Abstract
The Ebola epidemic in West Africa has caused significant morbidity and mortality. The outbreak has also disrupted health care services, including childhood vaccinations, creating a second public health crises. We project that after 6–18 months of disruptions, a large connected cluster of children unvaccinated for measles will accumulate across Guinea, Liberia, and Sierra Leone. This pool of susceptibility increases the expected size of a regional outbreak from 127,000 to 227,000 cases after 18 months, resulting in 2,000–16,000 additional deaths (comparable to the numbers of Ebola deaths reported thus far). There is a clear path to avoiding outbreaks of childhood vaccine-preventable diseases once the threat of Ebola begins to recede: an aggressive regional vaccination campaign aimed at age groups left unprotected due to health care disruptions.
The current Ebola crisis in West Africa is one of the worst public health disasters in recent memory, having caused over 21,000 cases and 8,400 deaths as of the time of writing and raising the specter of a broader international crisis (1). However, there are signs of hope. Evidence shows that the number of cases is declining in Liberia (2), and sustained transmission has been confined to Guinea, Liberia, and Sierra Leone, despite several trans-national introductions including recent transmission in Mali. Stopping Ebola would be a triumph for the global health community and the public health agencies of the affected countries. But even after the last Ebola case recovers, the disruptions of local health systems caused by the outbreak could lead to a second infectious disease crisis that could kill as many as, if not more, than the original outbreak.
Through the combination of the WHO Expanded Programme on Immunization (EPI) and periodic supplemental immunization campaigns, annual childhood deaths from vaccine-preventable diseases have dropped from an estimated 0.9 million in 2000 to 0.4 million in 2010 (3). Measles is emblematic of this success; globally, estimated annual measles mortality has decreased from 499,000 to 102,000 since the year 2000 (4, 5). The Ebola affected countries have been an important part of this achievement: the three countries reported nearly 93,685 cases of measles in the decade between 1994 and 2003 (despite Sierra Leone not reporting in 4 years), and only 6,937 between 2004 and 2013 (in both periods it is likely that only a fraction of measles cases were reported to the WHO) (6). Despite this success, measles susceptibility has been growing in all three countries in recent years, and each planned a measles vaccination campaign prior to the Ebola outbreak.
Measles epidemics often follow humanitarian crises. Measles is one of the most transmissible infections, and immunization rates tend to be lower than for other EPI vaccines, due in part to the older age at which measles vaccine must be administered (9 months versus 6 weeks or younger for the first dose of other vaccines (7)). For this reason, explosive measles outbreaks are often an early result of health system failure. Outbreaks have followed disruptions due to war (e.g., the current conflict in Syria (8)), natural disasters (e.g., the eruptions of Mt. Pinatubo in 1991 (9)), and political crises (e.g., Haiti in the early 1990s (10)). The effects are most acute when measles epidemics are associated with famine or long term national instability: a survey of 595 households displaced due to the Ethiopian famine in 2000 found measles to be a contributing cause in 35 of 159 deaths (11), and after years of instability in the Demographic Republic of Congo the country experienced a measles outbreak of 294,455 cases and 5,045 deaths between 2010 and 2013 (12).
To understand how Ebola related health care disruptions are increasing the risk from measles, we estimated the spatial distribution of unvaccinated children and the measles susceptibility profile for each country before and after these disruptions. Geo-located, cross-sectional data from Demographic Health Surveys (DHS) in Guinea, Liberia, Sierra Leone, and surrounding countries was used to estimate vaccine coverage in each 5 km × 5 km grid cell using a hierarchical Bayesian model and spatial smoothing techniques. These rates were applied to spatially explicit data on population and birth cohort size to map the number of children between 9 months and 5 years of age who were unvaccinated against measles before Ebola related health care disruptions (Figure 1A) (13, 14). Forward projections of the number of unvaccinated children after 6, 12, and 18 months were generated by reducing the rate of routine vaccination by 75% for the specified duration (reductions of 25%, 50%, and 100% were also considered as a sensitivity analysis). Full population susceptibility on a national level at baseline and after 18 months of disruptions were then estimated by combining these estimates with the results of models which estimate the immune profile in each age cohort based on their experience of routine immunization, supplemental immunization activities (SIAs), and natural infection (using techniques from Simons et al. (5) and data reported to the WHO (6)). The expected size of any regional, post-disruption measles outbreak was then calculated using a phenomenological model of the previously observed relationship between population susceptibility and measles outbreak attack rates in the region. Finally, we estimated the number of resulting deaths by applying estimates of the case fatality ratio (CFR) for outbreak settings to the overall attack rates (15). Full methodological details are available in the supplement.
