Abstract
Antibody-mediated rejection (ABMR) is a leading cause of allograft loss. Treatment efficacy depends on accurate diagnosis at an early stage. However, sensitive and reliable markers of antibody-endothelium interaction during ABMR are not available for routine use. Using immunohistochemistry, we retrospectively studied the diagnostic value of three markers of endothelial-to-mesenchymal transition (EndMT), fascin1, vimentin, and heat shock protein 47, for ABMR in 53 renal transplant biopsy specimens, including 20 ABMR specimens, 24 cell-mediated rejection specimens, and nine normal grafts. We validated our results in an independent set of 74 unselected biopsy specimens. Endothelial cells of the peritubular capillaries in grafts with ABMR expressed fascin1, vimentin, and heat shock protein 47 strongly, whereas those from normal renal grafts did not. The level of EndMT marker expression was significantly associated with current ABMR criteria, including capillaritis, glomerulitis, peritubular capillary C4d deposition, and donor-specific antibodies. These markers allowed us to identify C4d-negative ABMR and to predict late occurrence of disease. EndMT markers were more specific than capillaritis for the diagnosis and prognosis of ABMR and predicted late (up to 4 years after biopsy) renal graft dysfunction and proteinuria. In the independent set of 74 renal graft biopsy specimens, the EndMT markers for the diagnosis of ABMR had a sensitivity of 100% and a specificity of 85%. Fascin1 expression in peritubular capillaries was also induced in a rat model of ABMR. In conclusion, EndMT markers are a sensitive and reliable diagnostic tool for detecting endothelial activation during ABMR and predicting late loss of allograft function.
Keywords: transplant pathology, endothelium, rejection, antibody-mediated rejection
Antibody-mediated rejection (ABMR) develops in transplant recipients with donor-specific alloantibodies that mainly target HLA antigens expressed at the surface of the graft endothelium1–5; it is currently a major cause of renal graft loss.6–9 The updated Banff classification includes the following diagnostic criteria for ABMR10: (1) donor-specific antibodies (DSA) for HLA antigens in the recipient’s plasma; (2) inflammation in the allograft microcirculation: peritubular capillaritis (ptc) and glomerulitis (g); (3) evidence of antibody interaction with vascular endothelium, as attested to by C4d deposits on the endothelial cells of peritubular capillaries (PTC), or of moderate microvascular inflammation, or of elevated endothelial injury–activated gene transcripts. As a marker, C4d is not sensitive, however. Detection of endothelial injury–activated gene transcripts should be especially helpful for the diagnosis of C4d-negative ABMR.4 However, endothelial injury–activated gene transcripts detection by microarray is a sophisticated and expensive technique not easily applicable in many centers. The ability to detect vascular endothelial activation during ABMR by immunohistochemistry would be very desirable.
In vitro and in vivo experiments,11–14 as well as human data from kidney recipients,4 concur to show that the binding of DSA to endothelial cells profoundly affects the endothelial transcriptome. Molecules involved in inflammation, coagulation, cell motility, and endothelial repair are synthesized, with phenotypic changes reminiscent of an endothelial-to-mesenchymal transition (EndMT).3,13,14 Just like its epithelial counterpart, EndMT occurs under different stimuli,15–17 which contribute to fibrogenesis in animal models of renal and cardiac fibrosis.18,19 The aim of our work was to test the hypothesis that expression of EndMT markers could serve as evidence of current endothelial reaction following DSA binding and/or complement activation and thus help to consolidate the diagnosis of ABMR in renal grafts. Among the potential endothelial activation indicators analyzed, we studied particularly the expression of three mesenchymal markers: (1) fascin1, an actin-bundling protein involved in cell motility; (2) vimentin, an intermediate filament; and (3) hsp47, a collagen-specific chaperone protein, in endothelial cells from PTC of renal grafts with and without ABMR. Our study reveals that EndMT markers accurately diagnose ABMR and predict long-term graft dysfunction.
