Abstract
Repeat expansion mutations cause at least 22 inherited neurological diseases. The complexity of repeat disease genetics and pathobiology has revealed unexpected shared themes and mechanistic pathways among the diseases, for example, RNA toxicity. Also, investigation of the polyglutamine diseases has identified post-translational modification as a key step in the pathogenic cascade, and has shown that the autophagy pathway plays an important role in the degradation of misfolded proteins – two themes likely to be relevant to the entire neurodegeneration field. Insights from repeat disease research are catalyzing new lines of study that should not only elucidate molecular mechanisms of disease, but also highlight opportunities for therapeutic intervention for these currently untreatable disorders.
A new type of human genetic disease mutation was unexpectedly discovered more than 18 years ago – expansion of a repeated microsatellite sequence. At least 22 inherited disorders, all involving the neuraxis, are now known to be caused by expanded repeats (Table 1). Repeat expansion diseases include some of the most common inherited diseases, such as Huntington’s disease (HD) and myotonic dystrophy. In 1991 two repeat expansion mutations – for the X-linked disorders fragile X syndrome of mental retardation (FMR1) and X-linked spinal & bulbar muscular atrophy (SBMA) – were reported to produce disease phenotypes by encoding proteins with expanded poly-amino acid tracts 1,2. We now recognize SBMA as the first member of a sub-category of repeat expansion disorders known as the “CAG / polyglutamine” repeat diseases. Although it was initially thought that FMRI was caused by an expanded polyarginie tract, further work indicated that the predicted translation initiation codon for the FMR1 protein was incorrect. Instead, the CGG repeat expansion in FMR1 is located in the 5’ UTR. The expansion markedly reduces the expression of the gene by promoting hypermethylation of FMR1’s CpG-rich promoter 3–5.
Table 1.
Clinical and molecular characteristics of inherited neurological repeat expansion disorders
| Disease | Main clinical features | Causal repeat |
Location of repeat within gene |
Mechanism / category of disease |
Comments |
|---|---|---|---|---|---|
| Dentatorubral-pallido- luysian atrophy (DRPLA) |
Seizures, choreoathetosis, ataxia, cognitive decline |
CAG | Coding region | Polyglutamine GOF | Very rare, most patients in Japan |
| Fragile X syndrome of mental retardation (FMR1) |
Mental retardation, facial dysmorphism, autism |
CGG | 5’UTR | Hypermethylation of promoter, LOF |
Most common inherited MR |
| Fragile X tremor ataxia syndrome (FXTAS) |
Ataxia, intention tremor, parkinsonism |
CGG | 5’UTR | RNA GOF | Premutation carriers only |
| FRAXE mental retardation (FMR2) |
Mental retardation, hyperactivity |
GCC | 5’UTR | LOF | Needs to be ruled out in X-linked MR |
| Friedreichs ataxia (FRDA) | Ataxia, sensory loss, weakness, diabetes mellitus, ardiomyopathy |
GAA | Intron | LOF, phenocopy of mitochondrial disease |
Most common inherited ataxia in Caucasian ethnicity |
| Huntingtons disease (HD) | Chorea, dystonia, cognitive decline, psychiatric disease |
CAG | Coding region | Polyglutamine GOF | One of the most common inherited diseases in humans |
| Huntingtons disease- like 2 (HDL2) |
Chorea, dystonia, cognitive decline |
CTG | 3 ‘UTR / coding | RNA GOF, poly amino acid GOF, and/or LOF? |
A striking phenocopy of HD |
| Oculopharyngeal muscular dystrophy (OPMD) |
Eyelid weakness, dysphagia, proximal limb weakness |
GCG | Coding region | Polyalanine GOF | Modest expansion causes disease |
| Myoclonic epilepsy type 1 [Unverricht-Lundborg] (Gene: CSTB) |
Photosensitive myoclonus, tonic-clonic seizures, cerebellar degeneration |
CCCCGCCCCGCG | Promoter | LOF | Rare autosomal recessive disorder, Finland / N. Africa |
| Myotonic dystrophy type1 (DM1) |
Muscle weakness, myotonia, cardiac-endocrine-GI disease, mental retardation |
CTG | 3’UTR | RNA GOF | A very common form of muscular dystrophy |
| Myotonic dystrophy type 2 (DM2) |
Muscle weakness, myotonia, cardiac-endocrine-GI disease |
CTG | Intron | RNA GOF | A striking phenocopy of DM1 |
| Spinobulbar muscular atrophy (SBMA) |
Proximal limb weakness, lower motor neuron disease |
CAG | Coding region | Polyglutamine GOF | Phenotype includes LOF androgen insensitivity |
| Spinocerebellar ataxia type1 (SCA1) |
Ataxia, dysarthria, spasticity, ophthalmoplegia |
CAG | Coding region | Polyglutamine GOF | Accounts for 6% of all dominant ataxia |
| Spinocerebellar ataxia type 2 (SCA2) |
Ataxia, slow eye movement, hyporeflexia, motor disease, occasional parkinsonism |
CAG | Coding region | Polyglutamine GOF | Ataxin-2 protein may not reside in the nucleus |
| Spinocerebellar ataxia type 3 (SCA3) |
Ataxia, dystonia, lower motor neuron disease |
CAG | Coding region | Polyglutamine GOF | Most common dominant ataxia |
| Spinocerebellar ataxia type 6 (SCA6) |
Ataxia, dysarthria, sensory loss, occasionally episodic |
CAG | Coding region | Polyglutamine GOF | Causal gene encodes asubunitof P/Q- type Ca++ channel |
| Spincerebellar ataxia type 7 (SCA7) |
Ataxia, dysarthria, cone-rod dystrophy retinal disease |
CAG | Coding region | Polyglutamine GOF | Clinically distinct as patients have retinal disease |
| Spinocerebellar ataxia type 8 (SCA8) |
Ataxia, dysarthria, nystagmus, spasticity |
CTG / CAG | Untranslated RNA / coding region |
RNA GOF and Polyglutamine GOF |
Many cases of reduced penetrance |
| Spinocerebellar ataxia type 10 (SCA10) |
Ataxia, dysarthria, seizures dysphagia |
ATTCT | Intron | RNA GOF? | Huge repeats; only Mexican ancestry? |
| Spinocerebellar ataxia type 12 (SCA12) |
Tremor, ataxia, spasticity, dementia |
CAG | Promoter / 5 UTR? | Unknown | Causal gene encodes a phosphatase |
| Spinocerebellar ataxia type 17 (SCA17) |
Ataxia, dementia, chorea, seizures, dystonia |
CAG | Coding region | Polyglutamine GOF | Gene: TATA-binding protein (TBP) |
| Syndromic / non-syndromic X-linked mental retardation (Gene: ARX) |
Mental retardation alone, with seizures, or with dysarthria and dystonia |
GCG | Coding region | Likely LOF | Associated with West or Partington syndrome |
GOF = gain-of-function; LOF = loss-of-function; MR = mental retardation; UTR = untranslated region
Since these seminal discoveries, a variety of different repeat expansion mutations have been identified (Table 1), and at least four mechanisms of repeat expansion disease are now known: loss-of-function of the gene containing the repeat; gain-of-function due to production of a protein containing a polyglutamine tract expansion; gain-of-function due to production of RNA containing an expanded CUG tract; and gain-of-function due to production of a protein containing a polyalanine tract expansion. Classification of the repeat expansion diseases into one of these four mechanistic categories typically reflects both the sequence composition of the repeat and the location of the repeat within a gene (Table 1).
However, despite these advances, there are still difficulties – as there were at the genesis of the field – in determining exactly how repeat expansion mutations cause inherited human diseases. Therefore, current classification schemes are at risk for becoming outdated in the future. Indeed, a number of recent findings have revealed the potential complexity of the molecular mechanisms underlying the pathogenesis of various repeat expansion diseases. For example, intensive investigation into the CAG – polyglutamine diseases has identified post-translational modification of disease proteins as a key step in the pathogenic cascade, and now suggests that the autophagy pathway serves an important role in the degradation of misfolded proteins. Molecular genetic studies, driven by recent advances in our understanding of the transcriptome, suggest that bidirectional transcription and chromatin structure could be involved in repeat disease pathology and genetic instability. In this Review we examine the most compelling of these paradigm-shifting advances, focusing on RNA toxicity, autophagy, post-translational modification, bidirectional transcription, and genomic structure. We consider advances in these areas for both the relevant sub-category of repeat expansion disease and the broader repeat disease field, and note how these findings might be translated into novel, widely-applicable therapies.
RNA as a driver of toxicity
A shared mechanism for DM1 and DM2
One of the most striking developments in the repeat disease field has been the realization that expanded repeats in transcripts can cause cellular toxicity and neurodegeneration by altering the splicing machinery. In 1992, a CTG repeat expansion in the 3’ UTR of a protein kinase gene was found to be the cause of myotonic dystrophy type 1 (DM1), the most common form of adult muscular dystrophy 6–8. This discovery posed a puzzling question: How does a dominantly inherited repeat expansion in the non-coding region of a gene produce a disease phenotype that affects many different tissues? Although some evidence emerged for reduced dosage of the DM1 protein kinase as the cause of certain DM1 features, haploinsufficiency could not account for most facets of DM1 pathophysiology 9. As the DM1 gene is located in a gene-rich region, some argued that DM1 resulted from altered expression of the DM1 protein kinase in combination with altered expression of adjacent genes, and proposed that the CTG repeat expansion mimicked a contiguous gene syndrome 10. This “field theory” of DM1 pathogenesis gained support when knock-out mice lacking the gene downstream of DM1, SIX5, were found to develop cataracts, which are also a feature of DM1 11,12.