Figure 1.
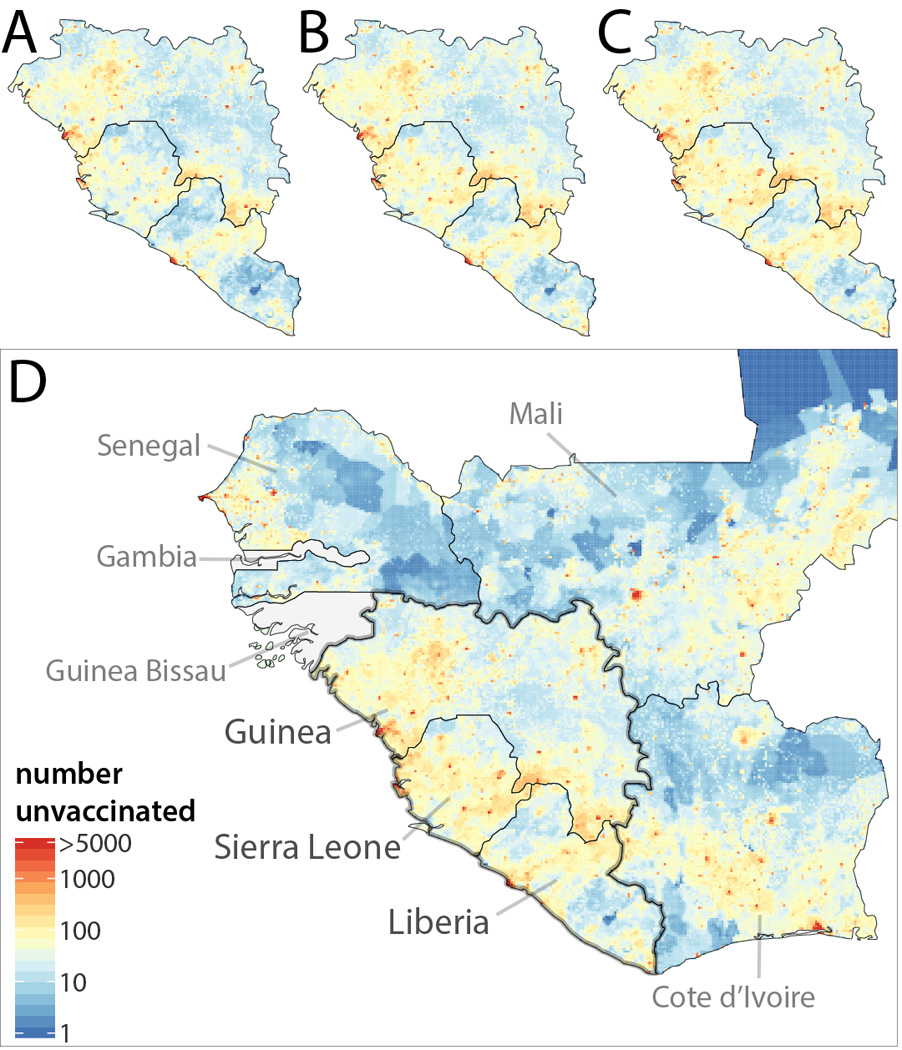
Number of children 9 to 59 months of age not vaccinated against measles in Guinea, Liberia, and Sierra Leone (A) before disruptions in vaccination due to Ebola, (B) after 6 months of a 75% reduction in measles vaccination rates nationally due to Ebola related health care disruptions, (C) after 12 months of disruptions, and (D) after 18 months of disruptions with numbers of unvaccinated children (without disruptions) in neighboring countries shown.