Results
EndMT Markers are Upregulated in Transplanted Kidneys with ABMR
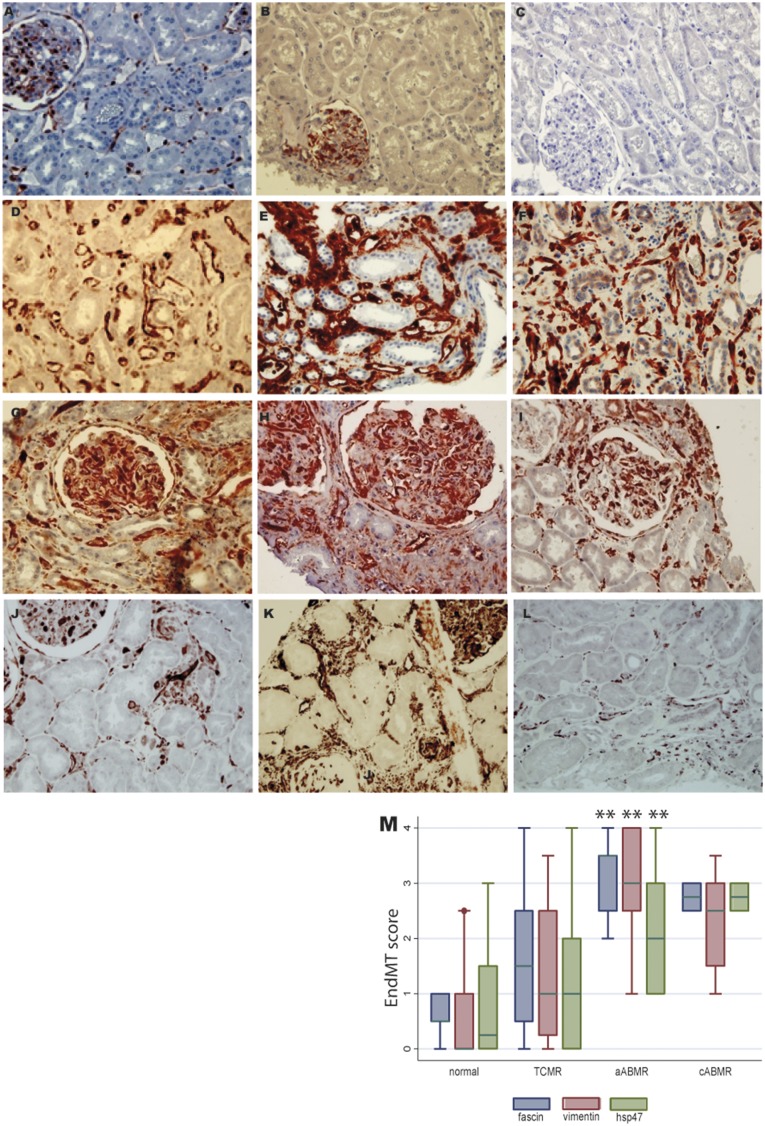
In normal kidneys and implantation biopsies, fascin1 expression was limited to the perinuclear zone in the endothelial cells lining the PTC and glomerular capillaries; these cells did not stain for vimentin or hsp47 (Figure 1, A–C).
Figure 1.
Expression of endothelial-to-mesenchymal transition markers in normal and in transplanted kidneys with ABMR. In normal kidneys, the expression of (A) fascin1 was limited to the perinuclear zone of glomerular and peritubular capillary endothelial cells. No staining for (B) vimentin and (C) Hsp47 was found in these cells. The peritubular zone and glomerular capillaries of the biopsies with aABMR showed strong and diffuse cytoplasmic expression of (D,G) fascin1, (E,H) vimentin, and (F,I) hsp47. Strong but focal staining of (J) fascin1, (K) vimentin, and (L) hsp47 was found in PTC in the cases with cABMR. (M) The level of EndMT marker expression in different groups of renal transplants in the biopsy study set (significantly higher levels of EndMT marker expression in the grafts with aABMR compared with those without in the study set of biopsies; **P<0.01).
In the first set of biopsies, we detected strong and diffuse cytoplasmic expression of fascin1, vimentin, and hsp47 in endothelial cells from both the PTC and glomerular capillaries of grafts with acute ABMR (aABMR) (Figure 1, D–I). This staining was strong but less diffuse in PTC of grafts with chronic ABMR (cABMR) (Figure 1, J–L). However, in five grafts with an active form of cABMR (with ptc, g, or chronic glomerulopathy [cg] and/or with diffuse C4d staining), the expression of EndMT markers was strong and extensive. These EndMT markers were not detected in seven of the nine biopsies from normal grafts or in 13 of the 24 with T lymphocyte–mediated rejection (TCMR). Our semi-quantification of the three EndMT markers in PTC is illustrated in Supplemental Figure 1. Endothelial expression of these markers was confirmed by double staining with vascular endothelial marker CD34 (Suplemental Figure 2). Figure 1M reports the mean score for each of these markers in the various groups.
Importantly, the endothelial expression of fascin was also assessed in normal rat kidneys, and in the transplanted kidneys from rats which had or had not previously (3 weeks earlier) received a blood transfusion from the same rat donor, and developed DSA pretransplant. While the native and normal kidneys from nontransplanted rats were not found to abnormally express fascin in PTC (the EndMT score was consistently 0, n=3), similarly to rats which had received a blood transfusion but no renal transplant (n=3), a strong staining was observed at day 7 posttransplant in rats subjected to this experimental model of ABMR. Expression of fascin was intense in the cytoplasm of endothelial cells lining PTC where inflammation was also obvious (n=5; EndMT score was 3 in two rats and 2 in the other three, Figure 2).
Figure 2.
EndMT markers in an experimental rat model of ABMR. EndMT markers were also found to be induced in PTC in a rat model of ABMR: in contrast with (A,B) control rat kidneys, where a very faint fascin staining draws a fine line under the glomerular endothelial cells and a few endothelial cells of peritubular capillaries, (C,D) rat kidneys with DSA-induced ABMR show a strong endothelial fascin expression in the PTC (arrows) filled with inflammatory cells.
