Using a novel method that allows assessment of cross-bridge cycling at body temperature in intact muscles obtained from end-stage failing as well as nonfailing human hearts, we show cross-bridge cycling rate to be length dependent in the right ventricle but not to be altered by disease.
Keywords: cross-bridge cycling kinetics, heart failure, trabeculae, muscle length, relaxation, muscle length
Abstract
Cross-bridge cycling rate is an important determinant of cardiac output, and its alteration can potentially contribute to reduced output in heart failure patients. Additionally, animal studies suggest that this rate can be regulated by muscle length. The purpose of this study was to investigate cross-bridge cycling rate and its regulation by muscle length under near-physiological conditions in intact right ventricular muscles of nonfailing and failing human hearts. We acquired freshly explanted nonfailing (n = 9) and failing (n = 10) human hearts. All experiments were performed on intact right ventricular cardiac trabeculae (n = 40) at physiological temperature and near the normal heart rate range. The failing myocardium showed the typical heart failure phenotype: a negative force-frequency relationship and β-adrenergic desensitization (P < 0.05), indicating the expected pathological myocardium in the right ventricles. We found that there exists a length-dependent regulation of cross-bridge cycling kinetics in human myocardium. Decreasing muscle length accelerated the rate of cross-bridge reattachment (ktr) in both nonfailing and failing myocardium (P < 0.05) equally; there were no major differences between nonfailing and failing myocardium at each respective length (P > 0.05), indicating that this regulatory mechanism is preserved in heart failure. Length-dependent assessment of twitch kinetics mirrored these findings; normalized dF/dt slowed down with increasing length of the muscle and was virtually identical in diseased tissue. This study shows for the first time that muscle length regulates cross-bridge kinetics in human myocardium under near-physiological conditions and that those kinetics are preserved in the right ventricular tissues of heart failure patients.
NEW & NOTEWORTHY
Using a novel method that allows assessment of cross-bridge cycling at body temperature in intact muscles obtained from end-stage failing as well as nonfailing human hearts, we show cross-bridge cycling rate to be length dependent in the right ventricle but not to be altered by disease.
heart failure is a complex disorder that currently affects 5.8 million patients, and this number is estimated to reach 8 million by 2030 in the United States (25). It is fundamental to have a precise and complete understanding of the alterations that occur in heart failure in order to better guide the identification of therapeutic targets. At the cellular level, various alterations can contribute to the decline in cardiac output in patients with heart failure. The rate of the cyclical interaction of myosin with actin, termed cross-bridge cycling rate, is an important determinant of cardiac output (34). Therefore, alterations in this cross-bridge cycling rate can potentially contribute to the pathophysiology of heart failure and can potentially be a therapeutic target. No current data are available on cross-bridge cycling rate of dynamically contracting human myocardium at normal physiological temperature, where it is known that temperature is a very potent modifier of contractile force and kinetics (11, 26, 37, 43).
The heart utilizes multiple mechanisms in order to fine-tune its pumping activity and adjust to the demands of the body. If and how the human heart regulates cross-bridge cycling kinetics is relatively unknown. The Frank-Starling relationship dictates that force is increased when the preload in the ventricles and hence cardiomyocyte length are increased. Some studies on animals show that muscle length also affects cross-bridge cycling kinetics, while others show a lack of such effect in various animal models (1, 12, 18, 21, 33, 37, 45). Cardiac studies in animal models, especially in small rodents, do not always translate to humans (31, 36). This is particularly important in regard to the contractile and cross-bridge cycling kinetics, since small animals have evolved to contract and relax several times faster than their human counterparts (36). In this study, we aimed to bridge these gaps in knowledge by studying the potential alterations in cross-bridge cycling rate, assessed by tension-redevelopment kinetics (6), during human heart failure and its regulation by muscle length. The novel aspect of this study is 1) the use of both freshly isolated nonfailing and failing human muscles and that 2) all experiments were performed in intact muscles and under conditions that are as close to the conditions of the body as experimentally possible for these types of studies. This will allow for a better understanding of the factors that determine cardiac output in humans and its alterations in heart failure.
METHODS
Human tissue collection.
Explanted hearts were obtained in the operating room, flushed immediately after removal from living donors, and thereafter transferred to the laboratory in cold cardioplegic solution containing (in mM) 110 NaCl, 16 KCl, 16 MgCl2, 10 NaHCO3, and 0.5 CaCl2. Hearts were procured and treated with identical protocols and timing regardless of their source.
All human tissues were experimented on with approval from the Institutional Review Board (IRB) of The Ohio State University and in accordance with the Declaration of Helsinki. Informed consent was acquired from cardiac transplant patients. Nontransplantable donor hearts (n = 9) were acquired from Lifeline of Ohio Organ Procurement; ejection fraction of these hearts, measured prior to procurement, was in the normal range, and none of the nonfailing hearts had signs of coronary bypass surgery or prior myocardial infarctions, while wall thicknesses, heart weights, and heart weight-to-body weight ratios were all in the normal range. End-stage failing hearts (n = 10) were acquired from patients undergoing cardiac transplantation at The Ohio State University Wexner Medical Center. All hearts, regardless of source, were processed with the same protocol. The characteristics of these hearts are provided in Table 1 and Table 2. An additional four nontransplantable donor hearts (not included in the tables) were used only for assessing the effects of temperature on the rate of tension redevelopment (ktr).
Table 1.
Characteristics of nonfailing hearts
| Heart | Age, yr | Sex | Race | Cause of Death | LVEF, % | Heart Weight, g |
|---|---|---|---|---|---|---|
| 685884 | 36 | Male | Caucasian | Fentanyl patch intoxication, cardiac arrest, anoxia | 60 | 415 |
| 474083 | 41 | Female | African-American | CVA, SAH | 55 | |
| 618200 | 58 | Female | Caucasian | Head trauma | 499 | |
| 481043 | 65 | Female | Caucasian | CVA, ICH | 451 | |
| 921821 | 22 | Male | African-American | DKA, cardiac arrest | 383 | |
| 947200 | 63 | Female | Caucasian | MVA, head trauma | 608 | |
| 476074 | 29 | Female | Caucasian | Anoxia, cardiac arrest | 50–55 | 271 |
| 240603 | 51 | Female | Caucasian | Blunt head trauma | 40–50 | 320 |
| 452192 | 55 | Female | African-American | CVA | 60 | 350 |
LVEF, left ventricular ejection fraction; CVA, cerebral vascular accident; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; DKA, diabetic ketoacidosis; MVA, motor vehicle accident.
Table 2.