We estimate that at the start of the Ebola crisis there were 778,000 (95% Credible Interval (CrI): 715,00–915,000) unvaccinated children in the three countries (Figure 1A). These children reside in a large contiguous cluster that crosses national boundaries. With every month of health care disruptions, we estimate that the number of children between 9 months and 5 years of age who are not vaccinated against measles increases by an average of 19,514 (assuming a 75% reduction in vaccination rates nationally), reaching 964,346 (95% CrI: 862,682–1,129,026) after 6 months (Figure 1B), 1,068,833 (95% CrI: 914,108–1,288,857) after 12 months (Figure 1C), and 1,129,376 (95% CrI: 934,926–1,409,052) after 18 months (Figure 1D). The results of variations in the spatial distribution and magnitude of disruptions in vaccination are shown in the supplement. This growing cluster of measles susceptibility abuts non-Ebola affected countries (e.g., Cote d’Ivoire) and regional cross border migration is frequent (16, 17), thus placing their populations at risk (Figure 1D).
This increase in unvaccinated children occurs on top of an already growing pool of measles susceptibility in the three countries (Figure 2) resulting from years of suboptimal routine vaccination (6). After 18 months of disruptions, a large cohort of susceptible children will have entered the population. This growth in susceptibility increases the risk and projected size of regional measles outbreaks. If vaccination had continued at pre-Ebola rates, a generalized measles outbreak would have caused 126,868 (plausible range: 84,833–181,769) cases. However, the projected outbreak size increases to 227,484 (153,458–321,702) cases after 18 months of disruptions, resulting in a projected 5,209 (1,757–16,173) additional deaths from measles (15). Measles mortality could be at the high end of this range due to the limited health care services and increased prevalence of malnutrition and vitamin A deficiency associated with the Ebola outbreak (18).
Figure 2.
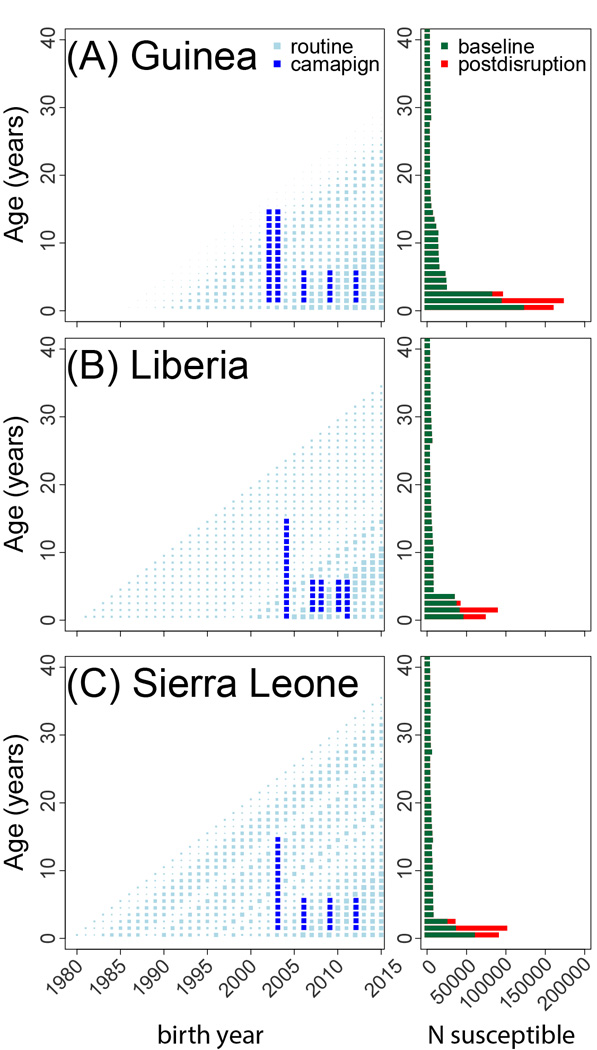
The left panel of each figure indicates the history of routine vaccination (light blue squares, size indicates coverage) and SIA campaigns (dark blue squares) for each age cohort by year in (A) Guinea, (B) Liberia, and (C) Sierra Leone. The right panel indicates the projected number susceptible to measles in each age cohort from 0 to 40 years of age: projected 2015 age distribution of measles susceptibility if no Ebola related disruptions to vaccination occurred (green) and after 18 months of Ebola related health care disruptions (red).