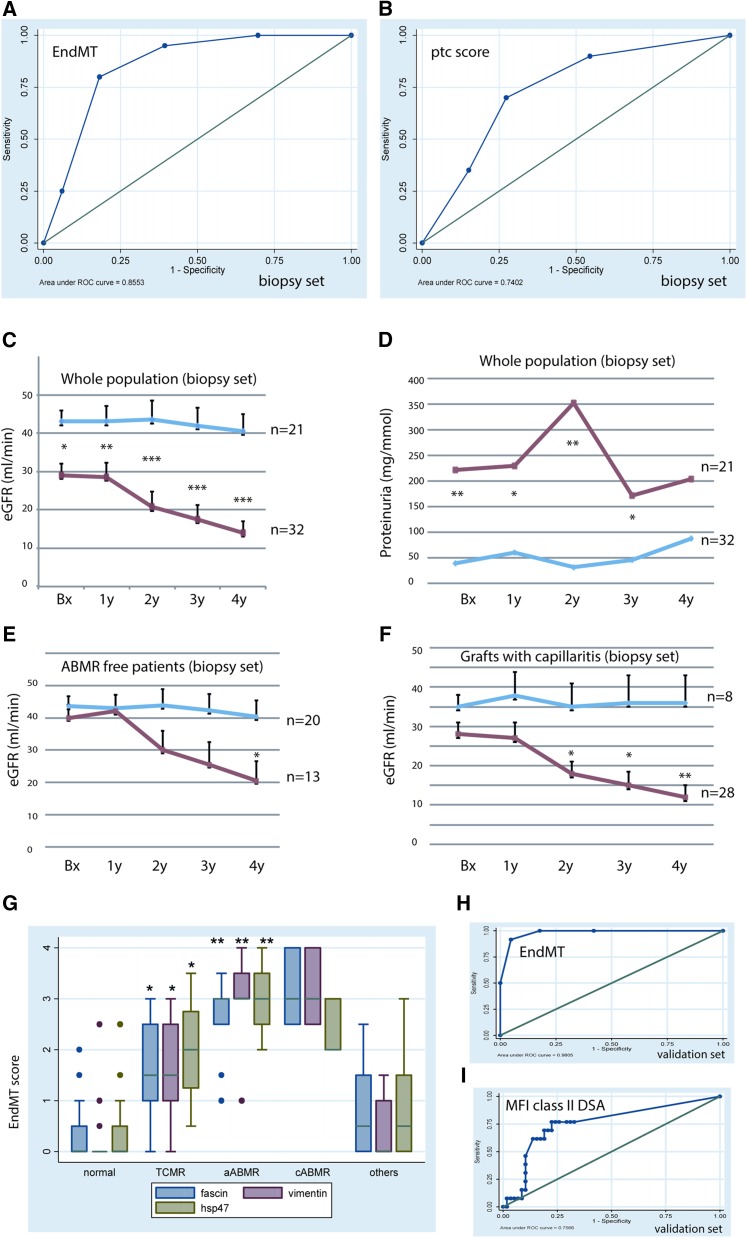
The level of PTC EndMT marker expression was significantly correlated with microcirculation inflammatory scores (i.e., with the ptc score: Rho=0.6, P<0.001; with the g scores: Rho=0.5, P<0.001), but not with the interstitial inflammation (i) or tubulitis (t) scores. The EndMT score was significantly higher in C4d-positive than in C4d-negative biopsy samples (mean=3.44±0.18 versus 1.8±0.24, P=0.002). Receiver operating characteristic (ROC) curve analysis revealed that EndMT markers did better than ptc for the diagnosis of ABMR at the time of biopsy: the area under the curve (AUC) calculated for these markers was 0.855 [95% confidence interval (95% CI), 0.76 to 0.95]; Figure 3A). An EndMT cutoff score of 2 yielded a sensitivity of 95% CI and a specificity of 60.6% for the diagnosis of ABMR. Note that the AUC for each EndMT marker analyzed separately, although lower than the combined AUC for the three markers together, was nonetheless 0.84 for fascin alone, 0.83 for vimentin, and 0.80 for HSP47. Each was thus better as a diagnostic tool than ptc, which had an AUC of only 0.74 [95% CI, 0.6 to 0.87] (Figure 3B).
Figure 3.
Predictive value of EndMT markers for the diagnosis and prognosis of ABMR. ROC curves for the diagnosis of ABMR in the biopsy study set, by (A) EndMT markers, or (B) ptc. With EndMT markers, the AUC was 0.855. When the cutoff point was set at 2, the sensitivity for ABMR diagnosis was 95% and the specificity was 60.6%. With ptc, the AUC was 0.74. (C) the evolution of graft function, and (D) the evolution of proteinuria according to peritubular EndMT marker expression in the biopsy study set. The blue curves describe the evolution of EndMT– grafts, the right curves that of EndMT+ grafts. (E) The evolution of eGFR in patients supposedly free from ABMR at the time of the biopsy (patients whose graft expressed EndMT markers at biopsy but were not then diagnosed with ABMR underwent a progressive deterioration of graft function up to 4 years postbiopsy). (F) In patients with capillaritis, EndMT markers were also predictive of a deterioration (capillaritis without EndMT was not of bad prognostic value). (C–F) *P<0.05; **P<0.01; ***P<0.001, for the comparison between EndMT– and EndMT+ grafts. (G) The level of EndMT marker expression in the different groups of renal transplants in the biopsy validation set: here again, the expression of EndMT markers was significantly higher in the grafts with aABMR compared with those without; *P<0.001 for the comparison of TCMR versus normal grafts; **P<0.0001 for aABMR versus normal grafts or grafts with TCMR. (H, I) The ROC curve for the diagnosis of ABMR by (H) EndMT markers, or by (I) DSA against HLA class II in the biopsy validation set: the AUC was 0.98 (95% CI, 95 to 100) for EndMT markers. A cutoff point at a score of 2 yielded a sensitivity of 100%, and a specificity of 85% for the diagnosis of ABMR. The AUC was 0.757 (95% CI, 0.61 to 0.9) when ABMR was diagnosed by the presence of DSA against HLA class II. The cutoff point for MFI 500 had a sensitivity of 77% and a specificity of 71% for the diagnosis of ABMR. MFI, mean fluorescence intensity.