Characteristics of failing hearts
| Heart | Age, yr | Sex | Race | Etiology | RV Function | Heart Weight, g |
|---|---|---|---|---|---|---|
| 303122 | 51 | Female | Caucasian | DCM | Hypokinetic | 508 |
| 709841 | 50 | Male | Caucasian | ICM | Severely reduced | 714 |
| 450564 | 30 | Male | Caucasian | DCM | Severely reduced | 482 |
| 328163 | 63 | Male | Caucasian | ICM | Normal | 506 |
| 479062 | 50 | Male | Caucasian | ICM | Moderately reduced | 636 |
| 323104 | 63 | Male | Caucasian | Nonischemic | Moderately reduced | 543 |
| 537263 | 62 | Male | African-American | ICM | Severely reduced | 427 |
| 522421 | 56 | Female | Caucasian | DCM | Moderately reduced | 470 |
| 214010 | 64 | Male | Caucasian | ICM | Moderately reduced | 495 |
| 597750 | 67 | Male | Caucasian | ICM | Moderately reduced | 630 |
Of note, heart 328163 had apparent normal right ventricular (RV) function based on echocardiography. Of the 2 muscles investigated, 1 indeed had a positive force-frequency relationship, while the other was flat. DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy.
Trabecula isolation.
Within 30 min, the right ventricle of each heart was transferred from the cardioplegic solution to a cold modified Krebs-Henseleit solution (K-H) previously bubbled with 95% O2-5% CO2 containing (in mM) 137 NaCl, 5 KCl, 0.25 CaCl2, 20 NaHCO3, 1.2 NaH2PO4, 1.2 MgSO4, 10 dextrose, and 20 2,3-butanedione monoxime (BDM) with a pH of 7.4. Small and linear right ventricular trabeculae were isolated and kept in this solution at 0–4°C until the time of the experiment (0–8 h). Muscles were placed in custom-made setups as previously described for animal models (37), and the perfusion solution was changed to another modified K-H without BDM and containing 0.25 mM CaCl2. This solution was maintained at 37°C and continuously bubbled with 95% O2-5% CO2, resulting in a pH of 7.4. Stimulation was initiated at baseline frequency of either 0.5 Hz (muscles used to assess baseline cardiac function) or 1 Hz (muscles used for ktr experiments), and the CaCl2 concentration of the solution was incrementally and slowly raised to 2 mM over the time course of ∼15 min. Muscles were gradually stretched until an increase in developed force was not matched by an increase in resting tension. This length, designated as optimal length (Lopt), corresponds to sarcomere length of ∼2.2 μm, which is near or at the in vivo sarcomere length at end-diastole (41).
Baseline trabecula function.
Right ventricular trabeculae were stabilized at Lopt and 0.5 Hz for ∼15 min. In the endocardial wall of human hearts, the smallest trabeculae are virtually always highly fibrogenous. Good contracting trabeculae have a size that is typically just over 150 μm. This size, in combination with ultrastructure (more fibrotic than small rodents), does not allow for unambiguous detection of sarcomere length with laser diffraction (30). In very rare cases (outliers, not representative of the average endocardial trabecula) a measurable sarcomere length pattern can be seen at rest, but this inevitable disappears during contraction. Thus length variations are set and denoted in percentage of Lopt rather than absolute sarcomere length. After contractile and kinetic data were collected at this optimal length, the length-tension relationship was determined at constant frequency of 0.5 Hz from L90 (90% of Lopt, corresponding to in vivo sarcomere length similar to the end-systolic phase) to Lopt. The force-frequency relationship (FFR) was determined at Lopt from 0.5 Hz to 3 Hz. The β-adrenergic response was determined by adding incremental amounts of isoproterenol for a final concentration of 1 μM at Lopt and 0.5 Hz. Data from multiple muscles of the same heart were averaged and used for the final analysis. Nonfailing muscles (n = 10 from 8 hearts) were 288 ± 32 μm in width, 191 ± 22 μm in thickness, and 3.7 ± 0.5 mm in length. Failing muscles (n = 13 from 9 hearts) were 538 ± 44 μm in width, 358 ± 29 μm in thickness, and 3.2 ± 0.4 mm in length. At the baseline stimulation rate of 0.5 Hz, muscle thickness did not adversely impact contractile strength; the correlation coefficient between muscle thickness and force (R2) was only 0.08. At higher frequency, this correlation was still very weak (R2 = 0.28) and thus does not impact data significantly.
ktr experiments in intact cardiac trabeculae.
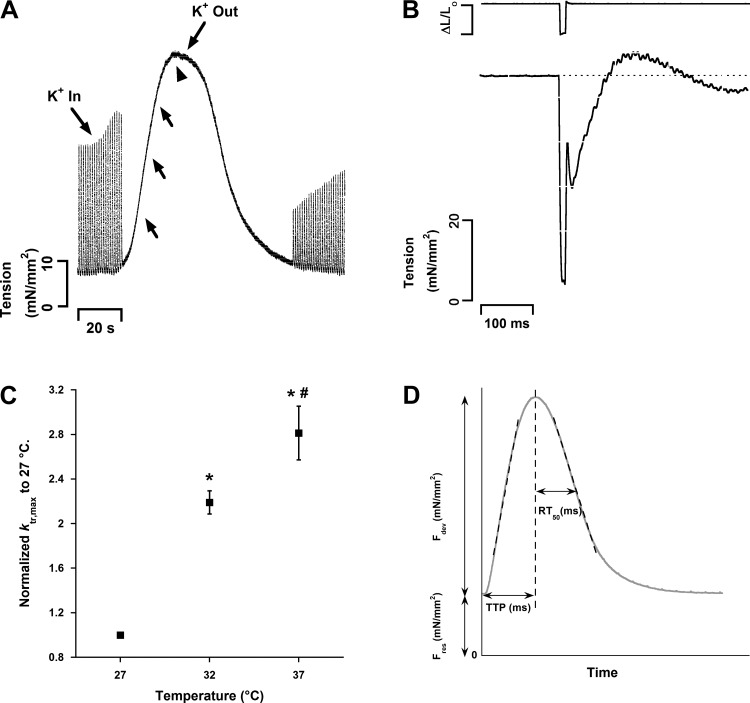
Nonfailing right ventricular trabeculae (n = 9 from 9 hearts) used for ktr experiments had an average width of 406 ± 50 μm, thickness of 267 ± 34 μm, and length of 2.7 ± 0.4 mm. The failing right ventricular muscles (n = 10 from 10 hearts) had an average width of 352 ± 26 μm, thickness of 234 ± 17 μm, and length of 3.3 ± 0.3 mm. Two of the muscles were previously subjected to the length-tension (at 0.5 Hz) and FFR (from 0.5 Hz to 3 Hz) experiments as described above. Muscles were stabilized at Lopt and 1 Hz for 10–15 min. In some of the muscles (nonfailing: n = 7 of 9, failing: n = 7 of 10), a brief FFR (1 Hz, 1.5 Hz, 2 Hz) was initially determined before any ktr experiments. Thereafter, muscles were restabilized at 1 Hz for 10–15 min, and this frequency was used for the remainder of the experiments. Muscles were exposed to a high-K+/Ca2+, low-Na+ solution containing (in mM) 20.6 NaCl, 121.4 KCl, 6 CaCl2, 20 NaHCO3, 1.2 MgSO4, 1.2 NaH2PO4, and 10 dextrose in order to induce a contracture (Fig. 1A). During this contracture, ktr maneuver was performed at submaximal (a subset of muscles) and maximal (all muscles) tension levels as previously described for rats and rabbits (37). Figure 1B shows a typical ktr tracing. The solution was switched back to K-H, muscles were stabilized at new lengths, L95 (95% of Lopt) and L90 (90% of Lopt), for 10–15 min, and ktr experiments were performed at each of these lengths as described above. Thereafter, muscles were restabilized at Lopt, and ktr experiments were performed at this length for a second time (designated Lopt,repeat) in order to compare to the first Lopt measurement and assess reproducibility.
