Abstract
Lithium and valproate modulate disturbances in intracellular calcium homeostasis implicated in the pathophysiology of bipolar disorder, but the molecular mechanisms are not fully understood. Two subtypes of transient receptor potential (TRP) channel family, i.e. TRPC3 and TRPM2, are potential candidates involved in calcium signaling and implicated in the pathophysiology of bipolar disorder. This study was designed to investigate whether mood stabilizers such as lithium and valproate affect the expression of TRPC3 and TRPM2. Rats were treated with intraperitoneal injections of lithium (2 mEq/kg b.i.d.) or valproate (300 mg/kg b.i.d.) acutely (for 24 h) or chronically (for 4 weeks). The changes in mRNA and protein levels of TRPC3 and TRPM2 were measured with real-time polymerase chain reaction and western blotting. The chronic administration of lithium and valproate significantly reduced levels of TRPC3 by 19.7% and 19.3%, respectively. No change was detected in the mRNA level of this channel. Neither acute nor chronic treatment with lithium or valproate had any effect on TRPM2 levels. The results suggest that downregulation of the TRPC3 channel is an important shared mechanism by which lithium and valproate can modulate calcium disturbances, whereas the TRPM2 channel does not appear to be affected by mood stabilizers, at least under non stressed conditions.
Keywords: TRPC3, TRPM2, Lithium, Valproate, Bipolar disorder, Cerebral cortex
INTRODUCTION
Ample evidence has indicated that disturbances in intracellular calcium homeostasis (ICH) play an important role in the pathophysiology of bipolar disorder (BD) (1,2,3) and may be relevant to the altered neuroplasticity and cellular adaptation observed in this disease (4). The mechanism underlying disturbances in calcium homeostasis in BD has not been fully elucidated, although recent findings have suggested a role for a family of nonselective calcium channels known as transient receptor potential (TRP) channels (5,6). Among the members of TRP family, much attention has been given to the subtypes canonical 3 (TRPC3) and melastatin 2 (TRPM2) in the pathophysiology of BD. TRPC3 is probably involved in the increased response to lysophosphatidic acid-stimulated calcium flow dynamics in B lymphoblast cells (BLCL) from patients with BD (5). Chronic treatment with therapeutic concentrations of lithium were found to decrease TRPC3 protein levels in BLCL from patients with BD more than in healthy individuals (7). Linkage studies in humans have identified one of the susceptibility loci for BD on chromosomal region 21q22.3 (8), where the TRPM2 gene has been mapped (9). A significant correlation has also been shown between the observed calcium imbalance in BLCL from patients with BD and a single nucleotide polymorphism on intron 19 of the TRPM2 gene (10). Furthermore, a significant decrease in TRPM2 mRNA levels was observed in BLCL from patients with BD who had high basal intracellular calcium levels (6).
Mood stabilizers such as lithium and valproate are the main drugs for the treatment of BD, and the modulation of ICH has been proposed to form part of their therapeutic action. Both drugs modify calcium signaling and homeostasis in neuronal cell models and in unexcitable cells. Chronic treatment with lithium decreased calcium influx through the N-methyl-D-aspartate receptor in neuronal cell culture (11). Lithium also decreased basal and agonist-stimulated calcium levels in a variety of cell lines such as astrocytes (12), C6 glioma cells (13), and rat GH3 pituitary cells (14). Furthermore, chronic but not acute treatment of BLCL from patients with lithium at therapeutic concentrations attenuated calcium entry by lysophophatidic acid (an index of receptor-operated calcium entry) or thapsigargin (an index of store-operated calcium entry) (15). Other reports indicated that sodium valproate regulated agonist-stimulated calcium responses in rat C6 glioma cells (16) and human platelets (17). Valproate affects cellular components related to calcium signaling such as protein kinase C and calcium/calmodulin-dependent protein kinase II (4,18). These findings suggest the potential importance of modifications in ICH disturbances in the mechanism of action of this mood-stabilizing drug.
Considering the role of TRPC3 and TRPM2 channels in the ICH disturbances and pathophysiology of BD, it can be hypothesized that mood stabilizers modify ICH through their effects on these channels. The few relevant studies published to date tested the effect of lithium (but not valproate) on TRPC3 and TRPM2 in BLCL (7) and human olfactory neuroepithelial-derived cell lines (19). Therefore, this study was designed to evaluate the in vivo effects of lithium and sodium valproate on the expression of TRPC3 and TRPM2 channels in the rat cerebral cortex.