The main risk factors significantly associated with EndMT marker expression were prolonged cold ischemia time (Rho=0.283, P=0.04) and a history of delayed graft function (mean EndMT scores=2.8±0.3 versus 1.54±0.2, P=0.001 for those with or without delayed graft function). No significant association between time since transplant and the level of EndMT marker expression was found in this set of biopsies (Rho=0.186, P=0.2). EndMT scores were very strongly associated with graft dysfunction (Rho=–0.454, P<0.001) and proteinuria (Rho=0.5, P<0.001) at the time of biopsy. Importantly, they predicted late graft dysfunction (Rho=–0.69, P<0.001) very accurately, as well as proteinuria (Rho=0.39, P=0.03) up to four years after biopsy (Table 1 and Figure 3, C and D).
Table 1.
Spearman’s correlation between the level of EndMT marker expression and the graft dysfunction or proteinuria (biopsy study set)
| Spearman’s correlation of EndMT with: | Rho | P Value |
|---|---|---|
| eGFR at time of biopsy | –0.454 | <0.001 |
| eGFR 1 year after biopsy | –0.43 | <0.002 |
| eGFR 2 year after biopsy | –0.61 | <0.001 |
| eGFR 3 year after biopsy | –0.634 | <0.001 |
| eGFR 4 year after biopsy | –0.69 | <0.001 |
| Proteinuria at time of biopsy | 0.5 | <0.002 |
| Proteinuria 1 year after biopsy | 0.31 | <0.05 |
| Proteinuria 2 year after biopsy | 0.6 | <0.001 |
| Proteinuria 3 year after biopsy | 0.341 | 0.06 |
| Proteinuria 4 year after biopsy | 0.39 | 0.03 |
EndMT is a Sensitive and Accurate Diagnostic Marker of ABMR and a Prognostic Marker of Graft Dysfunction
As progressive loss of graft function and proteinuria are two common features of ABMR, we monitored the patients after the biopsy. The detection of EndMT markers in 13 of 33 patients considered ABMR free at the time of the biopsy (11 had been diagnosed with TCMR and two had a normal histology) suggests that endothelial cell activation was present but unrecognized. As shown in Figure 3E, these 33 patients with or without EndMT marker expression had the same eGFR at the time of biopsy (m=40±4 versus 43.7±3 ml/min, P<0.7) as they did a year later. The function of the grafts with EndMT marker expression (n=13) then deteriorated, however, and by two years postbiopsy their eGFR was lower and continued to decline up to 4 years postbiopsy (20.5 versus 40.3±5 ml/min, P=0.02). Likewise, proteinuria was significantly higher at 2 years postbiopsy in the patients carrying an EndMT-positive graft than those EndMT-negative (207±76 versus 30±16 mg/mmol creatinine, P=0.002). Importantly, 14 (six EndMT-positive, eight EndMT-negative) patients in this group of 33 patients supposedly free from ABMR diagnosis at time of biopsy had a subsequent biopsy (months or years later); of the six previously EndMT-positive grafts, five had patent ABMR lesions, while none of the eight that were previously EndMT-negative did. Although this obviously does not prove that the ABMR diagnosed on the second biopsy was already happening at the time of the first one, these numbers do suggest that EndMT markers detect low-grade, subhistologic antibody-mediated injury at an early stage. C4d deposits were detected in only 45% of grafts diagnosed with aABMR in this set of biopsies. Interestingly, markers of EndMT were positive in both C4d-positive and C4d-negative ABMR cases (mean=3.44±0.5 versus 2.94±0.95, P=0.186): fascin1 staining was positive in 96% of ABMR cases, vimentin in 80%, and hsp47 in 68%. Logistic regression model found EndMT to be the best independent marker of ABMR or its late occurrence, with an odds ratio (OR) of 15 (95% CI, 1.3 to 172, P=0.03), after adjustment for C4d, capillaritis, HLA antibodies, time since transplant, and eGFR at biopsy. The likelihood-ratio (LR) test confirmed the value of adding EndMT detection to obtain an accurate diagnosis of ABMR-associated endothelial activation (LR χ2 of 11.93, P<0.001).