Fig. 1.
A: typical K+ contracture in a human trabecula (heart 328163, muscle RV1). Arrowhead shows when maximum rate of cross-bridge reattachment (ktr,max) was measured. Arrows show the approximate time of assessment of ktr during submaximal activation levels; note that these varied highly from muscle to muscle. No ktr maneuver was performed in this particular contracture. B: representative ktr tracing in a human trabecula (heart 618200, muscle RV4). The movement of the motor is shown at top. Note the transient overshoot during force redevelopment while the position of the motor is constant. All ktr tracings were fit to the equation described in methods from the residual tension after the maneuver to the onset of maximum tension during the overshoot. C: ktr measurements are temperature dependent in intact human right ventricular trabeculae. All measurements were made during the maximal tension level of the K+ contracture. n = 4 hearts (1 trabeculae per heart). P < 0.05: *vs. 27°C, #vs. 32°C. D: typical twitch tracing of a human right ventricular trabeculae (heart 685884, muscle RV1) at optimal length. Developed force (Fdev) corresponds to the tension that is generated during muscle contraction, and resting force (Fres) corresponds to the passive tension of the trabeculae. Time to peak (TTP) is the time it takes for the muscle to reach peak force development from onset of stimulation. RT50 is the time it takes for the muscle to relax from peak developed force to 50% of the force. +dF/dt and −dF/dt are the maximal velocity of force development and relaxation, respectively (expressed as mN·mm−2·s−1). +dF/dt and −dF/dt can be normalized to the developed force, resulting in +dF/dt/Fmax and −dF/dt/Fmax, respectively (not shown). The unit of these measurements is second−1.
The temperature dependence of ktr was assessed at maximal K+ contracture tension in another set of nonfailing donor hearts, not used for previous experiments, at temperatures of 37°C, 32°C, and 27°C. Muscles were stabilized at Lopt, 1 Hz, and each of these temperatures for 15 min prior to ktr experiments.
Statistical and data analysis.
Tension and kinetic measurements were collected and analyzed with custom-made programs in LabVIEW (National Instruments). Muscle tensions were normalized to the cross-sectional area of the muscles and expressed as millinewtons per square millimeter. The ktr tracings were analyzed from the time of the residual tension, remaining after the slack-restretch maneuver (not different between failing and nonfailing at Lopt), to when the muscle reaches maximum tension during the overshoot. Origin 7 software (OriginLab) was used to fit ktr tracings to the equation F = Fmax[1 − e−ktr(t)] + Fres and calculate EC50 of isoproterenol with the equation F = Fmin + Fmax[Fn/(Fn + EC50)]. Statistical analysis (KaleidaGraph) was performed with unpaired t-test, two-way ANOVA, or one-way ANOVA for repeated measures followed by Bonferroni post hoc test when appropriate. Statistical significance was set at P < 0.05. All data are shown as means ± SE.
RESULTS
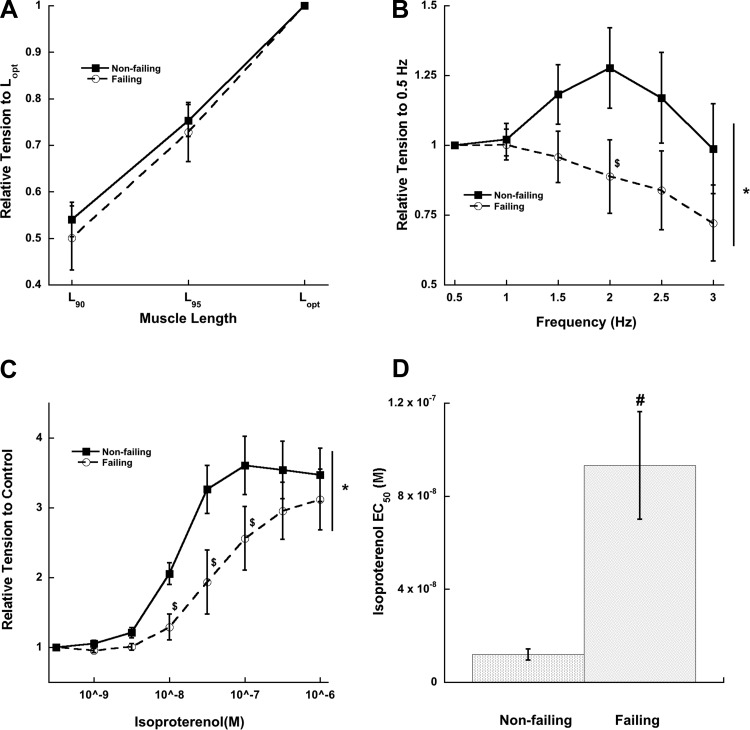
Length-tension relationship, force-frequency relationship, and β-adrenergic response in right ventricular myocardium.
In agreement with previous studies on length-dependent force development in human myocardium (27, 47), increasing muscle length stepwise from L90 to L95 to Lopt increased developed tensions in both nonfailing and failing right ventricular muscles to the same degree at a baseline frequency of 0.5 Hz (Fig. 2A). The FFR of nonfailing right ventricular myocardium was positive (up to 2 Hz), while failing right ventricular myocardium had a negative response (Fig. 2B). Both nonfailing and failing right ventricular myocardium responded positively to increasing isoproterenol concentrations (Fig. 2C); however, the EC50 of nonfailing muscles was significantly lower than that of failing muscles, indicating β-adrenergic desensitization and/or downregulation in the latter group (Fig. 2D).
