Abstract
The cellular and molecular mechanisms underlying adaptive changes to physiological stress within the intestinal epithelium remain poorly understood. Here, we show that PTEN, a negative regulator of the PI3K→AKT→mTORC1 signaling pathway, is an important regulator of dormant intestinal stem cells (dISCs). Acute nutrient deprivation leads to transient PTEN phosphorylation within d-ISCs and a corresponding increase in their number. This release of PTEN inhibition renders d-ISCs functionally poised to contribute to the regenerative response during re-feeding via cell-autonomous activation of the PI3K→AKT→mTORC1 pathway. Consistent with its role in mediating cell survival, PTEN is required for d-ISC maintenance at baseline, and intestines lacking PTEN have diminished regenerative capacity following irradiation. Our results highlight a PTEN-dependent mechanism for d-ISC maintenance and further demonstrate the role of d-ISCs in the intestinal response to stress.
Keywords: dormant, quiescent, intestinal stem cells, PTEN, fasting, MTORC1
INTRODUCTION
The physiological response to fasting has had enormous selective pressure throughout evolution and has been linked to disease prevention and improved clinical outcomes for many conditions (Longo and Mattson, 2014). Additionally, clinicians have increasingly recognized the value of providing even small volume enteral feeds to maintain and augment the recovery of the critically ill patient by preserving bowel mucosal integrity, thereby helping to prevent enteral-systemic bacterial translocation. Despite its relevance to human health, surprisingly little is known regarding how fasting affects metabolically dynamic and expensive tissues such as the gut. Some insight comes from animals that feed sporadically, such as snakes and hibernating mammals, which exhibit an astonishing capacity to modulate the intestinal mucosa in response to the presence or absence of enteral nutrition (Dunel-Erb et al., 2001; Secor et al., 1994). Similarly, humans and rodents that undergo periods of fasting or receive all their nutrition intravenously experience marked intestinal atrophy (Chappell et al., 2003). These findings underscore the requirement of luminal nutrition for intestinal maintenance and highlight an important energy-conserving mechanism. Under fed conditions, energy consumed by the intestinal mucosa represents ~15% of the total basal metabolic rate (Aiello, 1995) and following an extended fast, up to 25% of newly available calories are committed to restoring the intestinal mucosa (Secor et al., 1994), emphasizing the critical importance of intestinal homeostasis to the body. The cellular and molecular mechanisms underlying intestinal adaptation to fasting and re-feeding however remain poorly characterized. We propose that this process is regulated in part at the level of intestinal stem cells (ISCs).
Intestinal adaptation to fasting involves dramatic alterations in cell number, cycling frequency, and cell turnover, suggesting fundamental changes in stem/progenitor cell function (Dunel-Erb et al., 2001). In mice, a diverse array of ISC markers has been described and used to characterize this functionally heterogeneous population (Carlone and Breault, 2012; Goodell et al., 2015; Ritsma et al., 2014; Takeda et al., 2011; Tao et al., 2015; Yan et al., 2012). The spectrum of ISCs includes rapidly cycling crypt base columnar ISCs (r-ISCs) marked by Lgr5 expression, and slowly cycling, relatively dormant ISCs (d-ISCs) marked by mTert (telomerase), Bmi1, Lrig1, HopX and Dclk1 (Barker et al., 2007; Montgomery et al., 2011; Powell et al., 2012; Sangiorgi and Capecchi, 2008; Takeda et al., 2011; Westphalen et al., 2014). R-ISCs play a dominant role during daily intestinal maintenance and are sensitive to intestinal stress and injury (Barker et al., 2007; Carlone and Breault, 2012; Metcalfe et al., 2014; Ritsma et al., 2014), In contrast, d-ISCs, located in the “+4” supra-Paneth position, are resistant to stress and are activated upon injury to restore homeostasis (Metcalfe et al., 2014; Montgomery et al., 2011; Powell et al., 2012; Ritsma et al., 2014; Sangiorgi and Capecchi, 2008; Takeda et al., 2011; Tian et al., 2011). Adding additional complexity, recent data suggest a level of cellular plasticity within the ISC population, thereby allowing for inter-conversion between compartments (Goodell et al., 2015; Munoz et al., 2012; Ritsma et al., 2014; Takeda et al., 2011). While there are between twelve and sixteen r-ISCs in a single small intestinal crypt (Lopez-Garcia et al., 2010; Snippert et al., 2010), only one to two d-ISCs are typically present, underscoring their reserve role in intestinal maintenance (Breault et al., 2008; Powell et al., 2012; Sangiorgi and Capecchi, 2008; Takeda et al., 2011). To date, most studies looking at the stress response of ISCs have focused on radiation-induced injury (Kirsch et al., 2010; Potten, 2004; Roche et al., 2015), a potent but pathological insult. In contrast, few studies have examined the ISC response to more subtle yet common, physiological stressors such as acute nutrient deprivation.
To date, studies investigating the role of nutrients in ISC regulation have yielded conflicting results. In Drosophila, ISCs are largely unaffected by acute nutrient deprivation, but upon re-feeding are activated to proliferate in response to canonical insulin signaling (Choi et al., 2011; O’Brien et al., 2011). In contrast, long-term caloric restriction in mice leads to activation of r-ISC self-renewal, mediated indirectly by decreased mTORC1 signaling in neighboring Paneth cells (Yilmaz et al., 2012). How dISCs respond to changes in nutrient levels either during an acute fast or upon re-feeding remains unknown. Given that these cells are intrinsically stress responsive, it will be of particular interest to delineate how acute changes in nutrient levels specifically affect this quiescent population.