Capillaritis, which is currently a major criterion for ABMR diagnosis, has been found to be an important risk factor associated with long-term graft function loss. However, among the 36 patients with capillaritis, those without EndMT marker expression (n=8) kept a very stable graft function up to 4 years after biopsy, only those (n=28) with EndMT-positive grafts showed progressive deterioration of their graft function (mean eGFR at 4 years of 12±3, versus 36±7 ml/min, P<0.01; Figure 3F). Proteinuria was also significantly higher after 2 years in patients with both ptc and EndMT than in those with ptc but not EndMT (359±105 versus 1.6±1.6 mg/mmol creatine, P=0.004). This finding makes it clear that ptc alone lacks specificity for indicating endothelial activation and thus for diagnosing ABMR and predicting long-term graft dysfunction. Logistic regression analysis found that the EndMT marker was the best independent predictor of poor graft function 4 years after biopsy, with an OR of 6 (95% CI, 1.7 to 21; P<0.01), after adjustment for other known predictors of graft outcome such as ptc, graft fibrosis ci score, donor age, time since transplant, delayed graft function, and eGFR at biopsy. The LR test further confirmed the value of adding EndMT marker detection to the model for predicting long-term graft function loss (LR χ2 of 17.2, P<0.001). This is confirmation of the diagnostic value of EndMT markers for ABMR in an independent validation cohort.
In the validation cohort, EndMT scores were significantly higher in grafts diagnosed with ABMR than in those with TCMR, borderline lesions, nonimmunologic lesions, or no lesions (Figure 3G). Mean EndMT scores were also significantly higher in grafts from patients with higher DSA titers against class II HLA at biopsy (mean fluorescence intensity≥500, n=27, mean EndMT score=1.7) compared with those without or with lower DSA titers (mean fluorescence intensity<500, n=44, mean EndMT score=0.7, P<0.01). However, no difference in EndMT level was found regarding the class I DSA. Here again, mean EndMT scores were higher in C4d-positive than -negative grafts (3.3±0.44 versus 1.12±0.44, P=0.02). The level of EndMT marker expression was significantly correlated with ptc (Rho=0.68, P<0.001), and with g score (Rho=0.55, P<0.001). The high sensitivity and specificity of EndMT markers for the diagnosis of ABMR was confirmed in this set of patients by ROC curve analysis (Figure 3H): the AUC was 0.98 [95% CI, 0.956 to 1.0], with a sensitivity of 100% and a specificity of 85% for ABMR. In contrast, the AUC for DSA alone was 0.757 (Figure 3I). Again, prolonged cold ischemia time was significantly associated with EndMT marker expression (Rho=0.37, P=0.003). The level of EndMT marker expression was significantly higher in the patients with a history of delayed graft function compared with those without (m=1.7±1.4 versus 0.89±1.1, P=0.009). The patients with EndMT expression at biopsy, compared with those without such expression, had significantly lower graft function at biopsy (mean eGFR=21.7±5 versus 70±24, P=0.04), at 1 year (21.5±6 versus 68±26, P=0.05), and at 2 years (18±7 versus 58.7±16, P=0.03) after biopsy.
Discussion
In this study, we show in two independent sets of renal graft biopsies that EndMT is an interesting new marker to detect endothelial activation. In the context of kidney transplantation, this endothelial activation is presumably induced, during ABMR, by antibody interaction with vascular endothelial cells, yet whether EndMT is directly or indirectly (via microvascular inflammation) induced by DSA will require further studies. Nevertheless, the expression of three mesenchymal proteins, namely fascin1, vimentin, and hsp47, was mainly detected in the endothelial cells of capillaries from allografts with ABMR. In addition, these EndMT markers were more sensitive and more specific than the criteria currently used to diagnose ABMR and had good prognostic value for predicting future graft dysfunction. Interestingly, EndMT was also reproduced in an experimental in vivo model of ABMR, in rats, where DSA had been elicited by blood transfusions.
EndMT is an essential process for the atrioventricular cushion formation during embryonic development of the heart. EndMT was first described by Elisabeth Zeisberg et al. as an instrumental process causing fibrosis in the heart in mouse models of pressure overload and allogenic transplantation,18 and was later found to promote renal fibrogenesis in mice.19 After birth, EndMT occurs in several pathologic conditions such as tissue ischemia, complement activation, and inflammatory cytokine stimulation. Activation of TGFβ, Akt, and/or mTOR pathways has been shown to induce EndMT.15–17,20 As such, it could be an interesting indicator of vascular endothelial cellular activation.
ABMR is a prototypical model of endothelial injury,4 through either complement activation or complement-independent pathways.11–14 Here, patients with DSA anti-HLA antibodies against class II molecules had elevated levels of EndMT markers, and the PTC expressed fascin de novo in an experimental model of ABMR. These findings confirm that these DSAs are harmful for endothelial cells that survive immunologic injury. The detection by EndMT markers of C4d-negative cases of ABMR, especially chronic ABMR, is of particular clinical interest, because the latter is characterized by a low frequency of C4d positivity in PTC. Although EndMT marker expression was less extensive in chronic than acute ABMR cases in our study, staining was strong and diffuse in cases of “active” cABMR. This is probably because the endothelial phenotypical changes outlast C4d deposition. Thus, this result emphasizes the usefulness of EndMT markers for graft surveillance because they appear to reflect an active endothelial cell status, one calling for therapeutic intervention.