Fig. 2.
Length-tension relationship, force-frequency relationship, and β-adrenergic stimulation in nonfailing vs. failing right ventricular myocardium. A: length-tension relationship is not different (at 0.5 Hz stimulation frequency) between nonfailing and failing myocardium (ANOVA, P > 0.05). Lopt, optimal length; L90, 90% of Lopt; L95, 95% of Lopt. B: force-frequency relationship is negative in failing myocardium. C: β-adrenergic response is shifted to the right in failing myocardium. D: EC50 is significantly greater in failing myocardium. *P < 0.05 as determined with 2-way ANOVA; $post hoc t-test indicating a significant difference of P < 0.05 between failing and nonfailing groups; #P < 0.05 as determined with unpaired t-test between nonfailing and failing groups. n = 8 nonfailing hearts, n = 8–9 failing hearts (1–3 trabeculae/heart).
Kinetics of contraction are unaffected in right ventricle of failing myocardium and slow down with increasing muscle length.
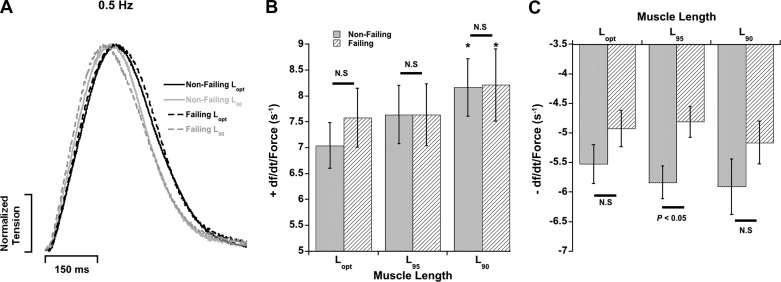
We analyzed kinetic parameters of the same right ventricular muscles in Fig. 2A, which also showed blunted FFR and β-adrenergic desensitization. Schematic definitions of these kinetic parameters are provided in Fig. 1D. The maximal rate of tension rise (+dF/dt) and maximal rate of tension decline (−dF/dt) were normalized to the developed twitch tensions in order to obtain a pure kinetic parameter in units of second−1 (28, 29) (Fig. 3). Within each group, +dF/dt/Fmax accelerated as muscles were shortened from Lopt to L90 (P < 0.05); however, there were no differences between nonfailing and failing myocardium at each respective muscle length at stimulation frequency of 0.5 Hz (P > 0.05). The relaxation parameter −dF/dt/Fdev, with exception of L95 (P < 0.05), was also similar between the two groups. No major alterations in other kinetic parameters were observed between nonfailing and failing myocardium (P > 0.05). Additional kinetic parameters of these muscles are provided in Table 3.
Fig. 3.
Contraction and relaxation parameters from the muscles in Fig. 2A. A: original representative recordings of single twitches (normalized) in failing and nonfailing human myocardium stimulated at 0.5 Hz at Lopt and L90. B: the kinetic parameter +dF/dt/Fdev corresponding to the kinetics of contraction speed-up at muscle length is decreased, while there is no difference between nonfailing and failing myocardium at each length. C: the kinetic parameter −dF/dt/Fdev corresponding to the kinetics of twitch relaxation at multiple muscle lengths. Measurements made at stimulation frequency of 0.5 Hz. *P < 0.05 vs. Lopt of same heart group. N.S., P > 0.05 between nonfailing and failing at each length. n = 8 nonfailing hearts, n = 9 failing hearts (1–3 trabeculae/heart).
Table 3.
Twitch characteristics of muscle from Fig. 2A
| TTP, ms | +dF/dt, mN·mm−2·s−1 | +dF/dt/F, s−1 | RT50, ms | −dF/dt, mN·mm−2·s−1 | −dF/dt/F, s−1 | |
|---|---|---|---|---|---|---|
| Nonfailing | ||||||
| Lopt | 219.3 ± 15.0 | 109.4 ± 24.7 | 7.0 ± 0.4 | 142.4 ± 11.4 | −100.4 ± 30.1 | −5.5 ± 0.3 |
| L95 | 210.5 ± 15.6 | 81.8 ± 14.4* | 7.6 ± 0.6 | 130.3 ± 10.5 | −72.0 ± 19.3 | −5.8 ± 0.3 |
| L90 | 193.9 ± 13.0*† | 64.0 ± 11.8* | 8.2 ± 0.6* | 126.8 ± 7.8* | −51.0 ± 12.9* | −5.9 ± 0.5 |
| Failing | ||||||
| Lopt | 211.2 ± 13.8 | 104.1 ± 26.3 | 7.6 ± 0.6 | 153.1 ± 9.5 | −70.2 ± 17.7 | −4.9 ± 0.3 |
| L95 | 204.8 ± 15.8 | 71.9 ± 16.3* | 7.6 ± 0.6 | 143.1 ± 8.1 | −46.8 ± 11.5* | −4.8 ± 0.3§ |
| L90 | 195.8 ± 14.4 | 51.9 ± 12.1* | 8.2 ± 0.7* | 140.4 ± 8.6 | −31.5 ± 6.8* | −5.2 ± 0.4 |
All parameters are means ± SE (measured at 0.5 Hz); n = 8 nonfailing, n = 9 failing (1–3 trabeculae/heart). TTP, time to peak force; +dF/dt, maximal velocity of contraction; +dF/dt/F, maximal velocity of contraction normalized to developed force; RT50, time from peak force to 50% relaxation; −dF/dt, maximal velocity of relaxation; −dF/dt/F, maximal velocity of relaxation normalized to developed force; Lopt, optimal length; L95, 95% of Lopt; L90, 90% of Lopt. P < 0.05 as determined with ANOVA for repeated measures with Bonferroni post hoc analysis:
vs. Lopt,
vs. L95 of the same group.
P < 0.05 vs. nonfailing of the same length as determined with unpaired t-test.
ktr measurements are temperature dependent.
We utilized ktr experiments during K+ contracture to assess cross-bridge cycling kinetics. For further characterization, we first determined the relationship between temperature and this kinetic parameter. Reducing temperature from 37°C to 32°C and then further to 27°C decreased ktr in our human nonfailing muscles from 18.1 ± 2.1 s−1 to 14.1 ± 1.4 s−1 and to 6.6 ± 0.9 s−1, respectively (n = 4; ANOVA, P < 0.05). This reflects a Q10 of ∼2.8 (Fig. 1C). This is close to the Q10 values (range of ∼2–3) that have been reported previously for ktr in intact and permeabilized rat cardiac preparations (11, 19, 37). Furthermore, a previous report indicated that the minimum frequency of dynamic stiffness has a minimum Q10 of 2.7 in human intact preparations (43). The effect of temperature on our ktr recordings in humans suggests that this parameter is measuring a cellular process, most likely cross-bridge cycling kinetics.