However, the field theory of DM1 pathogenesis could not easily be reconciled with a DM1 phenocopy caused by a different genetic locus 13. This disorder, known as myotonic dystrophy type 2 (DM2), has a phenotype similar to DM1. A distinction is occasional congenital presentation and mental retardation in DM1 patients who receive large CTG repeat expansions because of maternal anticipation 14,15. DM2 is caused by expansion of a CCTG tetranucleotide repeat in the zinc-finger protein 9 (ZNF9) gene 16, the normal function of which does not match any of the genes at the DM1 locus. Hence, the parsimonious conclusion was that RNA transcripts containing a CUG repeat expansion – whether in a triplet repeat – or a CCUG repeat – initiate a shared pathogenic cascade.
A role for RNA toxicity in DM1 had been first suggested by RNA foci in cells from patients with DM1 17, and indistinguishable RNA foci were found in samples from patients with DM2 16. The validity of the RNA toxicity model was strongly supported by a mouse model of myotonic dystrophy in which a 250 CTG repeat in the 3’ UTR of an unrelated transgene - skeletal actin -was shown to cause myotonia and myopathy 18. In another study, preceding the identification of DM2, altered splicing owing to increased function of the CUG-binding protein (CUG-BP) emerged as a plausible mechanism for the RNA gain-of-function toxicity 19. Subsequent work has implicated reduced function of muscleblind 1 (Mbnl1), owing to its sequestration into CUG repeat rich foci, as a contributing factor in the splicing abnormalities in DM1 and DM2 20. Mbnl1 is one of a family of three proteins (Mbnl1, Mbnl2, and Mbnl3) that have been shown to bind specifically to double-stranded RNA hairpins formed by CUG repeats 21. Although increased CUG-repeat containing RNAs appear to increase CUG-BP levels, the production of CUG and CCUG ribonuclear foci by expanded CUG-repeat containing RNAs results in Mbnl1 sequestration and depleted function. Therefore, the model is of increased CUG-BP function combined with decreased Mbnl1 function. Studies of the Drosophila melanogaster muscleblind mutant show that loss-of-function of Mbnl prevents terminal differentiation of retinal and muscle cells 22,23. In mammals, Mbnl1 serves an analogous function, as it favors the splicing of target genes into adult isoforms so, in DM1 and DM2, reduced Mbnl1 function allows fetal isoform production to persist in adult tissues, and affects the expression level of many target transcripts 24. A very recent study found that loss of Mbnl1 can explain the majority of splicing alterations in DM1, and identified extracellular matrix genes as a common target of expanded CUG-repeat containing RNAs, linking DM1 pathogenesis with other connective tissue diseases and muscular dystrophies 24. The pleiotropic phenotype of the myotonic dystrophies is thus believed to result from altered splicing of genes whose protein products function in pathways that are linked to disease features. For example, altered splicing of the chloride channel gene and insulin receptor genes are linked to myotonia and glucose intolerance, respectively.
FXTAS: two mechanisms at the FMR1 locus
Advances in our understanding of myotonic dystrophy pathogenesis set the stage for the characterization of other RNA gain-of-function repeat diseases. One of the most intriguing of these has been the fragile X-tremor ataxia syndrome (FXTAS) 25,26. This disorder occurs primarily in male carriers of the fragile X syndrome premutation allele, and appears to result from an entirely different molecular mechanism than fragile X syndrome. CGG expansions exceeding ~200 repeats produce a mental retardation phenotype by reducing expression of the FMR1 gene, whereas CGG expansions of 55 – 200 repeats result in higher expression of a transcript containing the CGG tracts. The RNA molecules with the expanded CGG tract initiate a cascade of events that culminate in central nervous system (CNS) neurodegeneration, which is characterized by ubiquitin-positive inclusions in the nuclei of neurons and glia 27. Studies in D. melanogaster showed that CGG repeat expansions are sufficient to produce neurodegeneration 28, and features of FXTAS histopathology were also recapitulated in a knock-in mouse model 29. In light of the CUG-BP model of myotonic dystrophy, researchers investigated the function of CGG RNA binding proteins. Through a combination of biochemical and genetic approaches, mainly in the fly, three proteins were found that bind CGG repeats and have reduced function in the disease models: Pur α, hnRNP A2 / B1, and CUGBP1 30,31. The extensive work on myotonic dystrophy and FXTAS, which combined molecular approaches with model organism studies and proteomics, has emphasized that mutant transcripts can produce neuronal dysfunction by disturbing the balance and availability of RNA-binding proteins. Altered function of these proteins seems to be the crux of the molecular pathology in these diseases (Figure 1).
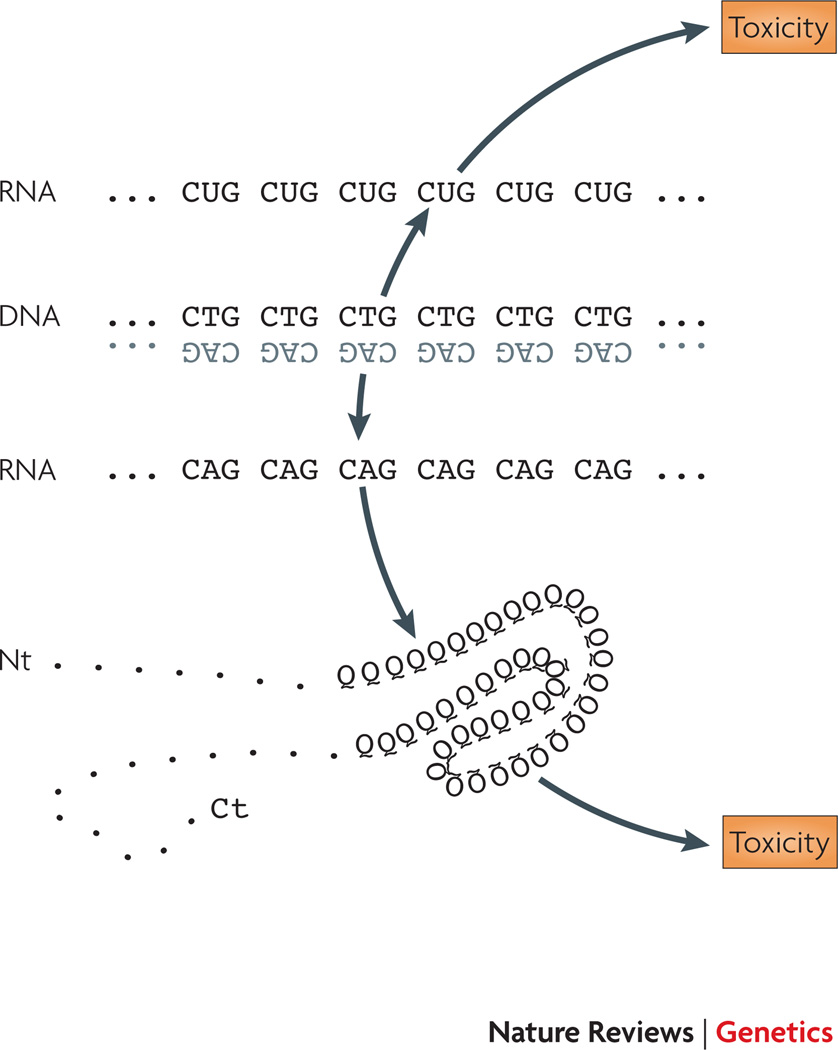
Figure 1. RNA toxicity in repeat expansion disease.
An important mechanism that is now well-established for many repeat expansion diseases is the toxicity of RNAs containing expanded repeat sequences. In these diseases, the toxic RNAs interact with different RNA binding proteins (coloured shaped) to produce disease. This is a “trans-dominant” model of RNA toxicity: interaction of mutant RNA with RNA binding proteins is envisioned to interfere with the functions of the interacting proteins, which leads to abnormalities in the pathways regulated by the RNA binding proteins.
Toxic RNAs in CAG/polyglutamine disease?
If uninterrupted CUG repeat expansions in RNA can produce neurotoxicity, is it possible that other types of repeat expansions also exert their toxic effects at the RNA level? In the case of the CAG / polyglutamine repeat diseases (Table 1), extensive work has shown that protein toxicity rather than RNA toxicity is principally responsible for the disease. Evidence against CAG-RNA toxicity include studies of SCA1 and SBMA transgenic mice in which production of mutant protein and mutant RNA did not yield a neurodegenerative phenotype when the mutant protein could not enter the nucleus, (either because of mutation in the nuclear localization signal or lack of ligand binding)30–33. Furthermore, in D. melanogaster no evidence for general CAG RNA toxicity was found34. However, the recent identification of muscleblind as a modifier of disease toxicity in the SCA3 fly model prompted Li et al. to reexamine this question and, contrary to earlier work, they demonstrated that untranslated RNAs containing tracts of 100 or 250 CAGs can cause retinal degeneration and neuronal dysfunction in D. melanogaster, although interrupted CAGCAA tracts were non-pathogenic 35. These researchers did not find evidence for altered splicing involving muscleblind, so the basis of CAG-RNA toxicity and of the modifier effect remains unclear. Therefore, whether CAG-RNA toxicity contributes to any, or all, of the nine canonical polyglutamine repeat diseases remains highly controversial. At the same time, considerable progress has been made in understanding the mechanistic basis of polyglutamine proteotoxicity, and sophisticated models of disease pathogenesis, involving protein modification and interaction, have now been proposed.