MATERIALS AND METHODS
Animals
All animals were handled in accordance with guidelines for animal care and with the approval of the Ethics Committee of Shiraz University of Medical Sciences. Male Sprague-Dawley rats (200-250 g) were kept for 1 week before the beginning of the study to allow them to acclimate to their new environment. They were housed in a room with 12 h light/dark cycles at 22 ± 2 °C. Animals had free access to water and food throughout the study.
Experimental design and dosing regimens
A pilot study was conducted to determine the appropriate dose of lithium and valproate for both acute and chronic treatments, in order to reach clinically relevant serum concentrations of each drug (0.5-1.2 mEq/L for lithium and 50-100 μg/ml for valproate) (20).
Acute treatment
Thirty-six rats were randomly allocated to three groups (12 animals per group) to receive lithium chloride, sodium valproate or normal saline (control group) for 24 h. Based on the pilot study, the rats were given a single intraperitoneal (i.p.) injection of lithium chloride (10 mEq/kg), sodium valproate (500 mg/kg) or normal saline (2.5 ml/kg) as a loading dose, followed by two i.p. injections of 2 mEq/kg lithium, 300 mg/kg valproate or 2.5 ml/kg normal saline in 24 h (21).
Chronic treatment
Thirty-six rats were randomly assigned to three groups (12 animals per group). Based on the pilot study, the rats were given lithium chloride (2 mEq/kg), sodium valproate (300 mg/kg) or normal saline (2.5 ml/kg) by i.p. injection twice a day for 4 weeks (22). Rats in the lithium treatment group were provided access to normal saline to compensate for the potential renal toxicity of lithium.
Preparation of animal blood samples and cortices
The animals were anaesthetized by chloroform 2 h after their last injection. Heart blood was collected and serum was separated and stored at −20 °C to measure drug levels. Immediately after blood samples were obtained, the animals were killed by neck dislocation and the whole brain was removed from the skull. The cortex was immediately dissected on ice (23) and stored at −80 °C until the use.
Determination of lithium and valproate serum levels
Drug serum levels were measured to ensure that the drugs had reached the relevant therapeutic serum levels. Flame photometry was used to measure the serum levels of lithium chloride, and gas chromatography was used for sodium valproate.
Western blotting
Western blotting was used to determine TRPC3 and TRPM2 protein levels in the rat cerebral cortex according to standard protocols (24) with some modifications. Briefly, 100 mg the cortex tissue was homogenized in NP-40 lysis buffer for 30 s at 4 °C with a homogenizer (Eberbach Corporation, Ann Arbor, MI, USA). After centrifugation at 11000 × g and 4 °C for 20 min, the supernatant was used to determine total protein concentration with the Bradford method (25). Lysate containing 20 μg of total protein was mixed with Laemmli sample buffer (1:1), heated to 95 °C for 3 min and applied to a polyacrylamide gel (4% stacking and 7.5% resolving gels), and then blotted onto nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). For immunodetection of TRPC3 protein, the membranes were blocked with 10% bovine serum albumin (BSA) for 1 h at room temperature, incubated with anti-TRPC3 antibody (1:1000) (Abcam, Cambridge, UK) overnight at 4 °C, and then incubated with protein A-horseradish peroxidase (1:1000) (Abcam) for 45 min at room temperature. Immunodetection of TRPM2 was done with a blocking solution of 5% skim milk and 1% BSA for 1 h at room temperature, followed by incubation with anti-TRPM2 primary antibody (1:1000) (Abcam) overnight at 4 °C, and then incubated with horseradish peroxidase-conjugated antibody to the primary antibody (1:10000) (Abcam) for 45 min at room temperature. To detect β-actin as the internal standard, membranes were blocked with 5% skim milk for 1 h at room temperature and then incubated with anti-β-actin antibody (1:10000) (Biolegend, San Diego, CA, USA) overnight at 4 °C. This was followed by incubation with horseradish peroxidase-conjugated antibody to the primary antibody (1:10000) (Abcam) for 45 min at room temperature. The primary and secondary antibodies were diluted in phosphate-buffered saline with Tween 20. An enhanced chemiluminescence kit (GE Healthcare) was used to detect the protein of interest on the membrane, which was then exposed to X-ray film (Kodak, New York, NY, USA). The bands were visualized on X-ray film and converted into digital images with a Packard Scanjet G2710 scanner. The relative optical density of bands was analyzed with ImageJ v1.47 software. All samples were run in duplicate and a common pooled homogenate was loaded on all gels to control for intra-gel variability. The signals of interest were normalized against β-actin (as the internal control) and then normalized against the common pool sample. The results are presented as normalized signal intensity in arbitrary units.