The molecular mechanisms underlying the regulation of d-ISC quiescence and activation remain poorly understood. PTEN (phosphatase and tensin homologue), a negative regulator of the PI3K→AKT→mTORC1 signaling pathway, is an essential regulator of numerous cellular processes including survival, proliferation, and energy metabolism. In addition, PTEN has been implicated in the maintenance of quiescent cell numbers (Di Cristofano and Pandolfi, 2000; Shen et al., 2007; Song et al., 2012); however, its complete loss has been associated with cellular senescence, providing a block to unrestrained cellular growth (Chen et al., 2005; Papa et al., 2014; Song et al., 2012). PTEN is also a known negative regulator of genes associated with ISC self-renewal and maintenance, including mTert (telomerase reverse transcriptase) (Kang et al., 1999; Kyo and Inoue, 2002; You et al., 2007; Zhou et al., 2006). In the intestine, PTEN is an important regulator of homeostasis (Langlois et al., 2009) with loss of function mutations giving rise to the PTEN hamartoma tumor syndrome (Hobert and Eng, 2009). In addition, PTEN is negatively regulated by phosphorylation (Ross and Gericke, 2009; Vazquez et al., 2001; Vazquez et al., 2000) and both PTEN and its inactive isoform, phospho-PTEN (pPTEN), have been shown to mark a discrete population of DNA label-retaining cells in the “+4” crypt position (He et al., 2004). Finally, PTEN has recently been shown to function as a gatekeeper of the fed-fasting transition in adipose tissue (Nelson et al., 2014), raising the possibility that it may serve a similar function in the intestine.
Here, we show that the physiological stress of fasting leads to the transient inhibition of PTEN in mTert-expressing d-ISCs and a corresponding increase in their number. In addition, fasting renders d-ISCs functionally poised to contribute to the regenerative response during re-feeding. This response is mediated by cell-autonomous activation of the PI3K→AKT→mTORC1 pathway, made possible by the inhibition of PTEN. Consistent with its role as a regulator of cell survival, we also show that PTEN is required for d-ISC maintenance at baseline and that intestines lacking PTEN have diminished regenerative capacity following irradiation.
RESULTS
Fasting increases d-ISC numbers
We have previously identified mTert expression as a marker for d-ISCs under baseline fed conditions (Montgomery et al., 2011). Using a high-resolution, three dimensional imaging technique that allows for the analysis of endogenous GFP fluorescence in freshly isolated intestinal crypts, we were able to identify at least one mTert-GFP+ cell in up to two-thirds of all crypts (Figures 1A, S1A), supporting the physiologically relevant role of these cells as dormant ISCs.
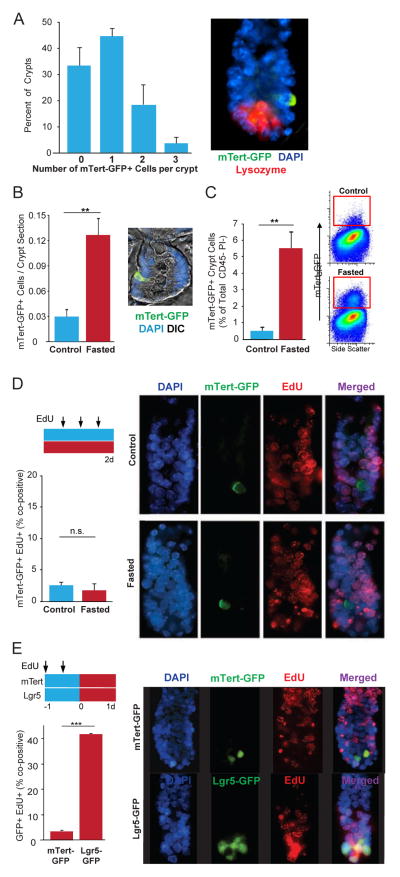
FIGURE 1. d-ISCs increase in number during fasting.
(A) Percentage of individual intestinal crypts from control (fed) mTert-GFP mice containing 0–3 labeled cells. N=3 animals. Representative image of isolated crypt showing endogenous mTert-GFP expression in the “+4” supra-Paneth position (arrow). Lysozyme (red) immunostaining identifies Paneth cells, 20X. (B) Number of mTert-GFP+ d-ISCs per crypt section from control (fed) and fasted mice. Representative image from a control mouse showing GFP+ cell (arrow) identified by immunofluorescent analysis, 60X. N=3, 6. (C) Percentage of mTert-GFP+ cells per total CD45−PI− crypt cells from control (fed) and fasted mice. Representative FACS plots showing GFP+ cells in boxes. N=3, 3. (D) Percentage of mTert-GFP+ cells co-expressing EdU in isolated crypts from control (fed) and fasted mice. Schematic of treatment regimen is shown. Representative optical sections showing non-cycling mTert-GFP+ cells (arrowheads) in both control (fed) and fasted crypts in DAPI, mTert-GFP, EdU (Click-iT®) and merged channels, 63X. N=3, 3. (E) Percentage of mTert-GFP+ and Lgr5-GFP+ cells co-expressing EdU in isolated crypts from fasted mice. Schematic of treatment regimen is shown. Representative optical sections of isolated crypts from mTert-GFP and Lgr5-GFP mice showing actively cycling (arrows) and non-cycling (arrowheads) ISCs in DAPI, endogenous GFP, EdU (Click-iT®) and merged channels, 60x. N=3, 3. Mean±SEM, Student’s t-test, **p<0.01, ***p<0.001, n.s. not significant.