Twitch contractile kinetics and tensions in muscles used for ktr experiments.
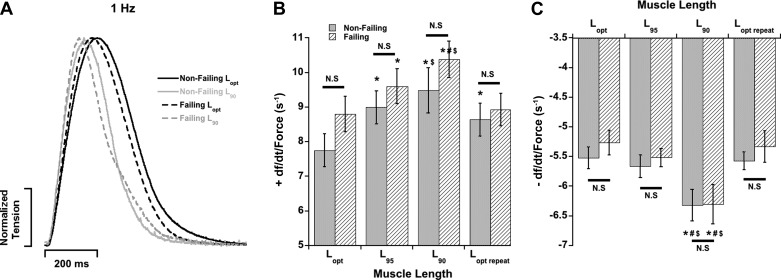
In parallel, a separate set of muscles was used for assessing the effects of muscle length on cross-bridge cycling kinetics by utilizing the ktr experiments. FFR measurements in a subset of these muscles also showed that nonfailing right ventricular myocardium had a positive FFR (from 1 Hz to 2 Hz) while failing right ventricular muscles had a flat FFR within the same frequency range (data not shown). Contractile parameters were measured in these muscles after they were stabilized at each muscle length at 1 Hz and before K+ contracture and ktr experiments. The +dF/dt/Fdev parameter was sped up as muscle lengths were decreased at a stimulation frequency of 1 Hz (Fig. 4) similar to 0.5 Hz (Fig. 3). Additionally, −dF/dt/Fdev also sped up at 1 Hz as muscle lengths were decreased. Furthermore, both +dF/dt/Fdev and −dF/dt/Fdev were similar between nonfailing and failing myocardium at each respective muscle length. Muscle length regulated the tension developed by these muscles, similar to the previous muscles shown in Fig. 2 (Table 4). Active developed (Fdev) and resting (Frest) twitch tensions were not significantly different between nonfailing and failing hearts at each respective muscle length (P > 0.05). Within each group, Fdev and Frest decreased as muscles were shortened from Lopt to L90 (Table 4). We did observe some rundown in developed tension over the long time course of the ktr experiments. This was quantified by repeating the experiments at Lopt for a second time (Lopt,repeat). Although this rundown seemed more pronounced and statistically significant in the nonfailing group (P < 0.05), the failing group did not reach statistical significance (P = 0.1083). This can partially be explained by our recent study (35) that shows that twitch-dependent rundown is an excitation-contraction (EC) coupling phenomenon and may likely be caused by deteriorating sarcoplasmic reticulum (SR) function. The nonfailing myocardium has a potential larger possibility for a loss of SR function, as this is already reduced in failing myocardium, as reflected by the negative FFR (24, 44).
Fig. 4.
Muscle length regulates kinetics of contraction and relaxation in both nonfailing and failing right ventricular myocardium. A: original representative recordings of single twitches (normalized) in failing and nonfailing human myocardium stimulated at 1 Hz at Lopt and L90. B: the contraction kinetic parameter +dF/dt/Fdev is accelerated as muscle length is decreased in both groups. C: the relaxation kinetic parameter −dF/dt/Fdev is accelerated as muscle length is decreased in both groups. There is no difference in either kinetic parameter between nonfailing and failing human myocardium at each respective muscle length. Measurements made at stimulation frequency of 1 Hz. P < 0.05: *vs. Lopt, #vs. L95, $vs. Lopt,repeat of same heart group. N.S., P > 0.05 between nonfailing and failing at each length. n = 9 nonfailing hearts, n = 10 failing hearts (1 trabecula/heart).
Table 4.
Twitch characteristics of muscle utilized for ktr experiments
| Fdev,mN/mm2 | Frest, mN/mm2 | TTP, ms | +dF/dt mN·mm−2·s−1 | +dF/dt/F, s−1 | RT50, ms | −dF/dt, mN·mm−2·s−1 | −dF/dt/F, s−1 | |
|---|---|---|---|---|---|---|---|---|
| Nonfailing | ||||||||
| Lopt | 23.2 ± 1.7 | 7.5 ± 0.7 | 212.6 ± 12.1 | 179.8 ± 16.6 | 7.8 ± 0.5 | 137.0 ± 4.0 | −127.6 ± 9.1 | −5.5 ± 0.2 |
| L95 | 9.3 ± 1.1* | 4.2 ± 0.5*‡ | 174.7 ± 8.7* | 84.8 ± 11.8* | 9.0 ± 0.5* | 123.9 ± 5.6* | −51.8 ± 5.4* | −5.7 ± 0.2 |
| L90 | 4.9 ± 0.6*†‡ | 2.4 ± 0.3*†‡ | 155.4 ± 7.1*†‡ | 48.6 ± 8.1*†‡ | 9.5 ± 0.7*‡ | 114.7 ± 5.3*‡ | −30.8 ± 3.7*†‡ | −6.3 ± 0.3*†‡ |
| Lopt,repeat | 8.9 ± 1.1* | 6.8 ± 0.8 | 181.0 ± 8.7* | 79.1 ± 12.1* | 8.6 ± 0.5* | 126.7 ± 3.7 | −49.0 ± 5.7* | −5.6 ± 0.2 |
| Failing | ||||||||
| Lopt | 18.5 ± 3.0 | 10.6 ± 1.6 | 181.0 ± 10.8 | 154.8 ± 21.0 | 8.8 ± 0.5 | 136.4 ± 5.9 | −96.6 ± 15.3 | −5.3 ± 0.2 |
| L95 | 10.8 ± 2.3*‡ | 5.7 ± 1.4*‡ | 159.3 ± 8.8*‡ | 96.7 ± 17.2* | 9.6 ± 0.5*‡ | 120.4 ± 4.4* | −59.8 ± 12.9* | −5.5 ± 0.2 |
| L90 | 7.3 ± 1.7*‡ | 3.7 ± 1.8*‡ | 147.7 ± 8.3*‡ | 71.7 ± 14.1*‡ | 10.4 ± 0.5*†‡ | 109.8 ± 2.7*‡ | −43.8 ± 9.3*‡ | −6.3 ± 0.3*†‡ |
| Lopt,repeat | 14.9 ± 3.4 | 10.6 ± 2.6 | 177.3 ± 10.5 | 127.6 ± 27.3 | 8.9 ± 0.5 | 126.0 ± 6.1 | −78.9 ± 17.7 | −5.3 ± 0.3 |
All parameters were means ± SE (measured at 1 Hz); n = 9 nonfailing, n = 10 failing (1 trabecula/heart). Fdev, active developed force (i.e., total minus resting); Frest, resting force (lowest force during a twitch); +dF/dt/F, maximal velocity of contraction normalized to active developed force; −dF/dt/F, maximal velocity of relaxation normalized to active developed force. P < 0.05 as determined with ANOVA for repeated measures with Bonferroni post hoc analysis:
vs. Lopt,
vs. L95,
vs. Lopt,repeat of the same group.
ktr is unchanged in failing myocardium; muscle length modulates ktr.
