantly reduces the efficacy of azole monotherapy [49], but controlled trials that compare azole-resistant with azole-susceptible cases in relation to treatment success have not been performed. Nevertheless, it seems important to identify patients with azole-resistant Aspergillus disease as early as possible to initiate effective therapy. Furthermore, azole resistance mechanisms generally reduce the activity of all azoles. In vitro susceptibility testing of TR34/L98H isolates showed that 99.6% of isolates were resistant to itraconazole, 92.4% to voriconazole, and 97.8% to posaconazole. For TR46/Y121F/T289A 100% of isolates were resistant to voriconazole, whereas 82.7% were resistant to itraconazole and 94.9% to posaconazole [50]. The recently introduced new azole isavuconazole also had high MICs in strains with reduced susceptibilities to other triazoles, mirroring changes in voriconazole susceptibility [5]. These results indicate that the clinical role of azoles in azole-resistant aspergillosis will, at best, be very limited.
In the absence of management guidelines, an expert panel recently discussed the approach they would use in patients with documented azole-resistant Aspergillus disease, or in regions where azole resistance has been reported [51]. As clinical evidence is generally lacking, the panel members relied on anecdotal experience, preclinical studies, and expert opinion with respect to treatment decisions. Most experts recommended moving away from azole monotherapy in patients in whom azole resistance was documented, switching to liposomal amphotericin B or voriconazole in combination with an echinocandin. In areas with confirmed environmental resistance, the threshold at which first-line therapy with azole monotherapy should be avoided was the subject of much debate, but most experts would consider moving away from azole monotherapy when resistance rates exceeded 10%. In that situation, azole-echinocandin combination therapy or liposomal amphotericin B was deemed an appropriate alternative choice [51]. It is therefore important to determine if azole resistance is present in a hospital by regular resistance testing of (stored) clinical A. fumigatus isolates. Most surveillance studies indicate that the frequency of azole resistance is still below the 10% threshold [52]. These studies relied on screening of unselected clinical A. fumigatus isolates, through, for instance, the use of agar plates supplemented with different azoles [52]. Although this approach is useful to determine the frequency of resistance, the role of environmental mutations, and trends over time, 2 recent Dutch studies indicated that the frequency of resistance may vary considerably between departments or risk groups within the same hospital. In one study, a resistance rate of 26% was found in A. fumigatus culture-positive patients in the intensive care unit, which was higher than in all other departments in the hospital (14%; P = .06) [53]. The authors suggested that patients with (undiagnosed) azole-resistant invasive aspergillosis might fail azole therapy while in the department. Subsequent clinical deterioration of the patient requires intensive care support where cultures become positive due to progressive disease. Another study reported the highest azole resistance rates in hematology patients, when primary A. fumigatus cultures were analyzed for resistance [54]. Therefore, general resistance surveillance might not reflect resistance rates in specific high-risk patient groups and detailed audits will be required to determine which primary treatment strategy would be appropriate.
A “POSTAZOLE” ERA?
Compared with antibacterial resistance, the looming problem of azole resistance in A. fumigatus may seem relatively insignificant as the number of patients affected is low and the question can be raised if drug resistance in an opportunistic pathogen is altogether a threat to public health. After all, Aspergillus diseases affect only specific patient groups with chronic lung disease or those with immunosuppression. Although the number of azole-resistant cases is still low, there is every reason to assume that new azole resistance mechanisms will continue to emerge in the environment and rapidly migrate across the world, as has been the case with TR34/L98H and TR46/Y121F/T289A [12, 13, 24]. Increasing azole resistance rates will challenge our current primary treatment recommendation (ie, voriconazole monotherapy), necessitating alternative treatment strategies such as azole-echinocandin combination therapy or liposomal amphotericin B in hospitals or wards where the 10% resistance threshold is exceeded [51]. In addition, the number of cases of breakthrough aspergillosis in patients on azole prophylaxis will increase and certain manifestations of invasive aspergillosis, such as central nervous system aspergillosis, will be virtually untreatable as the use of voriconazole will be precluded. The advances made with the clinical use of the azole class will be at least partly lost and, unless new drug targets are discovered, the overall mortality of Aspergillus diseases will increase (Table 3).
Table 3.