ABMR is the leading cause of long-term graft loss. Remarkably, in the cases previously diagnosed as TCMR or even in those without histologic lesions, the presence of EndMT marker expression was associated with progressive graft dysfunction and increased proteinuria during follow-up, which is compatible with indolent, subhistologic ABMR.5,21–23 Indeed, the diagnosis of ABMR was found in subsequent biopsies in a majority of those grafts with EndMT marker expression when a second biopsy was performed. In contrast, those with EndMT-negative grafts maintained very stable graft function for up to 4 years after the biopsy and none of those grafts had ABMR in subsequent biopsies. This strongly suggests that EndMT-positive grafts had ABMR at an early stage escaping the routine staining diagnostic procedure because of the poor capacity of diagnostic criteria at that time (2005–2009), which in turn probably contributes to the “false” low specificity of EndMT markers for the diagnosis of ABMR in this population, as shown in the ROC analysis. Thus, in the presence of DSA, EndMT detection may provide a new tool, an evidence of current interaction of endothelial cells to DSA, and consolidate the diagnosis of ABMR as required by Banff classification. In the future, accurate early diagnosis of ABMR with EndMT markers might increase the likelihood of detecting and properly treating ABMR before extensive graft fibrosis develops, although this point needs further study.
Peritubular capillaritis, an important pathologic feature in ABMR, is known to be closely associated with long-term graft dysfunction. However, some reports showed that ptc at an early stage of transplantation was not specific for ABMR.24,25 In the present study, we show that patients with both capillaritis and EndMT are the ones whose graft function progressively declined, while without EndMT, graft function remained stable up to 4 years after the biopsy.
In the present study, we found a significant association between a prolonged ischemia time and a history of delayed graft function with EndMT. These results extend our current knowledge on the role of ischemia reperfusion injury in endothelial cell activation. This in turn may trigger innate and adaptative immune responses in the graft.26 Activated endothelial cells might thus be involved in graft rejection and contribute to progressive graft loss.27
Before EndMT markers can be envisioned as a routine diagnostic tool for ABMR, several points relevant to our work must be discussed, and further work is necessary. First, patients were included in our study on the basis of the availability of leftover biopsy material, and our results must be confirmed on a prospective basis. Second, EndMT markers do not flag ABMR-specific reactions in endothelial cells and are probably expressed in other pathologic conditions characterized by endothelial injury. For example, we have observed that nontransplanted patients suffering from thrombotic microangiopathy in their native kidneys also exhibit an intense positivity for these markers (data not shown). Therefore, we believe that EndMT positivity is not restricted to ABMR, but more generally reflects endothelial injury, which may or may not be due to anti-HLA antibodies. Third, immunohistochemical semiquantification of markers is always a matter of debate. Nonetheless, immunohistochemistry offers a way of detecting precise phenotypic changes in a specific cell type (here, endothelial cells), and this technique can be practiced easily and routinely in most hospitals. Finally, the assessment of three different markers may seem burdensome. We found that fascin1 staining was the easiest to observe, which could make it the main EndMT marker. However, there are cases where fascin staining is difficult to interpret, possibly because of fixation issues. In these cases, HSP47 and vimentin (normally absent in endothelial cells) are helpful markers.
In conclusion, we report here that three mesenchymal proteins, fascin1, vimentin, and hsp47, are expressed at upregulated levels in endothelial PTC cells during ABMR. This expression suggests that DSA binding with/without complement activation activates the endothelial-to-mesenchymal transition. These endothelial phenotypic changes detectable by immunohistochemistry can be used routinely as evidence of a DSA-triggered endothelial reaction after transplantation to help diagnose ABMR and for graft monitoring. Early diagnosis of ABMR using EndMT markers might improve the efficacy of treatment, and help to prevent chronic DSA-induced lesions such as allograft glomerulopathy, interstitial fibrosis, tubular atrophy, and graft loss. Accurate diagnosis may also help prevent unnecessary treatment of patients without ABMR.
Concise Methods
Patients and Sample Collection
We retrospectively analyzed two sets of allograft biopsies taken from kidney recipients transplanted in our center. Their demographic data are shown in Table 2. All patients provided informed consent to participate in the study. Renal morphologic scores were assessed with the 2007 Banff working classification for renal graft pathology.28 Applying a case-control strategy, we used the first set of 53 biopsies, taken during 2005–2009 from grafts 10 days to 7.12 years after transplantation (36 for cause and 17 for graft surveillance), to study the diagnostic value of EndMT markers for ABMR. These markers were thus measured in 20 renal biopsies from patients diagnosed with acute (n=14) or chronic (n=6) ABMR (aABMR and cABMR); five of the six cABMR cases were active, according to the 2013 Banff classification.10 They were compared with the marker levels found in 33 control biopsies, including 24 diagnosed as TCMR or borderline lesions, and nine as normal at the time of biopsy. For this first set, the diagnosis of aABMR was based on: (1) a biopsy showing ptc with or without diffuse C4d staining on frozen tissue and anti-HLA class I or II antibodies detected by ELISA (adequate samples for retrospective analysis with single-antigen technology were not available for post hoc analysis); (2) the administration of treatment intended to control ABMR (typically, a combination of steroids, plasma exchange with intravenous immunoglobulins, and rituximab). The diagnosis of cABMR was based on the presence of cg (Banff chronic glomerulopathy score) in the biopsy and other clinical criteria. A second set of 74 biopsies was used to validate the results obtained from the first set of biopsies. These samples were collected during 2010–2011; we applied a cohort study strategy but were restricted to leftover samples from patients undergoing either graft control or indicated biopsies, taken from 6 days to 5.5 years after transplantation. This set finally consisted of nine biopsies showing aABMR (two with ABMR and TCMR), three cABMR (two active forms), nine TCMR or borderline lesions, 24 with diagnoses other than rejection, and another 29 samples with no lesions. DSA were detected by single-bead technology. Positive or negative control samples for different EndMT marker expression used in this study were: the healthy vicinity of three renal carcinomas, three implantation biopsies (negative control), and three renal grafts removed because of incurable ABMR leading to loss of graft function (positive control).