Real time polymerase chain reaction
Real-time polymerase chain reaction (PCR) was used to determine TRPC3 and TRPM2 mRNA levels. Total RNA was extracted from 100 mg of rat cerebral cortex by phenol/chloroform extraction method with TriPure isolation reagent (Roche Applied Science, Penzberg, Germany) according to the manufacturer's instructions (26). Complementary DNA was synthesized from 1 μg of total extracted RNA with the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer's guidelines. Gene-specific primer pairs were designed with Primer Express v.2 (Applied Biosystems, Foster City,CA, USA).
The primers for TRPC3 and TRPM2 were designed with an intron-insertion approach, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers spanned exon-exon junctions to exclude amplification of genomic DNA.
Real-time PCR was done in triplicate with SYBR Premix Ex Taq II (Takara, Shiga, Japan) to determine TRPC3 and TRPM2 mRNA levels, which were calibrated to GAPDH with the following forward and reverse primers:
For TRPC3: 5′CTGGATTGCACCTTGTACCAGG3′ and 5′GCAGACCCAGGAAGATGATGAA3′; for TRPM2: 5′AAGTTGCCTCAATCCGAGCAC3′ and 5′CAAGGTCTCAAAGGTCACCCA3′; and for GAPDH, 5′CGTGATCGAGGGCTGTTGG3′ and 5′CTGCTTCAGTTGGCCTTTCG3′.
The real time PCR conditions were: 30 s at 95 °C, 40 cycles of 5 s at 95 °C, and 34 s at 60 °C. After amplification, the melting curve was obtained by collecting fluorescence data while the temperature was increased from 60 °C to 95 °C over 1 min.
The mean threshold cycles of the samples were used to calculate expression ratios of experimental groups to the control group.
Statistical analyses
The differences in mean of TRPC3 and TRPM2 normalized intensities among the three groups were analyzed by one way ANOVA followed by Dunnett's t-test with SPSS software (v.16). The data from western blots are presented as the mean ± SD. Differences between treated and control groups in mRNA expression ratios for TRPC3 and TRPM2 were analyzed with the pair wise and TRPM2 were analyzed with the pair wise fixed reallocation randomization test using REST-384 software (v.2). The data for real-time PCR are expressed as the mean ± SEM of fold change relative to the vehicle group. P values less than 0.05 were considered statistically significant.
RESULTS
Serum concentrations of drugs
Lithium serum concentrations were 0.77 ± 0.22 mEq/L (n=12) in acute treatments and 0.83 ± 0.25 (n=12) mEq/L in chronic treatments. The serum concentrations of valproate were 76 ± 10 μg/mL (n=12) in acute treatments and 67 ± 14 (n=12) μg/ml in chronic treatments.
Characterization of TRPC3, TRPM2, and β-actin immunolabeled proteins
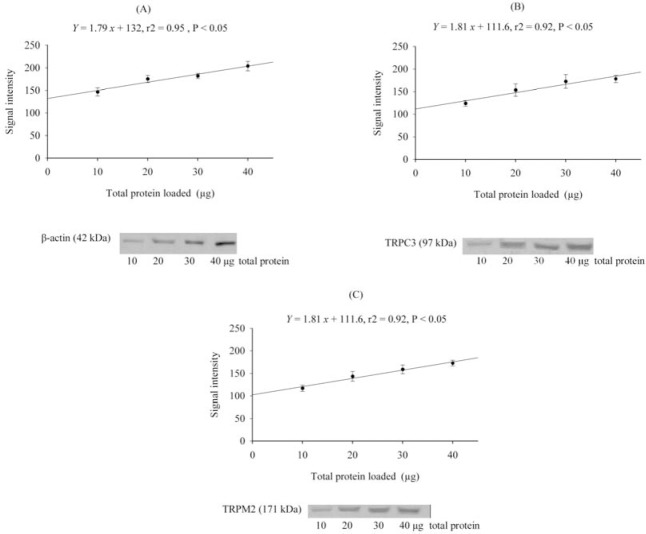
In the immunoblots, immunoreactive bands were detected at the expected molecular masses of 97 kDa for TRPC3, 171 kDa for TRPM2, and 42 kDa for β-actin (Fig. 1).
Fig. 1.