Reported Mortality Rates in Patients With Invasive Aspergillosis in Different Time Periods
|
Aspergillus Disease | ||||
|---|---|---|---|---|
| Era | IA | Comment | CNS IA | Comment |
| c-AmB era | 65% [2] | 122 of 187 patients receiving c-AmB died. | 95%–100% [3] | Literature review |
| 71.6% [55] | 187 of 261 patients with IA died. | 99% [56] | Review of 141 cases of CNS IA in immunocompromised patients, of whom 140 died. | |
| Azole era | 27.5% [57] | 9-wk mortality: 39 of 142 patients receiving voriconazole monotherapy. | 45.6% [7] | Retrospective analysis of 81 patients with CNS IA treated with voriconazole |
| 28.5% [58] | Population-based study analyzing 8563 aspergillosis cases in France. | 35.4% [59] | Literature review: 4 of 11 patients with CNS IA who received voriconazole monotherapy. | |
| Azole resistant | 100% [44] | Culture-positive patients with proven and probable IPA treated with voriconazole (5/5) | 86% [24, 44, 60] | 7 cases of azole-resistant CNS IA have been reported, of which 6 were fatal. |
| 88% [45] | 8 HSCT patients with culture-positive, azole-resistant IA, of whom 7 died. | |||
| 100% [54] | ICU patients with culture-positive azole-resistant IA died (10/10), compared with 21 of 28 (75%) with azole-susceptible IA. | |||
Abbreviations: c-AmB, conventional amphotericin B; CNS, central nervous system; HSCT, hematopoietic stem cell transplant; IA, invasive aspergillosis; ICU, intensive care unit; IPA, invasive pulmonary aspergillosis.
RETAINING THE AZOLE CLASS
In medicine we are confronted with the consequences of azole resistance selection in the environment, and given the prominent role of azole compounds both for management of fungal disease in humans and animals and for food production, the optimum strategy to overcome azole resistance would be to aim to retain the use of azoles for both applications. Measures that prohibit the use of specific azoles in the environment may severely compromise global food production and may not be effective. Although 5 azole fungicides were identified that might play a role in the emergence of resistance mutations [11, 20, 22], many azole fungicides show activity against A. fumigatus and thus may contribute to providing an environment with a selective advantage for azole-resistant strains, thus facilitating its persistence and spread.
An integrated approach focusing on clinical management, public health surveillance programs, and resistance selection in the environment is necessary to improve the survival of patients with azole-resistant aspergillosis, to track the emergence and spread of resistance mechanisms, and to understand how azole resistance develops in A. fumigatus in the environment.
Investigations in the environment should incorporate all applications of azoles including those in agriculture, biocides, and medicine. Recently, 2 Cyp51A point mutations, G54 and M220, were recovered from the environment in Germany [15], Romania, India, and Tanzania [16]. These mutations were previously considered to be associated with the patient route of resistance development, but the recovery from the environment suggests that these mutations either develop in the environment or that through use of azoles in hospitals and veterinary practices these mutations migrate to the environment. By understanding how azole resistance develops and persists in the environment, effective measures can be designed and implemented that prevent resistance development. It was suggested that the application of azole fungicides is crucial for the risk of resistance selection in A. fumigatus rather than the volume of use [61]. If this is the case, changes in current practices may reduce the risk of resistance selection without losing the azole class as a whole. An integrated approach would require an international and multidisciplinary collaboration including healthcare professionals, epidemiologists, researchers from agricultural and veterinary medicine, mycologists, and experts in fungal genetics. Furthermore, governments and other policy makers should recognize that action is urgently warranted if we want to retain the clinical use of azoles and evade a “postazole” era. However, if we are successful in preventing azole resistance selection in the environment, only time will tell if the clinical burden of azole-resistant Aspergillus disease will also diminish.