To assess the effect of acute nutrient deprivation on d-ISCs, mTert-GFP mice were fasted for 48 hours with ad libitum access to water, which led to a 20% weight loss, a reduction in villus height and villus and crypt cell number, decreased crypt cell proliferation, and increased crypt cell apoptosis (Figures S1B–H). Despite these dynamic changes, we observed a dramatic increase in the number of mTert-GFP+ cells in response to fasting (Figures 1B, 1C). To determine if this increase was due to enhanced cell proliferation, EdU-labeling studies were performed. Analysis of 364 mTert-GFP+ cells from 250 crypts showed no increase in the low number (2%) of co-positive mTert-GFP+ EdU+ cells between fed and fasted mice (Figure 1D), indicating that the marked increase in the number of mTert-GFP+ cells in response to fasting occurred via a proliferation-independent mechanism.
It has also been suggested that r-ISCs express mTert at high levels (Munoz et al., 2012), raising the possibility that the increase in mTert+ ISCs after fasting may be due to a specific increase in mTert expression in the r-ISC population. To investigate this, we examined the position of mTert-GFP+ cells within the crypts of fed and fasted mice. If fasting induced r-ISCs to express mTert, then the mean distribution of GFP+ cells should shift from the supra-Paneth (+4) position (Montgomery et al., 2011) towards the base of the crypt where r-ISCs are located (Barker et al., 2007). However, analysis of fasted mTert-GFP mice revealed no change in the distribution of GFP+ cells within the crypt, with the peak incidence still at the “+4” position (Figure S1I). To further confirm that fasting did not induce mTert-GFP expression within r-ISCs, mTert-GFP mice were treated with 2 doses of EdU 12 hours apart prior to fasting. Since r-ISCs are rapidly cycling, we reasoned that increased levels of EdU+ mTert-GFP+ cells should be detected if mTert expression was induced within these cells upon fasting. Instead, we observed that less than 4% of fasted mTert-GFP+ cells co-expressed EdU, consistent with the general quiescent nature of these cells (Figure 1E) (Montgomery et al., 2011). As expected, direct analysis of r-ISCs, using Lgr5-GFP-ires-CreER (Lgr5-GFP) reporter mice, showed a significant proportion of fasted Lgr5-GFP+ cells remained EdU+, consistent with their rapid cycling (Figure 1E) (Barker et al., 2007). In addition, there was no significant change in the total number of Lgr5-GFP+ cells with fasting (Figure S1J), however, there was a moderate decrease in the overall fraction that were actively cycling (Figure S1K). Together, these results confirm that the increased number of mTert-GFP+ cells in response to fasting occurs independently of the r-ISC population.
PTEN regulates the fed-fasting transition in d-ISCs
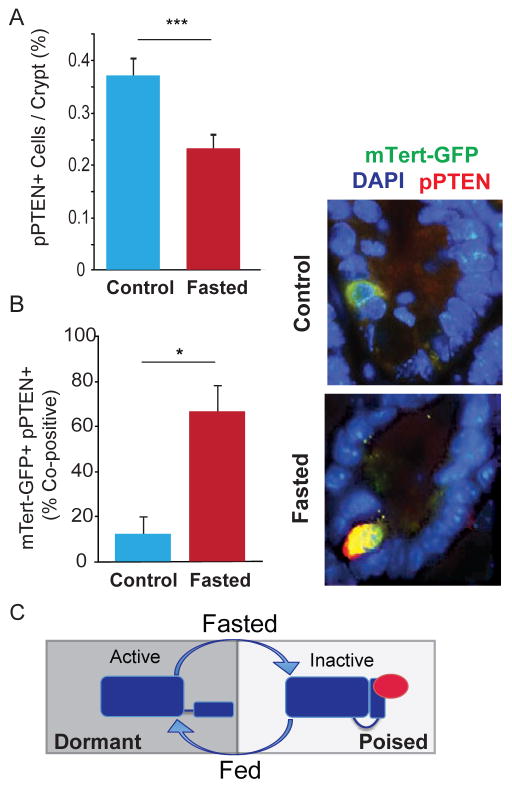
To further investigate the mechanism underlying the increased number of mTert-GFP+ d-ISCs with fasting, we examined the role of PTEN within these quiescent cells. Because PTEN and its inactive isoform, pPTEN, are expressed in slowly cycling “+4” crypt cells (He et al., 2004; Montgomery et al., 2011), and can function as gatekeepers of the fed-fasting transition (Nelson et al., 2014), we reasoned that they may also regulate d-ISCs. Analysis of pPTEN immunostaining in intestinal crypts revealed a general decrease in the total number of pPTEN-expressing crypt cells with fasting (Figure 2A). In contrast, analysis of pPTEN staining specifically within the mTert-GFP+ population revealed a dramatic increase in pPTEN co-staining during fasting, with >60% of all mTert-GFP+ cells co-expressing pPTEN, a >5 fold increase from baseline (Figure 2B, S3A). Together, these results demonstrate selective inactivation of PTEN within the mTert-GFP+ d-ISC population. In addition, given that PTEN is a known negative regulator of telomerase (Kang et al., 1999; Kyo and Inoue, 2002; You et al., 2007; Zhou et al., 2006), inactivation of PTEN likely leads to de-repression of the mTert locus accounting for the increased frequency of mTert-GFP cells with fasting. Finally, consistent with a role for PTEN/pPTEN as gatekeepers of the fed-fasting transition, these results reveal that fasting alters PTEN status in d-ISCs, rendering them functionally poised for lineage contribution upon re-feeding (Fig. 2C model).