The linear range of protein detection (r2> 0.90) for A; β-actin, B; TRPC3 and C; TRPM2 versus 10-40 μg of total protein extracted from rat cerebral cortex.
As shown, there was a linear relationship between the signal intensity of β-actin, TRPC3 and TRPM2 and total protein loaded in the range of 10-40 μg. The within-blot coefficient of variance was 5.3%, and the between-blot coefficient of variance was 7.5%.
Effects of lithium on TRPC3 and TRPM2 protein levels
Acute treatment with lithium decreased TRPC3 protein levels by 3.8% (P=0.24) and TRPM2 protein levels by 1.2% (P=0.76) in comparison to the control group. However, these changes were not statistically significant (Figs. 2A and 2C). Chronic lithium treatment significantly decreased TRPC3 protein levels by 19.7% compared to the control group (P<0.001) (Fig. 2B). Chronic lithium treatment did not significantly alter TRPM2 protein level in comparison to the control group (P=0.92) (Fig. 2D). Acute (2.7%, P=0.10) and chronic (2.4%, P=0.22) lithium treatments had no significant effects on β-actin levels compared to the control group.
Fig. 2.
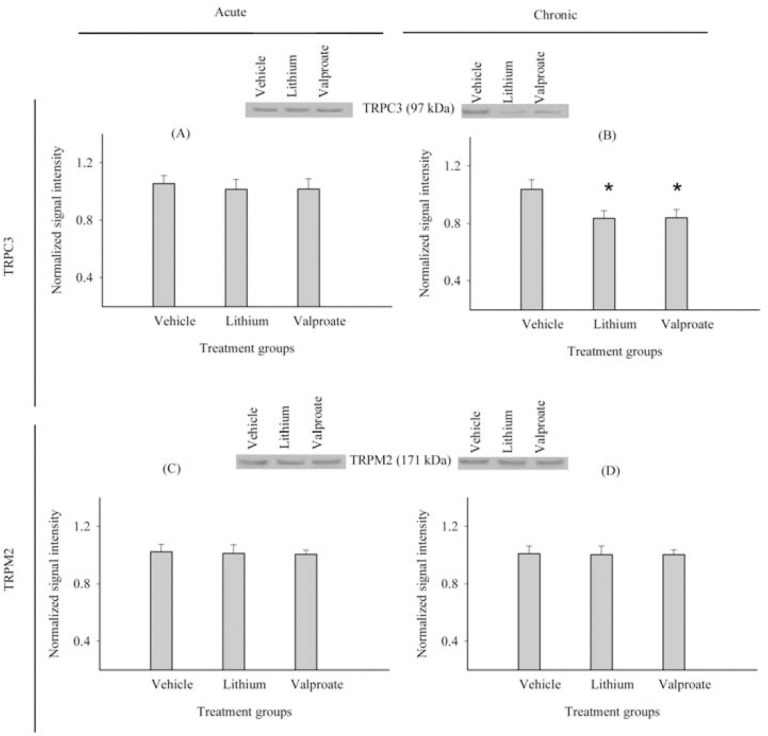
Effects of acute and chronic treatment with lithium or sodium valproate versus normal saline on A, B; TRPC3 and C, D; TRPM2 protein levels in rat cerebral cortex. Bar graphs represent the mean ± SD (n=12 per group) of TRPC3/TRPM2 signal intensities normalized against β-actin as an internal control and pooled lysates (arbitrary units). *P<0.05 significantly different compared to vehicle.
Effects of valproate on TRPC3 and TRPM2 protein levels
Acute treatment with sodium valproate caused slight decreases in TRPC3 (3.5%) (P=0.27) and TRPM2 (1.7%) (P=0.58) protein levels in the rat cerebral cortex compared to the control group (Figs. 2A and 2C). The chronic administration of valproate significantly decreased (19.3%) TRPC3 protein levels in the rat cerebral cortex compared to the control group (P<0.001) (Fig. 2B).
Chronic valproate treatment resulted in a small, non-significant decrease in TRPM2 protein levels (0.05%) (P=0.94) in comparison to the control group (Fig. 2D). β-actin levels were not significantly changed by acute (1.5%, P=0.45) or chronic (1.9%, P=0.37) treatment with valproate in comparison to the control group.