Autophagy in polyglutamine disease
“Autophagy”, literally “self-eating”, is a catabolic process in which cell constituents such as organelles and proteins are delivered to the lysosomal compartment for degradation (BOX 1). Defects in autophagy genes underlie an array of human disease ranging from cancer to autoimmune disease to neurodegeneration 36. It has recently become evident that autophagy plays an important role in polyglutamine disease pathogenesis, and possibly in the pathogenesis of other repeat expansion diseases as well. This has garnered substantial interest since the process of autophagy is amenable to pharmacologic manipulation, thus creating optimism about the possibility of targeting autophagy for therapeutic benefit. But is autophagy activated or impaired in polyglutamine disease? And should the aim be to activate autophagy or to suppress it? Answers to these questions have evolved as the role of autophagy in disease has been illuminated.
Box 1. Autophagy.
Autophagy refers to a set of biological processes in which cell constituents, such as organelles and proteins, are delivered to the lysosomal compartment for degradation. Autophagy may be broadly divided into three types: microautophagy consists of direct engulfment of small volumes of cytosol by lysosomes; chaperone-mediated autophagy (CMA) involves receptor-mediated translocation of proteins into the lysosomal lumen; and macroautophagy (described below). Autophagy is an evolutionarily conserved process for rapid mobilization of macromolecules when nutrient availability is limited 134. Amino acid starvation, for example, induces autophagy and results in increased degradation of non-essential proteins to provide amino acids for the synthesis of essential proteins. In lower organisms, autophagy is not essential when nutrients are abundant but in mammals, the role of autophagy has broadened to include additional functions beyond adapting to starvation. These include essential roles in development, immunity, and tumor suppression, among others 36.
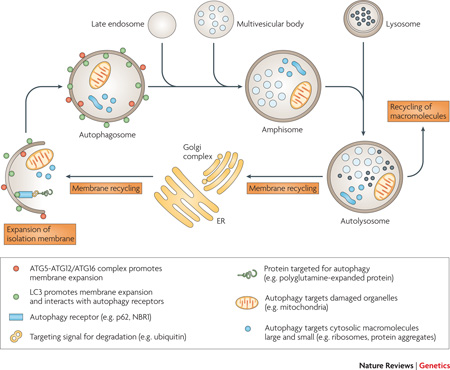
The first step in macroautophagy involves expansion of an isolation membrane that engulfs a portion of the cell; this membrane eventually fuses to form a new double-membraned structure known as an autophagosome (see figure). The source of membrane is not clear, but it might arise from endoplasmic reticulum or the Golgi apparatus. The process of autophagy is controlled by parallel activation cascades that involve ubiquitin-like (UBL) protein modification, strikingly similar to the activation cascade that regulates the ubiquitin proteasome system. The first arm of the cascade produces a large (~350 kDa) multimeric complex (ATG12-ATG5/ATG16) which is thought to act as a structural support for membrane expansion. A second arm of the cascade conjugates the microtubule-associated protein light chain 3 alpha (LC3) with the phospholipid phosphotidylethanolamine (PE). As PE is a component of the autophagosomal membrane, the lipidation reaction results in studding of the inner and outer membranes of autophagosomes with LC3. LC3 also contributes to membrane expansion but and additional important function is the ability to bind autophagy receptors such as sequestosome 1 (SQSTM1, also known as p62) permitting selective autophagy.
Once formed, new autophagosomes move through a stepwise maturation process that results in acidification and delivery of lysosomal hydrolases, permitting degradation of the luminal contents. In mammals, autophagosomes first fuse with endosomes and multivesicular bodies to form amphisomes, which subsequently fuse with lysosomes to create degradative vacuoles termed autolysosomes. Finally, the breakdown products from the autolysosome are translocated back across the lysosomal membrane for reuse in metabolic processes within the cytosol or in some cases are extruded from the cell, and membrane is recycled.
The association between autophagy and the repeat expansion diseases was first made during the study of brains from patients with HD in which mutant huntingtin was found to be associated with accumulated autophagic vacuoles. This finding was corroborated by examination of lymphoblasts from HD patients and in a mouse model of HD37–40 Hallmarks of autophagy have now been reported in animal models of other repeat expansion diseases, including SCA1, SCA7 and SBMA41, 42,43 Initially, the accumulation of autophagic vacuoles in dying neurons in HD and related diseases was interpreted as evidence that autophagy could contribute to cell death44. This hypothesis was supported by evidence that autophagy provides a non-apoptotic mode of programmed cell death – termed type II programmed cell death in some literature – in some circumstances. For example, autophagy is reported to mediate cell death in the Lurcher mouse model of cerebellar degeneration45. Subsequently, the role of autophagy has been extensively examined in animal models of polyglutamine disease and most empirical evidence suggests that autophagy is usually neuroprotective in these settings (reviewed in46).
Neuroprotective Autophagy
Autophagy might provide neuroprotection through accelerated turnover of misfolded disease proteins. Indeed, it has been shown that poly(A) binding protein 2 with an expanded alanine tract (the disease protein in oculopharyngeal muscular dystrophy) and at least some polyglutamine-expanded proteins are delivered to autophagic vacuoles38,47 and degraded by autophagy in vitro48,49. Further support for this view comes from studies in Drosophila models of SBMA and HD, in which genetic ablation of autophagy leads to greater accumulation of polyglutamine disease proteins and increased neurodegeneration50,51 Moreover, pharmacological augmentation of autophagy using small molecules such as rapamycin, lithium, or trehalose, or genetic augmentation by over-expression of HDAC6, results in accelerated turnover of expanded polyglutamine protein and reduced neurodegeneration in Drosophila and mouse models of polyglutamine disease52,53,54,55,56–58.
A caveat to these studies is that virtually all have been performed in models based on over-expression of exogenous mutant protein, which is precisely the scenario in which autophagy would be expected to have greatest effect. It remains important to examine the role of autophagy further in animal models that more faithfully recapitulate endogenous protein expression levels and patterns, using knock-in or BAC transgenic approaches, for example. Nevertheless, the view of autophagy as a neuroprotective process is consistent with the emerging appreciation that autophagy is cytoprotective in numerous contexts - such as conditions of oxidative stress, growth factor deficiency or nutrient limitation - through accelerated turnover of damaged organelles and maintenance of metabolic homeostasis by mobilization of intracellular energy stores59,60.
Is autophagy a disease target?
Despite compelling evidence that autophagy affords neuroprotection by degrading disease proteins, the question remains: why is this protection incomplete? Is it possible that autophagy is not only a modifier of disease but also a target of disease? It has been suggested that the increased numbers of neuronal autophagic vacuoles in some repeat expansion diseases may reflect a defect in autophagy flux rather than autophagy induction61. It is now appreciated that there is significant basal autophagy in many mammalian tissues. The demand for basal autophagy differs among tissues; it is particularly important in the liver and in post-mitotic cells such as neurons and myocytes62,63,64,65. Indeed, neurons are especially vulnerable to perturbations in the autophagy-lysosomal system – not only because they are highly metabolically active, but also because of their unique cellular architecture. In neurons, lysosomes are concentrated in the soma adjacent to the nucleus. Therefore, autophagosomes produced in dendrites, axons or synaptic terminal regions must be transported substantial distances to enable fusion with lysosomes, which makes autophagy in neurons particularly vulnerable to defects in vesicular trafficking66. The importance of basal autophagy in the CNS was shown by conditional knockout of key autophagy genes, which resulted in neurodegeneration with accumulation of ubiquitin-positive inclusions similar to that seen human neurodegenerative diseases (including many repeat expansion diseases) 67,68. The increased vulnerability of neurons to impairment of the autophagy-lysosomal system might account for the high frequency of neurological phenotypes produced by mutations that target the endosomal-lysosomal system69.
It remains to be shown convincingly whether autophagy is impaired in repeat expansion disease. Given the broad range of cellular abnormalities associated with polyglutamine diseases and other repeat expansion diseases, it would not be surprising to find impairment of autophagy. Impaired autophagy - a sort of ‘cellular indigestion’ – could result from overburdening the autophagy-lysosomal system with misfolded, aggregated protein that is difficult to degrade. Alternatively, autophagy could be directly impaired if a disease protein is important for the process of autophagy. As noted above, huntingtin associates with components of the endosomal-lysosomal system and is trafficked with autophagosomes in axons, which suggests that huntingtin might normally regulate autophagy8, 70. Recently, it was shown in vitro and in a mouse knock-in model of HD that polyglutamine expansion in huntingtin impairs the activity of the GTPase Rab11 and leads to a defect in endosome recycling 71,72. Thus, is it possible that polyglutamine expansion impairs a normal function of huntingtin and contributes to impaired autophagy? This would be consistent with the emerging theme that altered native protein function underlies polyglutamine disease pathogenesis.