FIGURE 2. Fasting leads to PTEN inactivation in d-ISCs.
(A) Percentage of total phospho-PTEN (pPTEN)+ cells per crypt in control (fed) and fasted mice. N= 5, 3. (B) Percentage of mTert-GFP+ crypt cells co-expressing pPTEN in control (fed) and fasted mice. Representative optical sections showing mTert-GFP+ (arrow) and pPTEN+ mTert-GFP+ (arrowhead) cells, 60X. N=6, 3. (C) Schematic illustration showing the dynamic regulation of PTEN by phosphorylation in the fasted and fed states. Mean±SEM, Student’s t-test, *p<0.05, ***p<0.001.
d-ISCs are functionally poised to contribute to regeneration during re-feeding
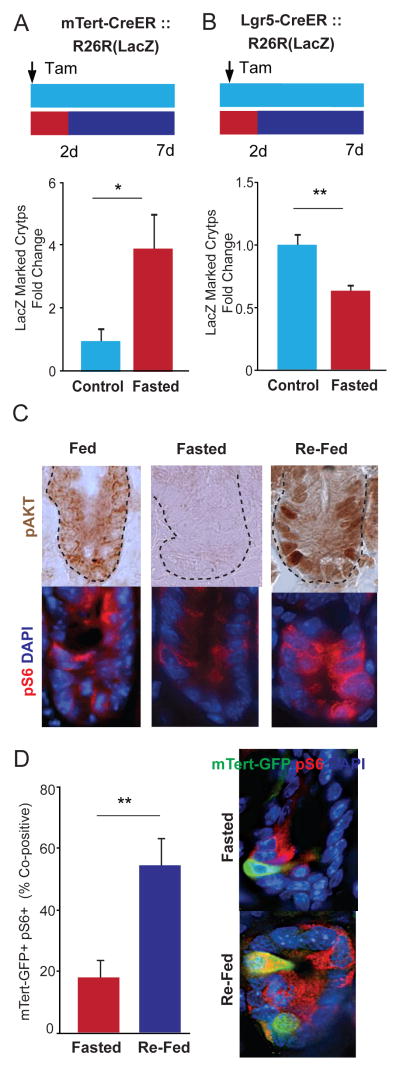
Remarkably, re-feeding for 24 hours was sufficient to reverse the morphological changes seen with fasting, underscoring the enormous regenerative capacity of this tissue (Figures S1B–F). To assess whether poised d-ISCs contribute to this regeneration, we performed lineage-tracing studies using mTert-CreER::R26R(LacZ) reporter mice to specifically label d-ISCs and their progeny (Montgomery et al., 2011). Mice received a single dose of tamoxifen and were either allowed to feed ad libitum for 7 days or were fasted for 48 hours then re-fed for the next 5 days. All marked crypt cells in the proximal 10cm of intestine (~200,000 crypts) were analyzed using our quantitative whole mount LacZ staining assay (Figure S2)(Montgomery et al., 2011). Consistent with the fasting-induced increase in mTert-GFP+ cells (Figures 1B, 1C, S3B), we observed a 4-fold increase in the number of LacZ-marked crypts and villi in fasted mice (Figure 3A, S3C) underscoring their contribution to tissue regeneration. By contrast, lineage-tracing analysis of r-ISCs using Lgr5-GFP-ires-CreER::R26R(LacZ) mice revealed a 40% decrease in LacZ-marked crypts after fasting (Figure 3B). Taken together, these data show that with fasting, d-ISCs become functionally poised to contribute to the intestinal lineage upon re-feeding.
FIGURE 3. d-ISCs show increased lineage contribution following fasting/re-feeding and activation of mTORC1 signaling.
(A) Quantitative whole mount analysis showing change in number of lineage-marked LacZ+ crypts from mTert-Cre::R26R(LacZ) control (fed) versus fasted/re-fed mice. Schematic of treatment regimen is shown above. N=6, 9. (B) Quantitative whole mount analysis showing change in number of lineage-marked LacZ+ crypts from Lgr5-Cre::R26R(LacZ) control (fed) versus fasted/re-fed mice. Schematic of treatment regimen is shown above. N=4, 4. (C) Phospho-AKT (pAKT) and phospho-S6 (pS6) immunostaining in fed, fasted (48h), and re-fed (10h) crypt sections. Representative images showing pAKT (DAB immunohistochemistry) and pS6 (red immunofluorescence), 60X. N= 3, 3. (D) Percentage of mTert-GFP+ crypt cells that co-express pS6 in fasted (48h) and re-fed (10h) mice. Representative optical sections of crypt sections showing mTert-GFP+ pS6− cells (arrows) and an mTert-GFP+ pS6+ cell (arrowhead) in DAPI, mTert-GFP, and pS6 merged channels, 60X. N=3, 3. Mean±SEM, Student’s t-test, *p<0.05, **p<0.01.