Characterization of TRPC3, TRPM2, and GAPDH mRNA
Amplification of TRPC3, TRPM2, and GAPDH cDNA with specific primer pairs yielded single bands on a 2% agarose gel at the expected sizes of 98 bp for TRPC3, 120 bp for TRPM2, and 97 bp for GAPDH. Standard curves of PCR amplification of the three target genes yielded an r2 > 0.99. The efficiency of PCR amplification of TRPC3, TRPM2, and GAPDH was calculated according to the standard curve slopes and were 1.90 ± 0.06 for TRPC3, 1.97 ± 0.09 for TRPM2, and 2.08 ± 0.12 for GAPDH.
Effects of acute and chronic lithium and valproate treatment on TRPC3 and TRPM2 mRNA levels
The acute treatment of rats with lithium or sodium valproate increased TRPC3 mRNA levels in the cerebral cortex by the factors of 1.20 ± 0.5 (P=0.52) (lithium) and 1.11 ± 0.42 (P=0.66) (valproate) compared to the vehicle. However, the observed changes were not statistically significant. Chronic treatment with lithium and valproate increased TRPC3 mRNA levels by the factors of 1.13 ± 0.39 (P=0.45) (lithium) and 1.08 ± 0.40 (P=0.59) (valproate) compared to the vehicle, but these changes were not statistically significant (Fig. 3A).
Fig. 3.
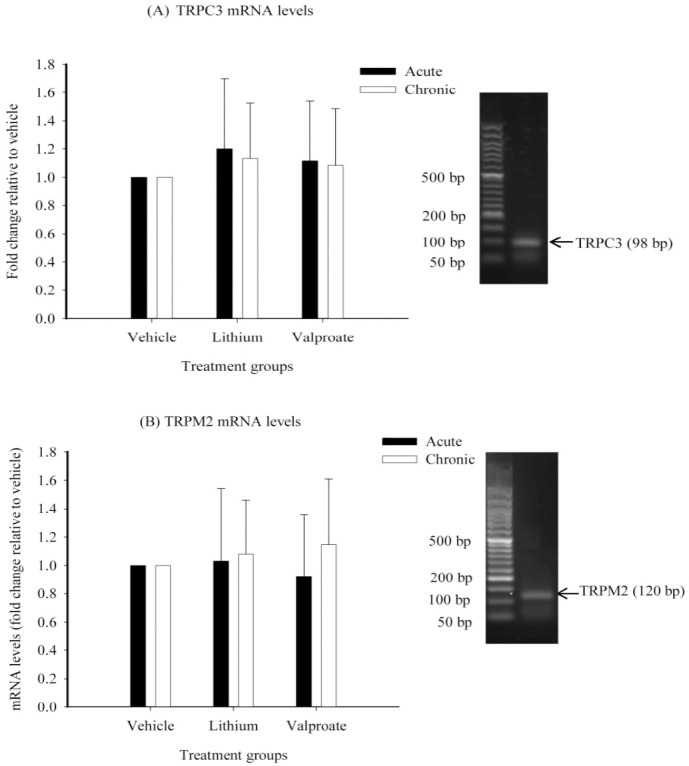
Effects of acute and chronic treatment with lithium and sodium valproate on mRNA levels of A; TRPC3 and B; TRPM2 in rat cerebral cortex. Bar graphs represent the GAPDH-normalized mean ± SEM of mRNA fold changes relative to vehicle (n=12 per group).
Acute treatment with lithium increased TRPM2 mRNA levels by a factor of 1.03 ± 0.51 (P=0.93) but valproate decreased it by a factor of 1.09 ± 0.44 (P=0.84) compared to the vehicle. However, these changes did not reach to a statistical significance. TRPM2 mRNA levels increased nonsignificantly after chronic treatment with lithium (by a factor of 1.08 ± 0.38) (P=0.62) or valproate (by a factor of 1.14 ± 0.46) (P=0.39) compared to the vehicle (Fig. 3B).
DISCUSSION
This study shows that chronic but not acute treatment with lithium or valproate at therapeutically relevant concentrations decreased TRPC3, but not TRPM2 protein levels in the rat cerebral cortex. Neither acute nor chronic treatment with lithium or valproate changed the TRPC3 or TRPM2 mRNA levels. These findings suggest that TRPC3 channels may be a common target of mood stabilizers in modulating disturbed ICH in people with BD.