The uncertain and rapidly changing Ebola situation in West Africa limits data availability and necessitates numerous analytic assumptions. Our assumption of a 75% reduction in routine vaccination rates is consistent with surveys of health care providers (19, 20) and the qualitative assessment of those who have been involved in the response; however, as there is no reliable information on current vaccination rates, we have considered reductions of 25%, 50%, and 100% in sensitivity analyses. An unusual age distribution of measles cases, poor or high quality medical care, and other factors may lead to CFRs that are higher or lower than the range used here, though our estimates are based on a comprehensive assessment of measles CFRs in outbreaks (15). There may be pre-existing pockets of susceptibility in older individuals not detected by our analysis for a variety of reasons (e.g., over-reporting of vaccination), hence campaign planning should not solely be based on the effects of the Ebola epidemic. The choice to project no further than 24 months into the future, and to focus on 18 months as the primary analysis, is necessarily somewhat arbitrary; however, it is unlikely that the region will remain measles free if disruptions continue much beyond 2 years and there is some evidence that the Ebola situation is beginning to improve (2).
Measles vaccine is just one of several childhood vaccines for which distribution may be limited by the Ebola outbreak. Similar reductions in the rate of vaccination would increase the number of children not receiving a pentavalent vaccine (PCV), Bacillus-Calmette-Guérin (BCG), and oral polio vaccine (OPV) by about 600,000 to 700,000. These setbacks have the potential to erode the substantial gains in the control of these diseases over recent decades, and a large population of children susceptible to poliovirus infection could threaten the Global Polio Eradication Initiative, should wild poliovirus be re-introduced.
Epidemics of measles are often and early result of interruptions in the delivery of public health services. However, childhood infections are not the only, or even the worst, potential health consequences of disruptions in the health care system. Malaria is the biggest infectious killer in sub-Saharan Africa, and lack of vector control interventions and proper diagnosis and treatment can increase malaria morbidity and mortality (these concerns have prompted Médecins Sans Frontières to conduct mass drug administration of anti-malarial drugs in some areas (21)). Provision of care to individuals with chronic infections, such as human immunodeficiency virus (HIV) and tuberculosis, are negatively impacted by the Ebola outbreak. Non-infectious causes of death increase significantly when medical care is not available (e.g., if maternal mortality rates rose to year 2000 levels, there would be an additional 4,454 deaths for each year of health care disruptions).