d-ISCs demonstrate cell-autonomous induction of mTORC1 signaling upon re-feeding
Because the transition from fasting to re-feeding results in a dramatic induction in circulating insulin levels and canonical PI3K→AKT→mTORC1 signaling, we next sought to investigate the role this pathway plays in the d-ISC response to the return of nutrients. Initially, we confirmed the effect of fasting and re-feeding on the phosphorylation of AKT and S6, a marker for mTORC1 signaling, in intestinal crypts. We detected modest diffuse staining of both phospho-AKT (pAKT) and phospho-S6 (pS6) in fed crypts, a very low level of both in fasted crypts, and a marked induction of both upon re-feeding (Figure 3C). Next, we performed co-immunohistochemistry for mTert-GFP and pS6 to determine whether re-feeding activated this pathway specifically in d-ISCs. In both the fed and fasted states, we observed an overall low level of d-ISC co-staining (GFP+ pS6+) (Figure 3D). With re-feeding however, a significant induction in the fraction (55%) of dual positive (GFP+ pS6+) cells was detected (Figure 3D) demonstrating that d-ISCs undergo cell-autonomous induction of mTORC1 signaling upon re-feeding. This is in contrast to r-ISCs in which mTORC1 signaling in adjacent Paneth cells regulates the response to calorie intake during long-term caloric restriction (Yilmaz et al., 2012). Combined, the above data show that the PI3K→AKT→mTORC1 pathway is altered in d-ISCs upon re-feeding indicating it functions in regulating lineage contribution. Activation of this pathway in d-ISCs was presumably due to the persistence of inactive PTEN (pPTEN+) within these cells in the re-fed state (compare Figure S3D with Figure 2B).
A subpopulation of d-ISCs is sensitive to the re-fed milieu
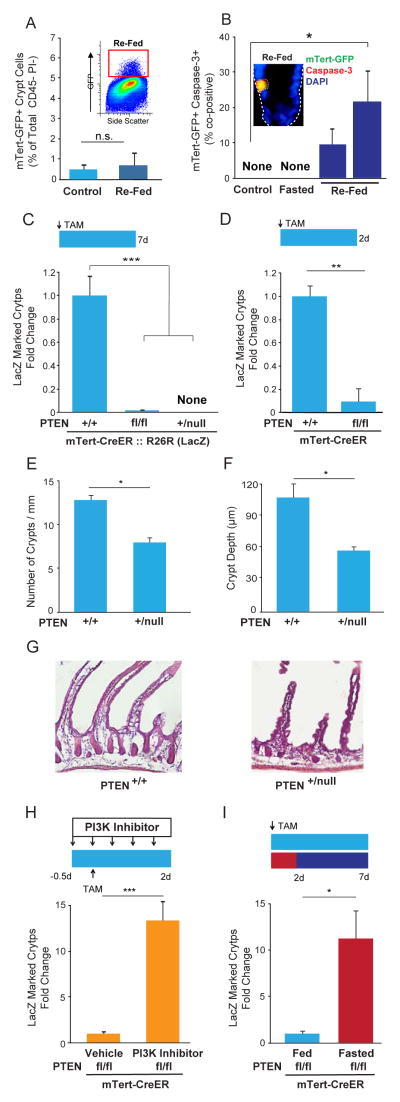
During our analysis of mTORC1 signaling in re-fed d-ISCs, we noted that re-feeding led to an apparent decrease in the overall number of mTert-GFP+ cells. To quantify this, we performed flow cytometric analysis on isolated crypt cells from mTert-GFP mice fasted for 48 hours and then re-fed for 24 hours, which showed a return to baseline levels of mTert-GFP+ cells (Figure 4A). In an effort to understand the mechanism(s) underlying this reduction, we assessed their rate of apoptosis using co-immunostaining for mTert-GFP and activated caspase-3 at 10 and 24 hours after re-feeding. Collectively, nearly 30% of mTert-GFP+ cells were found to be entering apoptosis at these time points, which is particularly notable given the absence of apoptosis in mTert-GFP+ cells from either fed or fasted mice (Figure 4B). These results demonstrate that a subpopulation of d-ISCs are sensitive to the re-fed milieu and suggest a potential negative role for active PI3K→AKT signaling in these cells.
FIGURE 4. PTEN is necessary for d-ISC maintenance and intestinal regeneration but is dispensable when PI3K signaling is low.