Levels of TRPC3 protein were significantly decreased after chronic treatment with lithium. In contrast, SY Tong (27) found that chronic lithium treatment (1 mM for 12 days) increased TRPC3 protein levels in rat primary cortical neuron cultures under oxidative stress conditions. The discrepancy may be due to differences in experimental designs, especially regarding in vitro versus in vivo experiments. Interactions between astroglial cells and neurons in an in vivo situation should be taken into account when comparing these results to in vitro studies. In this connection, astrocyte-neuron interactions have been shown to play a role in regulating ICH (28). In addition, the presence of TRPC3 channels and their involvement in store-operated calcium entry have been reported in astrocytes (29). Moreover, astrocytes have been shown to be a target of lithium action (30). Therefore, it is probable that the effect of lithium in decreasing TRPC3 in this study reflects the overall outcome of its effects on both neurons and astrocytes.
In the present study, chronic treatment with valproate had effects similar to lithium on TRPC3 protein levels. We found no published reports of the effect of valproate on the TRPC3 channel. According to our results, TRPC3 channels seem to be a common target of these two mood stabilizers in terms of their modulatory effects on ICH.
Lithium and valproate did not alter TRPC3 mRNA levels, but decreased TRPC3 protein levels in the rat cerebral cortex, which is in agreement with the study on the effects of chronic lithium treatment in BLCL (7). The discordance between changes in protein levels with no effect on mRNA levels was also reported in earlier research on the effects of lithium on bcl-2 protein in rat primary astrocyte cultures (31) and on brain-derived neurotrophic factor in the rat frontal cortex and hippocampus (32). The findings available to date suggest that the attenuating effect of lithium and valproate on TRPC3 protein is not mediated by their effect at the transcriptional level, but may rather be due to posttranslational modifications or changes in the stability of the TRPC3 protein. Both lithium and valproate are able to degrade some proteins (33,34), so further investigations are essential to determine how TRPC3 protein might be post transcriptionally targeted by these mood stabilizers. In the present study, lithium and valproate did not alter TRPM2 protein or mRNA levels in the rat cerebral cortex. In the absence of relevant in vivo studies, our findings suggest that TRPM2 may not play a role in the effect of these mood stabilizers on ICH. Conversely, it is possible that mood stabilizers may affect TRPM2 only under stressful conditions such as high intracellular calcium levels or oxidative stress. These conditions have been shown to affect TRPM2 expression in BLCL from patients with BD (6) and in rat primary cortical neuron cultures (35). In addition, one study reported that treatment with 1 mM lithium for 4 days reduced TRPM2 mRNA and protein levels in human olfactory neuroepithelial-derived progenitor cells under ionic stress induced by monensin (19). Therefore, the possibility that lithium and valproate might exert their effects on TRPM2 expression in the context of cellular stresses cannot be ruled out. This possibility should be addressed in future studies designed to examine the effects of lithium and valproate on TRPM2 under stressful conditions such as oxidative stress.
The finding that two structurally dissimilar antimanic agents decreased TRPC3 protein levels to the same extent led to the suggestion that the TRPC3 channel may be a potential common target in ICH modulation (15,36). This TRPC3-mediated modulatory effect on ICH may result in the downstream consequences that are related to the mood stabilizing activity of these drugs. The functional consequences of the decrease in TRPC3 protein levels by lithium and valproate could be explained by the following facts: 1) TRPC3 is apparently one of the molecular components of two calcium entry processes, i.e. receptor-operated and store-operated calcium entry (37,38,39,40), that are reportedly disturbed in BD (15,41). 2) Lithium and valproate have each been shown to modulate these calcium entry processes (15,36). Because it has not been possible thus far to measure intracellular calcium mobilization in the rat brain in vivo, the functional consequences of decreased TRPC3 protein levels in terms of the modulatory effect of lithium and valproate on ICH should be addressed in future in vitro studies.
Disturbances in ICH are associated with decreased neuroplasticity and cellular resilience in BD (42). The effect of mood stabilizers on neuroplasticity and neuronal resilience has been proposed to be relevant to their efficacy in treating BD. It is likely that lithium and valproate affect neuroplasticity by blunting calcium influx – an effect of their ability to decrease TRPC3 protein levels. In this connection, it has been observed that TRPC3 channel knockdown and inhibition by Pyr3 resulted in protection against calcium-mediated cell toxicity in a mouse model of acute pancreatitis (43). Hence, a reduction in TRPC3 protein levels by lithium and valproate might be involved in the neuroplastic effect of these drugs in BD.
CONCLUSION
Chronic treatment with lithium or valproate at their therapeutic concentrations reduced TRPC3 protein levels in the rat cerebral cortex. This implies that downregulation of the TRPC3 channel may be an important common mechanism by which lithium and valproate modulate calcium disturbances in people with bipolar disorder. Moreover, this downregulation may modify decreased neuroplasticity reported in bipolar disorder. However, lack of effect of lithium and valproate on TRPM2 suggests that this channel may not be involved in the regulation of calcium homeostasis by mood stabilizers, at least under normal conditions.