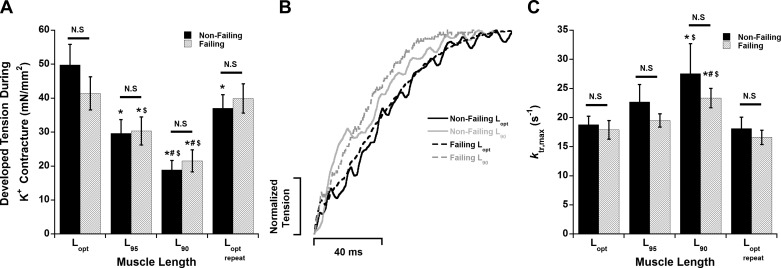
After stabilization and measurement of twitch parameters at a particular muscle length (Lopt, L95, L90, Lopt,repeat), ktr experiments were performed in order to assess cross-bridge cycling kinetics while the trabeculae were under a brief K+ contracture. These measurements were made after the muscles were stabilized at stimulation frequency of 1 Hz (equivalent to 60 beats/min). The maximal developed tension during K+ contracture decreased as muscles were shortened from Lopt to L95 to L90 (Fig. 5A). This tension was increased when muscles were restretched to Lopt,repeat. However, the tension of Lopt vs. Lopt,repeat was reduced in nonfailing samples (P < 0.05) but unchanged in failing samples (P = 1). There was no statistical difference (P > 0.05) in the K+ contracture tension between nonfailing and failing groups at each muscle length.
Fig. 5.
There is no difference in ktr,max during maximal activation between nonfailing and failing myocardium. A: decreasing muscle length results in a decrease in developed tension during the K+ contracture. B: representative force-redevelopment recordings in failing and nonfailing human myocardium at Lopt and L90. C: decreasing muscle length accelerates ktr in both nonfailing and failing myocardium. However, there is no difference between nonfailing and failing myocardium at each length. P < 0.05: *vs. Lopt, #vs. L95, $vs. Lopt,repeat of same heart group. N.S., P > 0.05 between nonfailing and failing human myocardium at each respective muscle length. n = 9 nonfailing hearts, n = 10 failing hearts (1 trabecula/heart).
In all of the muscles used for length studies, ktr was measured twice at Lopt during two separate K+ contractures, one toward the beginning and the other at the end of the experimental series. The ktr values of the first Lopt were not statistically different compared with Lopt,repeat in both the nonfailing and failing groups (P = 1 for both groups) (Fig. 5C). In both nonfailing and failing samples, ktr (during maximal K+ tension) was accelerated as muscles were shortened from Lopt to L90 (P < 0.05) and decreased as muscles were restretched back to Lopt,repeat (P < 0.05) (Fig. 5C). There were no significant differences (P > 0.05) in ktr values between nonfailing and failing groups at each muscle length (Fig. 3, B and C). The ratios of Fres (residual force following ktr) to FK+ (K+ contracture force before ktr) for the nonfailing group were 0.32 ± 0.03 (Lopt), 0.27 ± 0.06 (L95), 0.23 ± 0.07 (L90), and 0.30 ± 0.06 (Lopt,repeat) (P = 0.43). The ratios for the failing group were 0.18 ± 0.04 (Lopt), 0.2 ± 0.04 (L95), 0.2 ± 0.05 (L90), and 0.18 ± 0.06 (Lopt,repeat) (P = 0.97). We also measured ktr during submaximal K+ contracture tension levels in a subset of the muscles. During submaximal activation levels, ktr was not different between nonfailing and failing samples (data not shown).
DISCUSSION
A 2006 report by the National Heart, Lung, and Blood Institute recognized that current knowledge of the right ventricle in both health and disease is limited (46). The left and right ventricles have differences in structure, function, and response to stress and disease; hence, experiments specifically on the right ventricle are required (46). We report here for the first time that muscle length impacts on cross-bridge kinetics in intact human myocardial muscle preparations and that these kinetics are preserved in the right ventricular muscles of end-stage heart failure patients when assessed under near-physiological conditions.
The right ventricular myocardium of failing hearts in the present study had the expected blunted FFR and β-adrenergic desensitization, which are classical hallmarks that have been extensively observed in failing human myocardium (7, 9, 23, 38–40, 42). The mechanisms responsible for abnormal FFR have been attributed to alterations in calcium handling mechanisms (14) and that of β-adrenergic desensitization to receptor desensitization and/or downregulation following prolonged exposure to catecholamines (4, 7). Despite these typical pathological alterations, the twitch contractile kinetics were remarkably similar between nonfailing and failing myocardium. Various parameters corresponding to contractile and relaxation kinetics can be derived from the twitch contraction of an isolated linear myocardial muscle preparation. Of these parameters, +dF/dt/Fdev and −dF/dt/Fdev provide insight into purely kinetic processes (unit: s−1), as they are normalized by the amplitude of the force (28, 29). Our present data show that there were no differences in these parameters between the nonfailing and failing groups when measured at Lopt. These measurements were assessed at stimulation rates of 0.5 Hz (= 30 beats/min) and 1 Hz (= 60 beats/min) that are near or below the resting heart rates in humans. A retrospective analysis of previous studies on right ventricular muscles of failing human myocardium confirms these findings: Although they were not explicitly analyzed per muscle, the group-averaged values for +dF/dt/Fdev and −dF/dt/Fdev were either not different or very similar in failing vs. nonfailing muscles at near-resting heart rates (stimulation frequency of 0.5 Hz) (9, 42). We also extended such retrospective analysis to other studies that used left ventricular preparations and determined that these calculated kinetic parameters were also unaffected in the isolated left ventricular myocardium during heart failure at near-resting heart rates (stimulation frequencies of 0.5 Hz or 1 Hz) (22, 23, 40). Our study confirms that at near-resting heart rate, kinetics of contraction and relaxation of isolated human myocardium are not different in tissue from diseased hearts.