Note
Potential conflicts of interest. P. E. V. has received research grants from Astellas, Basilea, F2G, Gilead Sciences, Merck, and Pfizer; has been a consultant to Basilea, F2G, Gilead Sciences, Merck, and Pfizer; and has received speaker's fees from Basilea, Gilead Sciences, and Merck. J. F. M. has received grants from Astellas, Basilea, and Merck; has been a consultant to Astellas, Basilea, and Merck; and has received speaker's fees from Merck, United Medical, and Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015; 70:270–7. [DOI] [PubMed] [Google Scholar]
- 2.Patterson TF, Kirkpatrick WR, White M et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Aspergillus Study Group. Medicine (Baltimore) 2000; 79:250–60. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, Stevens DA. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis 1990; 12:1147–201. [DOI] [PubMed] [Google Scholar]
- 4.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 2001; 32:358–66. [DOI] [PubMed] [Google Scholar]
- 5.Miceli MH, Kauffman CA. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 2015; 61:1558–65. [DOI] [PubMed] [Google Scholar]
- 6.Walsh TJ, Anaissie EJ, Denning DW et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:327–60. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz S, Ruhnke M, Ribaud P et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 2005; 106:2641–5. [DOI] [PubMed] [Google Scholar]
- 8.Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma 2012; 49:423–33. [DOI] [PubMed] [Google Scholar]
- 9.Schweer KE, Bangard C, Hekmat K, Cornely OA. Chronic pulmonary aspergillosis. Mycoses 2014; 57:257–70. [DOI] [PubMed] [Google Scholar]
- 10.Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med 2007; 356:1481–3. [DOI] [PubMed] [Google Scholar]
- 11.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 2009; 9:789–95. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhary A, Kathuria S, Xu J, Meis JF. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 2013; 9:e1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snelders E, van der Lee HA, Kuijpers J et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 2008; 5:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camps SM, van der Linden JW, Li Y et al. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother 2012; 56:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bader O, Tünnermann J, Dudakova A et al. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother 2015; 59:4356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma C, Hagen F, Moroti R et al. Triazole resistant Aspergillus fumigatus harbouring G54 mutation: is it de novo or environmentally acquired? J Global Antimicrob Resist 2015; 3:69–73. [DOI] [PubMed] [Google Scholar]
- 17.Howard SJ, Pasqualotto AC, Anderson MJ et al. Major variations in Aspergillus fumigatus arising within aspergillomas in chronic pulmonary aspergillosis. Mycoses 2013; 56:434–41. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhary A, Sharma C, Hagen F, Meis JF. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol 2014; 9:697–711. [DOI] [PubMed] [Google Scholar]
- 19.Verweij PE, Kema GH, Zwaan B, Melchers WJ. Triazole fungicides and the selection of resistance to medical triazoles in the opportunistic mould Aspergillus fumigatus. Pest Manag Sci 2013; 69:165–70. [DOI] [PubMed] [Google Scholar]
- 20.Snelders E, Camps SM, Karawajczyk A et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 2012; 7:e31801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham CD, Reiss E, Hagen F, Meis JF, Lockhart SR. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg Infect Dis 2014; 20:1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Centre for Disease Control (ECDC). Technical report: risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species, 2013. Available at: http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?List=8db7286c-fe2d-476c-9133–18ff4cb1b568&ID=32 Accessed 28 August 2015.
- 23.Lazzarini C, Esposto MC, Prigitano A, Tortorano AM. 8th Congresso Nazionale Diagnostica E Terapia dell Micosi Opportunische, Genua, Italy, 25 June 2015. [Google Scholar]
- 24.van der Linden JW, Camps SM, Kampinga GA et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 2013; 57:513–20. [DOI] [PubMed] [Google Scholar]
- 25.Wiederhold NP, Garcia Gil V, Lindner J et al. Evaluation of Cyp51A mechanisms of azole resistance in Aspergillus fumigatus isolates from the United States. Mycoses 2015; 58(suppl 4):55. [Google Scholar]
- 26.Özmerdiven GE, Ak S, Ener B et al. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J Infect Chemother 2015; 21:581–6. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen E, Maertens J, De Bel A et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother 2015; 59:4569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. First detection of Aspergillus fumigatus azole-resistant strain due to cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes New Infect 2015; 6:33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 2015; 58:350–5. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol 2015; 6:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavergne RA, Morio F, Favennec L et al. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother 2015; 59:4331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhary A, Sharma C, van den Boom M et al. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 2014; 69:2979–83. [DOI] [PubMed] [Google Scholar]