Post-translational modification in polyglutamine disease
Non-polyglutamine determinants of disease
A striking feature of polyglutamine diseases is the selective vulnerability of the CNS despite widespread expression of many polyglutamine disease proteins in non-neural cell types. Yet, there is striking divergence in clinical phenotype among the polyglutamine diseases; neurologists can easily distinguish the movement disorder of HD from the weakness in SBMA or the ataxia in SCA1. The disease-specific features reflect selective loss of different populations of neurons: despite wide expression within the CNS, polyglutamine expansion in huntingtin selectively affects striatal neurons and cortical neurons, whereas the same genetic mutation in the androgen receptor or ataxin-1 targets motor neurons and Purkinje neurons, respectively. On the basis of these observations it was predicted that features other than polyglutamine, unique to each disease protein, must influence pathogenesis73. This prediction has been borne out in recent advances that have highlighted the importance of host protein context in polyglutamine disease pathogenesis. Principal among these have been insights into the influence of post-translational modification in pathogenesis and, in at least one case, in determining cell type specificity. Here we summarize some important insights into the role of post-translational phosphorylation, acetylation, and sumoylation in polyglutamine disease pathogenesis (Table 2).
Table 2.
Post-translational modifications of polyglutamine disease proteins
| Disease | Protein | Sumoylation | Acetylation | Phosphorylation |
|---|---|---|---|---|
| HD | Huntingtin | Lysines 6, 9, 15, 911,2 | Lysine 4443 | Serines 421, 434, 536, 1181, 1201, 2076, 2653, 26574 Threonine 35 |
| SBMA | AR | Lysines 385, 5116 | Lysines 630, 632, 6337 | Serine 215, 7928 |
| DRPLA | Atrophin-1 | Lysine(s) unknown9 | ND2 | Serine 734 |
| SCA 1 | Ataxin-1 | Lysines 16, 194, 610, 697, 746 |
ND2 | Serine 77610,11 Serine 239 Threonine 236 |
| SCA 2 | Ataxin-2 | ND2 | ND2 | Serines 860, 864 |
| SCA 3 | Ataxin-3 | Lysine(s) unknown12 | ND2 | Serine 256 |
| SCA 6 | CACNA1A | ND2 | ND2 | ND2 |
| SCA 7 | Ataxin-7 | ND2 | Lysine 25713 | Serines 840 849 Threonine 854 |
| SCA 17 | TBP-1 | ND1 | ND1 | ND1 |
Post-translational modifications for which empirical evidence indicates a role in pathogenesis are underlined.
ND1: This post-translational modification occurs, but there is no data pertaining to its role in disease pathogenesis.
ND2: There is no data pertaining to this post-translational modification.
Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington’s disease pathology. Science 2004; 304:100-4.
Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a Striatal Specific Protein, Mediates Mutant-Huntingtin Cytotoxicity. Science 2009; 324:1327-30.
Jeong H, Then F, Melia TJ, Mazzulli JR, Cui L, Savas JN, Voisine C, Paganetti P, Tanese N, Hart AC, Yamamoto A, Krainc D. Acetylation Targets Mutant Huntingtin to Autophagosomes for Degradation. Cell 2009; 137:60-72.
Schilling B, Gafni J, Torcassi C, Cong X, Row RH, LaFevre-Bernt MA, Cusack MP, Ratovitski T, Hirschhorn R, Ross CA, Gibson BW, Ellerby LM. Huntingtin phosphorylation sites mapped by mass
Aiken CT, Steffan JS, Guerrero CM, Khashwji H, Lukacsovich T, Simmons D, Purcell JM, Menhaji K, Zhu YZ, Green K, Laferla F, Huang L, Thompson LM, Marsh JL. Phosphorylation of threonine-3: Implications for huntingtin aggregation and neurotoxicity. J Biol Chem. 2009 Aug 26. [Epub ahead of print]
Mukherjee S, Thomas M, Dadgar N, Lieberman AP, Iniguez-Lluhi JA. Small Ubiquitin-like Modifier (SUMO) Modification of the Androgen Receptor Attenuates Polyglutamine-mediated Aggregation. Journal of Biological Chemistry 2009; 284:21296-306.
Thomas M, Dadgar N, Aphale A, Harrell JM, Kunkel R, Pratt WB, Lieberman AP. Androgen receptor acetylation site mutations cause trafficking defects, misfolding, and aggregation similar to expanded glutamine tracts. J Biol Chem. 2004 Feb 27;279(9):8389-95.
Palazzolo I, Burnett BG, Young JE, Brenne PL, La Spada AR, Fischbeck KH, Howell BW, Pennuto M. Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet. 2007 Jul 1;16(13):1593-603.
Terashima T, Kawai H, Fujitani M, Maeda K, Yasuda H. SUMO-1 co-localized with mutant atrophin-1 with expanded polyglutamines accelerates intranuclear aggregation and cell death. Neuroreport. 2002 Dec 3;13(17):2359-64.
Emamian ES, Kaytor MD, Duvick LA, Zu T, Tousey SK, Zoghbi HY, Clark HB, Orr HT. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003 May 8;38(3):375-87.
Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, Aitken A, Skoulakis EM, Orr HT, Botas J, Zoghbi HY. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003 May 16;113(4):457-68.
Shen L, Tang JG, Tang BS, Jiang H, Zhao GH, Xia K, Zhang YH, Cai F, Tan LM, Pan Q. Research on screening and identification of proteins interacting with ataxin-3. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005 Jun;22(3):242-7.
Phosphorylation of ataxin-1 maintains a balance
The importance of post-translational modification in polyglutamine disease was first illustrated by the Orr and Zoghbi laboratories in a series of papers examining ataxin-1 phosphorylation. Orr and colleagues detected polyglutamine length-dependent phosphorylation of ataxin-1 that mapped to serine 77674. Their interest was heightened after observing that an antibody specific for phospho-S776-ataxin-1 preferentially stained pathological, nuclear-localized ataxin-1 in their SCA1 mouse model. They generated transgenic mice expressing polyglutamine-expanded ataxin-1 with an alanine substituted for serine at position 776 (S776A) to prevent phosphorylation. The mice expressing polyglutamine-expanded ataxin-1-S776A had minimal behavioral and histopathological abnormalities, illustrating the importance of this phosphorylation in pathogenesis.
Phosphorylation alters the conformation of a target protein and can profoundly influence target protein function, often by regulating protein-protein interactions75. Ataxin-1 interacts with two discrete heterotypic protein complexes, one defined by the transcription factor Capicua and the other defined by the RNA-binding protein RBM17. Phosphorylation of ataxin-1 regulates the balance of ataxin-1 association with these distinct functional complexes. The evidence suggests that polyglutamine expansion upsets this balance and drives pathogenesis. Specifically, polyglutamine expansion promotes phosphorylation of S776, which favors association with the RBM17 complex and so might contribute to SCA1 neuropathology through a gain-of-function mechanism. Concomitantly, polyglutamine expansion attenuates the formation and function of the Capicua complex, contributing to SCA1 through a partial loss-of-function mechanism76,77. This discovery has important implications, as this model suggests that polyglutamine disease pathogenesis might involve subtle alteration of native protein function rather than an entirely novel gain of function. This insight highlights the importance of understanding the normal interactions and functions of each disease protein to understand the pathogenesis of each polyglutamine disease.
Phosphorylation and huntingtin trafficking
Huntingtin is subject to phosphorylation at multiple serines and phosphorylation of serine 421 (S421) has emerged as a particularly important determinant of HD pathogenesis. Phosphorylation of S421 has been reported to be carried out by multiple kinases, including the serine/threonine kinase Akt and the serum / glucocorticoid-induced kinase (SGK) 78–80. Significant levels of phospho-S421-huntingtin are present in normal human and mouse striatum, but are reduced by polyglutamine expansion. The basis of reduced phosphorylation is unclear, but is presumed to be owing to altered huntingtin conformation with polyglutamine expansion. Through the use of phosphorylation site mutants and manipulation of the relevant kinases, phosphorylation of S421 has been shown to substantially reduce toxicity in cultured striatal neurons and in a rodent model of Huntington’s disease78–81. What is the basis for this? Huntingtin normally associates with vesicle membranes and microtubules and contributes to endocytosis and transport of endocytic vesicles82–87. Indeed, huntingtin might mediate retrograde transport of signaling endosomes that carry brain-derived neurotrophic factor (BDNF)87. This is crucial, since BDNF signaling supplied by cortico-striatal projection neurons to the striatum - the brain region most severely affected in HD - is an important survival factor 88,89. Although it has been known for some time that polyglutamine expansion impairs the ability of huntingtin to support BDNF signaling, the basis for this was unclear87. The recent discovery that the phosphorylation status of S421 modulates the recruitment of motor proteins to endocytic vesicles and is a key determinant of huntingtin regulation of vesicular transport, and is altered by polyglutamine expansion, offers an explanation. Indeed, constitutive phosphorylation of S421 can overcome the BDNF signaling defect caused by polyglutamine expansion, and can suppress toxicity in HD models90,91. These data highlight a mechanism by altered post-translational modification owing to repeat expansion can affect the ratio of native protein-protein interactions. Therefore, as in the case of SCA1, the pathogenesis of HD appears to be mediated, at least in part, by alteration of native protein function.