The cardiac twitch contraction is dependent upon multiple processes ranging from action potential depolarization to calcium-induced calcium release and myofilament-based processes (3, 5, 28). Since force development is directly correlated to the number of cross bridges, and twitch kinetics are coupled to the cycling kinetics, we measured ktr, an index of cross-bridge cycling kinetics. This index was not different at Lopt between nonfailing and failing myocardium at maximal or submaximal activation levels, which is in accordance with our twitch data. A previous study investigated cross-bridge cycling kinetics in right ventricular muscles of heart failure patients (16). The results of this study showed that right ventricles of heart failure patients have lower minimal frequency of dynamic stiffness and unloaded shortening velocity (16), which indicates a decrease in cross-bridge cycling kinetics in contrast to the present study. However, this previous study, in contrast to the present study, was performed on permeabilized muscle preparations (where the cellular membrane and membrane-bound organelles are removed), at subphysiological temperature, and only in patients with idiopathic dilated cardiomyopathy (DCM). The difference between this study and our present results can possibly be attributed not only to the above-mentioned differences in experimental methodologies such as type of preparation and temperature but also to the type of heart failure. Most of the failing samples in the present study were classified as ischemic cardiomyopathy (ICM), and only three were classified as DCM. Several studies suggest that DCM and ICM human myocardium are different in regard to myofilament alterations (17), mitochondrial dysfunction (2), and protein expression (10). One of these studies comprehensively showed that alterations in various cardiac processes, such as β-adrenergic signaling, myofilament phosphorylation and function, and extracellular collagen content, are more pronounced in DCM than in ICM (17).
The role of muscle length in modulating ktr in animal models is controversial, with some studies showing either an effect or the lack of one (1, 13, 18, 21, 33, 37, 45). In this study, decreasing muscle length increased ktr to about the same extent in both nonfailing and failing human myocardium; hence, ktr at each muscle length was not statistically different between the two groups. This increase in ktr at shorter muscle lengths is in accordance with twitch kinetics, as they were generally accelerated as muscles were shortened from Lopt to L90 in both nonfailing and failing myocardium. Overall, the data show that muscle length is an important regulator of contractile and cross-bridge cycling kinetics in both nonfailing and failing human myocardium. Thus, regardless of muscle length, both the ktr and twitch kinetics were generally similar between nonfailing and failing myocardium. One previous study showed that length has no effect on ktr in nonfailing human myocardium (13). It should be noted that this human study was performed on permeabilized cardiomyocytes at subphysiological temperatures of 15°C and 25°C, while the present study was performed on intact multicellular preparations at body temperature (37°C). Cardiomyocyte permeabilization has the advantage of studying the sarcomeres in isolation from the rest of the cardiomyocytes. However, it does not provide complete information about the behavior of sarcomeres inside the cardiomyocytes where their activity can be regulated and altered. Furthermore, the permeabilization process has been shown to introduce artifacts such as drastic reduction in myofilament calcium sensitivity (15), and the lack of membranes can result in loss of constant-volume behavior, impacting myofilament spacing (32). The myofilaments in intact trabeculae are fully integrated into the cardiomyocytes, and their activity is under constant regulation. Experimental conditions such as temperature and extracellular ionic concentrations were chosen to mimic those of the body, while the intracellular conditions are regulated by the cardiomyocytes themselves. Still, most other studies on skinned fibers, albeit done in animal models and at low temperature, are in line with our findings and would indicate that length dependence of cross-bridge kinetics is indeed occurring and is predominantly a property of the myofilaments (1, 20, 33).
The implications of length-dependent changes in cross-bridge kinetics may contribute to the rapid pressure rise and fall in the ejecting ventricle, compared with the typically relatively slower kinetics in isometric muscles. As the ventricle ejects, sarcomere/muscle length is reduced, which according to our results could accelerate cross-bridge cycling kinetics. Likewise, at end-systolic length, comparable to L90 in our muscle studies, relaxation would initially occur with fast-cycling cross-bridges, which decelerate only when filling has started (i.e., when the muscle/sarcomere is relengthened). Furthermore, this length-dependent regulation also presents a potential mechanism whereby kinetics are actually reduced at the whole-organ level in the failing patients in contrast to the tissue level. There is an increase in end-diastolic pressure and/or volume in patients with heart failure that will effectively stretch the cardiomyocytes and cause them to function at longer muscle lengths. On one hand, this will aid to increase the force production and amount of pressure generation. On the other hand, it will in turn cause a reduction in the kinetics of cardiac contraction and contribute to reduced cardiac output in patients with heart failure. Therefore, while kinetics can be reduced in the right ventricles of heart failure patients, this is not necessarily due to the inherent dysfunction at the tissue level. Rather, the effect of muscle length at the organ level is the mechanism for this slowing phenomenon. Future studies are necessary in order to validate this proposed model.
In both failing and nonfailing tissue, we observed overshoot of the force during the ktr (Fig. 1B); this overshoot was significantly larger than in nearly identical studies on rabbit myocardium and is typically absent in small-rodent myocardium. The phenomenon of overshoot was discussed (8) to be due to filament compliance that may be greater in human myocardium than in small-rodent myocardium. In addition, there may be an active component to the overshoot; cross bridges can get synchronized during the ktr process, resulting in a transient greater-than-normal fraction of bound bridges compared with steady state. We believe the impact of this overshoot on our findings is minimized by the fact that the overshoot seemed similar in duration and magnitude in failing vs. nonfailing myocardium. Future studies could be directed at further determining this overshoot.
In summary, we conclude that there exists a length-dependent regulation of cross-bridge kinetics and twitch duration kinetics in human myocardium. With an increase in muscle length, cross-bridge cycling kinetics slow down, while twitch duration increases. This phenomenon of length-dependent regulation of kinetics is preserved in the right ventricle of end-stage failing hearts.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-113084 (P. M. L. Janssen) and American Heart Association Great Rivers Affiliate Pre-doctoral Fellowship 1148008 to N. Milani-Nejad.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.M.-N., P.F.B., R.S.H., A.K., P.J.M., and P.M.J. conception and design of research; N.M.-N., B.D.C., M.T.E., and J.-H.C. performed experiments; N.M.-N., B.D.C., M.T.E., J.-H.C., and P.M.J. analyzed data; N.M.-N., J.P.D., V.F., and P.M.J. interpreted results of experiments; N.M.-N. and P.M.J. prepared figures; N.M.-N. drafted manuscript; B.D.C. and P.M.J. edited and revised manuscript; B.D.C., M.T.E., J.P.D., J.-H.C., V.F., P.F.B., R.S.H., A.K., P.J.M., and P.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank the Lifeline of Ohio Organ Procurement organization and the Department of Cardiac Surgery of The Ohio State University Wexner Medical Center for providing the human hearts. We also thank Jennifer A. Conkle, Rachel L. Gearinger, Jason Murray, and Eric J. Schultz for their help and contribution with processing of tissues and performing some of the length-tension, FFR, and β-adrenergic stimulation studies.