ACKNOWLEDGMENTS
This study is based on work done by Sasan Zaeri in partial fulfillment of the requirements for his PhD thesis in pharmacology. This work was supported by a grant from Shiraz University of Medical Sciences (Grant No. 6368). We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the article.
REFERENCES
- 1.Dubovsky SL, Murphy J, Thomas M, Rademacher J. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry. 1992;149:118–120. doi: 10.1176/ajp.149.1.118. [DOI] [PubMed] [Google Scholar]
- 2.Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A, et al. High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am J Psychiatry. 1997;154:976–982. doi: 10.1176/ajp.154.7.976. [DOI] [PubMed] [Google Scholar]
- 3.Perova T, Wasserman MJ, Li PP, Warsh JJ. Hyperactive intracellular calcium dynamics in B lymphoblasts from patients with bipolar I disorder. Int J Neuropsychopharmacol. 2008;11:185–196. doi: 10.1017/S1461145707007973. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann RF, Schloesser RJ, Gould TD, Manji HK. Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol Neurobiol. 2005;32:173–202. doi: 10.1385/MN:32:2:173. [DOI] [PubMed] [Google Scholar]
- 5.Roedding AS, Li PP, Warsh JJ. Characterization of the transient receptor potential channels mediating lysophosphatidic acid-stimulated calcium mobilization in B lymphoblasts. Life Sci. 2006;80:89–97. doi: 10.1016/j.lfs.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Yoon IS, Li PP, Siu KP, Kennedy JL, Macciardi F, Cooke RG, et al. Altered TRPC7 gene expression in bipolar-I disorder. Biol Psychiatry. 2001;50:620–626. doi: 10.1016/s0006-3223(01)01077-0. [DOI] [PubMed] [Google Scholar]
- 7.Andreopoulos S, Wasserman M, Woo K, Li PP, Warsh JJ. Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharmacogenomics J. 2004;4:365–373. doi: 10.1038/sj.tpj.6500266. [DOI] [PubMed] [Google Scholar]
- 8.Straub RE, Lehner T, Luo Y, Loth JE, Shao W, Sharpe L, et al. A possible vulnerability locus for bipolar affective disorder on chromosome 21q22.3. Nat Genet. 1994;8:291–296. doi: 10.1038/ng1194-291. [DOI] [PubMed] [Google Scholar]
- 9.Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Macciardi F, Li PP, Yoon IS, Cooke RG, Hughes B, et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:36–43. doi: 10.1002/ajmg.b.30239. [DOI] [PubMed] [Google Scholar]
- 11.Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Hertz L. Inhibition of noradrenaline stimulated increase in [Ca2+]i in cultured astrocytes by chronic treatment with a therapeutically relevant lithium concentration. Brain Res. 1996;711:245–248. doi: 10.1016/0006-8993(95)01199-4. [DOI] [PubMed] [Google Scholar]
- 13.Yamaji T, Kagaya A, Uchitomi Y, Yokota N, Yamawaki S. Chronic treatment with antidepressants, verapamil, or lithium inhibits the serotonin-induced intracellular calcium response in individual C6 rat glioma cells. Life Sci. 1997;60:817–823. doi: 10.1016/s0024-3205(97)00010-6. [DOI] [PubMed] [Google Scholar]
- 14.Varney MA, Godfrey PP, Drummond AH, Watson SP. Chronic lithium treatment inhibits basal and agonist-stimulated responses in rat cerebral cortex and GH3 pituitary cells. Mol Pharmacol. 1992;42:671–678. [PubMed] [Google Scholar]
- 15.Wasserman MJ, Corson TW, Sibony D, Cooke RG, Parikh SV, Pennefather PS, et al. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology. 2004;29:759–769. doi: 10.1038/sj.npp.1300400. [DOI] [PubMed] [Google Scholar]
- 16.Yamaji T, Kagaya A, Uchitomi Y, Yokata N, Yamawaki S. Effects of carbamazepine and sodium valproate on 5-HT-induced calcium increase in individual C6 rat glioma cells. Neuropsychobiology. 1996;34:22–25. doi: 10.1159/000119286. [DOI] [PubMed] [Google Scholar]
- 17.Akimoto T, Kusumi I, Suzuki K, Masui T, Koyama T. Effects of valproate on serotonin-induced intracellular calcium mobilization in human platelets. J Psychiatry Neurosci. 2007;32:17–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Savina TA, Balashova OA, Shchipakina TG. Ca2+/calmodulin-dependent protein kinase II--a target for sodium valproate? Neurosci Behav Physiol. 2008;38:99–102. doi: 10.1007/s11055-008-0014-2. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Lei Z, Lu C, Roisen FJ, El-Mallakh RS. Effect of ionic stress on apoptosis and the expression of TRPM2 in human olfactory neuroepithelial-derived progenitors. World J Biol Psychiatry. 2010;11:972–984. doi: 10.3109/15622975.2010.507784. [DOI] [PubMed] [Google Scholar]