Table 2.
Patients’ clinical and morphologic data in the biopsy study and validation sets
| Characteristics | First setting n=53, case-control study | Second setting n=74, cohort study |
|---|---|---|
| Sex of patients men/women (men %) | 37/16 (70% men) | 41/33 (55.4% men) |
| Age of patients (years) | 48±15 | 50±14 |
| First graft | 48 (90%) | 61 (82.4%) |
| Deceased/living donor | 45/8 (85%) | 50/24 (67.6%) |
| Sex donor men/women (men %) | 25/28 (47.2% men) | 38/36 (51.4%) |
| Age of donor (years) | 54.5±18 | 54.6±15 |
| HLA class 1 AB at time of biopsy | 16/46 (35%) | |
| HLA class II AB at time of biopsy | 14/45 (31%) | |
| DSA HLA class 1 (MFI more than 500) at time of biopsy | 16/71 (22.5%) | |
| DSA HLA class II (MFI more than 500) at time of biopsy | 27/71 (38%) | |
| Cold ischemia time (minutes) | 1177±498 | 1002±488 |
| % CNI containing regimen | 48/53 (90.6%) | 74/74 (100%) |
| % Basiliximab induction therapy | 43/53 (81%) | 49/74 (66.2%) |
| % DGF and need hemodialysis | 18/53 (34%) | 19/74 (25.7%) |
| Biopsy for clinic cause/surveillance | 36/53 (68%) | 40/74 (54%) |
| Mean Banff score | ||
| g | 0.62±0.97 | 0.29±0.69 |
| ptc | 1.34±1.16 | 0.46±0.85 |
| i | 1.28±1.1 | 0.36±0.7 |
| t | 0.62±0.9 | 0.22±0.5 |
| v | 0.21±0.75 | 0.05±0.3 |
| cg | 0.235±0.76 | 0.08±0.4 |
| ci | 0.71±0.9 | 0.68±0.83 |
| ct | 0.4±0.63 | 0.55±0.8 |
| cv | 0.73±0.93 | 0.73±0.96 |
MFI, mean fluorescence intensity; i, mononuclear cell interstitial inflammation; t, tubulitis; v, intimal arteritis; ci, intersitial fibrosis; ct; tubular atrophy; cv, vascular fibrous intimal thickening.
Experimental Model of ABMR in Rats
An experimental model of ABMR was developed in rats, where DSA were elicited by transfusion of donor blood (Brown Norway RT1n) into a complete mismatch recipient (Lewis RT1l) 3 weeks prior to kidney transplantation,29 and the renal grafts were studied 7 days posttransplant. In the present study, paraffin-embedded sections of control or ABMR kidneys were used to detect the expression of EndMT markers in PTC.
Immunohistochemistry for EndMT Marker Detection
Immunohistochemistry was used to detect EndMT markers in paraffinized tissue, in both human and rat samples. Target retrieval was carried out by heating the tissue in citrate buffer. The sections were incubated overnight at 4°C with PBS containing mouse anti-fascin1 (Dako), vimentin (Diagnostic Biosystems), or hsp47 (Stressgen) antibodies. The immunoreactive proteins were then visualized with anti-mouse Histofine Simple Stain MAX PO and AEC+substrate–chromogen (Dako). As negative control, the primary antibody was replaced by an equal concentration of mouse IgG. The quality of the staining for each experiment was controlled by comparison with a positive (ABMR) and a negative (normal kidney) case. Double staining of three EndMT markers with endothelial marker CD34 was done according to the polink DS-MM kit (GBI Labs). Each EndMT marker was assessed three separate times in the PTC endothelial cells blinded to the clinical data. The semiquantitative assessment was scored as follows: no staining: 0; strong PTC cell staining in <10%: 1; in 10–24%: 2; 25–50%: 3; >50%: 4. Unless specifically mentioned for an individual marker, the highest of the three markers defined the EndMT score. Grafts with a score ≥2 (10% or more of PTC cells were strongly stained by any one of the three markers) were considered EndMT-positive. This cutoff was defined before any statistical analysis, based on our experience with EMT markers,30–33 and the level of EndMT marker expression observed in normal kidneys. The Kendall’s coefficient of concordance for EndMT score between different readings by one recorder was 0.97 (P<0.001), and between two recorders was 0.86 (P=0.001).