However, childhood vaccine-preventable diseases are an area where there is a clear, relatively inexpensive, and one-time intervention that could erase the impact of Ebola related health care disruptions. Coordinated campaigns across the three Ebola affected countries (and possibly neighboring countries) targeting those children who likely missed critical routine vaccinations during the Ebola epidemic with measles and polio vaccines, and potentially other life-saving childhood vaccines, could thwart a second public health disaster and avoid nearly 12,000 deaths from measles alone. Such a campaign should not only target those age groups likely missed during the Ebola crisis (children from birth to an age equal to the length of health care disruptions plus one year of age, see Figure 2), but also those groups where measles susceptibility was already suspected to be on the rise. Hence, at the very least a campaign should target children 6 months to 5 years of age (typical of the age range targeted by follow-up SIAs), perhaps extending the lower age range and administering childhood vaccines other than measles vaccine to the youngest children (e.g., those younger than the length of health care disruptions). The need for and logistics of simultaneous deployment of additional key public health interventions such as vitamin A supplementation and insecticide treated bed-nets should also be considered. Planning for such a vaccine campaign should begin now so that we can respond quickly when the situation stabilizes and minimize the time at risk for disease outbreaks. The growing pool of susceptible individuals in Guinea, Liberia, and Sierra Leone may place bordering countries at increased risk for a measles outbreak; they too may have suffered disruptions to their health care systems due to a focus on the threat from Ebola. These countries should carefully assess the performance of their vaccination programs, and consider campaigns to shore up any gaps.
Supplementary Material
ACKNOWLEGEMENTS
The authors would like to thank Matt Hanson for helping to organize the assessment of the impact of Ebola on measles vaccination and for motivating this work, and Wei Hao for helpful discussion in preparation of the manuscript.
FUNDING
This work is funded by the Bill & Melinda Gates Foundation (OPP1094793), the Science and Technology Directorate, Department of Homeland Security contract HSHQDC-12-C-00058 (B.T.G., C.J.E.M.), the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security and the Fogarty International Center, National Institutes of Health (C.J.E.M., B.T.G., A.J.T.). J.L. is supported by funding from NIH National Institute of Allergy and Infectious Disease (R01 AI102939). A.J.T. is supported by funding from NIH/NIAID (U19AI089674), and the Bill & Melinda Gates Foundation (OPP1106427, 1032350).
Footnotes
DHS data are made freely available by the DHS program to those filing a request (22). WorldPop project demographic data may be obtained from www.worldpop.org.uk. Data on measles incidence, routine vaccination coverage, and SIAs are available at www.who.int/immunization/monitoring_surveillance/data/en/. Ebola incidence data was extracted from publically available situation reports (23–25).
REFERENCES
- 1.World Health Organization. Ebola Situation report. 2015 (available at http://www.who.int/csr/disease/ebola/situation-reports/en/).
- 2.Nyenswah TG, et al. Evidence for declining numbers of Ebola cases — Montserrado County, Liberia, June-October 2014. MMWR. 2014 Nov 14;63:1–5. (Early release) [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global vaccine action plan 2011–2020. 2013
- 4.Liu L, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2014:6736. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 5.Simons E, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012;379:2173–2178. doi: 10.1016/S0140-6736(12)60522-4. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Data, statistics and graphics. 2015 (available at http://www.who.int/immunization/monitoring_surveillance/data/en/).
- 7.World Health Organization. WHO recommendations for routine immunization - summary tables. (available at http://www.who.int/immunization/policy/immunization_tables/en/).
- 8.Sharara SL, Kanj SS. War and infectious diseases: challenges of the Syrian civil war. PLoS Pathog. 2014;10:e1004438. doi: 10.1371/journal.ppat.1004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surmieda MR, et al. Surveillance in evacuation camps after the eruption of Mt. Pinatubo, Philippines. MMWR. CDC Surveill. Summ. 1992;41:9–12. [PubMed] [Google Scholar]
- 10.UNICEF. Sanctions: Children hard hit in Haiti. (available at http://www.unicef.org/sowc96/dsanctns.htm).