- 33.Astvad KM, Jensen RH, Hassan TM et al. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 2014; 58:5096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 2011; 55:4465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Pape P, Lavergne RA, Morio F, Alvarez C. Description of multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus in Colombia, South America, 2015. Emerg Infect Dis 2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard SJ, Cerar D, Anderson MJ et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 2009; 15:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CJ, Wang HC, Lee JC et al. Azole-resistant Aspergillus fumigatus isolates carrying TR34 /L98H mutations in Taiwan. Mycoses 2015; 58:544–9. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad S, Joseph L, Hagen F, Meis JF, Khan Z. Concomitant occurrence of itraconazole-resistant and -susceptible strains of Aspergillus fumigatus in routine cultures. J Antimicrob Chemother 2015; 70:412–5. [DOI] [PubMed] [Google Scholar]
- 39.Kurzyk EM, Nawrot U, Mroczynska M et al. Detection of clinical Aspergillus fumigatus isolates resistant to triazoles. Mycoses 2015; 58(suppl 4):53–4. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhary A, Kathuria S, Xu J et al. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR₃₄/L98H mutations in the cyp51A gene in India. PLoS One 2012; 7:e52871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camps SM, Rijs AJ, Klaassen CH et al. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J Clin Microbiol 2012; 50:2674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdolrasouli A, Rhodes J, Beale MA et al. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio 2015; 6:e00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mavridou E, Meletiadis J, Jancura P et al. Composite survival index to compare virulence changes in azole-resistant Aspergillus fumigatus clinical isolates. PLoS One 2013; 8:e72280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Linden JW, Snelders E, Kampinga GA et al. Clinical implications of azole resistance in Aspergillus fumigatus, the Netherlands, 2007–2009. Emerg Infect Dis 2011; 17:1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinmann J, Hamprecht A, Vehreschild MJ et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 2015; 70:1522–6. [DOI] [PubMed] [Google Scholar]
- 46.Denning DW, Park S, Lass-Florl C et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis 2011; 52:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Linden JW, Snelders E, Arends JP, Daenen SM, Melchers WJ, Verweij PE. Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. J Clin Microbiol 2010; 48:1478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong GL, van de Sande WW, Dingemans GJ et al. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol 2015; 53:868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepak AJ, Marchillo K, VanHecker J, Andes DR. Impact of in vivo triazole and echinocandin combination therapy for invasive pulmonary aspergillosis: enhanced efficacy against Cyp51 mutant isolates. Antimicrob Agents Chemother 2013; 57:5438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Ingen J, van der Lee HA, Rijs TA et al. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother 2015; 70:178–81. [DOI] [PubMed] [Google Scholar]
- 51.Verweij PE, Ananda-Rajah M, Andes D et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 2015; 21–22:30–40. [DOI] [PubMed] [Google Scholar]
- 52.van der Linden JW, Arendrup MC, Warris A et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 2015; 21:1041–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russcher A, van Paassen J, Dofferhoff PA, Kuijper EJ. High azole resistance rate of Aspergillus fumigatus at intensive care unit in a Dutch tertiary hospital. Ned Tijdschr Med Microbiol 2014; 22:S121. [Google Scholar]
- 54.Fuhren J, Voskuil WS, Boel CH et al. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J Antimicrob Chemother. 2015; 70:2894–8. [DOI] [PubMed] [Google Scholar]
- 55.White MH, Anaissie EJ, Kusne S et al. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis. Clin Infect Dis 1997; 24:635–42. [PubMed] [Google Scholar]
- 56.Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis 1996; 23:608–15. [DOI] [PubMed] [Google Scholar]
- 57.Marr KA, Schlamm HT, Herbrecht R et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 2015; 162:81–9. [DOI] [PubMed] [Google Scholar]
- 58.Bitar D, Lortholary O, Le Strat Y et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis 2014; 20:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kourkoumpetis TK, Desalermos A, Muhammed M, Mylonakis E. Central nervous system aspergillosis: a series of 14 cases from a general hospital and review of 123 cases from the literature. Medicine (Baltimore) 2012; 91:328–36. [DOI] [PubMed] [Google Scholar]
- 60.van der Linden JW, Jansen RR, Bresters D et al. Azole-resistant central nervous system aspergillosis. Clin Infect Dis 2009; 48:1111–3. [DOI] [PubMed] [Google Scholar]
- 61.Gisi U. Assessment of selection and resistance risk for demethylation inhibitor fungicides in Aspergillus fumigatus in agriculture and medicine: a critical review. Pest Manag Sci 2014; 70:352–64. [DOI] [PubMed] [Google Scholar]