(A) Percentage of mTert-GFP+ cells per total CD45−PI− crypt cells from control (fed) and re-fed (24h) mice. Representative re-fed FACS plot showing GFP+ cells in box. N=3, 3. (B) Percentage of mTert-GFP+ crypt cells co-expressing activated caspase-3 in control (fed) (0/39 GFP+ cells), fasted (0/139 GFP+ cells) and re-fed (10, 24h) mice. Representative optical section showing a GFP+ activated caspase-3+ cell (arrow) upon re-feeding, 60X. N= 3, 4, 4, 5. (C) Quantitative whole mount analysis showing change in lineage-marked LacZ+ crypts from mTert-Cre::R26R(LacZ) mice that were PTEN+/+, PTENfl/fl or PTEN+/null 7 days following tamoxifen treatment. Schematic of treatment regimen is shown above. N= 24, 19, 7. (D) Change in lineage-marked LacZ+ crypts from mTert-Cre::R26R(LacZ)::PTEN+/+ and mTert-Cre::R26R(LacZ)::PTENfl/fl mice 2 days after tamoxifen treatment. Schematic of treatment regimen is shown above. N= 4, 5. (E–G) Number of intestinal crypts per mm of intestine (E) and crypt depth (F) in PTEN+/+ (control) and PTEN+/null (G) mice 96 hours following 16Gy irradiation, N=3, 4. Representative H&E image of intestines, 20X. (H) Change in LacZ-marked crypts from mTert-Cre::R26R(LacZ)::PTENfl/fl mice treated with vehicle or PI3K-inhibitor (NVP-BKM120). Schematic of treatment regimen is shown above. N= 5, 4. (I) Change in LacZ marked crypts from mTert-Cre::R26R(LacZ)::PTENfl/fl mice under fed or fasted conditions. Schematic of treatment regimen is shown above. N=7, 9. Mean±SEM, Student’s t-test was used to compare groups of two. One-way ANOVA with Bonferroni post-hoc analysis was used to compare groups of three or more, *p<0.05, **p<0.01, ***p<0.001, n.s. not significant.
PTEN is essential for dormant ISC maintenance and intestinal regeneration
To further investigate both the potential sensitivity of d-ISCs to active PI3K→AKT signaling and the more general role of PTEN in d-ISC maintenance, we next asked how d-ISCs would respond to permanent PTEN loss under control fed conditions. We generated mTert-Cre::R26R(LacZ)::PTENfl/fl mice to conditionally delete PTEN in d-ISCs. In this system, tamoxifen-mediated nuclear translocation of CreER within mTert-expressing d-ISCs leads to concurrent activation of the R26R(LacZ) reporter allele and deletion of the floxed PTEN allele. Remarkably, whole mount LacZ analysis revealed near complete loss of lineage marking in these mice as compared to control mice with functionally intact PTEN (mTert-Cre::R26R(LacZ)::PTEN+/+) one week after tamoxifen administration (Figure 4C). Additional analysis at shorter time points revealed that loss of d-ISCs following PTEN deletion was evident as early as 2 days following tamoxifen induction, indicating a rapid and progressive depletion of these cells in fed animals (Figure 4D). Consistent with this, no LacZ-marked crypts were detected when lineage-tracing studies were performed with mice constitutively haploinsufficient for PTEN (mTert-Cre::R26R(LacZ)::PTEN+/null) (Figure 4C). These results show that PTEN is required for maintenance of the d-ISC population in the baseline fed state.
To functionally assess the impact of d-ISC loss following loss of PTEN, we utilized high dose radiation (16Gy), a model that obliterates r-ISCs (Barker et al., 2012; Marshman et al., 2001; Potten, 2004; Roche et al., 2015) while activating d-ISCs (Marshman et al., 2001; Montgomery et al., 2011; Potten, 2004; Powell et al., 2012; Roche et al., 2015; Yan et al., 2012). PTEN+/+ and PTEN+/null mice were irradiated and intestines were collected 96 hours later, during the peak of the regenerative response (Potten, 2004). To quantitate radiation-dependent damage to the stem cell compartment, intestinal sections were scored using the intestine-specific microcolony assay (Withers and Elkind, 1970). This analysis revealed a ~40% decrease in both crypt number and crypt depth in irradiated PTEN+/null mice as compared to controls (Figure 4E-G), suggesting decreased stem cell function in these mice during recovery from injury. Combined, these data underscore the critical role of PTEN in d-ISC maintenance and intestinal regeneration.
d-ISCs are sensitive to PI3K signaling and the fed milieu
Because PI3K→AKT signaling is negatively regulated by PTEN, we next investigated whether the loss of d-ISCs following conditional PTEN deletion was due to increased activity of this signaling pathway. mTert-CreER::R26R(LacZ)::PTENfl/fl mice were treated with tamoxifen and the selective class I PI3 kinase inhibitor, NVP-BKM120, and analyzed after 2 days. Whole mount LacZ analysis revealed that inhibition of PI3K signaling restored the number of LacZ-marked crypts despite the deletion of PTEN (Figure 4H). These results indicate that the loss of d-ISCs following PTEN deletion is due, in part, to increased PI3K→AKT signaling and indicate that d-ISCs are particularly sensitive to activation of this pathway.
Finally, given the low level of PI3K signaling with fasting (Figure 3C), we investigated whether fasting could rescue the PTEN-dependent loss of d-ISCs. Whole mount LacZ analysis of tamoxifen-treated fasted mTert-CreER::R26R(LacZ)::PTENfl/fl mice showed an increase in LacZ-marked crypts (Figure 4I) to levels comparable to wild-type PTEN mice. Genomic PCR analysis confirmed deletion of the floxed PTEN allele in LacZ-labeled cells (Figure S3E). Taken together, our results indicate that PTEN is required for the maintenance of dormant ISCs when PI3K/AKT signaling is active, but is dispensable when PI3K→AKT signaling is low.