Additional phosphorylation sites in huntingtin have been implicated as modifiers of disease, although less is known about how they are influenced by polyglutamine expansion or the mechanisms by they influence pathogenesis. For example, phosphorylation at S343 and S536 is associated with reduced cleavage of huntingtin, and at threonine 3 is associated with reduced aggregation of huntingtin N-terminal fragments92–94. Notably, the phosphorylation status of S13 and S16 has recently been implicated an important determinant of HD pathogenesis. Gu and colleagues generated transgenic mice that express full-length mutant huntingtin with S13 and S16 mutated to either aspartate (which mimics constitutive phosphorylation) or alanine (which is resistant to phosphorylation) 95. Both mutant proteins retain normal huntingtin function, as shown by their ability to rescue huntingtin knock-out phenotypes. Mice expressing the phosphorylation-resistant protein exhibited typical neurodegeneration with associated motor and behavioral phenotypes. By contrast, mice expressing the phosphomimetic protein did not exhibit neurodegeneration, illustrating the dramatic impact of this post-translational modification on toxicity. How might phosphorylation S13 and S16 influence pathogenesis? Thompson and colleagues recently found that these serines are phosphorylated by the I-kappaB kinase (IKK) complex. Through promoting modification of adjacent lysine residues, phosphorylation by IKK targets huntingtin for degradation by a process that requires both the proteasome and lysosome 96. Phosphorylation has also been implicated in influencing the toxicity of SCA3, DRPLA and SBMA, for example: phosphorylation of ataxin-3 at S256 by glycogen synthase kinase 3β reduces aggregation of polyglutamine-expanded atxain-397; phosphorylation of polyglutamine-expanded androgen receptor by MAPK at S516 is associated with increased cleavage and toxicity in cell models of SBMA 98; and S734 of atrophin-1 is phosphorylated by Jun-N-terminal kinase, although the significance of this in the pathogenesis of DRPLA has not yet been examined.
Acetylation as a determinant of stability
Post-translational protein acetylation takes place on the ε-amino group of lysines and prevents this group becoming positively charged, thus impacting the electrostatic properties of the protein (reviewed in 99). Acetylation has diverse consequences, including altered protein-DNA and protein-protein interactions. Acetylation can compete with ubiquitination at lysines residues, such that acetylation increases protein stability99. For example, acetylation of ataxin-7 at K257 was recently found to stabilize the protein and promote accumulation of the ataxin-7 N-terminal truncation product that is believed to be the toxic, disease-causing species 100. Acetylation can also, occasionally, reduce protein stability101.
Krainc and colleagues found that huntingtin stability is regulated by acetylation at K444102. Interestingly, they found that acetylation facilitates trafficking of mutant huntingtin into autophagosomes, thus serving as a signal for degradation by the autophagy-lysosomal system, rather than influencing proteasomal degradation. Acetylation accelerates clearance of the mutant huntingtin by autophagy and reverses its toxic effects in mouse primary striatal and cortical neurons and in a C. elegans model of HD. When polyglutamine-expanded huntingtin was rendered resistant to acetylation, the protein steadily accumulated and increased degeneration in cultured neurons and in mouse brain. This study provided the first evidence that acetylation can target proteins for autophagic degradation. However, the mechanism of targeting for degradation remains to be determined, as does the extent to which proteins other than huntingtin utilize this mechanism.
Sumoylation as a determinant of cell type vulnerability
Sumoylation is a reversible post-translational modification in which a small ubiquitin-like modifier (SUMO) is covalently conjugated to a lysine residue in a target protein. It provides an efficient way to modulate the subcellular localization, activity and stability of a wide variety of substrates. A role for sumoylation in polyglutamine disease was first suggested because of SUMO immunoreactivity in pathological inclusions characteristic of HD, SCA3 and DRPLA. Thompson and colleagues reported that sumoylation of huntingtin at K6 and K9 stabilizes N-terminal fragments of huntingtin, reduces its ability to form aggregates, and promotes its capacity to repress transcription 103. They also noted that sumoylation of a polyglutamine-expanded huntingtin fragment exacerbated neurodegeneration in a Drosophila model of HD.
Recently, huntingtin sumoylation was found to depend upon the small guanine nucleotide -binding protein Rhes by a mechanism independent of Rhes GTPase activity 104. Rhes preferentially binds polyglutamine-expanded huntingtin and promotes sumoylation, including at K9, K15 and K91. Sumoylation of mutant huntingtin at K15 and K91 correlated with reduced aggregation and increased cytotoxicity. Rhes is expressed only in the striatum, and endogenous Rhes co-purified with huntingtin in extracts from transgenic mice, suggesting that the interaction of these proteins takes place under physiological conditions. Thus, Rhes-huntingtin interactions may account for the localized neuropathology of HD, which is largely restricted to the corpus striatum. Other polyglutamine disease proteins - including ataxin-1, androgen receptor, TBP-1, and atrophin-1 – are sumoylated under physiological conditions, and in some cases this has been correlated with increased toxicity in cell culture disease models. The extent to which sumoylation influences pathogenesis in these diseases is yet to be explored, however.
Bidirectional transcription in repeat expansion disease
The two faces of SCA8
Spinocerebellar ataxia type 8 (SCA8) is a slowly progressive, dominantly inherited disease in which affected patients display pronounced signs of ataxia in combination with cortical spinal tract findings 105. The phenotype is highly variable, and cerebellar atrophy can be dramatic, though poorly correlated with the degree of ataxia. The unearthing of the SCA8 repeat expansion utilized a repeat-directed cloning method to pull out an expanded CTG repeat from a patient with ataxia, and this allowed researchers to demonstrate linkage to the repeat mutation in members of another very large ataxia family 106. The CTG repeat occurred in a gene that was transcribed and not translated, leading the team to propose that expression of the untranslated, expanded CTG repeat produced neurotoxicity at the RNA level. This hypothesis was not uniformly accepted, and required testing in a mouse model for its validation. Again both Drosophila and mouse models were generated, and the sufficiency of CTG expansion RNA toxicity was demonstrated 107,108. However, further analysis of the SCA8 BAC transgenic mice revealed an unexpected finding – 1C2-positive intra-nuclear inclusions of the type that would be detected in polyglutamine expansion diseases. As CTG is read as CAG in the anti-sense orientation, careful reexamination of the anti-sense sequence indicated potential translation of a protein consistently almost entirely of polyglutamine. Based upon a series of experiments with materials from SCA8 patients, SCA8 BAC mice, and cell culture models, these investigators demonstrated the existence of a transcript (ATXN8) encoding the predicted polyglutamine-rich ataxin-8 protein, and proposed that both polyglutamine pathology and RNA gain-of-function toxicity combine to yield the SCA8 phenotype. Recent work from this group has reinforced the CUG RNA toxicity of the ataxin-8 anti-sense transcript (ATXN8OS), and has found that the ATXN8OS transcript may sequester Mbnl1 protein 109. Mbnl1 sequestration was then shown to alter the splicing of gene whose product is a transporter of the inhibitory neurotransmitter GABA. The restricted expression of ATXN8OS is consistent with splicing defects occurring in only the cerebellum, although the extent of altered splicing beyond the GABA transporter gene in cerebellum is yet to be determined.
Whither HDL2?
Another repeat disease that is vying for a place among the difficult-to-classify repeat disorders is Huntington’s disease like-2 (HDL2). HDL2, as its name implies, appears to be a remarkably close phenocopy of Huntington’s disease 110. HDL2 is caused by a CTG repeat expansion occurring within the junctophilin-3 (JPH3) gene, such that affected individuals have 40 or more CTGs 110. Junctophilin-3 is a member of a family of proteins that link plasma membrane voltage sensors with intracellular ion channels 111. It remains unclear how the JPH3 CTG repeat expansion produces molecular pathology, as it occurs within the variably spliced exon 2A of the JPH3 gene, and three splice variants have been reported: one placing the CTG repeat in a polyleucine translational frame, one placing the CTG repeat in a polyalanine translational frame, and one localizing the CTG repeat to the 3’ UTR 112. Database queries do not reveal any evidence for transcription of the CAG repeat-containing strand; however, the presence of ubiquitin-positive neuronal intranuclear inclusions (that incidentally do NOT contain the huntingtin protein causal in HD) suggests the existence of a misfolded protein – whether it be polyglutamine or some other poly-amino acid tract. Careful study of HDL2 brain material has revealed evidence for RNA foci, and expression of JPH3 with an expanded CUG tract yields RNA foci containing Mbnl1 112. Furthermore, altered splicing of the genes encoding tau and amyloid precursor protein occurs in HDL2 frontal cortex. Thus, it appears that CUG RNA toxicity, together with some form of proteotoxicity, may combine in HDL2 to produce neurodegeneration. Hence, SCA8 and HDL2 appear to belong to a category of diseases involving simultaneous RNA gain-of-function and protein gain-of-function molecular pathology (Figure 2). In the case of HDL2, a third possible simultaneous mechanism is partial loss-of-function of junctophilin-3 protein, as JPH3 null mice and heterozygous null mice display impaired coordination and shortened lifespan 113, and HDL2 patients express reduced levels of JPH3 112. JPH3 loss-of-function, however, does not yield RNA foci or ubiquitin-positive inclusions in JPH3 knock-out mice, indicating that JPH3 haploinsufficiency alone can not account for HDL2.