- 11.Salama P, et al. Malnutrition, measles, mortality, and the humanitarian response during a famine in Ehiopia. JAMA. 2001;286:563–571. doi: 10.1001/jama.286.5.563. [DOI] [PubMed] [Google Scholar]
- 12.Mancini S, et al. Description of a large measles epidemic in Democratic Republic of Congo, 2010–2013. Confl. Health. 2014;8:9. doi: 10.1186/1752-1505-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linard C, Gilbert M, Snow RW, Noor AM, Tatem AJ. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS One. 2012;7:e31743. doi: 10.1371/journal.pone.0031743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatem AJ, et al. Mapping for maternal and newborn health: the distributions of women of childbearing age, pregnancies and births. Int. J. Health Geogr. 2014;13:2. doi: 10.1186/1476-072X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int. J. Epidemiol. 2009;38:192–205. doi: 10.1093/ije/dyn224. [DOI] [PubMed] [Google Scholar]
- 16.Wesolowski A, et al. Commentary: Containing the Ebola Outbreak - the Potential and Challenge of Mobile Network Data. PLoS Curr. 2014 doi: 10.1371/currents.outbreaks.0177e7fcf52217b8b634376e2f3efc5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia AJ, Pindolia DF, Lopiano KK, Tatem AJ. Modeling internal migration flows in sub-Saharan Africa using census microdata. Migr. Stud. 2014:1–22. [Google Scholar]
- 18.McFerron W. Ebola Stokes Liberian Food Shortage as Farmers Eat Seeds. Bloom. News. 2014 (available at http://www.bloomberg.com/news/2014-11-20/ebola-stokes-liberian-food-shortage-as-farmers-eat-seeds.html). [Google Scholar]
- 19.Epicentre. Cross-sectional survey on health care capacity and utilization, safety and hygiene measures available in health structures, and attack rate among health facility staff during the Ebola outbreak, Monrovia, Liberia, August 2014. 2014 [Google Scholar]
- 20.Bolkan HA, Bash-Taqi DA, Samai M, Gerdin M, von Schreeb J. Ebola and Indirect Effects on Health Service Function in Sierra Leone. PLoS Curr. 2014;1 doi: 10.1371/currents.outbreaks.0307d588df619f9c9447f8ead5b72b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Médecins Sans Frontières. MSF Begins Malaria Program in Ebola-Ravaged Monrovia, Liberia. 2015 (available at http://www.doctorswithoutborders.org/article/msf-begins-malaria-program-ebola-ravaged-monrovia-liberia).
- 22.ICF International. Demographic and Health Surveys (various) Calverton, Maryland: ICF International; 2014. (available at http://dhsprogram.com/data/). [Google Scholar]
- 23.Ambassade de France à Conakry. Ebola : point de situation au 16 janvier 2015. 2015 (available at http://www.ambafrance-gn.org/Ebola-point-de-situation-au-16-1040).
- 24.L. M. of Health. Liberia Ebola Daily Sitrep no. 242 for 12th January 2015. 2015 (available at http://www.mohsw.gov.lr/documents/Sitrep 242 Jan 12th 2015.pdf).
- 25.Sierra Leone National Ebola Response Centre (NERC) EBOLA OUTBREAK UPDATES---January 16, 2015. 2015 (available at http://health.gov.sl/wp-content/uploads/2015/01/Ebola-Update-January-16-2015.pdf).
- 26.Stan Development Team. RStan: the R interface to Stan, Version 2.5. 2014 (available at http://mc-stan.org/rstan.html).
- 27.Lessler J, Metcalf CJE, Grais RF, Luquero FJ, Cummings DAT, Grenfell BT. Measuring the performance of vaccination programs using cross-sectional surveys. PloS Medicine. 2011;8:e1001110. doi: 10.1371/journal.pmed.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjørnstad ON, Finkenstadt B, Grenfell BT. Endemic and epidemic dynamics of measles: Estimating epidemiological scaling with a time series SIR model. Ecological Monographs. 2002;72:169–184. [Google Scholar]
- 29.Chen S, Fricks J, Ferrari MJ. Tracking measles infection through non-linear state space models. J R Stat Soc Ser C Appl Stat. 2011;61:117–134. [Google Scholar]
- 30.World Health Organization. WHO recommendations for routine immunization - summary tables. (available at http://www.who.int/gho/maternal_health/countries/en/).
- 31.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. (available at http://www.R-project.org/) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