- 20.Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. divalproex maintenance study group. Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- 21.Li R, Wing LL, Wyatt RJ, Kirch DG. Effects of haloperidol, lithium, and valproate on phosphoinositide turnover in rat brain. Pharmacol Biochem Behav. 1993;46:323–329. doi: 10.1016/0091-3057(93)90360-6. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell T, Rotzinger S, Nakashima TT, Hanstock CC, Ulrich M, Silverstone PH. Chronic lithium and sodium valproate both decrease the concentration of myo-inositol and increase the concentration of inositol monophosphates in rat brain. Brain Res. 2000;880:84–91. doi: 10.1016/s0006-8993(00)02797-9. [DOI] [PubMed] [Google Scholar]
- 23.Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- 24.Roedding AS, Gao AF, Wu AM, Li PP, Kish SJ, Warsh JJ. TRPC3 protein is expressed across the lifespan in human prefrontal cortex and cerebellum. Brain Res. 2009;1260:1–6. doi: 10.1016/j.brainres.2008.12.069. [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Tong S. The Role of oxidative stress on neural TRPC3, TRPC5, TRPC6 expression and/or function and relevance to bipolar disorder. Available from: http://hdl.handle.net/1807/32495 .
- 28.Zonta M, Carmignoto G. Calcium oscillations encoding neuron-to-astrocyte communication. J Physiol Paris. 2002;96:193–198. doi: 10.1016/s0928-4257(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 29.Shirakawa H, Sakimoto S, Nakao K, Sugishita A, Konno M, Iida S, et al. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyoo IK, Dager SR, Kim JE, Yoon SJ, Friedman SD, Dunner DL, et al. Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacology. 2010;35:1743–1750. doi: 10.1038/npp.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keshavarz M, Emamghoreishi M, Nekooeian AA, J JW, Zare HR. Increased bcl-2 protein levels in rat primary astrocyte culture following chronic lithium treatment. Iran J Med Sci. 2013;38:255–262. [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024:183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 33.Yin L, Joshi S, Wu N, Tong X, Lazar MA. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erb alpha. Proc Natl Acad Sci U S A. 2010;107:11614–11619. doi: 10.1073/pnas.1000438107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Convertini P, Shen M, Xu X, Lemoine F, de la Grange P, et al. Valproic acid causes proteasomal degradation of DICER and influences miRNA expression. PLoS One. 2013;8:e82895. doi: 10.1371/journal.pone.0082895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roedding AS, Tong SY, Au-Yeung W, Li PP, Warsh JJ. Chronic oxidative stress modulates TRPC3 and TRPM2 channel expression and function in rat primary cortical neurons: relevance to the pathophysiology of bipolar disorder. Brain Res. 2013;1517:16–27. doi: 10.1016/j.brainres.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Perova T, Kwan M, Li PP, Warsh JJ. Differential modulation of intracellular Ca2+ responses in B lymphoblasts by mood stabilizers. Int J Neuropsychopharmacol. 2010;13:693–702. doi: 10.1017/S1461145709000261. [DOI] [PubMed] [Google Scholar]
- 37.Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 38.Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Luckhoff A, et al. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- 39.Barritt GJ. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- 40.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 41.Hough C, Lu SJ, Davis CL, Chuang DM, Post RM. Elevated basal and thapsigargin-stimulated intracellular calcium of platelets and lymphocytes from bipolar affective disorder patients measured by a fluorometric microassay. Biol Psychiatry. 1999;46:247–255. doi: 10.1016/s0006-3223(98)00308-4. [DOI] [PubMed] [Google Scholar]
- 42.Manji HK, Moore GJ, Rajkowska G, Chen G. Neuroplasticity and cellular resilience in mood disorders. Mol Psychiatry. 2000;5:578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 43.Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, et al. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]