Figure 2. Sense and antisense toxicity.
For a subset of repeat expansion diseases, sense and anti-sense transcripts are produced from the the repeat region. In these cases, one transcript may cause toxicity through a RNA gain-of-function pathway and the other transcript, which is generated in opposite orientation, may yield a toxic poly amino-acid tract containing protein. In the example shown here for SCA8, a transcript containing a CUG repeat expansion is hypothesized to produce toxicity, and a polyglutamine protein encoded from an anti-sense transcript is predicted to simultaneously produce toxicity. Even for diseases that do not exhibit bidirectional overlapping transcription, such as SCA3, concomitant RNA toxicity from the RNA transcript containing the CAG repeat expansion has been proposed. Hence, one must now consider the potential toxic contribution of different gene products (RNA and protein) from the sense strand - as well as the potential toxic contribution of different gene products from anti-sense overlapping transcription.
Genomic structure and anti-sense transcription: At the cross-roads of gene expression and repeat disease?
Up to this point, we have considered the potential contribution of the transcriptional and translational gene products of the different repeat disease loci; however, unique aspects of the repeat disease loci are also coming into focus at the level of DNA sequence and chromatin, reflecting a trend in the study of many human genetic disorders – especially those where alterations in transcription are likely to lie at the heart of the disease process. Most repeat expansion diseases involve gene loci with highly polymorphic repeats in normal individuals. In such cases, both the median and mode for the normal repeat are quite large, even though the repeats are non-pathogenic. Early work in the repeat disease field demonstrated that CTG repeats are potent nucleosome positioning elements 114. That expanded repeats could impact the chromatin organization of the regions within which they reside thus seems quite plausible. CTCF (or the “CCCTC binding factor”) is an evolutionarily conserved zinc-finger DNA binding protein with activity in chromatin insulation, transcription regulation, and genomic imprinting 115. In 2001, Filippova et al. identified CTCF binding sites adjacent to and/or flanking a variety of triplet repeat loci, including DM1 and SCA7 116. At the DM1 locus, CTCF binding prevents the spread of heterochromatin into the DM1 gene by blocking antisense transcription 117. When the DM1 CTG repeat becomes hyper-expanded (as in congenital DM1), CTCF binding is lost and antisense transcription spreads out, resulting in heterochromatin formation throughout the DM1 and adjacent genes. One of us has studied the CTCF binding sites at the SCA7 locus, and has shown that abrogation of CTCF binding either by point mutation or methylation of the binding site promotes CAG repeat instability in SCA7 transgenic mice, both in the germline and in somatic tissues 118. Dramatic instability in the size of the CAG repeat tract is a prominent feature in SCA7, and this genetic instability occurs in most of the other repeat expansion diseases (reviewed in119).
As CTCF binding sites are uncovered by directed approaches (such as electrophoretic mobility shift assays) and discovery-driven approaches (such as ChIP-CHIP) 120–122, evidence for CTCF binding in close proximity to the repeat sequences themselves is emerging. In some cases, CTCF binding appears associated with anti-sense transcription 117,123, but in DM1 and Friedreich’s ataxia, CTCF depletion may permit production of an anti-sense transcript, resulting in heterochromatin formation and gene silencing 117,124 At the same time, surveys of mammalian transcriptomes are uncovering tremendous numbers and varieties of non-coding RNAs, including anti-sense transcripts at the start sites of sense transcription and in adjacent 5’ promoter regions 125–128. This phenomenon appears prominent at repeat disease loci. For example, at the fragile X locus, an anti-sense transcript overlapping the CGG repeat in opposite orientation has been identified 129. The anti-sense FMR1 transcript (ASFMR1) is up-regulated in patients carrying premutations alleles, and appears silenced in full mutation carriers. This pattern of altered anti-sense transcription may characterize a number of repeat disease loci, and raises important questions about the role of coordinate or disconnected non-coding RNA transcription and sense disease gene transcription in the face of repeat length and chromatin structure change. CTCF and non-coding transcript regulation of genomic architecture could be important for understanding transcription dysregulation in many repeat expansion diseases, especially as evidence mounts for alteration of epigenetic regulatory processes in certain of the polyQ diseases 130,131. We thus expect that considerable work on this topic will emerge over the next few years.
Conclusions
Enormous strides have been made since the initial discovery of a novel, esoteric type of mutation as the cause of SBMA and the fragile X syndrome. Since that time, pathogenic repeat expansions have been found to underlie 22 distinct human neurological diseases. Research in this field has revealed incredible unexpected complexity, including toxicities mediated at the protein, RNA, and genomic level. Perturbations of numerous cellular functions have been implicated, including transcription, RNA splicing, RNA translation, mitochondrial function, and protein quality control systems in addition to specific neuronal functions such as axonal transport and synaptic activity. This complexity has lured a remarkably diverse group of scientists to the field, catalyzing unprecedented interdisciplinary approaches to elucidating the mechanisms of disease. As a result, research in the repeat expansion field has often been at the vanguard of key advances in molecular genetics. For example, repeat disease research continues to play a leading role in understanding the basis of defective RNA metabolism and autophagy in human disease. A nagging question for the repeat disease field is why the development of meaningful treatments has not kept pace with the remarkable trajectory of research advance in the study of mechanisms and pathways. While this disconnect applies to most neurological disorders, the repeat diseases offer a wonderful opportunity for rationale therapy development – since definitive diagnosis based upon repeat allele expansion is the norm. Discoveries over the last few years have yielded a promising crop of candidate targets and pathways, and have led to some very encouraging therapeutic developments, including the use of oligonucleotide and morpholino anti-sense repeats targeted against the DM1 CTG expansion 132,133. Yet, there can be no doubt that successful application of one or more of these strategies, as a therapy for a repeat expansion disease in affected patients, represents the most crucial advance that could come out of this field.
Acknowledgments
The authors’ repeat disease research is supported by the NIH (R01 NS041648, R01 GM059356 & R01 EY014061 to A.R.L., and R01 NS053825 & R01 AG031587 to J.P.T.) and by grants from the Muscular Dystrophy Association to A.R.L. and J.P.T.