REFERENCES
- 1.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J 87: 1784–1794, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja P, Wanagat J, Wang Z, Wang Y, Liem DA, Ping P, Antoshechkin IA, Margulies KB, Maclellan WR. Divergent mitochondrial biogenesis responses in human cardiomyopathy. Circulation 127: 1957–1967, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic, 2001. [Google Scholar]
- 5.Biesiadecki BJ, Davis JP, Ziolo MT, Janssen PM. Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophys Rev 6: 273–289, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83: 3542–3546, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Campbell KS. Filament compliance effects can explain tension overshoots during force development. Biophys J 91: 4102–4109, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary KW, Rossman EI, Piacentino V 3rd, Kenessey A, Weber C, Gaughan JP, Ojamaa K, Klein I, Bers DM, Houser SR, Margulies KB. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol 44: 837–845, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cortes R, Rivera M, Rosello-Lleti E, Martinez-Dolz L, Almenar L, Azorin I, Lago F, Gonzalez-Juanatey JR, Portoles M. Differences in MEF2 and NFAT transcriptional pathways according to human heart failure aetiology. PLoS One 7: e30915, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Tombe PP, Stienen GJ. Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J Physiol 584: 591–600, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebus JP, Stienen GJ. Effects of 2,3-butanedione monoxime on cross-bridge kinetics in rat cardiac muscle. Pflügers Arch 432: 921–929, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Edes IF, Czuriga D, Csanyi G, Chlopicki S, Recchia FA, Borbely A, Galajda Z, Edes I, van der Velden J, Stienen GJ, Papp Z. Rate of tension redevelopment is not modulated by sarcomere length in permeabilized human, murine, and porcine cardiomyocytes. Am J Physiol Regul Integr Comp Physiol 293: R20–R29, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol 500: 73–86, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Gao WD, Backx PH, Azan-Backx M, Marban E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circ Res 74: 408–415, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Hajjar RJ, Gwathmey JK. Cross-bridge dynamics in human ventricular myocardium. Regulation of contractility in the failing heart. Circulation 86: 1819–1826, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Hamdani N, Borbely A, Veenstra SP, Kooij V, Vrydag W, Zaremba R, Dos Remedios C, Niessen HW, Michel MC, Paulus WJ, Stienen GJ, van der Velden J. More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. J Muscle Res Cell Motil 31: 289–301, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock WO, Martyn DA, Huntsman LL. Ca2+ and segment length dependence of isometric force kinetics in intact ferret cardiac muscle. Circ Res 73: 603–611, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Hancock WO, Martyn DA, Huntsman LL, Gordon AM. Influence of Ca2+ on force redevelopment kinetics in skinned rat myocardium. Biophys J 70: 2819–2829, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanft LM, McDonald KS. Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres. J Physiol 588: 2891–2903, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanft LM, McDonald KS. Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 296: H1524–H1531, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasenfuss G, Mulieri LA, Leavitt BJ, Allen PD, Haeberle JR, Alpert NR. Alteration of contractile function and excitation-contraction coupling in dilated cardiomyopathy. Circ Res 70: 1225–1232, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75: 434–442, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Hasenfuss G, Reinecke H, Studer R, Pieske B, Meyer M, Drexler H, Just H. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res Cardiol 91: 17–22, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 6: 606–619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiranandani N, Varian KD, Monasky MM, Janssen PM. Frequency-dependent contractile response of isolated cardiac trabeculae under hypo-, normo-, and hyperthermic conditions. J Appl Physiol (1985) 100: 1727–1732, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Holubarsch C, Ruf T, Goldstein DJ, Ashton RC, Nickl W, Pieske B, Pioch K, Ludemann J, Wiesner S, Hasenfuss G, Posival H, Just H, Burkhoff D. Existence of the Frank-Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation 94: 683–689, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol 299: H1741–H1749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol 299: H1092–H1099, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen PM, de Tombe PP. Uncontrolled sarcomere shortening increases intracellular Ca2+ transient in rat cardiac trabeculae. Am J Physiol Heart Circ Physiol 272: H1892–H1897, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Jweied E, deTombe P, Buttrick PM. The use of human cardiac tissue in biophysical research: the risks of translation. J Mol Cell Cardiol 42: 722–726, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90: 59–65, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Korte FS, McDonald KS. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J Physiol 581: 725–739, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald KS. The interdependence of Ca2+ activation, sarcomere length, and power output in the heart. Pflügers Arch 462: 61–67, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milani-Nejad N, Brunello L, Gyorke S, Janssen PM. Decrease in sarcoplasmic reticulum calcium content, not myofilament function, contributes to muscle twitch force decline in isolated cardiac trabeculae. J Muscle Res Cell Motil 35: 225–234, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther 141: 235–249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PM. Effect of muscle length on cross-bridge kinetics in intact cardiac trabeculae at body temperature. J Gen Physiol 141: 133–139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation 85: 1743–1750, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res 85: 38–46, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest 98: 764–776, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol Heart Circ Physiol 263: H293–H306, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Rossman EI, Petre RE, Chaudhary KW, Piacentino V 3rd, Janssen PM, Gaughan JP, Houser SR, Margulies KB. Abnormal frequency-dependent responses represent the pathophysiologic signature of contractile failure in human myocardium. J Mol Cell Cardiol 36: 33–42, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Ruf T, Schulte-Baukloh H, Ludemann J, Posival H, Beyersdorf F, Just H, Holubarsch C. Alterations of cross-bridge kinetics in human atrial and ventricular myocardium. Cardiovasc Res 40: 580–590, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Schlotthauer K, Schattmann J, Bers DM, Maier LS, Schutt U, Minami K, Just H, Hasenfuss G, Pieske B. Frequency-dependent changes in contribution of SR Ca2+ to Ca2+ transients in failing human myocardium assessed with ryanodine. J Mol Cell Cardiol 30: 1285–1294, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Stelzer JE, Moss RL. Contributions of stretch activation to length-dependent contraction in murine myocardium. J Gen Physiol 128: 461–471, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Weil J, Eschenhagen T, Hirt S, Magnussen O, Mittmann C, Remmers U, Scholz H. Preserved Frank-Starling mechanism in human end stage heart failure. Cardiovasc Res 37: 541–548, 1998. [DOI] [PubMed] [Google Scholar]