DISCUSSION
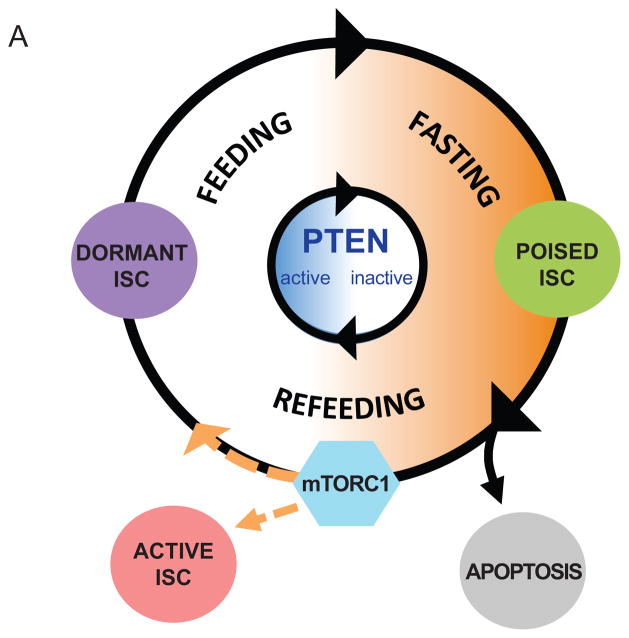
Our data support a model (Figure 5) where fasting leads to the inhibition of PTEN and activation of mTert expression in d-ISCs, rendering them functionally poised to contribute to intestinal regeneration. Repression of PTEN in these cells subsequently allows for cell-autonomous activation of the PI3K→AKT→mTORC1 signaling pathway upon the return of enteral nutrition. With re-feeding, these activated d-ISCs undergo one of three fates: 1) lineage contribution to intestinal renewal as active stem cells, 2) initiation of programmed cell death, or 3) return to dormancy. Together, these events contribute to the homeostatic response and ensure that d-ISC numbers return to a pre-fasting baseline.
FIGURE 5. Model of d-ISC regulation by PTEN and nutrient status.
(A) Model in which fasting leads to the inhibition of PTEN and subsequent activation of mTert expression in d-ISCs, rendering them functionally poised to contribute to intestinal regeneration.
The notion of stem cell activation refers to the transition of a cell from a resting (dormant) state to a metabolically active state whereby cells become functionally poised. The mechanisms underlying stem cell activation, however, remain largely unknown. Recently, Rando and colleagues (Rodgers et al., 2014) described the “Galert state” of stem cell activation, a transitional stage in the cell cycle between Go and G1. In their model, dormant muscle stem cells enter Galert in response to a remote tissue injury; then, depending on the physiological need, these cells either contribute directly to the muscle lineage or return to the dormant state. Our data indicate that d-ISCs behave in a similar fashion in response to the stress of fasting and re-feeding. Moreover, resolution of the Galert state in both dormant muscle stem cells (Rodgers et al., 2014) and d-ISCs is mediated by an mTORC1-dependent mechanism. The extent to which other dormant stem cell populations utilize the Galert state remains to be determined.
The mechanism(s) responsible for maintaining dormant ISC numbers are complex and include factors such as PTEN, which plays a central role in the regulation of numerous cellular processes including survival, proliferation, and energy metabolism (Song et al., 2012). PTEN is best known for its ability to suppress the PI3K→AKT pathway through its lipid phosphatase activity (Song et al., 2012), although increasing evidence also points to an expanding role for phosphatase-independent effects of PTEN on cellular regulation including functions within the nucleus (Lee et al., 1999; Song et al., 2011; Song et al., 2012; Trotman et al., 2007). Here we show that PTEN is required for maintenance of d-ISC numbers under baseline (fed) conditions, that loss of PTEN results in the rapid loss of d-ISCs, and that PTEN is critical for intestinal regeneration following severe injury. Interestingly, both fasting and inhibition of PI3K→AKT signaling prevent the PTEN-mediated loss of d-ISCs, which implies that PTEN is required for the maintenance of dormant ISCs when PI3K→AKT signaling is active, but is dispensable when PI3K→AKT signaling is low. A similar response to the loss of PTEN has also been shown for dormant hematopoietic stem cells (d-HSCs), which initially demonstrate cell cycle activation and enhanced lineage contribution followed by premature exhaustion and stem cell loss (Yilmaz et al., 2006). This effect has been linked, at least in part, to an enhanced rate of protein synthesis within d-HSCs following loss of PTEN (Signer et al., 2014). Analogous results have been shown in the prostate, where complete loss of PTEN leads to a “fail-safe” mechanism of cellular senescence, thus protecting the organism from unrestrained cellular growth (Chen et al., 2005; Papa et al., 2014; Song et al., 2012).
While in most cells active PI3K→AKT signaling is essential for growth and survival, our studies imply that this pathway may have deleterious effects in specific cellular contexts. The evolution of these cellular and molecular mechanisms may represent a conserved strategy to cope with physiological stress in metabolically expensive tissues. A better understanding of these mechanisms will contribute to improved strategies to promote tissue regeneration.
METHODS
Detailed methods and raw data are available in Supplemental Materials.
Statistical Analysis
Two-tailed Student’s t-test was used to compare groups of two and one-way ANOVA with Bonferroni post-hoc analysis was used to compare groups of three or more. Statistical significance was set at p<0.05 unless otherwise noted.