Glossary
- Haploinsufficiency
A condition in a diploid organism in which a single functional copy of a gene results in a phenotype, such as a disease
- Contiguous gene syndrome
A multi-symptom disorder that is caused by a deletion of a large sequence of DNA that encodes several genes
- Genocopy
A genotype that determines a phenotype closely similar to that determined by a different gene
- Anticipation
The tendency of certain diseases to have an earlier age of onset and increasing severity in successive generations
- Myotonia
The failure of muscle to relax immediately after voluntary contraction has stopped
- Myopathy
A disease of the muscle
- Premutation
An unstable mutation that has no phenotypic effect but that is highly likely to mutate to a full mutation during transmission through the germ line, as is seen with some expanding trinucleotide repeats
- Inclusions
Accumulations of proteins and other materials that are visualized as discrete entities at the light microscope level often after the application of special stains or antibodies
- Post-translational modification
A covalent chemical modification of a protein that takes place subsequent to translation
- Endocytosis
The process whereby cells engulfs extracellular material through invagination of the plasma membrane to create an endocytic vesicle
- Neurotrophic factor
A small protein that promotes the growth and/or survival of neurons
- Nucleosome
The basic unit of chromatin. A nucleosome contains approximately 146 bp of DNA wrapped around a histone octamer
- Heterochromatin
Parts of chromosomes with an unusual degree of contraction and that consequently have different staining properties from the euchromatin at nuclear divisions. Largely composed of repetitive DNA, heterochromatin forms dark bands after Giemsa staining
References
- 1.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 2.Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 3.Ashley CT, et al. Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet. 1993;4:244–251. doi: 10.1038/ng0793-244. [DOI] [PubMed] [Google Scholar]
- 4.Bell MV, et al. Physical mapping across the fragile X: hypermethylation and clinical expression of the fragile X syndrome. Cell. 1991;64:861–866. doi: 10.1016/0092-8674(91)90514-y. [DOI] [PubMed] [Google Scholar]
- 5.Heitz D, et al. Isolation of sequences that span the fragile X and identification of a fragile X-related CpG island. Science. 1991;251:1236–1239. doi: 10.1126/science.2006411. [DOI] [PubMed] [Google Scholar]
- 6.Aslanidis C, et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992;355:548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- 7.Brook JD, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3’ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 8.Harley HG, et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992;355:545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- 9.Jansen G, et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996;13:316–324. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- 10.Harris S, Moncrieff C, Johnson K. Myotonic dystrophy: will the real gene please step forward! Hum Mol Genet 5 Spec No. 1996:1417–1423. doi: 10.1093/hmg/5.supplement_1.1417. [DOI] [PubMed] [Google Scholar]
- 11.Klesert TR, et al. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25:105–109. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar PS, et al. Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet. 2000;25:110–114. doi: 10.1038/75500. [DOI] [PubMed] [Google Scholar]
- 13.Ranum LP, Rasmussen PF, Benzow KA, Koob MD, Day JW. Genetic mapping of a second myotonic dystrophy locus. Nat Genet. 1998;19:196–198. doi: 10.1038/570. [DOI] [PubMed] [Google Scholar]
- 14.Ricker K, et al. Proximal myotonic myopathy. Clinical features of a multisystem disorder similar to myotonic dystrophy. Arch Neurol. 1995;52:25–31. doi: 10.1001/archneur.1995.00540250029009. [DOI] [PubMed] [Google Scholar]
- 15.Day JW, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60:657–664. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 16.Liquori CL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9 . Science. 2001;293:864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 17.Timchenko LT. Myotonic dystrophy: the role of RNA CUG triplet repeats. Am J Hum Genet. 1999;64:360–364. doi: 10.1086/302268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mankodi A, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289:1769–1773. doi: 10.1126/science.289.5485.1769. This paper demonstrates that expression of a CUG repeat expansion in a non-repeat disease RNA is sufficient to produce a myotonic dystrophy-like phenotype in mice. This was an important step in validating the RNA gain-of-function toxicity model
- 19. Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:37–741. doi: 10.1126/science.280.5364.737. This study revealed a role for altered splicing in the pathogenesis of myotonic dystrophy, and also offered an explanation for how the DM1 gene defect could affect a variety of different cell types and tissues
- 20. Kanadia RN, et al. A muscleblind knockout model for myotonic dystrophy. Science. 2003;302:1978–1980. doi: 10.1126/science.1088583. This work implicated muscleblind in the splicing pathology caused by the CUG repeat expansions in myotonic dystrophy, and provided evidence for the genesis and nature of the splicing alterations in this disease
- 21.Miller JW, et al. Recruitment of human muscleblind proteins to (CUG) (n) expansions associated with myotonic dystrophy. Embo J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artero R, et al. The muscleblind gene participates in the organization of Z-bands and epidermal attachments of Drosophila muscles and is regulated by Dmef2. Dev Biol. 1998;195:131–143. doi: 10.1006/dbio.1997.8833. [DOI] [PubMed] [Google Scholar]
- 23.Begemann G, et al. muscleblind, a gene required for photoreceptor differentiation in Drosophila, encodes novel nuclear Cys3His-type zinc-finger-containing proteins. Development. 1997;124:4321–4331. doi: 10.1242/dev.124.21.4321. [DOI] [PubMed] [Google Scholar]
- 24.Du H, et al. Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy. Nat Struct Mol Biol. 2010 doi: 10.1038/nsmb.1720. <aop>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagerman RJ, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 26.Jacquemont S, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco CM, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 28.Jin P, et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 29.Willemsen R, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 30.Jin P, et al. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sofola OA, et al. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsuno M, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–854. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 33.Klement IA, et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine- induced disease in SCA1 transgenic mice. Cell. 1998;95:41–53. doi: 10.1016/s0092-8674(00)81781-x. [DOI] [PubMed] [Google Scholar]
- 34.McLeod CJ, OKeefe LV, Richards RI. The pathogenic agent in Drosophila models of ‘polyglutamine’ diseases. Hum Mol Genet. 2005;14:1041–1048. doi: 10.1093/hmg/ddi096. [DOI] [PubMed] [Google Scholar]
- 35.Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008;453:1107–1111. doi: 10.1038/nature06909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 37.Sapp E, et al. Huntingtin localization in brains of normal and Huntington’s disease patients. Ann Neurol. 1997;42:604–612. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- 38.Kegel KB, et al. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 2000;20:7268–7278. doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen A, et al. Expanded CAG repeats in exon 1 of the Huntingtons disease gene stimulate dopamine-mediated striatal neuron autophagy and degeneration. Human Molecular Genetics. 2001;10:1243–1254. doi: 10.1093/hmg/10.12.1243. [DOI] [PubMed] [Google Scholar]
- 40.Nagata E, Sawa A, Ross CA, Snyder SH. Autophagosome-like vacuole formation in Huntington’s disease lymphoblasts. Neuroreport. 2004;15:1325–1328. doi: 10.1097/01.wnr.0000127073.66692.8f. [DOI] [PubMed] [Google Scholar]
- 41.Vig PJ, Shao Q, Subramony SH, Lopez ME, Safaya E. Bergmann glial S100B activates myo-inositol monophosphatase 1 and Co-localizes to purkinje cell vacuoles in SCA1 transgenic mice. Cerebellum. 2009;8:231–244. doi: 10.1007/s12311-009-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zander C, et al. Similarities between spinocerebellar ataxia type 7 (SCA7) cell models and human brain: proteins recruited in inclusions and activation of caspase-3. Hum Mol Genet. 2001;10:2569–2579. doi: 10.1093/hmg/10.22.2569. [DOI] [PubMed] [Google Scholar]
- 43.Montie HL, et al. Cytoplasmic retention of polyglutamine-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2009;18:1937–1950. doi: 10.1093/hmg/ddp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 45.Yue Z, et al. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 46.Nedelsky NB, Todd PK, Taylor JP. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2008;1782:691–699. doi: 10.1016/j.bbadis.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor JP, et al. Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet. 2003;12:749–757. doi: 10.1093/hmg/ddg074. [DOI] [PubMed] [Google Scholar]
- 48.Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 49.Qin ZH, et al. Autophagy regulates the processing of amino terminal huntingtin fragments. Hum Mol Genet. 2003;12:3231–3244. doi: 10.1093/hmg/ddg346. [DOI] [PubMed] [Google Scholar]
- 50.Berger Z, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Human Molecular Genetics. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 51.Pandey UB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 52. Ravikumar B, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. This study demonstrated that pharmacological induction of autophagy ameliorated neurodegeneration in animal models of HD
- 53.Pandey UB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 54.Pandey UB, Batlevi Y, Baehrecke EH, Taylor JP. HDAC6 at the intersection of autophagy, the ubiquitin-proteasome system and neurodegeneration. Autophagy. 2007;3:643–645. doi: 10.4161/auto.5050. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar S, et al. A rational mechanism for combination treatment of Huntingtons disease using lithium and rapamycin. Hum Mol Genet. 2008;17:170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka M, et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med. 2004;10:148–154. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 57.Davies JE, Sarkar S, Rubinsztein DC. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum Mol Genet. 2006;15:23–31. doi: 10.1093/hmg/ddi422. [DOI] [PubMed] [Google Scholar]
- 58.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 59.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxidants & Redox Signaling. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 60.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 61.McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008;16:75–84. doi: 10.1159/000109761. [DOI] [PubMed] [Google Scholar]
- 62. Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. This study, and the related study by Hara et al, demonstrated the importance of basal, quality control autophagy in the central nervous system. Impairment of basal autophagy was found to cause neurodegeneration with accumulation of ubiquitin-positive inclusions
- 63.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 64.Komatsu M, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. Journal of Cell Biology. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Komatsu M, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollenbeck PJ. Products of Endocytosis and Autophagy Are Retrieved from Axons by Regulated Retrograde Organelle Transport. Journal of Cell Biology. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 68.Kamaya H, Hayes JJ, Jr, Ueda I. Dissociation constants of local anesthetics and their temperature dependence. Anesth Analg. 1983;62:1025–1030. [PubMed] [Google Scholar]