Statistical Analyses
t Tests were used to compare EndMT marker staining between two groups according to transplant-related diseases and to compare graft function or proteinuria according to EndMT expression. Statistical significance was defined as P<0.05, with P assessed by a two-sided nonparametric Mann–Whitney test. The associations of each EndMT marker with the Banff histologic scores, graft function, and proteinuria were evaluated with Spearman’s rank correlation analysis. The sensitivity and specificity of the EndMT markers, ptc score, and DSA for the diagnosis of ABMR were determined by ROC curve analysis. Logistic regression analysis was used to confirm the independent association of different clinical or morphologic findings, including EndMT, associated with ABMR or with poor graft function at 4 years, and to determine their adjusted ORs and 95% CI. The cutoff value for the poor graft function at 4 years after biopsy was the median at this time point (defined before statistical analysis), which turned out to be 25 ml/min. An LR test was used to compare the suitability of two logistic regression models (with or without EndMT markers) for diagnosing ABMR and predicting poor graft function at 4 years. Stata 8 was used for data analysis.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ben Naimi, and Ms. Elsa Akil, platform manager, from Anygenes, Ms. Edith Baugey for excellent technical assistance in immunohistochemistry.
Alexandre Hertig is the recipient of a Contrat d’Interface Hospitalier, with Inserm (2014–2017).
We would like to thank the “Office du Transfert de Technologie & des Partenariats Industriels de Assistance Publique de Hôpitaux de Paris” and Serge Lapointe, “Fasken Martineau, Agents de brevets et marques de commerce, Montréal (QC) Canada” for their assistance in preparing the patent application to the US Patent and Trademark Office for EndMT: endothelial activation biomarker characterizing antibody-mediated rejection.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R01DK092454-04 (to A.D.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070679/-/DCSupplemental.
References
- 1.Cai J, Terasaki PI: Humoral theory of transplantation: mechanism, prevention, and treatment. Hum Immunol 66: 334–342, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Terasaki P, Mizutani K: Antibody mediated rejection: update 2006. Clin J Am Soc Nephrol 1: 400–403, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Colvin RB, Smith RN: Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5: 807–817, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 7.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ: Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF: Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol 168: 5415–5423, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Lepin EJ, Zhang Q, Zhang X, Jindra PT, Hong LS, Ayele P, Peralta MV, Gjertson DW, Kobashigawa JA, Wallace WD, Fishbein MC, Reed EF: Phosphorylated S6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant 6: 1560–1571, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Jindra PT, Hsueh A, Hong L, Gjertson D, Shen XD, Gao F, Dang J, Mischel PS, Baldwin WM, 3rd, Fishbein MC, Kupiec-Weglinski JW, Reed EF: Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol 180: 2214–2224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler ME, Souda P, Jin YP, Whitelegge JP, Reed EF: Characterization of the endothelial cell cytoskeleton following HLA class I ligation. PLoS ONE 7: e29472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, Fiocchi C: Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol 179: 2660–2673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu P, Huang L, Ge X, Yan F, Wu R, Ao Q: Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int J Exp Pathol 87: 463–474, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curci C, Castellano G, Stasi A, Divella C, Loverre A, Gigante M, Simone S, Cariello M, Montinaro V, Lucarelli G, Ditonno P, Battaglia M, Crovace A, Staffieri F, Oortwijn B, van Amersfoort E, Gesualdo L, Grandaliano G: Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol Dial Transplant 29: 799–808, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R: Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R: Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, Walker SJ, Vanhoutte D, Heymans S, Grieve DJ, Cave AC, Brewer AC, Zhang M, Shah AM: Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J Am Coll Cardiol 63: 2734–2741, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Wavamunno MD, O’Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, Nankivell BJ: Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 7: 2757–2768, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Colvin RB: Pathology of chronic humoral rejection. Contrib Nephrol 162: 75–86, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Haas M: Pathologic features of antibody-mediated rejection in renal allografts: an expanding spectrum. Curr Opin Nephrol Hypertens 21: 264–271, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF: A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant 12: 1168–1179, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Halloran PF, Sellares J: Microcirculation lesions alone are not reliable for identifying antibody-mediated rejection. Am J Transplant 13: 1931–1932, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Menke J, Sollinger D, Schamberger B, Heemann U, Lutz J: The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant 19: 395–400, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Dormond O, Dufour M, Seto T, Bruneau S, Briscoe DM: Targeting the intragraft microenvironment and the development of chronic allograft rejection. Hum Immunol 73: 1261–1268, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Huang G, Wilson NA, Reese SR, Jacobson LM, Zhong W, Djamali A: Characterization of transfusion-elicited acute antibody-mediated rejection in a rat model of kidney transplantation. Am J Transplant 14: 1061–1072, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC: Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant 6: 2937–2946, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Hertig A, Anglicheau D, Verine J, Pallet N, Touzot M, Ancel PY, Mesnard L, Brousse N, Baugey E, Glotz D, Legendre C, Rondeau E, Xu-Dubois YC: Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol 19: 1584–1591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazzan M, Hertig A, Buob D, Copin MC, Noël C, Rondeau E, Dubois-Xu YC: Epithelial-to-mesenchymal transition predicts cyclosporine nephrotoxicity in renal transplant recipients. J Am Soc Nephrol 22: 1375–1381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu-Dubois YC, Galichon P, Brocheriou I, Baugey E, Morichon R, Jouanneau C, Ouali N, Rondeau E, Hertig A: Expression of the transcriptional regulator snail1 in kidney transplants displaying epithelial-to-mesenchymal transition features. Nephrol Dial Transplant 29: 2136–2144, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.