Study Approval
All animal procedures were approved by the Boston Children’s Hospital IACUC.
Supplementary Material
Acknowledgments
We thank members of the Breault laboratory, J. Majzoub, W. Lencer, M. White, M. Neutra, R. Grand, S. Bonner-Weir, N. Kalaany, D. Esposito, P. Karpowicz, X. He and L. Cantley for helpful discussions. H. Wu provided the PTENflox mice. This research was supported by NIH F32DK091995, T32DK007477, 5K12HD5289610, Harvard Medical School Shore Fellowship, Boston Children’s Hospital Wolpow/Rubin IBD Fellowship, NASPGHAN Nestlé Nutrition Award, and HDDC Pilot & Feasibility Award (to CAR), F32DK107108 (to MSS), R01DK084056 and HSCI Junior Faculty Award, the Timothy Murphy Fund, the IDDRC P30HD18655 and the HDDC P30DK034854 (to DTB).
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
Author Contributions
CAR, RKM, DLC and DTB designed the experiments. CAR, MSS, LTD, DCT, HT, DMA, LJ, BBW, HDR, RKM, and AT carried out the experiments. CAR and DTB analyzed the data, and CAR, DLC, and DTB wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello LWP. The Expensive-Tissue Hypothesis. Current Anthropology. 1995;36:199–221. [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ, Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci USA. 2008;105:10420–10425. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlone DL, Breault DT. Tales from the crypt: the expanding role of slow cycling intestinal stem cells. Cell Stem Cell. 2012;10:2–4. doi: 10.1016/j.stem.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell VL, Thompson MD, Jeschke MG, Chung DH, Thompson JC, Wolf SE. Effects of incremental starvation on gut mucosa. Dig Dis Sci. 2003;48:765–769. doi: 10.1023/a:1022849112100. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci USA. 2011;108:18702–18707. doi: 10.1073/pnas.1109348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Dunel-Erb S, Chevalier C, Laurent P, Bach A, Decrock F, Le Maho Y. Restoration of the jejunal mucosa in rats refed after prolonged fasting. Comparative biochemistry and physiology. 2001;129:933–947. doi: 10.1016/s1095-6433(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Nguyen H, Shroyer N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat Rev Mol Cell Biol. 2015;16:299–309. doi: 10.1038/nrm3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009;11:687–694. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Santiago PM, di Tomaso E, Sullivan JM, Hou WS, Dayton T, Jeffords LB, Sodha P, Mercer KL, Cohen R, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S, Inoue M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene. 2002;21:688–697. doi: 10.1038/sj.onc.1205163. [DOI] [PubMed] [Google Scholar]
- Langlois MJ, Roy SA, Auclair BA, Jones C, Boudreau F, Carrier JC, Rivard N, Perreault N. Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB journal. 2009;23:1835–1844. doi: 10.1096/fj.08-123125. [DOI] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Marshman E, Ottewell PD, Potten CS, Watson AJ. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol. 2001;195:285–292. doi: 10.1002/path.967. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. Embo J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson OL, Jansen HT, Galbreath E, Morgenstern K, Gehring JL, Rigano KS, Lee J, Gong J, Shaywitz AJ, Vella CA, et al. Grizzly Bears Exhibit Augmented Insulin Sensitivity while Obese Prior to a Reversible Insulin Resistance during Hibernation. Cell Metab. 2014;20:376–382. doi: 10.1016/j.cmet.2014.07.008. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A, Wan L, Bonora M, Salmena L, Song MS, Hobbs RM, Lunardi A, Webster K, Ng C, Newton RH, et al. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell. 2014;157:595–610. doi: 10.1016/j.cell.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiation research. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. SOX9 Maintains Reserve Stem Cells and Preserves Radio-resistance in Mouse Small Intestine. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AH, Gericke A. Phosphorylation keeps PTEN phosphatase closed for business. Proc Natl Acad Sci U S A. 2009;106:1297–1298. doi: 10.1073/pnas.0812473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor SM, Stein ED, Diamond J. Rapid upregulation of snake intestine in response to feeding: a new model of intestinal adaptation. Am J Physiol. 1994;266:G695–705. doi: 10.1152/ajpgi.1994.266.4.G695. [DOI] [PubMed] [Google Scholar]
- Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Signer RA, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature. 2014;509:49–54. doi: 10.1038/nature13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S, Tang D, Morita Y, Sperka T, Omrani O, Lechel A, Sakk V, Kraus J, Kestler HA, Kuhl M, et al. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. Embo J. 2015;34:624–640. doi: 10.15252/embj.201490700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. International journal of radiation biology and related studies in physics, chemistry, and medicine. 1970;17:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- You Y, Geng X, Zhao P, Fu Z, Wang C, Chao S, Liu N, Lu A, Gardner K, Pu P, et al. Evaluation of combination gene therapy with PTEN and antisense hTERT for malignant glioma in vitro and xenografts. Cellular and molecular life sciences: CMLS. 2007;64:621–631. doi: 10.1007/s00018-007-6424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Bae-Jump VL, Whang YE, Gehrig PA, Boggess JF. The PTEN tumor suppressor inhibits telomerase activity in endometrial cancer cells by decreasing hTERT mRNA levels. Gynecologic oncology. 2006;101:305–310. doi: 10.1016/j.ygyno.2005.10.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.