- 69.Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4:590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- 70.Atwal RS, et al. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Human Molecular Genetics. 2007;16:2600–2615. doi: 10.1093/hmg/ddm217. [DOI] [PubMed] [Google Scholar]
- 71.Li X, et al. Mutant Huntingtin Impairs Vesicle Formation from Recycling Endosomes by Interfering with Rab11 Activity. Mol Cell Biol. 2009 doi: 10.1128/MCB.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, et al. Disruption of Rab11 activity in a knock-in mouse model of Huntington’s disease. Neurobiol Dis. 2009;36:374–383. doi: 10.1016/j.nbd.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.La Spada AR, Taylor JP. Polyglutamines placed into context. Neuron. 2003;38:681–684. doi: 10.1016/s0896-6273(03)00328-3. [DOI] [PubMed] [Google Scholar]
- 74.Emamian ES, et al. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003;38:375–387. doi: 10.1016/s0896-6273(03)00258-7. [DOI] [PubMed] [Google Scholar]
- 75.Johnson LN, Lewis RJ. Structural basis for control by phosphorylation. Chem Rev. 2001;101:2209–2242. doi: 10.1021/cr000225s. [DOI] [PubMed] [Google Scholar]
- 76.Chen HK, et al. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–468. doi: 10.1016/s0092-8674(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 77. Lim J, et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452:713–718. doi: 10.1038/nature06731. This study demonstrated that polyglutamine expansion shifts the ratio of ataxin-1 between two alternative protein complexes. This discovery has important implications, suggests that polyglutamine disease pathogenesis might involve subtle alteration of native protein function rather than an entirely novel gain of function
- 78.Humbert S, et al. The IGF-1/Akt pathway is neuroprotective in Huntingtons disease and involves huntingtin phosphorylation by Akt. Developmental Cell. 2002;2:831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 79.Rangone H, et al. The serum- and glucocorticoid-induced kinase SGK inhibits mutant huntingtin-induced toxicity by phosphorylating serine 421 of huntingtin. European Journal of Neuroscience. 2004;19:273–279. doi: 10.1111/j.0953-816x.2003.03131.x. [DOI] [PubMed] [Google Scholar]
- 80.Warby SC, et al. Huntingtin phosphorylation on serine 421 is significantly reduced in the striatum and by polyglutamine expansion in vivo. Human Molecular Genetics. 2005;14:1569–1577. doi: 10.1093/hmg/ddi165. [DOI] [PubMed] [Google Scholar]
- 81.Pardo R, et al. Inhibition of calcineurin by FK506 protects against polyglutamine-huntingtin toxicity through an increase of huntingtin phosphorylation at S421. Journal of Neuroscience. 2006;26:1635–1645. doi: 10.1523/JNEUROSCI.3706-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Difiglia M, et al. Huntingtin Is a Cytoplasmic Protein Associated with Vesicles in Human and Rat-Brain Neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 83.Engelender S, et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150(Glued) subunit of dynactin. Human Molecular Genetics. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 84.Kalchman MA, et al. HIP1, a human homologue of S-cerevisiae Sla2p, interacts with membrane-associated huntingtin in the brain. Nature Genetics. 1997;16:44–53. doi: 10.1038/ng0597-44. [DOI] [PubMed] [Google Scholar]
- 85.Li SH, Gutekunst CA, Hersch SM, Li XJ. Interaction of Huntingtin-associated protein with dynactin P150(Glued) Journal of Neuroscience. 1998;18:1261–1269. doi: 10.1523/JNEUROSCI.18-04-01261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li XJ, et al. A Huntingtin-Associated Protein Enriched in Brain with Implications for Pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- 87.Gauthier LR, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 88.Altar CA, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 89.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. Journal of Neuroscience. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zala D, et al. Phosphorylation of mutant huntingtin at S421 restores anterograde and retrograde transport in neurons. Human Molecular Genetics. 2008;17:3837–3846. doi: 10.1093/hmg/ddn281. [DOI] [PubMed] [Google Scholar]
- 91.Colin E, et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. Embo Journal. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Warby SC, et al. Phosphorylation of huntingtin reduces the accumulation of its nuclear fragments. Mol Cell Neurosci. 2009;40:121–127. doi: 10.1016/j.mcn.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Aiken CT, et al. Phosphorylation of threonine-3: Implications for huntingtin aggregation and neurotoxicity. J Biol Chem. 2009 doi: 10.1074/jbc.M109.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schilling B, et al. Huntingtin phosphorylation sites mapped by mass spectrometry. Modulation of cleavage and toxicity. J Biol Chem. 2006;281:23686–23697. doi: 10.1074/jbc.M513507200. [DOI] [PubMed] [Google Scholar]
- 95.Gu M, Yang XW. Record pending. 2009 [Google Scholar]
- 96.Thompson LM, et al. IKK phosphorylates Huntingtin and targets it for degradation by the proteasome and lysosome. J Cell Biol. 2009;187:1083–1099. doi: 10.1083/jcb.200909067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fei E, et al. Phosphorylation of ataxin-3 by glycogen synthase kinase 3beta at serine 256 regulates the aggregation of ataxin-3. Biochem Biophys Res Commun. 2007;357:487–492. doi: 10.1016/j.bbrc.2007.03.160. [DOI] [PubMed] [Google Scholar]
- 98.LaFevre-Bernt MA, Ellerby LM. Kennedy’s disease Phosphorylation of the polyglutamine-expanded form of androgen receptor regulates its cleavage by caspase-3 and enhances cell death. J Biol Chem. 2003;278:34918–34924. doi: 10.1074/jbc.M302841200. [DOI] [PubMed] [Google Scholar]
- 99.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of nonhistone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 100.Mookerjee S, et al. Posttranslational modification of ataxin-7 at lysine 257 prevents autophagy-mediated turnover of an N-terminal caspase-7 cleavage fragment. J Neurosci. 2009;29:15134–15144. doi: 10.1523/JNEUROSCI.4720-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeong JW, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 102.Jeong H, et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72. doi: 10.1016/j.cell.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steffan JS, et al. SUMO modification of Huntingtin and Huntingtons disease pathology. Science. 2004;304:100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- 104.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Day JW, Schut LJ, Moseley ML, Durand AC, Ranum LP. Spinocerebellar ataxia type 8: clinical features in a large family. Neurology. 2000;55:649–657. doi: 10.1212/wnl.55.5.649. [DOI] [PubMed] [Google Scholar]
- 106.Koob MD, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8) Nat Genet. 1999;21:379–384. doi: 10.1038/7710. [DOI] [PubMed] [Google Scholar]
- 107. Moseley ML, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006;38:758–769. doi: 10.1038/ng1827. This paper validated that expansion of the SCA8 triplet repeat is sufficient to produce neurological disease, and demonstrated that transcription of a non-coding RNA on one strand and transcription of a coding CAG RNA on the opposite, resulting in the production of a polyglutamine protein, both occur at the SCA8 disease locus
- 108.Mutsuddi M, Marshall CM, Benzow KA, Koob MD, Rebay I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14:302–308. doi: 10.1016/j.cub.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 109.Daughters RS, et al. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5:e1000600. doi: 10.1371/journal.pgen.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Margolis RL, et al. A disorder similar to Huntington’s disease is associated with a novel CAG repeat expansion. Ann Neurol. 2001;50:373–380. doi: 10.1002/ana.1312. [DOI] [PubMed] [Google Scholar]
- 111.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 112.Rudnicki DD, et al. Huntingtons disease--like 2 is associated with CUG repeat-containing RNA foci. Ann Neurol. 2007;61:272–282. doi: 10.1002/ana.21081. [DOI] [PubMed] [Google Scholar]
- 113.Nishi M, et al. Motor discoordination in mutant mice lacking junctophilin type 3. Biochem Biophys Res Commun. 2002;292:318–324. doi: 10.1006/bbrc.2002.6649. [DOI] [PubMed] [Google Scholar]
- 114.Wang YH, Gellibolian R, Shimizu M, Wells RD, Griffith J. Long CCG triplet repeat blocks exclude nucleosomes: a possible mechanism for the nature of fragile sites in chromosomes. J Mol Biol. 1996;263:511–516. doi: 10.1006/jmbi.1996.0593. [DOI] [PubMed] [Google Scholar]
- 115.Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–527. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- 116. Filippova GN, et al. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat Genet. 2001;28:335–343. doi: 10.1038/ng570. This study indicated that altered CTCF binding at the DM1 locus could be involved in this disease. It also implicated CTCF in a trinucleotide repeat disease for the first time, and suggested that CTCF binding sites are commonly associated with repeat tracts susceptible to disease-causing expansion
- 117.Cho DH, et al. Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF. Mol Cell. 2005;20:483–489. doi: 10.1016/j.molcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Libby RT, et al. CTCF cis-regulates trinucleotide repeat instability in an epigenetic manner: a novel basis for mutational hot spot determination. PLoS Genet. 2008;4:e1000257. doi: 10.1371/journal.pgen.1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pearson CE, Nichol Edamura K, Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005;6:729–742. doi: 10.1038/nrg1689. [DOI] [PubMed] [Google Scholar]
- 120.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 121.Kim TH, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nguyen P, et al. CTCFL/BORIS is a methylation-independent DNA-binding protein that preferentially binds to the paternal H19 differentially methylated region. Cancer Res. 2008;68:5546–5551. doi: 10.1158/0008-5472.CAN-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chao W, Huynh KD, Spencer RJ, Davidow LS, Lee JT. CTCF, a candidate transacting factor for X-inactivation choice. Science. 2002;295:345–347. doi: 10.1126/science.1065982. [DOI] [PubMed] [Google Scholar]
- 124.De Biase I, Chutake YK, Rindler PM, Bidichandani SI. Epigenetic silencing in Friedreich ataxia is associated with depletion of CTCF (CCCTC-binding factor) and antisense transcription. PLoS One. 2009;4:e7914. doi: 10.1371/journal.pone.0007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He Y, Vogelstein B, Velculescu VE, Papadopoulos N, Kinzler KW. The antisense transcriptomes of human cells. Science. 2008;322:1855–1857. doi: 10.1126/science.1163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Preker P, et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]
- 128.Seila AC, et al. Divergent transcription from active promoters. Science. 2008;322:1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ladd PD, et al. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007;16:3174–3187. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- 130.Seong IS, et al. Huntingtin facilitates polycomb repressive complex 2. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim MO, et al. Altered histone monoubiquitylation mediated by mutant huntingtin induces transcriptional dysregulation. J Neurosci. 2008;28:3947–3957. doi: 10.1523/JNEUROSCI.5667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mulders SA, et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc Natl Acad Sci USA. 2009;106:13915–13920. doi: 10.1073/pnas.0905780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wheeler TM, et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science. 2009;325:336–339. doi: 10.1126/science.1173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nedelsky NB, Todd PK, Taylor JP. Autophagy and the ubiquitin-proteasome system: collaborators in neuroprotection. Biochim Biophys Acta. 2008;1782:691–699. doi: 10.1016/j.bbadis.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]